PD-1, a member of the immunoglobulin gene superfamily, was found to be involved in programmed cell death and subsequently demonstrated to be critical for the negative regulation of T cells. Its ligands PD-L1 (B7-H1) and PD-L2 (B7-DC) interact with PD-1 and negatively regulate T cell-mediated immune responses.1 CD80 also interacts with PD-L1 as a counterreceptor. Moreover, PD-L1 can serve as a receptor to transmit signals to T cells and tumor cells to resist immune-mediated destruction.1 Therefore, PD-L1 can act as both a ligand and a receptor for immunoregulatory functions. This commentary will focus on the roles of various cellular sources of PD-L1 in regulating antitumor immunity.
In addition to tumor cells, activated immune cells, such as T cells, B cells, and natural killer (NK) cells, often express PD-L1.1 PD-L1 is also commonly expressed on myeloid cells, including macrophages, myeloid-derived suppressor cells, and dendritic cells (DCs), in the tumor microenvironment.1 The expression of PD-L1 can be upregulated in both tumor cells and normal cells by many cytokines, especially interferon-γ.1 The contribution of PD-L1-expressing cells to antitumor T cell suppression is critical for stratifying patients for their response to anti-PD-1/PD-L1 immunotherapy and for understanding PD-L1-mediated inhibitory mechanisms in detail.
Soon after the discovery of the inhibitory role of PD-1 in autoimmune disease,1 it was found that ectopically expressed PD-L1 in tumor cells played a T cell-inhibitory role in a syngeneic tumor model.2–4 Subsequently, PD-L1 on tumor cells has been widely used as a biomarker or companion diagnostic for checkpoint blockade therapy. However, almost half of patients positive for tumor PD-L1 expression fail to respond, while some patients with PD-L1-negative tumors still respond to PD-L1 blockade, suggesting that the working model of how PD-1/PD-L1 signaling affects immune responses might be more complicated.5 Therefore, in recent years, numerous studies have focused on exploring the important roles of PD-L1 from different cell sources. Kleinovink et al., Munn et al., and Tang et al. showed that PD-L1 expression on host cells is essential for PD-L1-mediated T cell suppression.6–8 However, Juneja et al. revealed that PD-L1 on tumor cells is sufficient for immune evasion.9 Noguchi et al. and Lau et al. concluded that both tumor cell PD-L1 and host cell PD-L1 are required to mediate the suppression of antitumor immunity.10,11
To dissect the role of PD-L1 derived from different cell types in inhibiting the antitumor T cell response, PD-L1−/− mice and PD-L1−/− tumor cells are commonly used to exclude the contribution of PD-L1 from nontumor cells or tumor cells, respectively. Genetic disruption of PD-L1 in mice may cause more activated CD4+ and CD8+ T cell phenotypes in the tumor microenvironment. Similarly, in these models, tumor cell lines are deficient in PD-L1 during the entire tumor development period, which may lead to a different tumor microenvironment. To overcome these limitations, we established tumor models using mPD-L1-expressing tumors in hPD-L1 KI mice or hPD-L1-expressing tumors in WT mice. In our models, the immunosuppressive roles of PD-L1 in both tumor cells and nontumor cells existed during the entire tumor development and immunotherapy periods, which better resembled the natural setting. We also took advantage of anti-mPD-L1 and anti-hPD-L1 antibodies with no cross-reactivity to selectively block tumor- or nontumor-derived PD-L1. Our data indicated that blocking PD-L1 on nontumor or tumor cells could effectively reactivate tumor-specific CD8+ T cell responses and inhibit tumor growth in the tumor microenvironment. In addition, both tumor-derived PD-L1 and nontumor-derived PD-L1 contributed to T cell inhibition in a nonredundant manner, and blocking both sources of PD-L1 achieved synergy and resulted in the maximum antitumor effect. In addition, the expression ratio of PD-L1tumor/PD-L1nontumor determined their relative contributions to immune suppression.12
Since the expression of PD-L1 on nontumor cells is as important as that on tumor cells, there is increasing research investigating nontumor cellular sources of PD-L1, such as macrophages, NK cells, and DCs. Peng et al. and Oh et al. found that DCs represent a critical source of PD-L1 for inhibiting antitumor immunity by using mouse models with specific ablation of PD-L1 in DCs. Although macrophages are the major source of PD-L1 in tumors, DC-derived PD-L1 is critical for the regulation of the antitumor T cell response. Ablation of PD-L1 in DCs but not in macrophages enhances antitumor T cell responses and inhibits tumor growth. In addition to its typical role in regulating T cell function, PD-L1 has recently been reported to regulate the functions of DCs and NK cells.13,14 Mayoux et al. demonstrated that DCs are a critical target of PD-L1 blockade treatment. Blocking PD-L1 on DCs dissociates B7.1/PD-L1 in cis interactions to enhance CD28 costimulation of T cells and induce T cell priming and proliferation.15 Dong et al. showed that NK cells upregulate PD-L1 expression via the PI3K/AKT/NF-κB pathway. PD-L1+ NK cells showed remarkably enhanced antitumor activity compared to PD-L1− NK cells. PD-L1 blockade could further enhance PD-L1+ NK cell function through the p38 signaling pathway. This provides a potential mechanism for the antitumor efficacy of anti-PD-L1 therapy in PD-L1− tumors.16
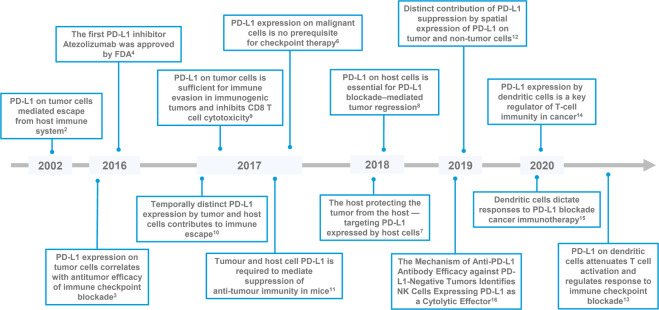
Overall, these findings shed new light on both the clinical prediction of responders and the understanding of the underlying mechanisms of PD-1/PD-L1 blockade-mediated antitumor immunity (Fig. 1).
Fig. 1. Timeline of some key discoveries and advances related to PD-L1 in antitumor immunity.
Blue boxes represent critical studies or advances related to PD-L1. PD-L1 programmed cell death 1 ligand 1, FDA the Food and Drug Administration, NK cells natural killer cells
Acknowledgements
X.Y. was supported by the National Natural Science Foundation of China (81971467).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: X Zhang, Y Huang
References
- 1.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 2.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott DF, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J. Clin. Oncol. 2016;34:833–842. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 4.Villaruz LC, Socinski MA. The clinical utility of PD-L1 testing in selecting non-small cell lung cancer patients for PD1/PD-L1-directed therapy. Clin. Pharm. Ther. 2016;100:212–214. doi: 10.1002/cpt.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr. Opin. Pharm. 2015;23:32–38. doi: 10.1016/j.coph.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinovink, J. W. et al. PD-L1 expression on malignant cells is no prerequisite for checkpoint therapy. Oncoimmunology6, e1294299 (2017). [DOI] [PMC free article] [PubMed]
- 7.Munn DH. The host protecting the tumor from the host - targeting PD‑L1 expressed by host cells. J. Clin. Invest. 2018;128:570–572. doi: 10.1172/JCI99047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang H, et al. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J. Clin. Invest. 2018;128:580–588. doi: 10.1172/JCI96061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juneja VR, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Exp. Med. 2017;214:895–904. doi: 10.1084/jem.20160801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noguchi T, et al. Temporally Distinct PD-L1 Expression by Tumor and Host Cells Contributes to Immune Escape. Cancer Immunol. Res. 2017;5:106–117. doi: 10.1158/2326-6066.CIR-16-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau, J. et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat. Commun.8, 14572 (2017). [DOI] [PMC free article] [PubMed]
- 12.Zhang X, et al. Distinct contribution of PD-L1 suppression by spatial expression of PD-L1 on tumor and non-tumor cells. Cell Mol. Immunol. 2019;16:392–400. doi: 10.1038/s41423-018-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng Q, et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 2020;11:4835. doi: 10.1038/s41467-020-18570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh SA, et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer. 2020;1:681–691. doi: 10.1038/s43018-020-0075-x. [DOI] [PubMed] [Google Scholar]
- 15.Mayoux, M. et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci. Transl. Med.12, eaav 7431 (2020). [DOI] [PubMed]
- 16.Dong W, et al. The mechanism of anti-PD-L1 antibody efficacy against PD-L1-negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Disco. 2019;9:1422–1437. doi: 10.1158/2159-8290.CD-18-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]