Abstract
Introduction
Dose escalation and reduction of biologic treatments are frequent in clinical practice. The aim of this systematic review is to summarise evidence on dose adjustment of biologic treatments for moderate-to-severe plaque psoriasis in the real-world.
Methods
A systematic review of real-world evidence on dose adjustment of biologics for plaque psoriasis was performed. Searches were conducted in BIOSIS Previews®, Embase®, International Pharmaceutical Abstracts, MEDLINE®, and SciSearch® in March 2020. Real-world studies that reported biologic dose adjustment for moderate-to-severe plaque psoriasis were included.
Results
The search identified 162 papers, and 20 studies with 30,912 patients were included from 2014 to 2020. More studies reported on dose escalation than dose reduction. For adalimumab, 3–54% of patients had dose reduction while 0–37% had dose escalation. For infliximab, only two studies reported a dose reduction, with rates of 22–29%, while dose escalation rates varied from 14 to 67%. Dose reduction rates of 5–49% were reported for etanercept while 0–55% of patients had doses escalated. For ustekinumab, dose escalation and reduction rates ranged from 3 to 37% and 7 to 42%, respectively. Two studies reported on dose adjustment for secukinumab; in one 52% of patients initiated on 150 mg instead of the recommended 300 mg, while another reported no dose increase.
Conclusions
Dose adjustment of biologics for psoriasis is common, with escalation more frequently reported than reduction. Dose escalation may have economic and safety consequences, while dose reduction may impact efficacy. These aspects are important to consider when making decisions on treatment dosing.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-021-00559-z.
Keywords: Plaque psoriasis, Dosing, Adalimumab, Etanercept, Infliximab, Secukinumab, Ustekinumab
Key Summary Points
| This systematic review summarised evidence on the use of dose adjustment of biologic treatments for moderate-to-severe plaque psoriasis in the real-world setting |
| More studies reported on dose escalation than dose reduction: |
| For adalimumab, 3–54% of the patients had a dose reduction while 0–37% had a dose escalation |
| For infliximab, only two studies reported a dose reduction with rates of 22–29% while dose escalation rates varied from 14 to 67% |
| Dose reduction rates of 5–49% were seen for etanercept while 0–55% of patients had their dose escalated |
| For ustekinumab, the dose escalation and reduction rates ranged from 3–37% and 7–42%, respectively |
| Only two studies reported on dose adjustment for secukinumab; one reported that 52% of patients initiated on a lower dose of 150 mg instead of the recommended 300 mg while one reported no dose increase with secukinumab |
| Dose escalation and reduction of biologic treatments is a frequent occurrence in clinical practice. Dose escalation will typically have economic consequences and safety may be negatively impacted, while dose reduction may have negative consequences on efficacy. These aspects are important to consider when making decisions on treatment dosing |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14627169.
Introduction
Biologic treatments have resulted in significant improvements in outcomes for patients with moderate-to-severe plaque psoriasis [1, 2]. Several biologics are now available and include the tumour necrosis factor (TNF)-α inhibitors, adalimumab, etanercept and infliximab, and the interleukin (IL)-12/23 inhibitor, ustekinumab. More recently, IL-23 inhibitors, including guselkumab, tildrakizumab, and risankizumab, and IL-17 inhibitors, including secukinumab and ixekizumab, which bind to the IL-17 ligand, and brodalumab, which targets the IL-17-receptor A, have been approved. IL-17 and IL-23 inhibitors have shown improved efficacy compared with TNF-α inhibitors or ustekinumab in clinical trials with complete skin clearance now considered a realistic goal for many patients [3–6].
Standard approved dosing regimens of these targeted biologic agents have been established in clinical trials. However, real-world experience indicates that alternative off-label dosing regimens are frequently used and that dose adjustment of biologic treatments is a frequent occurrence in clinical practice [7–9]. Dose escalation may entail increasing the medication given per single dose or shortening the interval between doses. This is most often done in an effort to address an inadequate primary response or because of a secondary loss of response over time, which may be related to the development of anti-drug antibodies. Dose escalation has been reported to result in improved efficacy with some biologic treatments [10]. However, higher doses may be associated with an increased risk of treatment-related adverse events. The prolonged use of increased doses may also represent a significant economic burden, since dose escalation strategies may have a considerable impact on treatment costs.
Physicians may also consider dose reduction by lowering the medication per dose or lengthening the dosing interval. This may be done to identify the lowest effective dose that will maintain a clinical response, typically in patients who have an adequate sustained response. This may help to minimize the risk of adverse events or may be done in response to other concurrent events, such as the need for surgery with the risk of infection or vaccination. Dose reduction may also be motivated by a desire or need to reduce treatment costs. However, dose reduction has been associated with the risk of decreased efficacy [10].
Clinical guidelines recommend dose escalation in patients who have an inadequate primary response that may be due to insufficient drug exposure, although this may be associated with an increased risk of infection, and, depending on the drug, may be off-licence and not funded [11]. However, knowledge of the extent to which dose adjustment is used in clinical practice is limited. The aim of this systematic review was to summarise evidence on the use of dose adjustment of biologic treatments for moderate-to-severe plaque psoriasis in the real-world setting.
Methods
A systematic review of real-world evidence on dose adjustment of biologics for plaque psoriasis was performed. Searches were conducted in BIOSIS Previews®, Embase®, International Pharmaceutical Abstracts, MEDLINE®, and SciSearch® on March 4, 2020. The search was limited to English language publications with no limits regarding publication date. Conference abstracts were excluded. The search strategy is shown in the supplementary material. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Inclusion criteria were real-world studies that reported dose adjustment for adult patients with moderate-to-severe plaque psoriasis treated with an approved biologic (adalimumab, brodalumab, etanercept, guselkumab, infliximab, ixekizumab, risankizumab, secukinumab, tildrakizumab, or ustekinumab). Dose adjustment was defined as any change in dose or dosing interval from the product prescribing information (Table 1) and there was no limit regarding study duration/length of follow-up. Titles were screened and abstracts were assessed for inclusion by one reviewer, with a second reviewer independently performing a quality assurance check of 40% of records. Full texts were independently assessed for inclusion by two reviewers. Reference lists of all included studies were also reviewed to identify any additional relevant publications. Studies were excluded if no doses were reported, dose adjustment was not included per biologic, it was not clear how many patients were included, or dose was not reported for a specific number of patients. Dose adjustment rates were extracted with data extraction being carried out by one reviewer and checked by a second reviewer.
Table 1.
Approved dosing regimen
| Product | Recommended dosing regimen |
|---|---|
| Adalimumab | 80 mg starting dose, followed after 1 week by 40 mg every 2 weeks. Beyond 16 weeks, patients with inadequate response to 40 mg every 2 weeks may benefit from an increase in dosage to 40 mg every week or 80 mg every 2 weeks |
| Etanercept | 25 mg twice weekly OR 50 mg once weekly OR 50 mg twice weekly for 12 weeks followed by 25 mg twice weekly OR 50 mg once weekly for up to 24 weeks. Treatment should be discontinued in patients who show no response after 12 weeks |
| Infliximab | 5 mg/kg starting dose, then 5 mg/kg after 2 weeks, followed by 5 mg/kg after 4 weeks, then 5 mg/kg every 8 weeks. Treatment should be discontinued in patients who show no response after 14 weeks |
| Secukinumab | 300 mg once weekly for 5 weeks, then maintenance 300 mg every month. Each 300 mg dose is given as two injections of 150 mg |
| Ustekinumab |
For bodyweight < 100 kg: Initially 45 mg, then 45 mg after 4 weeks, then 45 mg every 12 weeks For bodyweight ≥ 100 kg: Initially 90 mg, then 90 mg after 4 weeks, then 90 mg every 12 weeks |
Results
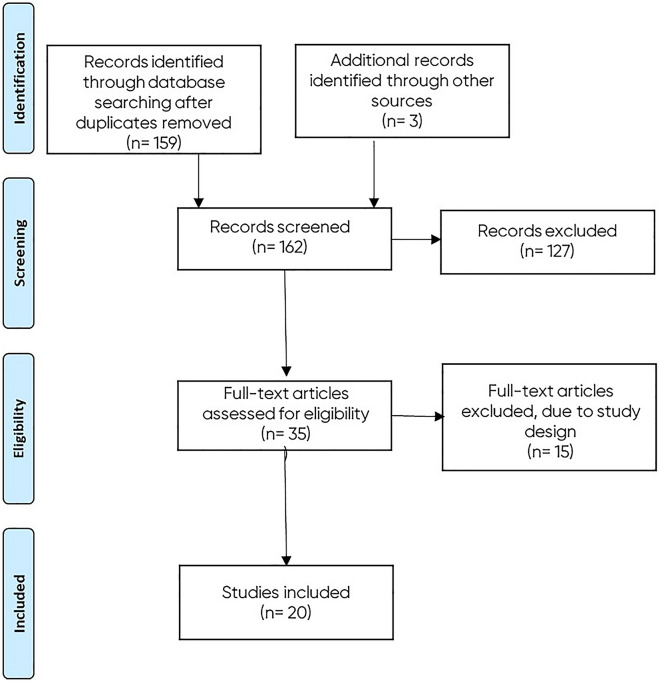
A total of 159 records were identified in the initial database searches (after removal of duplicates). Of these, 32 full-text articles were reviewed for inclusion, and three additional records were identified. Fifteen of these 35 were excluded because of their study design (Fig. 1).
Fig. 1.
Flow chart of included studies
Twenty studies were included from 2014 to 2020 reporting on a total of 30,912 patients [7–9, 12–28]. Studies were conducted in the USA (n = 9), Spain (n = 3), Denmark (n = 2), Italy (n = 2), UK (n = 2), Canada (n = 1), and The Netherlands (n = 1). Details of these 20 studies are summarised in Table 2. Patient characteristics are reported in Supplementary Table 1.
Table 2.
Summary of included studies
| References | Study design (database) | Patients (N) | Dose adjustment definition | Treatments | Dose escalation (%) | Dose reduction (%) |
|---|---|---|---|---|---|---|
| Bewley (UK) [12] | Retrospective cohort study (Quintiles IMS Hospital Treatment Insights database) | 362 | > 30% increase in the average daily dose or decrease in the dosing interval compared with the posology in UK SPC | ETA (n = 60) | 20 | NR |
| UST (n = 44) | 18 | NR | ||||
| INF (n = 83) | 28 | NR | ||||
| ADA (n = 175) | 14 | NR | ||||
| Cai (USA) [13] | Retrospective Claims study (HealthCore Integrated Research database) | 374 | Higher dose than index biologic | UST (n = 119) | 6.9 | 2.7 |
| Cao (USA) [14] | Retrospective, observational study (Truven Health MarketScan Commercial Claims and Encounters and Medicare Supplemental Coordination of Benefits databases) | 1000 | Change from 45 to 90 mg or 90 to 45 mg | UST (n = 1000) | 19.3 | 5.1 |
| Carrascosa (Spain) [7] | Observational, cross-sectional study (BIOBADADERM registry) | 637 | Higher or lower than EMA | ETA (n = 126) | 7.9 | 33.3 |
| UST (n = 230) | 10.4 | 30.9 | ||||
| INF (n = 51) | 13.7 | 29.4 | ||||
| ADA (n = 230) | 2.2 | 41.3 | ||||
| Carter (USA) [15] | Retrospective study (Truven Health MarketScan Commercial Claims and Encounters and Medicare Supplemental Databases) | 7527 |
UST: higher than the patients’ initial dose ADA and ETA: average weekly dose in the maintenance period > 15% higher than the maintenance dose recommended on the product label |
ETA (n = 4011) | 30.9 | NR |
| UST (n = 583) | 18.2 | NR | ||||
| ADA (n = 2933) | 7.8 | NR | ||||
| Egeberg (USA) [16] | Cohort study (DERMBIO registry) | 2161 | Higher than EMA label | ETA (n = 579) | 39 (≤ 24 wks); 35.1 (25–52 wks) | NR |
| UST (n = 1055) | 20 (≤ 24 wks); 46.2 (25–52 wks) | NR | ||||
| INF (n = 333) | 22.7 (≤ 24 wks); 56.7 (25–52 wks) | NR | ||||
| ADA (n = 1332) | 3.5 (≤ 24 wks); 0.9 (25–52 wks) | NR | ||||
| SEC (n = 196) | 0 | NR | ||||
| Esposito (Italy) [9] | Retrospective, observational study (digital databases and/or medical records) | 350 | Shortening/lengthening of dosing interval and/or increasing/reduction of the drug per dose per single administration | ETA (n = 175) | 2.9 | 12.6 |
| UST (n = 40) | 7.5 | 0 | ||||
| INF (n = 49) | 24.5 | 22.4 | ||||
| ADA (n = 86) | 0 | 16.3 | ||||
| Esposito (Italy) [17] | Retrospective, observational study (medical records) | 115 | Dose in terms of frequency or administration variation | ETA (n = 106) | 10.4 | NR |
| INF (n = 9) | 33.3 | NR | ||||
| Feldman (USA) [18] | Retrospective study (MarketScan Commercial Encounters Database) | 4039 | Dose escalation or reduction was defined as the patient experiencing a dose increase or decrease of at least 25% following the titration window | ETA (n = 2452) | 41 | 48.7 |
| UST (n = 195) | 35.9 | 37.4 | ||||
| ADA (n = 1662) | 36.6 | 53.7 | ||||
| Feldman (USA) [19] | Retrospective cohort study (Truven Health MarketScan Commercial Encounters Database) | 3310 | 10% higher than indicated in the label for ≥ 180 days (consecutive/non-consecutive) over a 12-month period following the maintenance period | ETA (n = 1443) | 20 | NR |
| UST (n = 420) | 14.8 | NR | ||||
| ADA (n = 1447) | 2.6 | NR | ||||
| Gulliver (Canada) [20] | Observational, retrospective (Newfoundland and Labrador Centre for Health databases and The Newfoundland and Labrador Medical Care Plan (MCP) Fee-for-Service Physician Claims Database) | 248 | Increase dose/frequency | ETA (n = 47) | 25.5 | NR |
| UST (n = 54) | 16.7 | NR | ||||
| INF (n = 61) | 14.8 | NR | ||||
| ADA (n = 86) | 12.8 | NR | ||||
| Iskandar (UK) [8] | Observational cohort study (BADBIR registry) | 2980 | Change in average weekly dose | ETA (n = 996) | 11.4 | 5.2 |
| UST (n = 309) | 17.7 | 30.0 | ||||
| ADA (n = 1675) | 4.5 | 2.5 | ||||
| Lee (USA) [21] | Retrospective chart review (medical charts) | 34 | Any treatment regimen that differed from FDA-approved dosing | ETA (n = 34) | 29.4 | 11.8 |
| Luber (USA) [22] | Retrospective cohort study (medical records) | 93 | Increasing dose from 5 to 10 mg/kg or increasing infusion frequency from every 8 weeks to every 4 weeks | INF (n = 93) | 66.7 | NR |
| Romero-Jimenez (Spain) [23] | Observational, longitudinal and retrospective study (clinical histories) | 62 | Shortened or lengthened dosage interval compared with SPC | UST (n = 62) | 22.6 | 22.6 |
| Sanz-Gil (Spain) [24] | Retrospective, observational chart review (outpatient pharmacy unit database) | 74 | Lengthened dose interval, increased frequency of administration | ETA (n = 20) | 0 | 10 |
| UST (n = 33) | 15 | 9 | ||||
| ADA (n = 21) | 0 | 24 | ||||
| Schwensen (Denmark) [25] | Retrospective study (patient records and DERMBIO registry) | 69 | Patients initiated on 150 mg instead of recommended 300 mg | SEC (n = 69) | NR | 52.2 |
| Wilder (USA) [26] | Retrospective review (patient charts) | 119 | Increase in the dose of UST to 90 mg and/or administration more frequently than every 12 weeks | UST (n = 119) | 42 | NR |
| Wu (USA) [27] | Retrospective observational study (Truven Health Analytics MarketScan Databases) | 6732 | Increase of the dose between two consecutive prescription fills of at least 40 mg for ADA users and at least 45 mg for UST users | UST (n = 1795) | 19.5 (biologic naïve); 20.6 (biologic experienced) | NR |
| ADA (n = 4937) | 8.6 (biologic naïve); 11.0 (biologic experienced) | NR | ||||
| Zweegers (NL) [28] | Prospective study (BioCAPTURE registry) | 356 | Higher than EMA label | ETA (n = 245) | 55.1 | NR |
| UST (n = 90) | 17 | NR | ||||
| ADA (n = 178) | 31.5 | NR |
ADA adalimumab, EMA European Medicines Agency, ETA etanercept, FDA US Food and Drug Administration, INF infliximab, NR not reported, SEC secukinumab, SPC Summary of Product Characteristics, UST ustekinumab
There was no consistent definition of dose escalation or dose reduction across studies; some studies considered any dose change from the recommended label dosing or dosing interval as a dose adjustment whereas some used a percentage increase or decrease, e.g. with a threshold of 10–30% as a dose adjustment. Time periods over which changes in dosing were assessed also varied considerably between studies, which is likely to have affected the potential for dose adjustment.
More studies reporting dose adjustment were identified for ustekinumab (n = 16; dose escalation only, n = 7, escalation and reduction, n = 9), etanercept (n = 13; dose escalation only, n = 7, escalation and reduction, n = 6), and adalimumab (n = 12; dose escalation only, n = 7, dose escalation and reduction, n = 5) than infliximab (n = 7; dose escalation only, n = 5, escalation and reduction, n = 2). Only two studies reported data on secukinumab (n = 2; dose escalation only, n = 1, dose reduction only, n = 1). Dose escalation was reported more frequently than dose reduction (19 versus 10 studies). No eligible studies were identified for brodalumab, guselkumab, ixekizumab, risankizumab, or tildrakizumab.
Adalimumab
The proportion of patients who underwent an adalimumab dose escalation ranged from 0 [9, 24] to 36.6% [18] across 12 studies. Shortening the dosing interval was the most widely used strategy in the dose escalation of adalimumab [7]. In the Egeberg et al. [16] study, the proportion of patients in the adalimumab cohort (n = 1332) on an escalated dose decreased from 3.5% during the first 24 weeks of treatment to 0.9% during maintenance therapy (25–52 weeks). Long-term data (5 years of treatment) showed that adalimumab dose escalations higher than the EMA label were higher at 5 years versus 1 year of treatment (39.3% vs. 31.5%) [28].
The proportion of patients who underwent an adalimumab dose reduction ranged from 2.5 [8] to 53.7% [18] across five studies. Among patients treated with adalimumab over a 3-year period in the Esposito et al. [9] study who had a dose reduction (16.3%), 12 had a dose interruption and two had an interval increase to > 14 days. Extending the dosing interval was the strategy used in all patients who underwent an adalimumab dose reduction. Adalimumab was the drug with the highest percentage of dose-reduced patients in the study by Carrascosa et al. [7], with extending the dosing interval the strategy used in all patients who underwent an adalimumab dose reduction. In patients who had a dose reduction (24%) in the study by Sanz-Gil et al. [24], their dose intervals were lengthened from every 2 weeks to 3, and in one patient to every 4 weeks. Motivations for dose reduction included prolonged remission (71.4%), patient choice (14.2%), other reasons (7.1%), and concomitant event (7.1%) [9].
Etanercept
The proportion of patients who underwent an etanercept dose escalation ranged from 0 [24] to 55.1% [28] across 13 studies. All patients treated with etanercept over a 3-year period in the Esposito et al. [9] study who had a dose escalation (2.9%) had a dose increase from 25 to 50 mg twice weekly. The administration of an increased dose while maintaining the interval was the most frequent strategy in the Carrascosa et al. [7] study (80%). In contrast, most patients had a decrease in the dosing interval in the Lee et al. [21] study. In the Egeberg et al. [16] study, the proportion of patients on an escalated dose reduced from 39.0% during the first 24 weeks of treatment to 35.1% during maintenance therapy (25–52 weeks). Long-term data (5 years of treatment) showed that etanercept dose escalations higher than the EMA label were higher at 5 years versus 1 year of treatment (71.4% vs 55.1%) [28]. All dose escalations in the Lee et al. and Esposito et al. [9, 21] studies were because of inadequate efficacy. In the Lee et al. [21] study, 30% of patients achieved better results with the 50 mg twice weekly dosing versus 25 mg twice weekly and two patients maintained this benefit when they reverted back to the lower dose.
The proportion of patients who underwent an etanercept dose reduction ranged from 5.2 [8] to 48.7% [18] across six studies. Among patients treated with etanercept over a 3-year period in the Esposito et al. [9] study who had a dose reduction (12.6%), seven had a dose interruption, six had an interval increase to > 8 days, and nine had their dose reduced to 25 mg weekly. Extending the dosing interval was the most common strategy used in 79% of patients who underwent an etanercept dose reduction in the Carrascosa et al. [7] study. In patients who had a dose reduction (10%) in the Sanz-Gil et al. [24] study, dose was reduced to once every 2 weeks. Dose reductions (11.8%, n = 4) occurred less often than dose escalations in the retrospective chart review by Lee et al. [21]. Motivations for dose reduction in the Esposito et al. [9] study included prolonged remission (59.1%), patient choice (18.1%), other reasons (13.6%), or a concomitant event (9.1%).
Infliximab
The proportion of patients who underwent an infliximab dose escalation ranged from 13.7 [7] to 66.7% [22] across seven studies. Among patients treated with infliximab over a 3-year period in the Esposito et al. [9] study who had a dose escalation (24.5%), 11 had an interval decrease to < 8 weeks and 1 had their dose increased to > 5 mg. In one study, shortening the dosing interval was reported as the most widely used strategy in the dose escalation of infliximab (100%; n = 7) [7]. Luber et al. [22] reported that the most frequent dose escalation strategy for infliximab was increasing from 5 mg/kg every 8 weeks to 5 mg/kg every 4 weeks to 10 mg/kg every 4 weeks (21%; n = 13/62). In the Egeberg et al. [16] study, the proportion of patients on an escalated dose increased from 22.7% during the first 24 weeks of treatment to 56.7% during maintenance therapy (25–52 weeks).
The proportion of patients who underwent an infliximab dose reduction ranged from 22.5 [9] to 29.4% [7] across two studies. Among patients treated with infliximab over a 3-year period in the Esposito et al. [9] study who had a dose reduction (22.5%), two had a dose interruption, and nine had an interval increase to > 8 weeks. One study showed that the most common strategy for infliximab dose reduction was to decrease the dose administered below the recommended range (73%) [7].
Ustekinumab
The proportion of patients who underwent an ustekinumab dose escalation ranged from 6.9 [13] to 42% [26] across 16 studies. Among patients treated with ustekinumab over a 3-year period in the Esposito et al. [9] study who had a dose increase (7.5%), one had an interval decrease to < 12 weeks, and two had their dose increased to 90 mg (patients with body weight < 100 kg). Shortening the dosing interval was the most common dose escalation strategy to escalate ustekinumab [7]. In the Egeberg et al. [16] study, the proportion of patients on an escalated dose increased from 20% during the first 24 weeks of treatment to 46.2% during maintenance therapy (25–52 weeks). This is consistent with findings from the Cao et al. [14] study, which demonstrated that ustekinumab dose escalation was more likely to happen during the maintenance period: (second dose, 1.4% versus fifth dose, 19.3%). Subgroup analysis findings suggested that biologic-experienced patients were more likely to receive an increased dose during their treatment than biologic-naïve patients [14]. Long-term data (5 years of treatment), showed that ustekinumab dose escalations higher than the EMA label were higher at 5 years versus 1 year of treatment (24%/n = 22 vs. 17%/n = 15) [28].
The proportion of patients who underwent an ustekinumab dose reduction ranged from 2.7 [13] to 37.4% [18] across eight studies. Extending the dosing interval was the most common strategy used to reduce the ustekinumab dose (100%) in the Carrascosa et al. [7] study; this is in line with results from the Iskandar et al. [8] study. In contrast to dose escalation, the likelihood of a dose reduction from ustekinumab 90–45 mg was low and stable in the maintenance phase (second dose, 6.1% versus fifth dose, 5.1%) [14].
Secukinumab
Only two studies reported on dose adjustment for secukinumab. One study reported that, of 195 patients, none initiated treatment with doses higher than the label and no dose increases occurred during maintenance therapy [16]. Another study reported that 52% of patients (n = 36) initiated treatment with the lower dose of 150 mg instead of 300 mg [25]. This occurred at a single centre and was largely attributed to poor tolerability of previous treatment.
Discussion
Results from this systematic review of 20 real-world studies published between 2014 and 2020, including over 30,000 patients, showed that dose adjustment of biologic therapies for the treatment of psoriasis is frequent. For example, a cross-sectional study of over 600 patients in Spain found that 42% of patients treated for at least 6 months with the same biologic were receiving an off-label dose [7]. Similarly, data from Italy reported 20% of 350 patients needed dose adjustment over a 3-year period [9]. This suggests that an inadequate response or dissatisfaction with standard dosing of biologic agents is common among patients with moderate-to-severe psoriasis. Most studies were retrospective and based on data from various sources including disease registries, hospital case records or insurance claims databases. Definitions of dose adjustment differed across studies, with some reporting any change from the label-approved dosing regimen and some requiring a change in average dose of between 10 and 30%.
Dose escalation was investigated and reported in more studies than dose reduction (n = 19 versus n = 10, respectively), highlighting the need to provide more evidence on dose reduction strategies, specifically the aims and reasons behind employing this strategy. In studies that investigated both dose escalations and reductions (n = 8), three found dose escalations more frequent, two found dose reductions most frequent, one found no difference, and three showed varying frequencies depending on the biologic drug. Overall, dose escalation was reported to range from zero to two-thirds of patients and dose reductions from 2.5% to just over half of patients. This wide variation reflects the differences in study design, including which treatments were assessed, the time period and duration assessed, healthcare setting, as well as the definition of dose adjustment used. Only one study used the time trend method for analysing results which they believe provides the most comprehensive information on dosing patterns in clinical practice as it examines dose escalation/reduction relative to the recommended cumulative dose [8]. Consequently, the varied methods limit the ability to draw meaningful comparisons across the studies. However, some trends were observed.
Shortening the dose interval seems to be the most frequent strategy for dose escalation of adalimumab, infliximab, and ustekinumab. This could be because this approach is more flexible while dose variation is limited by available marketed doses. In contrast, increasing the dose was the most frequent strategy for etanercept dose escalation. The absence of dose escalations for secukinumab may reflect that the use of this drug is relatively new to physicians; however, Egeberg et al. [16] noted that the markedly short drug survival of secukinumab is unlikely to be explained by the lack of dose escalation. Dose escalations were less frequent in the maintenance phase (25–52 weeks) than the initial phase (≤ 24 weeks) of adalimumab and etanercept treatment, but more frequent in the maintenance phase than the initial phase for infliximab and ustekinumab treatment. Long-term data (5 years of treatment) showed that dose escalations are more frequent at 5 years versus 1 year of treatment for adalimumab, etanercept, and ustekinumab. Dose escalations in patients who did not switch treatment in the Feldman et al. [18] study were frequently followed by dose reductions or discontinuations. This suggests that exceeding standard doses of biologics may not be sustainable. The main motivation to dose-escalate was inefficacy. Dose escalation may improve effectiveness for patients with severe disease who have an inadequate response to standard dosing regimens.
Increasing the dose interval and dose interruptions were the most common strategies for dose reduction for all drugs analysed except for infliximab and also secukinumab, although this is based on a single study. Luber et al. [22] concluded that first decreasing the infliximab infusion interval followed by increasing the dose tends to allow for longer duration of infliximab therapy than first increasing the dose followed by decreasing the infusion interval. In the 5-year retrospective study by Sanz-Gil et al. [24] dosing intervals were more likely to be widened for adalimumab and etanercept and more likely to be reduced for ustekinumab. The main motivation for dose reductions was prolonged remission. Dose reductions achieving the lowest effective dose limit any unnecessary drug exposure and in turn lower the risk of dose-dependant adverse events; additional advantages include an improvement in the patient’s quality of life due to fewer injections and reductions in healthcare resources and costs. However, the theoretical risk with dose reductions includes decreased efficacy, and longer dosing intervals may increase the risk of anti-drug antibody formation [10].
A small number of studies investigated efficacy outcomes of dose adjustments. However, these analyses largely reflected that dose reduction generally occurred in patients with a good response while lower efficacy, which may have been associated with more severe disease, was associated with dose escalation rather than showing the effect of dose adjustment itself on outcomes [9, 23]. Results from the Schwensen et al. [25] study indicated that dose reduction of secukinumab may not have a detrimental effect on efficacy, but this needs to be confirmed in further studies. In a previous systematic review of 23 prospective clinical trials that evaluated changes in dosing regimens of biologics for patients with psoriasis who were non-responders, dose escalation with adalimumab, etanercept, and ustekinumab usually resulted in greater efficacy than standard dosing regimens, whereas dose reduction resulted in decreased efficacy [10]. However, this was based on data from clinical trials rather than a real-world setting. Another recent study that included 303 treatments reported that efficacy and drug survival were similar in patients with dose reduction compared with patients with standard dose [29].
Limited safety data regarding dose adjustments were available in the studies analysed. One study of secukinumab found no statistical differences in adverse events between the 150 and 300 mg groups [25]. Dose escalations may be associated with a higher rate of adverse events than standard dosing or dose reductions, but whether there is a clinically significant difference remains to be confirmed.
Above-label doses are associated with a significant annual extra cost per patient. In a USA-based study, increased doses of biologic therapy (≥ 10% over the labelled dose for ≥ 180 days) resulted in additional annual costs per patient of $19,458 for etanercept, $18,972 for adalimumab, and $21,045 for ustekinumab [19]. In the UK, the increased dose (≥ 30% above the labelled dose) was associated with a mean annual extra cost per patient of £7936 for adalimumab, £5912 for etanercept, £2422 for ustekinumab and £2275 for infliximab [12]. However, avoidance of escalating doses may be associated with lower costs but could have negative impact on treatment efficacy. Given that psoriasis treatments are chronic and the high cost of biologics, it is essential to conduct economic studies on the long-term use of these drugs including the impact of dose adjustment [24].
Willingness to adjust doses may also be influenced by healthcare reimbursement systems. For example, in Spain, drugs are exclusively delivered by hospital-owned pharmacies and are wholly financed by the regional public health system, in which most regions do not usually permit dose escalation. Similarly, in Italy, the situation can vary between regions, with choice of biologic drug and any dose adjustment not influenced by reimbursement issues in some but not all areas. In addition, pricing may have an effect on dose adjustment; for instance, different doses may be priced at a similar level, meaning no cost restraint on increasing dose. In the UK, Iskandar et al. [8] suggested that the low proportion of patients with a cumulative dose higher than the recommended cumulative dose is likely to be related to the system of public funding for biologic therapies. Although UK guidelines recommended dose escalation in patients with an inadequate primary response due to inadequate dose exposure before switching, it is also noted that it may be off-licence and not reimbursed [11]. This may encourage switching to an alternative treatment rather than escalating the dose of the current treatment. A similar situation also exists in other countries.
Changes in the therapeutic landscape over time, with an increasing number and type of available treatments, might also have been expected to influence the extent of dose adjustment, with the decision to adjust doses becoming less frequent as more alternative options become available. However, this observation could not be confirmed given the limited number and heterogeneity of included studies. In addition, the introduction of biosimilars to treat psoriasis may also have an effect on dose adjustment of biologics. The first biosimilars to treat psoriasis were approved in Europe in 2013 (infliximab), with several adalimumab and etanercept biosimilars approved since 2016. Biosimilars typically have lower drug prices and so may have the effect of increasing access to treatments for many patients. The lower price of biosimilars may also mean fewer cost restraints and less reluctance to increase dose for cost reasons, encouraging greater dose escalation. However, only one of the studies in this review specifically reported biosimilar use and did not report separate dose adjustment data [16].
One aspect of dose adjustment that was not considered in this review is intermittent dosing, although a small number of studies included interrupted dosing within the definition of dose reduction. A recent review identified 18 reports of intermittent use of biologic therapies and concluded that the majority of patients (≥ 60%) were generally able to re-establish disease control following re-treatment and that safety profiles were similar with intermittent and continuous dosing [30]. However, an earlier systematic review reported that withdrawal of treatment typically led to an increase in disease activity, and retreatment did not result in equivalent response rates to initial therapy for most biologics [10]. As such, the potential role of intermittent dosing remains unclear. Other limitations of this analysis include that many of the studies were retrospective or observational in nature, and as such, unobserved confounding and patient selection bias could exist. In addition, many studies included data on claims for a filled prescription, which does not guarantee that the drug was administered to the patient.
The frequent use of dose adjustment may also suggest a need to develop a more personalised therapeutic approach to the use of biologics, especially given the increasing number of treatment options. Several features have been shown to influence response to biologic therapy, including genetic, immune, and environmental factors [31, 32]. In the future, it may be possible to assign patients with psoriasis to different anti-psoriatic drugs based on disease endotype and predictive biomarkers in order to reduce immunosuppressive-related side effects and maximise treatment efficacy.
Conclusion
Dose adjustments of biologics for psoriasis are common in clinical practice. However, despite the widespread occurrence of dose adjustments, few studies have reported on this subject. High variations were seen in the reported dose adjustment rates, and no direct comparisons could be made since the study design, definition of dose adjustments, and patient populations varied. Dose escalation will typically have economic consequences and safety may be negatively impacted, while dose reduction may have negative consequences on efficacy. These aspects are important to consider when making decisions on treatment dosing. More studies are required to assess the effect of dose adjustments on efficacy and safety, as well as its economic impact, to better inform clinical decision making.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This analysis and manuscript development, including the journal’s Rapid Service Fee, was funded by Leo Pharma A/S.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance were provided by Andy Bond of Spirit Medical Communications Group Ltd., funded by Leo Pharma A/S.
Disclosures
Alessio Gambardella and Gaetano Licata have nothing to disclose. Anne Sohrt is an employee of LEO Pharma A/S.
Compliance with Ethics Guidelines
This was a systematic review of the literature and, as such, no ethical approval was required. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–269. doi: 10.1001/jamadermatol.2019.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sbidian E, Chaimani A, Afach S, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2020;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 4.Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409. doi: 10.1016/j.jaad.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 6.Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431. doi: 10.1016/j.jaad.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 7.Carrascosa JM, Garcia-Doval I, Pérez-Zafrilla B, et al. Use of off-label doses is frequent in biologic therapy for moderate to severe psoriasis: a cross-sectional study in clinical practice. J Dermatolog Treat. 2015;26:502–506. doi: 10.3109/09546634.2015.1034070. [DOI] [PubMed] [Google Scholar]
- 8.Iskandar IYK, Ashcroft DM, Warren RB, et al. Patterns of biologic therapy use in the management of psoriasis: cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR) Br J Dermatol. 2017;176:1297–1307. doi: 10.1111/bjd.15027. [DOI] [PubMed] [Google Scholar]
- 9.Esposito M, Gisondi P, Conti A, et al. Dose adjustment of biologic therapies for psoriasis in dermatological practice: a retrospective study. J Eur Acad Dermatol Venereol. 2017;31:863–869. doi: 10.1111/jdv.14145. [DOI] [PubMed] [Google Scholar]
- 10.Brezinski EA, Armstrong AW. Off-label biologic regimens in psoriasis: a systematic review of efficacy and safety of dose escalation, reduction, and interrupted biologic therapy. PLoS ONE. 2012;7(4):e33486. doi: 10.1371/journal.pone.0033486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith CH, Yiu ZZN, Bale T, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2020: a rapid update. Br J Dermatol. 2020 doi: 10.1111/bjd.19039. [DOI] [PubMed] [Google Scholar]
- 12.Bewley A, Miglio C, Tian H, Gilloteau I, Whitehead J, Hermans R. Dose increase beyond labelled dose of biologics is associated with incremental pharmacy costs: results from a real-world study in the UK. J Dermatolog Treat. 2019;30:376–382. doi: 10.1080/09546634.2018.1524820. [DOI] [PubMed] [Google Scholar]
- 13.Cai Q, Carter C, AbuDagga A, Schenkel B, Jones M, Tan H. Real-world dosing and utilization of ustekinumab among patients with psoriasis. Am J Pharm Benefits. 2014;6(3):129–136. [Google Scholar]
- 14.Cao Z, Carter C, Wilson KL, Schenkel B. Ustekinumab dosing, persistence, and discontinuation patterns in patients with moderate-to-severe psoriasis. J Dermatolog Treat. 2015;26:113–120. doi: 10.3109/09546634.2014.883059. [DOI] [PubMed] [Google Scholar]
- 15.Carter C, Wilson KL, Smith D, Lee S. Comparative treatment patternsamong psoriasis patients using adalimumab, etanercept, or ustekinumab. Am J Pharm Benefits. 2016;8:191–198. [Google Scholar]
- 16.Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178:509–519. doi: 10.1111/bjd.16102. [DOI] [PubMed] [Google Scholar]
- 17.Esposito M, Prignano F, Rongioletti F, et al. Efficacy and safety of adalimumab after failure of other anti-TNFα agents for plaque-type psoriasis: clinician behavior in real life clinical practice. J Dermatolog Treat. 2019;30:441–445. doi: 10.1080/09546634.2018.1529382. [DOI] [PubMed] [Google Scholar]
- 18.Feldman SR, Zhao Y, Navaratnam P, Friedman HS, Lu J, Tran MH. Patterns of medication utilization and costs associated with the use of etanercept, adalimumab, and ustekinumab in the management of moderate-to-severe psoriasis. J Manag Care Spec Pharm. 2015;21:201–209. doi: 10.18553/jmcp.2015.21.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman SR, Zhao Y, Zhou H, Herrera V, Tian H, Li Y. Economic impact of above-label dosing with etanercept, adalimumab, or ustekinumab in patients with psoriasis. J Manag Care Spec Pharm. 2017;23:583–589. doi: 10.18553/jmcp.2017.23.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulliver WP, Randell S, Gulliver S, Gregory V, Nagle S, Chambenoit O. Biologic therapy utilization in patients with moderate to severe psoriasis and psoriatic arthritis: an observational summary of biologic therapy use in a clinical setting. J Cutan Med Surg. 2018;22:567–576. doi: 10.1177/1203475418786712. [DOI] [PubMed] [Google Scholar]
- 21.Lee EB, Thomas LW, Vegeberg A, Wu JJ. Dosage adjustments in patients with psoriasis on etanercept: a retrospective chart review. J Psoriasis Psoriatic Arthritis. 2020;5:52–53. doi: 10.1177/2475530320910466. [DOI] [Google Scholar]
- 22.Luber AJ, Tsui CL, Heinecke GM, Lebwohl MG, Levitt JO. Long-term durability and dose escalation patterns in infliximab therapy for psoriasis. J Am Acad Dermatol. 2014;70:525–532. doi: 10.1016/j.jaad.2013.10.059. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Jimenez RM, Escudero-Vilaplana V, Baniandres Rodriguez O, García Martín E, Mateos Mayo A, Sanjurjo SM. Association between clinical factors and dose modification strategies in the treatment with ustekinumab for moderate-to-severe plaque psoriasis. J Dermatolog Treat. 2018;29:792–796. doi: 10.1080/09546634.2018.1466978. [DOI] [PubMed] [Google Scholar]
- 24.Sanz-Gil R, Pellicer A, Montesinos MC, Valcuende-Cavero F. Improved effectiveness from individualized dosing of self-administered biologics for the treatment of moderate-to-severe psoriasis: a 5-year retrospective chart review from a Spanish University Hospital. J Dermatolog Treat. 2020;31:370–377. doi: 10.1080/09546634.2019.1602246. [DOI] [PubMed] [Google Scholar]
- 25.Schwensen JF, Clemmensen A, Sand C, et al. Effectiveness and safety of secukinumab in 69 patients with moderate to severe plaque psoriasis: a retrospective multicenter study. Dermatol Ther. 2017 doi: 10.1111/dth.12550. [DOI] [PubMed] [Google Scholar]
- 26.Wilder EG, Patel M, Hebeler K, Menter A. Ustekinumab treatment for psoriasis in 119 patients maintained on therapy for a minimum of one year: a review. J Drugs Dermatol. 2014;13:905–910. [PubMed] [Google Scholar]
- 27.Wu JJ, Guérin A, Gauthier G, Sundaram M. Healthcare resource utilization, healthcare costs and dose escalation in psoriasis patients initiated on ustekinumab versus adalimumab: a retrospective claim study. J Dermatolog Treat. 2017;28:290–298. doi: 10.1080/09546634.2016.1247946. [DOI] [PubMed] [Google Scholar]
- 28.Zweegers J, Groenewoud JMM, van den Reek JMPA, et al. Comparison of the 1- and 5-year effectiveness of adalimumab, etanercept and ustekinumab in patients with psoriasis in daily clinical practice: results from the prospective BioCAPTURE registry. Br J Dermatol. 2017;176:1001–1009. doi: 10.1111/bjd.15023. [DOI] [PubMed] [Google Scholar]
- 29.Llamas-Velasco M, Daudén E. Reduced doses of biological therapies in psoriasis may increase efficiency without decreasing drug survival. Dermatol Ther. 2020;33(6):e14134. doi: 10.1111/dth.14134. [DOI] [PubMed] [Google Scholar]
- 30.Al-Hammadi A, Ruszczak Z, Magariños G, Chu CY, El Dershaby Y, Tarcha N. Intermittent use of biologic agents for the treatment of psoriasis in adults. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16803.10.1111/jdv.16803. [DOI] [PubMed] [Google Scholar]
- 31.Membrive Jiménez C, Pérez Ramírez C, Sánchez Martín A, et al. Influence of genetic polymorphisms on response to biologics in moderate-to-severe psoriasis. J Pers Med. 2021;11(4):293. doi: 10.3390/jpm11040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ovejero-Benito MC, Reolid A, Sánchez-Jiménez P, et al. Histone modifications associated with biological drug response in moderate-to-severe psoriasis. Exp Dermatol. 2018;27(12):1361–1371. doi: 10.1111/exd.13790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.