Abstract
Introduction
Cholestasis is a liver disease caused by a malfunction of the hepato-biliary system. Oxidative stress as a systemic complication is the main characteristic of cholestasis. The aim of this study was to evaluate the anti-inflammatory and hepatoprotective effects of Portulaca oleracea (PO) methanolic extract on liver dysfunction and tissue damage induced by bile duct ligation (BDL) in rats.
Materials and methods
Twenty-eight male Wistar rats were randomly divided into four groups: sham control (SC), BDL alone, SC plus 500 mg/kg methanolic extract of PO orally for 1 week, and BDL plus 500 mg/kg methanolic extract of PO orally for 1 week. After 1 week, the animals were anesthetized, and the liver and blood samples were taken from each animal. Biochemical parameters, oxidative stress biomarkers, histopathological changes, as well as the gene expression of IL-1, TNF-α, TGF-β, and α-SMA have been evaluated.
Results
The methanolic extract of PO at a dose of 500 mg/kg significantly decreased the plasma levels of aminotransferases, alkaline phosphatase as compared to BDL group (P < 0.05), while it had no significant effect on the levels of oxidative stress markers in the hepatic tissue. The plasma level of malondialdehyde and ferric-reducing antioxidant power were markedly elevated in the BDL group in comparison to SC group (P < 0.05), while treatment with PO significantly reduced these markers (P < 0.05). The administration of PO attenuated hydroxyproline content, bile duct proliferation, and inflammation score in the cholestatic liver in contrast to non-treated BDL rats (P < 0.05). Moreover, the methanolic extract of PO markedly declined the expression of TNF-α and TGF-β pro inflammatory genes in contrast to BDL rats.
Conclusions
Taken together, our findings showed that PO attenuated liver injury by decreasing liver function tests, inflammation, and hydroxyproline content. As a result, it is suggested that PO can be applied in cholestatic liver damage as a therapeutic or adjuvant agent.
Keywords: Cholestasis, Portulaca oleracea, Oxidative stress, Anti-inflammatory agents, Bile ducts, Rats
Cholestasis; Portulaca oleracea; Oxidative stress, Anti-inflammatory agents, Bile ducts, Rats.
1. Introduction
The liver as the largest organ in the body plays an important role in the body's metabolic functions that include synthesis and metabolism, glucose homeostasis, bile production, inactivation, detoxification of various factors, and regulation of immune responses [1]. Liver diseases include hepatitis, obstructive jaundice, or cholestasis, liver cirrhosis, alcoholic and non-alcoholic fatty liver, and liver cancers [2]. Cholestasis is a liver disease initiated by malfunction of the hepatobiliary system. It is the result of the buildup of bile fatty acids and other toxins in the plasma and liver. Cholestasis may be caused because of impaired bile secretion by hepatocytes and bile duct cells (intrahepatic cholestasis) or by the obstruction of bile ducts that carry bile from the liver to the intestine (extrahepatic cholestasis). Retention of hydrophobic bile salts in cholestasis causes damage to hepatocytes, followed by liver fibrosis, and liver cirrhosis [3, 4]. Cirrhosis is the last stage of liver disease and one of the top ten causes of death worldwide with an estimated annual incidence of 14.1 per 100,000 people [5]. Numerous factors can cause this disease, which include inflammation, viral infections, autoimmune diseases, gallstones, pancreatic, and liver tumors [6].
Oxidative stress as a systemic complication is the main characteristic of cholestasis. An increase in lipid peroxidation or malondialdehyde (MDA) is seen in the plasma, kidneys, brain, and heart of laboratory rats 24 h after bile duct ligation (BDL). Oxidative stress is the result of an imbalance between the oxidative and antioxidant systems (vitamins A, C, E, and compounds such as beta-carotene and metabolites such as GSH). A number of enzymes play an antioxidant role, the most important of which are catalase (CAT), glutathione reductase (GR) [7], glutathione peroxidase (GPX), and superoxide dismutase (SOD) [8]. Oxidative stress causes dysfunction in production and secretion of bile salts in the bile canaliculi, dysfunction of skeletal actin arrangement of the cell, and disrupts the structure of strong joints. Increased levels of oxidative stress are observed in rodents and humans with bile duct disorders [7, 9].
Herbal plants are valuable natural resources that today are considered by the developed countries of the world as raw materials to become safe drugs for humans. Some herbs contain high amounts of antioxidants that can be effective in human health. Known as “Global Panacea,” Portulaca oleracea (PO) [10] or purslane is mentioned by the World Health Organization as one of the most widely used medicinal plants [11]. PO is an annual succulent in the Portulacaceae family, which may reach 40 cm in height in the seeding stages. This plant might be described as a superior food in the future due to its high nutritional and antioxidant properties. PO has fleshy leaves and small black seeds [12]. Compounds such as pectin, protein, carbohydrates, unsaturated fatty acids, various minerals including iron, copper, potassium, calcium, phosphorus, melatonin (MLT), and selenium are present in different parts of this plant [11, 13]. The antioxidant properties of this plant is mostly due to compounds such as flavonic acid [11]. This plant is rich in vitamins A, B1, and B2. Other benefits of this plant include vitamin C, niacinamide, and nicotinic acid. Its succulent leaves contain the most flavonoids and ascorbic acid and have a protective effect against free radicals. The extract of this plant has the highest content of phenolic acid and is also the richest source of omega-3 fatty acids. The phenolic compounds of this plant inhibit the peroxidation activities of H2O2 on fatty acids and thus reduce the amount of MDA [12, 14]. PO also has a wide range of biological effects that include antioxidant, analgesic, anti-inflammatory, neuroprotective, hepatoprotective, and anti-tumor. This plant is used to treat various diseases that include stomach diseases, inflammation of the liver, respiratory diseases, fever, headache, and ulcer. It is also known to regulate sugar and fat metabolism in the body [15, 16, 17].
For the laboratory model of hepatic cholestasis, the use of a reliable animal model has been suggested with BDL based on the induction of extrahepatic cholestasis in rat [18, 19]. This model is associated with oxidative stress and fibrosis. The plasma levels of important liver enzymes: aspartate aminotransferase (AST), alanine aminotransferase (ALT) [16], gamma-glutamyl transpeptidase (GGT), bilirubin, alkaline phosphatase (ALP), and lactate dehydrogenase are elevated in BDL rats. Obstructive jaundice and cholestasis occur about 1 week after BDL, which can be used to study therapeutic agents that have a protective effect on liver cholestasis [20]. Because of the antioxidant properties of PO and the role of oxidants in the development of hepatic cholestasis induced by BDL, the methanolic extract of this plant can be a suitable choice to investigate its possible protective effects against hepatic cholestasis induced by BDL.
2. Materials and methods
2.1. Chemicals
Thiobarbituric acid (TBA), Ethylenediaminetetraacetic acid (EDTA), 5, 5′-dithiols-(2-nitrobenzoic acid) (DTNB) and pentobarbital, were obtained from Sigma Chemical Co (St Louis, MO, USA). Formaldehyde, 2, 4-dinitrophenylhydrazine (DNPH) and trichloroacetic acid (TCA) were purchased from Merck (Germany).
2.2. Plant material and extraction
The plant has been collected from around the city of Yasuj and has been identified by a botanist. After the collection of PO samples, they were cleaned and to dry they were placed at room temperature away from light for several days. Then, they were chopped and prepared for extraction. Afterward, 100 g of the dried plant was drenched with 1,000 mL of 70% methanol solution. The resultant mixture was placed at a temperature of 37 °C for 48 h (after filtering). The prepared solution was filtered using a Whatman® Grade 1 filter paper. The prepared mixture was concentrated as much as possible by the rotary device under vacuum conditions. Finally, the extract was dried in an incubator at a temperature of 50 °C and refrigerated at -20 °C [21].
2.3. Animals
Three-month-old male Wistar rats weighing 200–250 g were obtained from the Animal House of Shahrekord University of Medical Sciences, Shahrekord, Iran, and kept at a 12:12 h light/dark cycle with free access to food and water for a week. The present study was approved by the Ethics Committee of Yasuj University of Medical Sciences, Yasuj, Iran, (code: IR.YUMS.REC.1398.159). The animals were handled according to the Principles of Laboratory Animal Care (NIH Publication No.86_23).
2.4. Experiment and procedure
In this study, Twenty-eight male Wistar rats were randomly divided into four groups with 7 animals in each group, which included the first (sham control [SC]), second (BDL alone), third (SC and 500 mg/kg methanolic extract of PO orally for 1 week) [22], and fourth (BDL and 500 mg/kg methanolic extract of PO orally for 1 week) groups. The SC and BDL groups received 0.5 ml normal saline and the extract was solved in normal saline. The BDL technique was used to induce cholestasis. After the induction of general anesthesia with drugs such as ketamine (50 mg/kg) -HCl and xylazine-HCl (10 mg/kg) in the experimental groups, the common bile duct of the animal was tied on both sides and cut into two same parts. Subsequently, bile secretion failed and led to cholestasis 1 week after the surgery. With the passage of time and damage caused by the accumulation of toxic bile acids in the liver, the fibrosis spread over the whole organ and cholestasis occurred (1 week after the surgery). At the same time. with an equal number of rats, all the operations except BDL were conducted. These rats were considered sham-operated [23].
In addition, 7 days after the surgery following taking blood samples in accordance with protocols approved by ethical considerations about working with laboratory animals, the rats were sacrificed by blood sampling of their hearts after the induction of anesthesia with ether. The blood samples were taken in heparinized tubes, and plasma samples were taken from them. Three parts of the liver tissue were isolated from animals: for histopathological examination, gene expression (stored in liquid nitrogen at -70 °C), and oxidative stress markers (frozen and homogenized with a homogenizer in phosphate-buffered saline (pH = 7.4 and 50 mM).
2.5. Biochemical analysis
To determine the extent of liver damage in the isolated plasma, critical liver enzyme parameters that included ALT [16], AST, ALP, total bilirubin, and total protein were assessed. The content of tissue hydroxyproline was analyzed according to Ehrlich's reagent [24].
2.6. Determination of oxidative stress
Ferric reducing ability of plasma (FRAP) was measured for the determination of plasma ability to reduce ferric ions (Fe3+). By the reduction of Fe3+ and their conversion to ferrous ions (Fe2+) at acidic pH in the existence of Tripyridyl-s-triazine (TPTZ), the blue Fe-TPTZ complex was produced. The resultant color intensity could be spectrophotometrically determined at a wavelength of 593 nm. This reaction was nonspecific and any molecule with the ability to reduce Fe3+ under the above-mentioned conditions could participate in it [25]. The serum concentration of nitric oxide metabolites (nitrite and nitrate content) was measured by the Griess method [10]. For the measurement of protein sulfhydryl amount, 150 μL of Tris base EDTA buffer, 790 μL of absolute methanol solution, and 50 μL of homogenized liver was added into the tube. After mixing, 50 μL of DTNB solution was added and vortexed again. In another tube, 50 μL of the homogenized tissue without DTNB reagent were mixed (blank sample). The reagents were added to the third tube; however, no spilled protein was added (reagent blank). The blank sample and reagent blank were omitted, the additional absorption of protein and DTNB reagent, respectively. Therefore, what remains was the light absorption of thiol groups. The test tubes were incubated at room temperature for 15 min, and then their OD was read at 412 nm [26]. To determine the protein carbonyl concentration, the reaction between 2,4-dinitrophenylhydrazine and carbonyl protein was done; as a result, its light absorption could be measured [10]. The content of MDA in was determined after the reaction with TBA and calculation of the light absorption of the red color at 412 nm [27].
2.7. Determination of antioxidant enzyme
CAT activity in red blood cells was measured by the Hugo Aebi technique. In this procedure, the decomposition rate of the H2O2 substrate at 240 nm was measured with a spectrophotometer [28]. SOD activity was measured based on its ability to inhibit the oxidation of nicotinamide adenine dinucleotide phosphate (NADPH). Nucleotide oxidation changes were measured by a spectrophotometer at 340 nm and physiological pH [29].
2.8. mRNA levels of IL-1, TNF-alpha, TGF-β and, α-SMA
Real-time polymerase chain reaction (RT-PCR) calculated liver IL-1, TNF-alpha, TGF-β and, α-SMA mRNA. Total RNA was isolated and cDNA was generated by total RNA and cDNA Synthesis kit (Sinaclon, Tehran, Iran). RT-PCR was done by the instrument Rotor Gene 3000 (Bio-Rad, USA). The PCR was completed in 40 cycles. Data of IL-1, TNF-alpha, TGF-β and, α-SMA were determined relative to GAPDH utilizing the 2–ΔCt formula.
2.9. HPLC analysis
A Knauer HPLC system (Berlin, Zehlendarf, Germany), consisting of a Knauer (2500 basic model) UV detector operating at the wavelength of 250 nm was used in this work. The applied column in the work was a C18 RP column (Eurospher 100-5 C18, 4 mm × 250 mm, 5 μm particle size). The methanol (55%), acetonitrile (15%), and water (30%, containing 0.5% of orthophosphoric acid) were selected as the mobile phase. After setting the flow rate to 1 mL min−1 and optimizing the chromatographic conditions, all tests were done at room temperature.
2.10. Histological evaluation
For histopathological examination, the liver tissue samples were first fixed in 10% formalin buffer. In the next step, dehydration of the samples was carried out using alcohol, and finally, the dehydrated samples were molded in paraffin. Then, 5-micron tissue sections were prepared and stained using hematoxylin and eosin.
2.11. Statistical evaluation
The data were analyzed by SPSS software (Version 16). The normality of data was determined and the one-way analysis of variance was used with Tukey's post hoc test. The data were stated as Mean ± SEM. P < 0.05 was described as significant for all data. The graphs were created in PRISM.
3. Results
3.1. Biochemical parameters
The liver injury was evaluated by the determination of the plasma levels of liver enzymes. As shown Figure 1, the plasma levels of AST, ALP, ALT, total protein, and total bilirubin were markedly increased in the BDL rat when compared with the SC group (P < 0.05). The methanolic extract of PO at a dose of 500 mg/kg significantly decreased the plasma levels of AST, ALT, and ALP in rats as compared to BDL group for 7 days (P < 0.05). There were no significant changes in the plasma levels of total bilirubin and total protein in comparison to the BDL rats.
Figure 1.
Effect of methanolic extract of Portulaca oleracea on plasma biochemical parameters in BDL-induced rats. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; BDL: Bile duct ligation; SC: Sham control; and PO: Portulaca oleracea. Each value shows the Mean ± SEM, ∗significant difference as compared to the SC group (p < 0.05), and #significant difference as compared to the BDL group (P < 0.05).
3.2. Liver tissue oxidative stress markers
Our finding showed a significant decrease of the total thiol (TSH) level and a significant increase of MDA in the BDL group as compared to the SC group (P < 0.05). Treatment of BDL rats with methanolic extract of PO at a dose of 500 mg/kg had no significant effect on the levels of oxidative stress markers in the hepatic tissue, which include MDA, FRAP, and TSH (Table 1).
Table 1.
Effect of methanolic extract of Portulaca oleracea on liver oxidative stress markers in BDL-induced rats.
| Groups | SC | BDL alone | SC + PO (500) mg/kg) | BDL + PO (500) |
|---|---|---|---|---|
| FRAP (μmol/g tissue) | 89.24 ± 6.35 | 81.55 ± 5.67 | 76.23 ± 3.88 | 104.21 ± 17.49 |
| TSH (μmol/g tissue) | 0.30 ± 0.008 | 0.24 ± 0.017∗ | 0.29 ± 0.015 | 0.27 ± 0.011 |
| MDA (nmol/g tissue) | 30.04 ± 1.01 | 32.69 ± 0.51∗ | 28.12 ± 0.28 | 32.13 ± 0.77 |
FRAP: The ferric reducing antioxidant power; TSH: total thiol group; MDA: malondialdehyde; BDL: Bile duct ligation; SC: Sham control; PO: Portulaca oleracea.
Each value represents the Mean ± SEM. ∗Significant difference as compared to the SC group (p < 0.05).
3.3. Plasma oxidative stress parameters
As shown in Table 2, the plasma levels of MDA and FRAP were significantly increased in the BDL group as compared to SC group (P < 0.05), while the treatment of BDL rats with methanolic extract of PO at a dose of 500 mg/kg significantly decreased these markers (P < 0.05).
Table 2.
Effect of methanolic extract of Portulaca oleracea on plasma oxidative stress markers in BDL-induced rats.
| Groups | SC | BDL alone | SC + PO (500) mg/kg) | BDL + PO (500) |
|---|---|---|---|---|
| FRAP (μmol/ml) | 416.91 ± 66.10 | 1376.18 ± 114.39∗ | 366.14 ± 84.55 | 1054.31 ± 107.47∗ |
| MDA (μmol/ml) | 388.62 ± 17.12 | 782.05 ± 54.71∗ | 394.23 ± 20.37 | 653.84 ± 35.58∗ |
FRAP: MDA: malondialdehyde; the ferric-reducing antioxidant power; BDL: Bile duct ligation; SC: Sham control; and PO: Portulaca oleracea.
Each value shows the Mean ± SEM. ∗Significant difference in comparison to the SC group (p < 0.05).
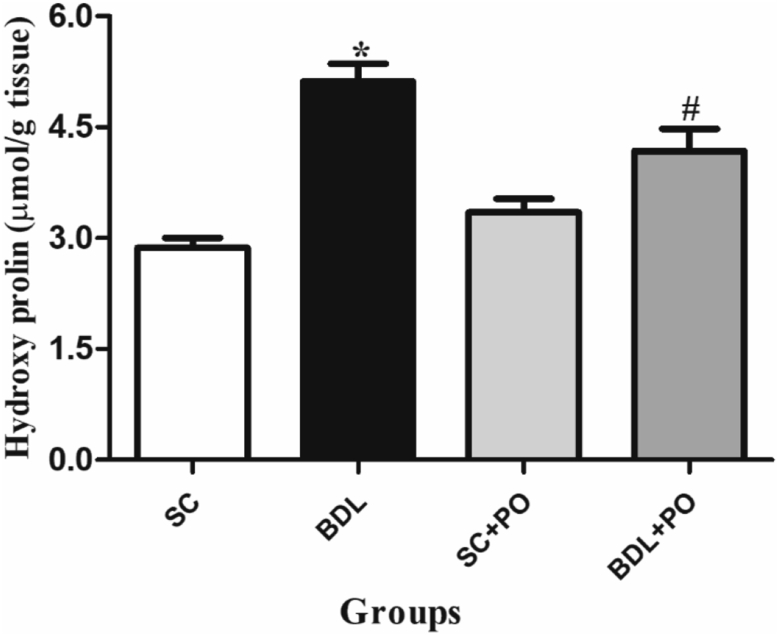
3.4. Hydroxyproline assay
There was a significant increase in hydroxyproline (μg/g dried tissue) content in the hepatic tissue after BDL in comparison to the SC group (P < 0.05). Administration of PO at a dose of 500 mg/kg attenuated hydroxyproline content in the cholestatic liver as contrast to the nontreated BDL group (P < 0.05) (Figure 2).
Figure 2.
Effect of methanolic extract of Portulaca oleracea on hydroxyproline content in the liver tissue (μmol/g tissue) of BDL-induced rats. BDL: Bile duct ligation; SC: Sham control; and PO: Portulaca oleracea Each value shows the Mean ± SEM; ∗significant difference in comparison to the SC group (p < 0.05) and #significant difference as compared to the BDL group (P < 0.05).
3.5. Antioxidant enzymes
As shown in Table 3, the activity of the CAT and SOD enzymes in the BDL group did not show any significant alteration as compared to other groups (P < 0.05).
Table 3.
Effect of methanolic extract of Portulaca oleracea on the antioxidant enzymes activity in BDL-induced rats.
| Groups | SC | BDL alone | SC + PO (500) mg/kg) | BDL + PO (500) mg/kg) |
|---|---|---|---|---|
| CAT (U/ml) | 8.76 ± 0.46 | 8.13 ± 0.53 | 8.68 ± 0.25 | 9.12 ± 0.40 |
| SOD (U/ml) | 188.21 ± 9.37 | 195.67 ± 10.23 | 173.47 ± 2.27 | 202.24 ± 13.55 |
CAT: Catalase; SOD: Superoxide dismutase. BDL: Bile duct ligation; SC: Sham control; and PO: Portulaca oleracea.
Each value shows the Mean ± SEM.
3.6. Histological examinations
SC group showed that normal cellular structures with certain hepatic cells and nuclei were clearly observed. Severe biliary duct hyperplasia, tissue fibrosis, and inflammatory infiltration of white blood cells were detected in the BDL group in comparison with the SC group (Figure 3). There was a significant increase in bile duct proliferation and inflammation score in the hepatic tissue after BDL in comparison to the SC rats (P < 0.05). Administration of PO at a dose of 500 mg/kg attenuated inflammation and bile duct proliferation score in the cholestatic liver in comparison to the non-treated BDL rats (P < 0.05) (Figure 4).
Figure 3.
Photomicrograph of rat liver stained with hematoxylin and eosin (10 ×). A: Sham control (SC), B: Bile duct ligated (BDL), C: SC treated with 500 mg/kg of the methanolic extract of Portulaca oleracea, and D: BDL treated with 500 mg/kg of the methanolic extract of Portulaca oleracea. In the BDL and BDL + PO group, bile duct proliferation (red arrow) and inflammation of liver tissue (blue arrow) are prominent at this picture.
Figure 4.
Effect of methanolic extract of Portulaca oleracea on bile duct proliferation (%) (A) and inflammation score of liver tissue (B). BDL, bile duct-ligation, SC, sham control; and PO, Portulaca oleracea ∗Markedly different from SC rats, P ≤ 0.05 and #Markedly different from BDL rats, P ≤ 0.05.
3.7. Gene expression
BDL significantly increased the expression of TGF-β, TNF-α, and IL-1 pro inflammatory genes, while it slightly increased SMA-α expression as compared to SC rats. Methanolic extract of PO in BDL rats markedly reduced the expression of TNF-α and TGF-β pro inflammatory genes in comparison to BDL alone rats (Figure 5).
Figure 5.
The effect of methanolic extract of Portulaca oleracea on the mRNA levels of IL-1, TNF-alpha, TGF-β and, α-SMA. BDL: Bile duct ligation; SC: Sham control; and PO: Portulaca oleracea. Each value shows the Mean ± SEM; ∗significant difference in comparison to the SC rats (p < 0.05) and #significant difference as compared to the BDL group (P < 0.05).
3.8. HPLC analysis
The HPLC overlay chromatogram and related calibration curves of MLT were prepared using the ranges of 0.05–20 mg L−1 (Figure 6a). The typical chromatogram of methanolic extract of PO are presented in Figure 6b. On the basis of obtained data injected samples of methanolic extract of PO, the concentration of MLT was determined as 53.97 ± 1.1 mg g−1.
Figure 6.
a) the HPLC overlay chromatogram and related calibration curves (inset in fig) obtained from the standard of melatonin (MLT) at 250 nm and b) in the methanolic extract of Portulaca oleracea.
4. Discussion
Cholestasis is a non-physiological condition that can be induced in rodents by surgical closure of the common bile duct or BDL. This rodent model is widely used to study cholestatic liver damage, fibrogenesis, and the effects of obstructive jaundice [30]. In this study, the hepatoprotective effect of methanolic extract of PO was evaluated in the hepatic cholestatic rat model [4]. The most sensitive biomarkers of cholestasis are elevated levels of ALT, ALP, AST, and total bilirubin, which reflect the metabolism of bile acids in the liver [17]. As expected, BDL significantly increased the plasma levels of ALT, ALP, AST, and total bilirubin, and total protein. Pishva et al (2018) have been reported that plasma level of AST, ALT, and ALP increased due to liver structural integrity destruction. It seems that these parameters were released to blood circulation because of cellular damage [31]. Generally, the elevation of AST is an indicator of liver damage in rats, while ALT elevation is more associated with necrosis status [32]. It is also known that BDL is associated with increased ALP activity, which is particularly characteristic of cholestatic disease. The ALP enzyme is mainly attached to the cell membrane, and this increase may be due to the accumulation of bile salts that damage the membrane, which results in cell lysis and eventually the release of the enzymes into the bloodstream [10]. Serum bilirubin analysis is one of the most common methods used to diagnose liver disease. In agreement with previous study [33], it seems that the increased level of total bilirubin in the BDL group is due to liver cell destruction and bile duct obstruction caused by BDL. In addition, the increase in total protein content was observed in the present study to be a self-healing mechanism in the process of liver regeneration during the response to the acute phase of BDL stress [34]. Traditional Chinese medicine has used Portulaca oleracea, also known as Ma-Chi-Xian, to resolve toxin and clear heat. Various pharmacological effects of this herb, which is commonly known in Chinese folklore medicine as "vegetable for long life," have been investigated and validated by scientific studies [11].
Previous study exhibited that PO is rich in vitamins, minerals, omega-3 fatty acids, and a wide range of phytochemicals such as alkaloids, terpenoids, flavonoids, and organic acids [35]. Also, we showed that MLT is present in the methanolic extract of Portulaca oleracea. Anti-hyperglycemic and anti-hyperlipidemic, renoprotective, and hepatoprotective effects are considered as the most important medicinal effects of PO [36]. Behravan et al (2011) exerts that PO extract prevented oxidative DNA damage, which is likely related to its antioxidant constituents [37]. There are several studies [38, 39, 40] that revealed the hepatoprotective effect of PO by diverse mechanisms. In a study by Prabhakaran et al. (2010), it has been shown that the administration of methanol extract of PO at oral dose of 200 and 400 mg/kg had remarkable hepatoprotective effects against D-galactosamine [38]. Ahmida et al. (2010) showed that PO inhibited paracetamol-induced hepatotoxicity by the limitation of the liver injury induced by paracetamol [39]. PO extract administration showed protective effects against CCl4-induced damage in rat liver by normalized liver function tests and SOD enzyme [40]. In our work, the methanolic extract of PO significantly decreased the levels of AST, ALP, and ALT in BDL rats as compared to BDL alone group. Decreased activity of liver enzymes after treatment with PO extract is a clear sign of protection against liver damage, accelerate the regeneration of parenchymal cells, and reduce the leakage of enzymes into circulation. Another inflammatory effect of cholestasis is the penetration of mast cells into the damaged bile ducts, which in turn induced a series of dynamic events such as biliary hyperplasia and excessive secretion of histamine [41] According to liver tissue samples, we conclude that methanolic extract of PO decreases liver injury as it significantly decreases bile duct proliferation and inflammation of the BDL group.
Oxidative stress is the main characteristic of cholestasis, which is due to the imbalance of the anti-oxidative and oxidative system. It has been reported that impaired oxidative stress regulation may play an important role in BDL-induced cholestatic damage [42]. In addition, Zhao et al (2015) showed that the buildup of bile acids in liver cells leads to apoptosis and necrosis and the stimulation of the formation of reactive oxygen species (ROSs) [11]. The ROSs can cause the oxidation of lipids and proteins. MDA, as a cytotoxic product of cells, is derived from lipid peroxidation and necrotic damage [17]. Samarghandian et al (2017) declared that MDA could merge with biomolecules and cause cellular disturbance. They demonstrated PO decrease in MDA and increased glutathione and FRAP level in streptozotocin-induced diabetic rats [16]. In our study, the administration of methanolic extract of PO significantly decreases FRAP and MDA serum levels of cholestatic rats. Several studies indicated that the PO extract is full of compounds with strong antioxidant activity such as flavonoids, saponins, tannins, minerals, melatonin, and ascorbic acid [11, 43, 44, 45]. In this sense, these compounds can inhibit lipid peroxidation and chelate metal ions by competing with and trapping free radicals.
Determination of hydroxyproline levels in the liver tissue is a standard and appropriate method that used to evaluate the amount of collagen production and the effectiveness of liver protective agents. Ligation of the bile ducts results in the reflux of bile acids toward the liver tissue, which subsequently destroys the liver cells and induces inflammatory responses characterized by the activation of Kupffer cells and accumulation of leukocytes. These inflammatory cells produce ROSs that can promote collagen deposition [10]. Treatment with the methanolic extract of PO significantly decreases the hydroxyproline level in the liver tissue of cholestatic rats. Apparently in the PO-treated group, hepatocyte destruction, necrosis, and the infiltration of inflammatory cells were improved, thus collagen deposition was significantly reduced.
Hepatic stellate cells are the main source of collagen production in liver injury [46]. On the other hand, evidence has shown that the activation of inflammatory signaling cascades is associated with oxidative stress [16]. Recent studies have emphasized the essential role of inflammation in the creation and progression of liver damage during cholestasis [44]. The cells involved in inflammatory responses are the population of resident macrophages in the liver (Kupffer cells). Active Kupffer cells can penetrate the liver parenchyma and induce the secretion of proinflammatory cytokines such as TNF-α and TGF-β. TNF-α, as an endogenous central mediator, regulates hepatic injury through direct cytotoxicity and inflammatory cascade formation [47]. Also, TGF-β is the strongest proinflammatory factor that plays a vital role in initiating and maintaining fibrogenesis. TGF-β accelerates the activation of liver stellate cells and stimulates their conversion to myofibroblasts [48]. Consistent with our findings, Ali et al. (2011) stated that PO had a prophylactic and therapeutic value on cholestasis-induced liver fibrosis by inhibiting oxidative stress, reducing the expression of profibrogenic cytokines, collagenolytic activity, and hepatic stellate cells activation [48]. In concordance with previous studies, ligation of the bile ducts significantly increased the expression of proinflammatory genes [49, 50, 51]. In our experiments, the administration of methanolic extract of PO significantly decreases the overexpression of TGF-β and TNF-α proinflammatory genes in BDL rats. PO probably protects liver cells from inflammatory damage caused by BDL by inhibiting inflammatory mediators and blocking hepatic peroxidants markers.
5. Conclusion
Altogether, our finding demonstrated that PO can significantly decrease liver enzymes like AST, ALT, and ALP. It can alleviate liver injury by a decrease in inflammation and hydroxyproline content. Finally, we could suggest that PO extract can be a good choice as a curative agent or adjunctive therapeutic agent for cholestatic liver damage.
Declarations
Author contribution statement
Amir Hossein Doustimotlagh: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Zahra Moslemi; Mina Bahrami; Zahra Daneshyar; Nasrin Shakeri; Arash Asfaram; Esmaeel Panahi kokhdan; Zahra Barmoudeh: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Ebrahim Hosseini, Mahboubeh Mansourian, Mahdieh Eftekhari: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Vice-Chancellor for Research of Yasuj University of Medical Sciences, I.R. Iran (Grant number: 980035).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Devi S. 2018. Structure and Function of Hepatic Parenchymal Cells. [Google Scholar]
- 2.Williams R. Global challenges in liver disease. Hepatology. 2006;44(3):521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 3.Costa ELdO., Azevedo GMd, Jr., Petroianu A. Morphological changes in the liver and kidneys of rats subjected to terminal ileum exclusion during obstructive cholestasis. Acta Cir. Bras. 2014;29(6):353–358. doi: 10.1590/s0102-86502014000600001. [DOI] [PubMed] [Google Scholar]
- 4.Lin T.-K., Huang L.-T., Huang Y.-H., Tiao M.-M., Tang K.-S., Liou C.-W. The effect of the red wine polyphenol resveratrol on a rat model of biliary obstructed cholestasis: involvement of anti-apoptotic signalling, mitochondrial biogenesis and the induction of autophagy. Apoptosis. 2012;17(8):871–879. doi: 10.1007/s10495-012-0732-3. [DOI] [PubMed] [Google Scholar]
- 5.Konerman M., Loomba R. 2016. The burden and Aetiology of Liver Cirrhosis, and the Risk of Death. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y., Zhou G., Wang J., Jia L., Zhang P., Li R. Paeoniflorin protects against ANIT-induced cholestasis by ameliorating oxidative stress in rats. Food Chem. Toxicol. 2013;58:242–248. doi: 10.1016/j.fct.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Purucker E., Winograd R., Roeb E., Matern S. Glutathione status in liver and plasma during development of biliary cirrhosis after bile duct ligation. Res. Exp. Med. 1998;198(4):167–174. doi: 10.1007/s004330050100. [DOI] [PubMed] [Google Scholar]
- 8.Ljubuncic P., Tanne Z., Bomzon A. Evidence of a systemic phenomenon for oxidative stress in cholestatic liver disease. Gut. 2000;47(5):710–716. doi: 10.1136/gut.47.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee S., Richert L., Augustijns P., Annaert P. Hepatocyte-based in vitro model for assessment of drug-induced cholestasis. Toxicol. Appl. Pharmacol. 2014;274(1):124–136. doi: 10.1016/j.taap.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 10.Sadeghi H., Azarmehr N., Razmkhah F., Sadeghi H., Danaei N., Omidifar N. The hydroalcoholic extract of watercress attenuates protein oxidation, oxidative stress, and liver damage after bile duct ligation in rats. J. Cell. Biochem. 2019;120(9):14875–14884. doi: 10.1002/jcb.28749. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y.-X., Xin H.-L., Rahman K., Wang S.-J., Peng C., Zhang H. Portulaca oleracea L.: a review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/925631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim Y.Y., Quah E.P. Antioxidant properties of different cultivars of Portulaca oleracea. Food Chem. 2007;103(3):734–740. [Google Scholar]
- 13.Park S.-H., Kim D.-K., Bae J.-H. The antioxidant effect of Portulaca oleracea extracts and its antimicrobial activity on Helicobacter pylori. Korean J. Food Nutr. 2011;24(3):306–311. [Google Scholar]
- 14.Kafi M., Rahimi Z. Effect of salinity and silicon on root characteristics, growth, water status, proline content and ion accumulation of purslane (Portulaca oleracea L.) Soil Sci. Plant Nutr. 2011;57(2):341–347. [Google Scholar]
- 15.Chen B., Zhou H., Zhao W., Zhou W., Yuan Q., Yang G. Effects of aqueous extract of Portulaca oleracea L. on oxidative stress and liver, spleen leptin, PARα and FAS mRNA expression in high-fat diet induced mice. Mol. Biol. Rep. 2012;39(8):7981–7988. doi: 10.1007/s11033-012-1644-6. [DOI] [PubMed] [Google Scholar]
- 16.Samarghandian S., Borji A., Farkhondeh T. Attenuation of oxidative stress and inflammation by Portulaca oleracea in streptozotocin-induced diabetic rats. J. Evid. Based Comp. Altern. Med. 2017;22(4):562–566. doi: 10.1177/2156587217692491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao J.-Y., Li H.-W., Liu F.-G., Li Y.-C., Tian S., Cao L.-H. Effects of Portulaca oleracea extract on acute alcoholic liver injury of rats. Molecules. 2019;24(16):2887. doi: 10.3390/molecules24162887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira F., Facincani I., Jorgetti V., Ramalho L.N., Volpon J.B., dos Reis L.M. Etiopathogenesis of hepatic osteodystrophy in Wistar rats with cholestatic liver disease. Calcif. Tissue Int. 2009;85(1):75–83. doi: 10.1007/s00223-009-9249-3. [DOI] [PubMed] [Google Scholar]
- 19.Pastor A., Collado P.S., Almar M., González-Gallego J. Antioxidant enzyme status in biliary obstructed rats: effects of N-acetylcysteine. J. Hepatol. 1997;27(2):363–370. doi: 10.1016/s0168-8278(97)80183-3. [DOI] [PubMed] [Google Scholar]
- 20.Ackerman Z., Weinreb M., Amir G., Pollak R.D. Bone mineral metabolism and histomorphometry in rats with cholestatic liver disease. Liver. 2002;22(2):166–172. doi: 10.1046/j.0106-9543.2002.01566.x. [DOI] [PubMed] [Google Scholar]
- 21.Nadimi M., Mohammadali Z., Madani M. 2013. The Effect of Aqueous and Ethanolic Extracts of Teucrium Polium on Candida Albicans and Two Species of Malassezia. [Google Scholar]
- 22.Anusha M., Venkateswarlu M., Prabhakaran V., Taj S.S., Kumari B.P., Ranganayakulu D. Hepatoprotective activity of aqueous extract of Portulaca oleracea in combination with lycopene in rats. Indian J. Pharmacol. 2011;43(5):563. doi: 10.4103/0253-7613.84973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doustimotlagh A.H., Dehpour A.R., Etemad-Moghadam S., Alaeddini M., Kheirandish Y., Golestani A. Nitrergic and opioidergic systems affect radiographic density and histomorphometric indices in bile-duct-ligated cirrhotic rats. Histol. Histopathol. 2017;32(7):743–749. doi: 10.14670/HH-11-836. [DOI] [PubMed] [Google Scholar]
- 24.Lin S.-Y., Wang Y.-Y., Chen W.-Y., Liao S.-L., Chou S.-T., Yang C.-P. Hepatoprotective activities of rosmarinic acid against extrahepatic cholestasis in rats. Food Chem. Toxicol. 2017;108:214–223. doi: 10.1016/j.fct.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Asemi Z., Jazayeri S., Najafi M., Samimi M., Shidfar F., Tabassi Z. Association between markers of systemic inflammation, oxidative stress, lipid profiles, and insulin resistance in pregnant women. ARYA Atheroscl. 2013;9(3):172. [PMC free article] [PubMed] [Google Scholar]
- 26.Habeeb A. [37] Reaction of protein sulfhydryl groups with Ellman's reagent. Methods Enzymol. 1972;25:457–464. doi: 10.1016/S0076-6879(72)25041-8. Elsevier. [DOI] [PubMed] [Google Scholar]
- 27.Ardjmand A., Shahaboddin M.E., Mazoochi T., Ghavipanjeh G. Ameliorative effects of cerebrolysin against isoproterenol-induced myocardial injury in male rats. Life Sci. 2019;227:187–192. doi: 10.1016/j.lfs.2019.04.056. [DOI] [PubMed] [Google Scholar]
- 28.Aebi H. 1984. Catalase in Vitro Methods Enzymol 105: 121–126. Find This Article Online. [DOI] [PubMed] [Google Scholar]
- 29.Paoletti F., Mocali A. [18] Determination of superoxide dismutase activity by purely chemical system based on NAD (P) H oOxidation. Methods Enzymol. 1990;186:209–220. doi: 10.1016/0076-6879(90)86110-h. Elsevier. [DOI] [PubMed] [Google Scholar]
- 30.Georgiev P., Jochum W., Heinrich S., Jang J., Nocito A., Dahm F. Characterization of time-related changes after experimental bile duct ligation. Br. J. Surg. 2008;95(5):646–656. doi: 10.1002/bjs.6050. [DOI] [PubMed] [Google Scholar]
- 31.Pishva S.P., Elaheh Mousavi E., Mousavi Z., Jaafari M.R., Dehpour A.R., Rezayat Sorkhabadi S.M. The effect of berberine nanomicelles on hepatic cirrhosis in bile duct-ligated rats. Nanomed. J. 2018;5(4):199–209. [Google Scholar]
- 32.Arya A., Azarmehr N., Mansourian M., Doustimotlagh A.H. Inactivation of the superoxide dismutase by malondialdehyde in the nonalcoholic fatty liver disease: a combined molecular docking approach to clinical studies. Arch. Physiol. Biochem. 2019:1–8. doi: 10.1080/13813455.2019.1659827. [DOI] [PubMed] [Google Scholar]
- 33.Hua W., Zhang S., Lu Q., Sun Y., Tan S., Chen F. Protective effects of n-Butanol extract and iridoid glycosides of Veronica ciliata Fisch. Against ANIT-induced cholestatic liver injury in mice. J. Ethnopharmacol. 2021;266:113432. doi: 10.1016/j.jep.2020.113432. [DOI] [PubMed] [Google Scholar]
- 34.Fang J., Luo L., Ke Z., Liu C., Yin L., Yao Y. Polydatin protects against acute cholestatic liver injury in mice via the inhibition of oxidative stress and endoplasmic reticulum stress. J. Funct. Foods. 2019;55:175–183. [Google Scholar]
- 35.Nemzer B., Al-Taher F., Abshiru N. Phytochemical composition and nutritional value of different plant parts in two cultivated and wild purslane (Portulaca oleracea L.) genotypes. Food Chem. 2020;320:126621. doi: 10.1016/j.foodchem.2020.126621. [DOI] [PubMed] [Google Scholar]
- 36.Iranshahy M., Javadi B., Iranshahi M., Jahanbakhsh S.P., Mahyari S., Hassani F.V. A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017;205:158–172. doi: 10.1016/j.jep.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Behravan J., Mosafa F., Soudmand N., Taghiabadi E., Razavi B.M., Karimi G. Protective effects of aqueous and ethanolic extracts of Portulaca oleracea L. aerial parts on H2O2-induced DNA damage in lymphocytes by comet assay. J. Acupunc. Meri. Stud. 2011;4(3):193–197. doi: 10.1016/j.jams.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Prabhakaran V., Kumar B.S.A., Shekar D.S., Nandeesh R., Subramanyam P., Ranganayakulu D. Evaluation of the hepatoprotective activity of Portulaca oleracea L. on D-galactosmaine-induced hepatic injury in rats. Bol. Latinoam. Caribe Plantas Med. Aromat. 2010;9(3):199–205. [Google Scholar]
- 39.Ahmida M.H. Evaluation of in vivo antioxidant and hepatoprotective activity of Portulaca oleracea L. against paracetamol-induced. Am. J. Pharmacol. Toxicol. 2010;5(4):167–176. [Google Scholar]
- 40.Eidi A., Mortazavi P., Moghadam J.Z., Mardani P.M. Hepatoprotective effects of Portulaca oleracea extract against CCl4-induced damage in rats. Pharmaceut. Biol. 2015;53(7):1042–1051. doi: 10.3109/13880209.2014.957783. [DOI] [PubMed] [Google Scholar]
- 41.Hargrove L., Kennedy L., Demieville J., Jones H., Meng F., DeMorrow S. Bile duct ligation–induced biliary hyperplasia, hepatic injury, and fibrosis are reduced in mast cell–deficient KitW-sh mice. Hepatology. 2017;65(6):1991–2004. doi: 10.1002/hep.29079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamza A.A. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem. Toxicol. 2010;48(1):345–355. doi: 10.1016/j.fct.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Erkan N. Antioxidant activity and phenolic compounds of fractions from Portulaca oleracea L. Food Chem. 2012;133(3):775–781. [Google Scholar]
- 44.Rahimi V.B., Ajam F., Rakhshandeh H., Askari V.R. A pharmacological review on Portulaca oleracea L.: focusing on anti-inflammatory, anti-oxidant, immuno-modulatory and antitumor activities. J. Pharmacopuncture. 2019;22(1):7. doi: 10.3831/KPI.2019.22.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derouiche S., Abbas K., Djermoune M. Polysaccharides and Ascorbic Acid Content and the Effect of Aqueous Extract of Portulaca Oleracea in High-Fat Diet-Induced Obesity, Dyslipidemia and Liver Damage in Albino Wistar Rats= Contenu des Polysaccharides et Acide Ascorbique et Effet de l'Extrait Aqueux de Portulaca Oleracea sur l'Obesite, la Dyslipidemie et les Dommages Hepatiques Induit par un Regime Hypergras chez les Rats Albino Wistar. Algerian J. Arid Environ. 2017;258(5779):1–11. [Google Scholar]
- 46.Duval F., Moreno-Cuevas J.E., González-Garza M.T., Rodríguez-Montalvo C., Cruz-Vega D.E. Protective mechanisms of medicinal plants targeting hepatic stellate cell activation and extracellular matrix deposition in liver fibrosis. Chin. Med. 2014;9(1):1–11. doi: 10.1186/s13020-014-0027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharifi-Rigi A., Heidarian E., Amini S.A. Protective and anti-inflammatory effects of hydroalcoholic leaf extract of Origanum vulgare on oxidative stress, TNF-α gene expression and liver histological changes in paraquat-induced hepatotoxicity in rats. Arch. Physiol. Biochem. 2019;125(1):56–63. doi: 10.1080/13813455.2018.1437186. [DOI] [PubMed] [Google Scholar]
- 48.Ali S.I., Said M.M., Hassan E.K.M. Prophylactic and curative effects of purslane on bile duct ligation-induced hepatic fibrosis in albino rats. Ann. Hepatol. 2016;10(3):340–346. [PubMed] [Google Scholar]
- 49.Gabele E., Froh M., Arteel G.E., Uesugi T., Hellerbrand C., Scholmerich J. TNFalpha is required for cholestasis-induced liver fibrosis in the mouse. Biochem. Biophys. Res. Commun. 2009;378(3):348–353. doi: 10.1016/j.bbrc.2008.10.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao R.Y., Shearn C.T., Orlicky D.J., Battista K.D., Alexeev E.E., Cartwright I.M. Bile acids modulate colonic MAdCAM-1 expression in a murine model of combined cholestasis and colitis. Mucosal Immunol. 2021;14(2):479–490. doi: 10.1038/s41385-020-00347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geier A., Dietrich C.G., Voigt S., Kim S.K., Gerloff T., Kullak-Ublick G.A. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology. 2003;38(2):345–354. doi: 10.1053/jhep.2003.50317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.