Abstract
In a sustained search for novel and effective antioxidants, a potential therapeutic leads against renal, and neurological disorders. Amongst the heterocycles, pyrazole and their derivatives have been extensively studied for their biological potencies, particularly to a larger extent for their antioxidant properties. Although many of pyrazole derivatives displayed antioxidant activities, still there is a need of developing efficient protocol for their synthesis, involving ecofriendly conditions, molecules of greater antioxidant efficacy and lesser toxicity, etc. In this context, the current study presents an amberlyst-15 catalysed efficient synthesis of 2-pyrazoline derivatives, 5(a-g) via (3 + 2) annulation of chalcones with phenylhydrazines. Structure proofs of new pyrazoles offered by spectral studies, and the molecular structure of compound 5d of the series by crystallographic studies, which revealed an intra molecular hydrogen bond interactions (C–H⋯N type), and stabilization by C–H...π and π---π molecular interactions. Of the series, compounds 5g and 5h show excellent DPPH (IC50 = 0.245 ± 0.01, and 0.284 ± 0.02 μM); and hydroxyl (IC50 = 0.905 ± 0.01, and 0.892 ± 0.01μM) radical scavenging activities comparable with respective controls, ascorbic acid (IC50 = 0.483 ± 0.01μM) and BHA (IC50 = 1.739 ± 0.01μM). The molecular docking and ADME/Tox studies indicate that, these compounds have good antioxidant activity through π-π stacking with Catalase via Try337 and Phe140, and therefore, might be lead antioxidants for further study.
Keywords: Amberlyst-15, Annulation, Antioxidant, Hydroxyl, Chalcone, Docking
Amberlyst-15, annulation, antioxidant, hydroxyl, chalcone, docking.
1. Introduction
Oxidative stress induced by the free radical damage cell membranes, and nucleic acids, which results in aging, cancer, atherosclerosis, and Alzheimer's disease [1]. The studies on small-molecules with antioxidant efficacy to prevent the deleterious effects is important field of research. The drug molecules that contains a pyrazole core remain as choice in medicinal chemistry towards more practical antioxidant agents [2]. The pyrazole derivatives were prepared in good to excellent chemical yields by the silver chloride catalyzed isomerization of α, β-acetylenic hydrazones in dichloromethane at room temperature [3]. The molecular iodine catalyzed reaction of α,β-unsaturated ketones and sulfonyl hydrazide [4], the four-component reaction of dialkyl acetylenedicarboxylates, isocyanides, ethyl acetoacetate and hydrazine/phenylhydrazine produce pyrazoles [5], The (3 + 2) annulation of chalcones with thiosemicarbazide [6], and hydrazines with ynone trifluoroborates [7] gave pyrazoles with regioselectivity. The Chloramine-T catalyzed 1,3-dipolar cycloaddition of hydrazones to alkenes form pyrazoles [8].
Several natural and synthetic pyrazole derivatives possess wide spectrum of pharmacological potentials with druggable properties [9]. For instance, the pyrazole analogs exhibit cytotoxicity against DLA cells, reduces tumour loads, and downregulates tumour progression proliferation [10]. The pyrazole derivatives have antidiabetic and anti-inflammatory [11], antimicrobial [12], and acetylcholinesterase inhibitor [13] properties. The Salvia officinalis L belongs to Lamiaceae family is known for its antioxidant and many pharmaceutical potencies, like anti-spasmodic, astringent, sedative, anti-hyperglycemic, and anti-inflammatory [14]. The complexes isolated from Black Sea marine inverterbrate tissues have displayed antioxidant, antimicrobial and mitogenic activities [15]. Main protease (Mpro) in the life cycle of SARS-CoV-2 mediate viral replication, transcription, and is a drug target for the virus, the computational results reveal a hypothesis for experimental validation [16].
The pyrazole motif is an important core in the development of antioxidants, that alone or combined with other pharmacophore shows high degree of antioxidant activity [17]. For instance, the N-formyl pyrazolines synthesized via Michael addition displayedd promising anticancer and antioxidant [18], and pyrazolo-pyrimidines possess cytotoxic and radical scavenging properties [19]. The reports on the curcumin pyrazole analogs indicated that these alleviate oxidative stress-induced PC12 neuronal damage abilities [20], and pyrazole-thiazoles show antimicrobial and antioxidant activities [21]. The prepared pyrazole-triazole hybrids displayed citotoxic and antioxidant [22], and pyrazole aldehydes have xanthine oxidase inhibitory [23] properties.
Although, a plenty of research on pyrazoles and their biological activities has been reported. Many of the methods adopted for the synthesis requires drastic reaction conditions, and produces less yields, and more toxic bi-products, etc. Furthermore, the most of the pyrazole derivatives exhibited lesser antioxidant properties comparable with the standards employed. In this context, in an attempt towards new therapeutics with greater antioxidant potentials and less toxicity, and to overcome drawbacks of synthetic procedure, the present work has been undertaken. The current study demonstrates the Amberlyst-15 catalyzed reaction of chalcones, and phenylhydrazine hydrochlorides at room temperature to get thienyl-pyrazoles in good yields. The structures of synthesized compounds were characterized through spectral analysis, and one compound by crystallographic studies. After the structural characterization, the new compounds were assessed for free radical scavenging activities. Furthermore, an in silico molecular docking and ADMET analysis was carried out to validate the results and their mode of action.
2. Experimental
2.1. Materials and methods
All the chemicals and reagents procured from Sigma Aldrich were used as received. Pre-coated silica gel-aluminum plates (Merck, F-254) used for thin-layer chromatography (TLC). IR Spectra were obtained on PerkinElmer FT-IR Spectrophotometer, 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were obtained on Agilent NMR spectrometer. Mass spectra were obtained on Lynx SCN781 spectrometer (TOF mode). Absorbance was recorded on ELICO SL 159 UV-Vis spectrophotometer. The un-corrected melting points were with Centex apparatus.
2.2. Synthesis of thienyl-pyrazoles, 5(a-i)
A mixture of chalcones, 3(a-f) (5 mmol), phenylhydrazine hydrochlorides, 4(a-b) (5 mmol), and Amberlyst-15 (10%, w/w) in acetonitrile (25 mL) was stirred at room temperature for 30–60 min. After the completion, the separated solid was filtered, washed with diethyl ether (2 × 20 mL), then treated with ethyl acetate (20 mL), and stirred for 10 min and again filtered. The filtrate was concentrated under vacuum, the solid formed was triturated in diethyl ether, filtered and dried to get the products 5(a-i). Alternatively, the reaction was carried out in 30% acetic acid under boiling conditions.
2.2.1. 5-(3-Methylthiophen-2-yl)-1,3-diphenyl-4,5-dihydro-1H-pyrazole, 5a
Obtained from 3-(3-methylthiophen-2-yl)-1-phenylprop-2-en-1-one, 3a (1.14g, 5 mmol) and phenylhydrazine hydrochloride, 4a (0.72g, 5 mmol); 1H NMR (CDCl3, δ ppm): 2.665 (s, 3H, CH3), 3.004–3.064 (dd, 1H, J = 6.8, 16.8Hz, C4-Ha), 3.731–3.803 (dd, 1H, J = 12.4 17.2Hz, C4-Hb), 5.190–5.237 (dd, 1H, J = 6.4, 12.4 Hz, C5–H), 6.70–6.818 (m, 5H, Ar–H), 6.998–7.063 (m, 4H, Ar–H), 7.208–7.245 (m, 3H, Ar–H); 13C NMR (CDCl3, δ ppm): 16.49 (1C, CH3), 43.661 (1C, C-4), 64.37 (1C, C-5), 105.14 (1C), 111.25 (1C), 119.14 (1C), 123.44 (1C), 125.33 (1C), 125.70 (1C), 126.071 (1C), 126.53 (1C), 127.58 (1C), 128.42 (1C), 128.72 (1C), 129.30 (1C), 129.84 (1C), 134.92 (1C), 141.40 (1C), 142.96 (1C), 145.23 (1C, C-3). MS (m/z): 318.0 (M+, 100); Anal. Calcd. (found) for C20H18N2S (%): C, 75.44 (75.32); H, 5.70 (5.67); N, 8.80 (8.75).
2.2.2. 3-(4-Fluorophenyl)-5-(3-methylthiophen-2-yl)-1-phenyl-4,5-dihydro-1H-pyrazole, 5b
Obtained from 1-(4-fluorophenyl)-3-(3-methylthiophen-2-yl)prop-2-en-1-one, 3b (1.23g, 5 mmol) and phenylhydrazine hydrochloride, 4a (0.72g, 5 mmol); 1H NMR (CDCl3, δ ppm): 2.83 (s, 3H, CH3), 3.01–3.069 (dd, 1H, J = 6.8, 16.8Hz, C4-Ha), 3.737–3.810 (dd, 1H, J = 12.4, 17.2Hz, C4-Hb), 5.197–5.245 (dd, 1H, J = 6.8, 12.4Hz, C5–H), 6.619–6.890 (m, 2H, Ar–H), 7.001–7.068 (m, 5H, Ar–H), 7.152–7.248 (m, 4H, Ar–H); 13C NMR (CDCl3, δ ppm): 13.68 (1C, CH3), 46.42 (1C, C-4), 63.72 (1C, C-5), 111.29 (1C), 113.56 (1C), 115.29 (1C), 116.26 (1C), 119.34 (1C), 121.96 (1C), 125.44 (1C), 126.08 (1C), 126.46 (1C), 127.47 (1C), 128.97 (1C), 129.87 (1C), 130.29 (1C), 130.46 (1C), 131.13 (1C), 131.80 (1C), 143.16 (1C), 163.51 (1C, C-3). MS (m/z): 336.0 (M+, 100); Anal. Calcd. (found) for C20H17FN2S (%): C, 71.40 (71.34); H, 5.05 (5.09); N, 8.33 (8.28).
2.2.3. 1-(3-Chlorophenyl)-3-(4-fluorophenyl)-5-(3-methylthiophen-2-yl)-4,5-dihydro-1H-pyrazole, 5c
Obtained from 1-(4-fluorophenyl)-3-(3-methylthiophen-2-yl)prop-2-en-1-one, 3b (1.23g, 5 mmol) and 3-chlorophenylhydrazine hydrochloride, 4b (0.89g, 5 mmol); 1H NMR (CDCl3, δ ppm): 2.310 (s, 3H, CH3), 3.014–3.073 (dd, 1H, J = 6.8,116.8Hz, C4-Ha), 3.713–3.786 (dd, 1H, J = 12.4, 17.6Hz, C4-Hb), 5.168–5.216 (dd, 1H, J = 6.8, 12.0Hz, C5–H), 6.699–6.809 (m, 4H, Ar–H), 6.995–7.249 (m, 6H, Ar–H); 13C NMR (CDCl3, δ ppm): 14.06 (1C, CH3), 43.72 (1C, C-4), 64.18 (1C, C-5), 111.26 (1C), 113.51 (1C), 119.07 (1C), 125.27 (1C), 125.62 (1C), 126.43 (2C), 127.37 (1C), 128.55 (1C), 129.02 (1C), 129.41 (1C), 131.54 (1C), 134.87 (1C), 137.67 (1C), 138.45 (1C), 142.95 (1C), 145.28 (1C, C-3). MS (m/z): 370.1 (M+, 100), 372.14 (M+2, 33); Anal. Calcd. (found) for C20H16ClFN2S (%): C, 64.77 (64.74); H, 4.35 (4.32); N, 7.55 (7.51).
2.2.4. 1-(3-Chlorophenyl)-3-(4-chlorophenyl)-5-(3-methylthiophen-2-yl)-4,5-dihydro-1H-pyrazole, 5d
Obtained from 1-(4-chlorophenyl)-3-(3-methylthiophen-2-yl)prop-2-en-1-one, 3c (1.31g, 5 mmol) and 3-chlorophenylhydrazine hydrochloride, 4b (0.89g, 5 mmol); 1H NMR (CDCl3, δ ppm): 2.249 (s, 3H, CH3), 2.940–2.998 (dd, 1H, J = 17.2, 6.4Hz, C4-Ha), 3.864–3.937 (dd, 1H, J = 12.0, 17.2Hz, C4-Hb), 5.497–5.543 (dd, 1H, J = 6.4, 12.4Hz, C5–H), 6.592–6.617 (m, 1H, Ar–H), 6.742–6.800 (m, 2H, Ar–H), 7.003–7.154 (m, 5H, Ar–H), 7.384–7.467 (m, 2H, Ar–H); 13C NMR (CDCl3, δ ppm): 13.78 (1C, CH3), 42.18 (1C, C-4), 60.68 (1C, C-5), 101.35 (1C), 105.84 (1C), 108.14 (1C), 110.73 (1C), 113.20 (1C), 119.08 (1C), 120.52 (1C), 126.43 (1C), 128.08 (1C), 129.90 (1C), 132.44 (1C), 134.13 (1C), 134.99 (1C), 137.30 (1C), 145.27 (1C), 148.05 (1C), 148.73 (1C, C-3). MS (m/z): 390.0 (M+4, 10), 388.0 (M+2, 63), 386.0 (M+, 100); Anal. Calcd. (found) for C20H16Cl2N2S (%): C, 62.02 (61.95); H, 4.16 (4.12); N, 7.23 (7.19).
2.2.5. 3-(3-Methoxyphenyl)-5-(3-methylthiophen-2-yl)-1-phenyl-4,5-dihydro-1H-pyrazole, 5e
Obtained from 1-(3-methoxyphenyl)-3-(3-methylthiophen-2-yl)prop-2-en-1-one, 3d (1.27g, 5 mmol) and phenylhydrazine hydrochloride, 4a (0.72g, 5 mmol); 1H NMR (CDCl3, δ ppm): 2.289 (s, 3H, CH3), 3.323–3.368 (dd, 1H, J = 4.4, 5.2Hz, C4-Ha), 3.816 (s, 3H, OCH3), 3.877–3.923 (dd, 1H, J = 11.6, 17.2Hz, C4-Hb), 5.696–5.736 (dd, 1H, J = 4.8, 12.0Hz, C5–H), 6.731 (s, 2H, Ar–H), 6.907–7.023 (m, 5H, Ar–H), 7.345–7.384 (t, 2H, Ar–H), 7.853 (d, 2H, Ar–H); 13C NMR (CDCl3, δ ppm): 13.90 (1C, CH3), 45.82 (1C, C-4), 54.45 (1C, OCH3), 55.43 (1C, C-5), 111.55 (1C), 120.60 (2C), 120.80 (1C), 122.22 (2C), 129.06 (2C), 130.12 (2C), 131.33 (2C), 133.34 (1C), 139.46 (1C), 151.99 (1C), 155.40 (1C), 158.10 (1C, C-3). MS (m/z): 348.0 (M+, 100); Anal. Calcd. (found) for C21H20N2OS (%): C, 72.38 (72.31); H, 5.79 (5.75); N, 8.04 (8.01).
2.2.6. 1-(3-Chlorophenyl)-3-(3-methoxyphenyl)-5-(3-methylthiophen-2-yl)-4,5-dihydro-1H-pyrazole, 5f
Obtained from 1-(3-methoxyphenyl)-3-(3-methylthiophen-2-yl)prop-2-en-1-one, 3d (1.27g, 5 mmol) and 3-chlorophenylhydrazine hydrochloride, 4b (0.89g, 5 mmol); 1H NMR (CDCl3, δ ppm): 2.287 (s, 3H, CH3), 3.161–3.217 (dd, 1H, J = 4.8, 17.6Hz, C4-Ha), 3.680–3.737 (dd, 1H, J = 5.2, 11.2Hz, C4-Hb), 3.827 (s, 3H, OCH3), 5.744–5.786 (dd, 1H, J = 4.8, 11.6Hz, C5–H), 6.736 (s, 1H, Ar–H), 6.821–7.030 (m, 4H, Ar–H), 7.206–7.322 (m, 5H, Ar–H); 13C NMR (CDCl3, δ ppm): 13.91 (1C, CH3), 42.71 (1C, C-4), 54.50 (1C, OCH3), 55.36 (1C, C-5), 111.39 (1C), 116.07 (2C), 119.13 (2C), 122.46 (2C), 129.74 (2C), 130.20 (2C), 132.63 (1C), 133.49 (1C), 139.10 (1C), 151.68 (1C), 155.26 (1C), 159.76 (1C, C-3), 162.93 (1C). MS (m/z): 384.0 (M+2, 33), 382.0 (M+, 100); Anal. Calcd. (found) for C21H19ClN2OS (%): C, 65.87 (65.81); H, 5.00 (4.96); N, 7.32 (7.28).
2.2.7. 5-(3-Methylthiophen-2-yl)-1-phenyl-3-(p-tolyl)-4,5-dihydro-1H-pyrazole, 5g
Obtained from 3-(3-methylthiophen-2-yl)-1-(p-tolyl)prop-2-en-1-one, 3e (1.42g, 10 mmol) and phenylhydrazine hydrochloride, 4a (0.72g, 5 mmol); 1H NMR (CDCl3, δ ppm): 2.298 (s, 3H, CH3), 2.378 (s, 3H, CH3), 3.152–3.213 (dd, 1H, J = 7.6, 16.8 Hz, C4-Ha), 3.768–3.841 (dd, 1H, J = 12.0, 20.8Hz, C4-Hb), 5.389–5.438 (dd, 1H, J = 7.6, 12.0Hz, C5–H), 6.744–6.841 (d, 3H, Ar–H), 7.047–7.247 (m, 6H, Ar–H), 7.609–7.629 (d, 2H, Ar–H); 13C NMR (CDCl3, δ ppm): 14.43 (1C, CH3), 42.06 (1C, C-4), 60.50 (1C, C-5), 110.72 (1C), 113.29 (1C), 119.05 (1C), 125.74 (1C), 125.90 (1C), 128.00 (1C), 129.18 (2C), 129.35 (1C), 129.82 (2C), 130.03 (1C), 132.42 (1C), 134.07 (1C), 134.97 (1C), 139.42 (1C), 145.24 (1C), 148.31 (1C, C-3). MS (m/z): 332.0 (M+, 100); Anal. Calcd. (found) for C21H20N2S (%): C, 75.87 (75.82); H, 6.06 (6.03); N, 8.43 (8.40).
2.2.8. 3-(3,4-Dimethoxyphenyl)-5-(3-methylthiophen-2-yl)-1-phenyl-4,5-dihydro-1H-pyrazole, 5h
Obtained from 1-(3,4-dimethoxyphenyl)-3-(3-methylthiophen-2-yl)prop-2-en-1-one, 3f (1.44g, 5 mmol) and phenylhydrazine hydrochloride, 4a (0.72g, 5 mmol); 1H NMR (CDCl3, δ ppm): 2.314 (s, 3H, CH3), 3.142–3.204 (dd, 1H, J = 8.0, 17.2Hz, C4-Ha), 3.722–3.762 (dd, 1H, J = 4.4, 13.2Hz, C4-Hb), 3.864 (s, 3H, OCH3), 5.425–5.475 (dd, 1H, J = 8.0, 12.0Hz, C5–H), 6.799–6.912 (m, 4H, Ar–H), 7.042–7.153 (m, 2H, Ar–H), 7.207–7.410 (d, 4H, Ar–H); 13C NMR (CDCl3, δ ppm): 13.95 (1C, CH3), 42.94 (1C, C-4), 55.44 (1C, OCH3), 55.47 (1C, OCH3), 59.47 (1C, C-5), 110.64 (1C), 112.82 (1C), 113.83 (1C), 114.20 (1C), 124.32 (1C), 126.27 (1C), 127.44 (1C), 128.91 (1C), 129.57 (1C), 130.12 (1C), 130.32 (1C), 133.92 (1C), 135.66 (1C), 139.72 (1C), 145.27 (1C), 147.25 (1C), 159.72 (1C, C-3). MS (m/z): 378.1 (100); Anal. Calcd. (found) for C22H22N2O2S (%): C, 69.81 (69.74); H, 5.86 (5.83); N, 7.40 (7.36).
2.2.9. 1-(3-Chlorophenyl)-3-(3,4-dimethoxyphenyl)-5-(3-methylthiophen-2-yl)-4,5-dihydro-1H-pyrazole, 5i
Obtained from 1-(3,4-dimethoxyphenyl)-3-(3-methylthiophen-2-yl)prop-2-en-1-one, 3f (1.44g, 5 mmol) and 3-chlorophenylhydrazine hydrochloride, 4b (0.89g, 5 mmol); 1H NMR (CDCl3, δ ppm): 2.304 (s, 3H, CH3), 3.146–3.207 (dd, 1H, J = 7.6, 17.2Hz, C4-Ha), 3.767–3.839 (dd, 1H, J = 12.0, 16.8Hz, C4-Hb), 3.903 (s, 3H, OCH3), 3.977 (s, 3H, OCH3), 5.379–5.428 (dd, 1H, J = 7.6, 12.0Hz, C5–H), 6.75–6.851 (m, 4H, Ar–H), 7.039–7.089 (m, 3H, Ar–H), 7.21–7.251 (m, 1H, Ar–H), 7.481 (s, 1H, Ar–H); 13C NMR (CDCl3, δ ppm): 13.92 (1C, CH3), 43.14 (1C, C-4), 55.93 (1C, OCH3), 55.99 (1C, OCH3), 59.10 (1C, C-5), 108.29 (1C), 110.62 (1C), 111.33 (1C), 113.85 (1C), 119.26 (1C), 119.39 (1C), 123.61 (1C), 125.20 (1C), 129.81 (1C), 130.34 (1C), 132.79 (1C), 134.66 (1C), 139.10 (1C), 146.32 (1C), 148.29 (1C), 149.18 (1C), 150.26 (1C, C-3). MS (m/z): 414.2 (M+, 33), 412.22 (M+, 100); Anal. Calcd. (found) for C22H21ClN2O2S (%): C, 63.99 (63.92); H, 5.13 (5.10); N, 6.78 (6.72).
2.3. X-ray diffraction studies
The brown colored prismatic defect free single crystal of approximate dimension 0.27 × 0.24 × 0.21 mm3 was chosen for X-ray diffraction studies. The X-ray intensity data of the molecule 5d were collected using Rigaku XtaLAB Mini diffractometer with X-ray generator operating at 50 kV, 12 mA and MoKα radiation. Data were collected with χ fixed at 54°, for different settings of ϕ (0° and 360°), the scan width of 0.5° with exposure time of 3 s and the sample to detector distance of 50 mm. The complete data sets were processed by CRYSTAL CLEAR [24]. The crystal structures were solved by SHELXS and SHELXL programs [25, 26]. The geometrical calculations were performed with PLATON [27]. The molecular and packing diagrams were generated using MERCURY [28].
2.4. Biological activity
2.4.1. DPPH radical scavenging activity
The activity of compounds 5(a–i) was performed by a Blois method [29], with different concentrations (2.5, 5.0, 7.5 and 10.0 μM) in methanol. The absorbance was read against blank at 517 nm.
2.4.2. Hydroxyl radical scavenging activity
The experiments were performed according to reported procedure [30]. A solution of phosphate buffer (0.1 mL); 2-deoxyribose (0.2 mL), compounds, 5(a–i) (2.5, 5.0, 7.5 and 10.0 μM), Hydrogen peroxide (0.1 mL, 10 mM), ascorbic acid (0.1 mL, 1 mM), EDTA (0.1 mL), and Ferric chloride (0.01 mL, 100 mM) was incubated at 37 °C for 60 min. After this, a cold 2.8% trichloroacetic acid (1mL) followed by 1% thiobarbituric acid (1mL, 1g/100mL of 0.05 N NaOH) were added, and kept for 15 min in boiling water. The absorbance measured at 535 nm.
2.5. Molecular docking studies
The co-ordinates of Catalase (CAT) (PDB id: 2CAG) and CuZn superoxide dismutase (CuZnSOD) (PDB id: 1CB4) were collected from the Brookhaven Protein Data Bank [31]. The minimal energy of ligands was computed by OPLS 2005. Proteins prepared by retrieving into workspace, and their structure were corrected by prime software module. Water molecules from CAT and CuZnSOD were removed beyond 5 Å from the hetero atoms. The interaction between the receptor, and water molecules were optimized during protein pepwizard [32]. OPLS 2005 force field applied to the protein to restrain minimization and RMSD of 0.30 Å was set to converge heavy atoms before start of docking. Each ligand was docked into the receptor grid of radii 20 Å, and the docking calculation was performed. ADME/Tox properties were calculated with ADME/Tox prediction program [33].
3. Results and discussion
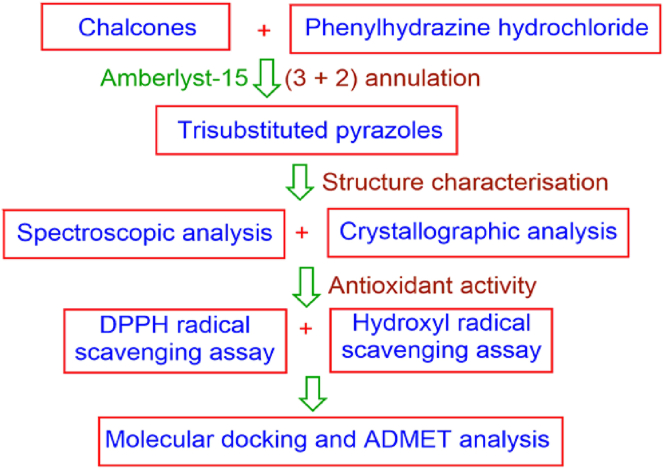
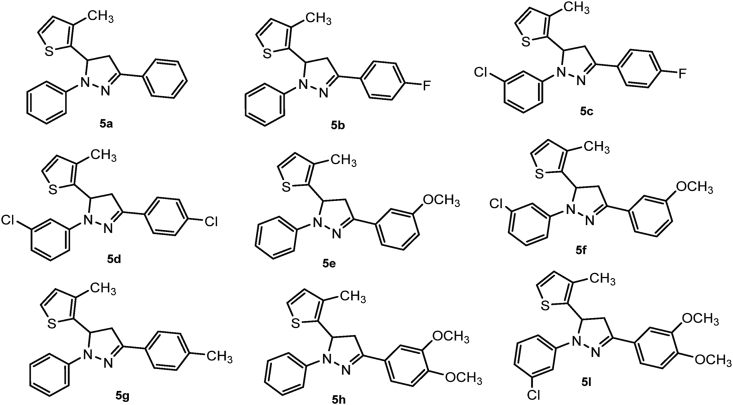
The current investigation presents the synthesis of new pyrazole derivatives, structural characterisation by spectral and crystallographic studies; which follows the assessment of new compounds for their antioxidant activities. Further, to substantiate with the experimental results, the molecular docking and ADMET analysis was performed to substantiate the experimental results. The flowchart showing the methodology is presented on Figure 1. A library of trisubstituted pyrazoles prepared were presented in Figure 2.
Figure 1.
The research methodology flowchart.
Figure 2.
The chemical structures of the synthesized compounds 5(a-i).
3.1. Chemistry
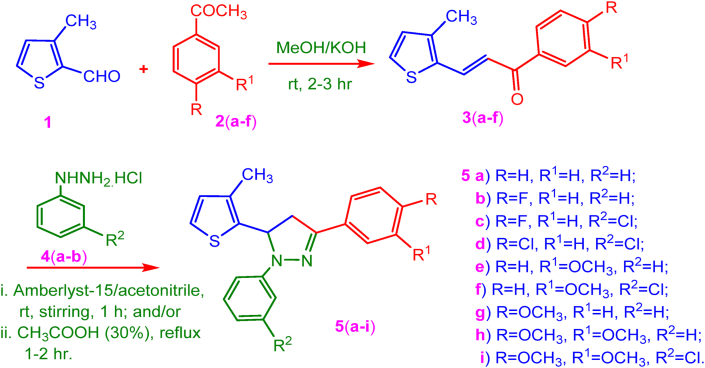
A two-step synthesis of different tri-substituted pyrazole products was performed. In the first step, the required chalcones, 3(a-f) were prepared from thiophene-2-aldehyde 1, with various substituted acetophenone, 2(a-f) in line with our earlier report [34]. In the second step, the chalcones 3(a-f) were subjected to Amberlyst-15 (10%, w/w) catalyzed (3 + 2) annulation reaction with phenylhydrazine hydrochloride 4(a-b) in acetonitrile as solvent. The reaction was performed under swirling conditions. The reaction produced thienyl-pyrazoles, 5(a-i) in good yields. In an alternative method, the compounds 5(a-i) were also obtained by reflux conditions in 30% acetic acid medium without an added catalyst. In this research, cyclocondensation of the chalcones, and phenylhydrazine hydrochlorides produced desired trisubstituted pyrazole derivatives (Figure 3).
Figure 3.
Synthetic route for thienyl-pyrazolines 5(a-i).
Amberlyst-15, (divinylbenzene-styrenesulfonic acid) is a versatile catalyst for the reactions under non-aqueous conditions, it promotes the reaction through its –SO3H proton, in esterification, Prins cyclization, and crossed-aldol condensation etc., [35]. We found that Amberlyst-15 catalyzed synthesis of pyrazole derivatives 5(a-i) occurs in less time with minimum heat energy compared, and an improved product yields (>4–9%) comparable to the conventional reflux conditions method in acetic acid (Table 1). The recovered catalyst can be re-used efficiently.
Table 1.
Reaction time and yields of Amberlyst-15 mediated and conventional synthesis.
| Compound | Amberlyst-15 mediated method |
Conventional Method |
Melting Point (oC) | ||
|---|---|---|---|---|---|
| Time (Min) | Yield (%) | Time (Min) | Yield (%) | ||
| 5a | 52 | 72 | 75 | 68 | 96–98 |
| 5b | 47 | 67 | 75 | 61 | 102–103 |
| 5c | 54 | 78 | 115 | 70 | 102–104 |
| 5d | 50 | 88 | 85 | 79 | 116–117 |
| 5e | 56 | 77 | 110 | 69 | 110–112 |
| 5f | 57 | 70 | 105 | 63 | 120–122 |
| 5g | 45 | 76 | 65 | 71 | 94–96 |
| 5h | 45 | 79 | 70 | 70 | 119–121 |
| 5i | 60 | 78 | 120 | 72 | 149–151 |
3.2. Spectroscopic studies
In the 1H NMR spectra, compounds 5(a-i) show that –CH2- group of the pyrazole ring diastereotopic; and failed to show the signals of C=C protons of 3(a-f), confirming the (3 + 2) annulation. In 1H NMR spectrum, compound 5i show a doublet of doublets for C4-Ha at δ 3.146–3.207 (J = 7.6, 17.2 Hz) ppm; C4-Hb at δ 3.767–3.839 (J= J = 12.0, 16.8 Hz) ppm, and C5–H at δ 5.379–5.428 (J= J = 7.6, 12.0 Hz) ppm. It shows singlets for CH3 at δ 2.304 ppm, for two OCH3 at δ 3.903, δ 3.977 ppm, and multiplet at δ 6.750–6.851 ppm, δ 7.039–7.089 ppm, δ 7.215–7.481 ppm for aromatic protons. In 13C NMR spectrum, it shows signals for C-4, C-5 and C-3 carbons of pyrazoline ring at δ 43.14, 59.10 and 150.26 ppm, respectively. It also shows signals for CH3 at δ 13.92 ppm, for two OCH3 carbons at δ 55.93 and δ 55.99 ppm. In mass spectrum, it shows peaks at m/z 415.2 (M+2) (34%), 413.2 (M+). It shows comparable CHN analysis data with the theoretical values. Analytical and spectral data (1H & 13C NMR, MS) for all compounds 5(a-i) were in full agreement with the proposed structures, and also structurally related pyrazoles [35]. Furthermore, the structure of one compound 5d, of the series was provided by crystallographic studies.
3.3. Single crystal X-ray diffraction studies
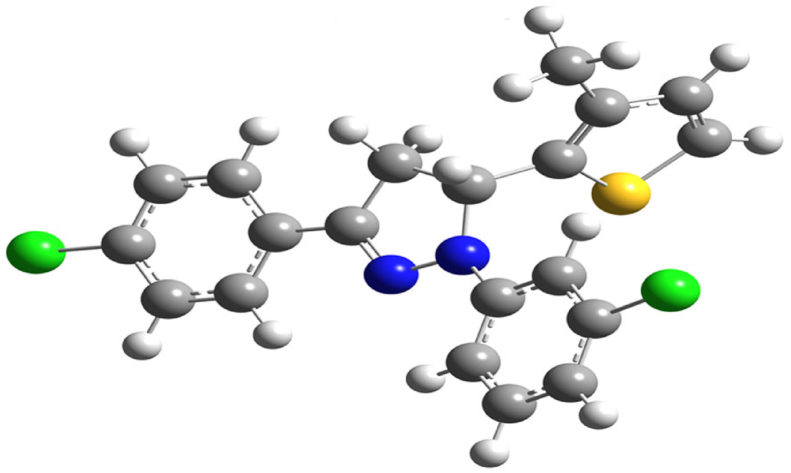
The crystallographic data and structure refinement data of 5d are depicted in Table 2. The ORTEP of the molecule with the thermal ellipsoids drawn with 50% probability is shown in Figure 4. The bond length, bond angles and torsion angles are summarized in Tables 3, 4 and 5. The compound has four aromatic rings, viz; a pyrazole, thiophene, and two chlorophenyl rings. Thiophene ring is planar (r.m.s. deviation is 0.007(5) Å) with maximum deviation (0.006(6) Å) observed for atoms C2 and C3. Pyrazole ring is little non-planar (r.m.s. deviation is 0.096(5) Å) with maximum deviation of 0.082(6) Å for atom C7. Among two chlorophenyl rings, 3-dichlorophenyl ring is planar (r.m.s. deviation is 0.010(6) Å) with maximum deviation (0.012(6) Å) observed for atom C17. Similarly, 4-chlorophenyl ring is also planar (r.m.s. deviation is 0.007(6) Å) with a deviation of 0.008(7) Å for atom C21. The methylthiophene ring is fixed at C7, 3-dichlorophenyl ring fixed at N11, 4-chlorophenyl ring is fixed at C9 and benzodioxole ring is fixed at C11 atom of the pyrazole ring. The chlorine atoms substituted to the corresponding phenyl rings are in the same plane of the rings.
Table 2.
Crystal structure data and refinement details of molecule 5d.
| Parameter | value |
|---|---|
| CCDC deposit No. | 1838509 |
| Empirical formula | C20H16N2SCl2 |
| Formula weight | 387.32 |
| Temperature | 293 K |
| Wavelength | 0.71073 Å |
| Crystal system, space group | Monoclinic, P21/a |
| Unit cell dimensions | a = 17.840(5) Å |
| b = 5.779(4) Å | |
| c = 19.270(7) Å | |
| α = 90◦ β = 112.20(2)◦ γ = 90◦ | |
| Volume | 1839.4(2) Å3 |
| Z | 4 |
| Density(calculated) | 1.399 Mg m −3 |
| Absorption coefficient | 0.471 mm −1 |
| F000 | 800 |
| Crystal size | 0.27 × 0.24 × 0.21 mm |
| θ range for data collection | 3.38o - 27.57o |
| Index ranges | −16 ≤ h ≤ 23 |
| −3 ≤ k ≤ 7 | |
| −24 ≤ l ≤ 25 | |
| Reflections collected | 6493 |
| Independent reflections | 4133 [Rint = 0.0921] |
| Absorption correction | multi-scan |
| Refinement method | Full matrix least-squares on F2 |
| Data/restraints/parameters | 4133/0/227 |
| Goodness-of-fit on F2 | 1.001 |
| Final [I > 2σ(I)] | R1 = 0.0778, wR2 = 0.1763 |
| R indices (all data) | R1 = 0.1873, wR2 = 0.2338 |
| Largest diff. peak and hole | 0.272 and −0.304 e Å −3 |
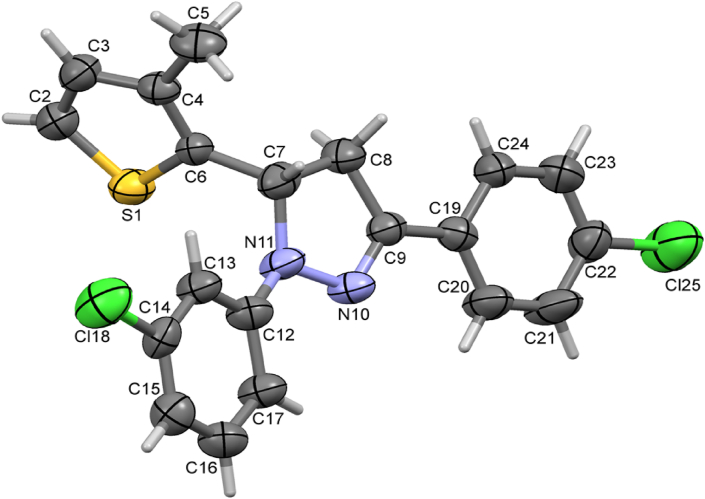
Figure 4.
ORTEP of the compound 5d with thermal ellipsoids drawn at 50% probability.
Table 3.
Bond lengths (Å) of compound 5d.
| Atoms | XRD | DFT | Atoms | XRD | DFT |
|---|---|---|---|---|---|
| Cl18–C14 | 1.743(6) | 1.764 | C8–C9 | 1.506(8) | 1.515 |
| Cl25–C22 | 1.737(6) | 1.757 | C9–C19 | 1.461(8) | 1.463 |
| S1–C2 | 1.701(6) | 1.733 | C12–C13 | 1.400(8) | 1.406 |
| S1–C6 | 1.723(5) | 1.751 | C12–C17 | 1.397(8) | 1.408 |
| N10–N11 | 1.414(5) | 1.371 | C13–C14 | 1.371(8) | 1.393 |
| N10–C9 | 1.295(7) | 1.292 | C14–C15 | 1.363(8) | 1.391 |
| N11–C7 | 1.477(7) | 1.488 | C15–C16 | 1.394(8) | 1.397 |
| N11–C12 | 1.390(7) | 1.401 | C16–C17 | 1.378(8) | 1.389 |
| C2–C3 | 1.370(8) | 1.364 | C19–C20 | 1.385(8) | 1.409 |
| C3–C4 | 1.413(8) | 1.436 | C19–C24 | 1.383(7) | 1.405 |
| C4–C5 | 1.503(8) | 1.507 | C20–C21 | 1.382(9) | 1.388 |
| C4–C6 | 1.371(8) | 1.377 | C21–C22 | 1.374(9) | 1.398 |
| C6–C7 | 1.497(8) | 1.505 | C22–C23 | 1.347(8) | 1.392 |
| C7–C8 | 1.553(8) | 1.556 | C23–C24 | 1.383(9) | 1.394 |
| Correlation Coefficient (CC) | 0.9929 | ||||
Table 4.
Bond angles (o) of compound 5d.
| Atoms | XRD | DFT | Atoms | XRD | DFT |
|---|---|---|---|---|---|
| C2–S1–C6 | 91.8(3) | 91.4 | N11–C12–C13 | 121.0(5) | 120.2 |
| N11–N10–C9 | 108.4(4) | 110.3 | N11–C12–C17 | 120.1(5) | 120.6 |
| N10–N11–C7 | 112.0(4) | 112.3 | C13–C12–C17 | 118.8(5) | 119.3 |
| N10–N11–C12 | 118.2(4) | 118.6 | C12–C13–C14 | 119.9(5) | 119.1 |
| C7–N11–C12 | 123.8(4) | 124.1 | Cl18–C14–C13 | 118.6(4) | 118.3 |
| S1–C2–C3 | 111.5(4) | 111.7 | Cl18–C14–C15 | 119.2(4) | 119.2 |
| C2–C3–C4 | 113.3(5) | 113.6 | C13–C14–C15 | 122.2(5) | 122.4 |
| C3–C4–C5 | 123.5(5) | 122.2 | C14–C15–C16 | 118.3(5) | 117.7 |
| C3–C4–C6 | 111.5(5) | 111.8 | C15–C16–C17 | 121.3(5) | 121.6 |
| C5–C4–C6 | 124.9(5) | 126 | C12–C17–C16 | 119.7(5) | 119.9 |
| S1–C6–C4 | 111.8(4) | 111.6 | C9–C19–C20 | 122.2(5) | 121 |
| S1–C6–C7 | 119.7(4) | 119.9 | C9–C19–C24 | 120.7(5) | 120.7 |
| C4–C6–C7 | 128.4(5) | 128.5 | C20–C19–C24 | 117.1(5) | 118.3 |
| N11–C7–C6 | 113.3(4) | 113.5 | C19–C20–C21 | 121.7(5) | 121 |
| N11–C7–C8 | 101.5(4) | 101.6 | C20–C21–C22 | 119.2(5) | 119.4 |
| C6–C7–C8 | 114.4(5) | 114.3 | Cl25–C22–C21 | 119.6(4) | 119.5 |
| C7–C8–C9 | 102.5(4) | 102.4 | Cl25–C22–C23 | 120.0(5) | 119.6 |
| N10–C9–C8 | 113.7(5) | 112.9 | C21–C22–C23 | 120.5(6) | 120.9 |
| N10–C9–C19 | 122.4(5) | 122.1 | C22–C23–C24 | 120.3(6) | 119.2 |
| C8–C9–C19 | 123.6(5) | 125 | C19–C24–C23 | 121.3(5) | 121.2 |
| Correlation Coefficient (CC) | 0.9954 | ||||
Table 5.
Torsion angles (o) of compound 5d.
| Atoms | XRD | DFT | Atoms | XRD | DFT |
|---|---|---|---|---|---|
| C6–S1–C2–C3 | -0.5 | -0.2 | N11–C7–C8–C9 | -12.8(5) | -6 |
| C2–S1–C6–C4 | 0.5(4) | 0.1 | C7–C8–C9–C19 | -176.0(5) | -177.9 |
| C2–S1–C6–C7 | -176.2(5) | -176.5 | C7–C8–C9–N10 | 9.7(6) | 3.5 |
| C9–N10–N11–C7 | -7.9(5) | -5.4 | N10–C9–C19–C24 | -177.7(5) | -180 |
| C9–N10–N11–C12 | -161.6(5) | -161.6 | C8–C9–C19–C20 | -170.8(5) | -178.6 |
| N11–N10–C9–C8 | -1.7(6) | 0.9 | C8–C9–C19–C24 | 8.5(8) | 1.6 |
| N11–N10–C9–C19 | -176.1(5) | -177.7 | N10–C9–C19–C20 | 3.1(8) | -0.2 |
| C12–N11–C7–C8 | 165.2(4) | 161.8 | N11–C12–C13–C14 | 174.5(5) | 179.5 |
| N10–N11–C12–C13 | 160.8(5) | 167.8 | C17–C12–C13–C14 | -1.5(8) | 0.3 |
| C7–N11–C12–C17 | -173.7(5) | -166.1 | N11–C12–C17–C16 | -173.7(5) | -179.4 |
| N10–N11–C7–C6 | 136.4(4) | 130.4 | C13–C12–C17–C16 | 2.4(8) | -0.1 |
| N10–N11–C7–C8 | 13.2(5) | 7.2 | C12–C13–C14–Cl18 | 176.9(4) | 179.8 |
| N10–N11–C12–C17 | -23.3(7) | -12.9 | C12–C13–C14–C15 | 0.6(8) | -0.2 |
| C12–N11–C7–C6 | -71.6(6) | -74.9 | Cl18–C14–C15–C16 | -176.8(4) | -180 |
| C7–N11–C12–C13 | 10.3(7) | 14.6 | C13–C14–C15–C16 | -0.6(8) | -0.1 |
| S1–C2–C3–C4 | 1.1(6) | 0.3 | C14–C15–C16–C17 | 1.5(8) | 0.2 |
| C2–C3–C4–C6 | -0.8(7) | -0.2 | C15–C16–C17–C12 | -2.4(8) | -0.1 |
| C2–C3–C4–C5 | 179.0(5) | 178.8 | C9–C19–C20–C21 | 178.6(6) | 179.6 |
| C5–C4–C6–S1 | -179.7(4) | -178.9 | C24–C19–C20–C21 | -0.7(9) | 0.2 |
| C5–C4–C6–C7 | -3.4(9) | -2.6 | C9–C19–C24–C23 | -179.3(5) | -179.7 |
| C3–C4–C6–S1 | 0.1(6) | 0.1 | C20–C19–C24–C23 | -0.1(8) | -0.1 |
| C3–C4–C6–C7 | 176.4(5) | 176.3 | C19–C20–C21–C22 | 1.5(9) | 0.2 |
| C4–C6–C7–N11 | 145.2(5) | 145.9 | C20–C21–C22–Cl25 | -179.7(5) | -180 |
| C4–C6–C7–C8 | -99.1(7) | -98.2 | C20–C21–C22–C23 | -1.6(10) | -0.1 |
| S1–C6–C7–C8 | 77.0(5) | 77.8 | Cl25–C22–C23–C24 | 179.0(5) | 180 |
| S1–C6–C7–N11 | -38.8(6) | -38.2 | C21–C22–C23–C24 | 0.9(10) | 0.1 |
| C6–C7–C8–C9 | -135.2(5) | -128.6 | C22–C23–C24–C19 | -0.1(9) | 0.1 |
| Correlation Coefficient (CC) | 0.9995 | ||||
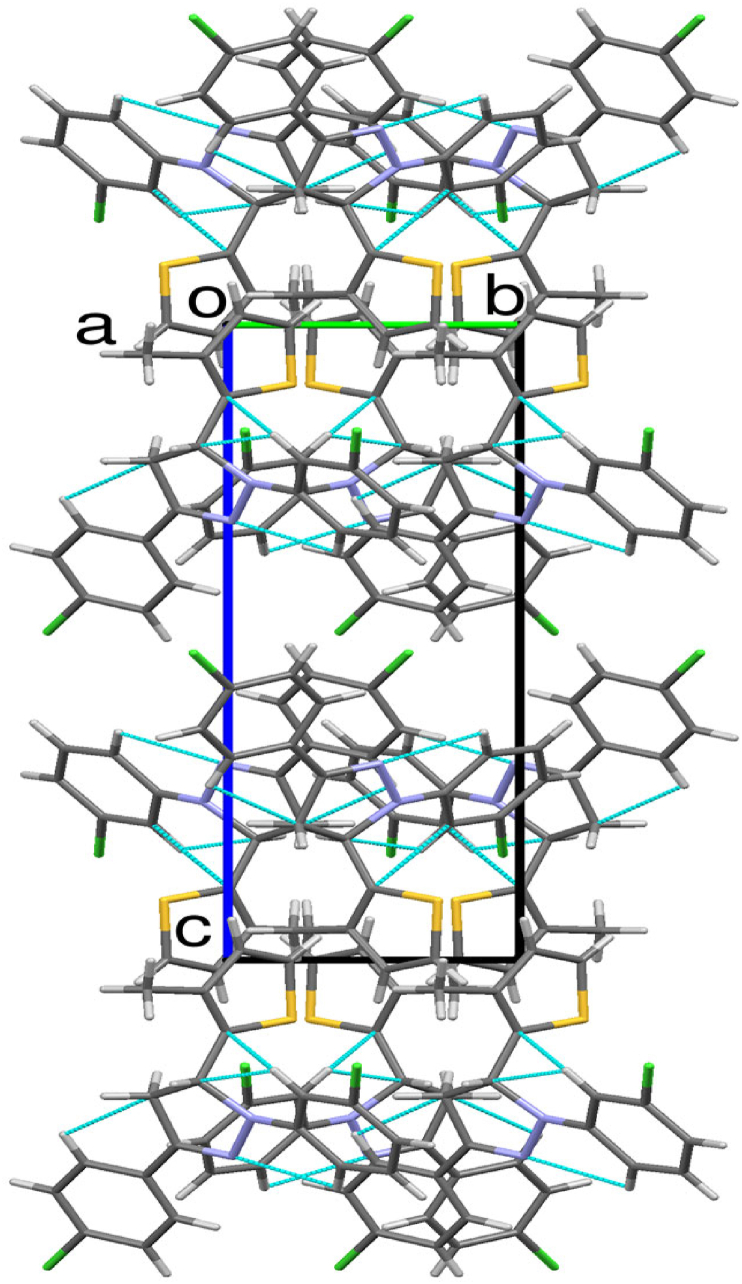
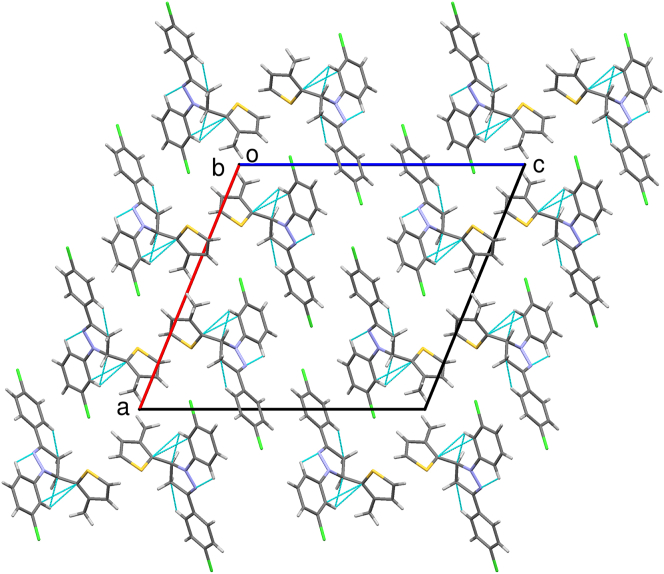
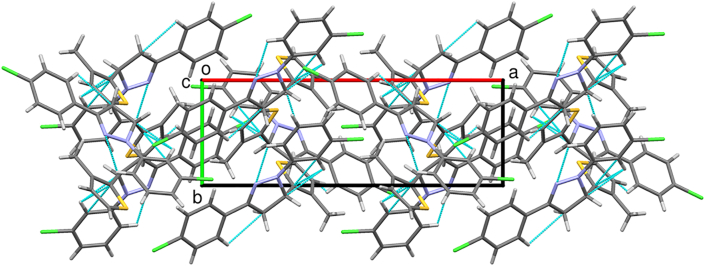
A dihedral angle of 76.3(3)o between the pyrazole and thiophene ring indicates that they lie in different planes. Similarly, the angle between thiophene to 3-chlorophenyl and 4-chlorophenyl rings are 86.5(3)o and 74.1(3)o respectively. The dihedral angle of 13.5(3)o, and 4.0(3)o between pyrazole ring with 3-chlorophenyl and 4-chlorophenyl rings indicates that these coplanar. The analyzed Cremer and Pople puckering parameters (Q = 0.136(6) Å and ϕ = 78(2)o) were in agreement with the structurally identical molecules [36, 37]. The ring has envelope conformation on C7 atom with rotation parameters value of P = 238.9(14)o and τ = 14.1(3)o. The C–H······N hydrogen bond and C–H····π and π····π interactions stabilized the structure: The C17–H17· · · N10 intramolecular hydrogen bond interactions forms five membered pseudo ring (N10/N11/C12/C17/H17). Also the other molecular interactions such as, C–H····π interaction: C2–H2····Cg1 with a C−Cg distance of 3.512(6) Å, H····Cg distance of 2.77 Å and C–H····Cg angle of 137o; C7–H7····Cg3 with a C−Cg distance of 3.735(6) Å, H· · · Cg distance of 2.93 Å and C–H····Cg angle of 140o; C15–H15····Cg4 with a C−Cg distance of 3.567(7) Å, H· · · Cg distance of 2.74 Å and C–H····Cg angle of 148o. π····π interaction: Cg2····Cg3 with a Cg − Cg distance of 3.956(4) Å, α = 13.5(3)o, β = 22.7o, γ = 28.5o and a perpendicular distance of Cg2 on ring Cg3 = 3.649(2) Å. The packing of the molecules viewed across a, b and c-axis showing the layered stacking are depicted in Figures 5, 6, and 7, respectively.
Figure 5.
A perspective view of a supramolecular network by hydrogen bond interactions of the molecules 5d along the a-axis.
Figure 6.
A perspective view of a supramolecular network by hydrogen bond interactions of the molecules 5d along the b-axis.
Figure 7.
A perspective view of a supramolecular network by hydrogen bond interactions of the molecules 5d along the c-axis.
3.4. Hirshfeld surface analysis
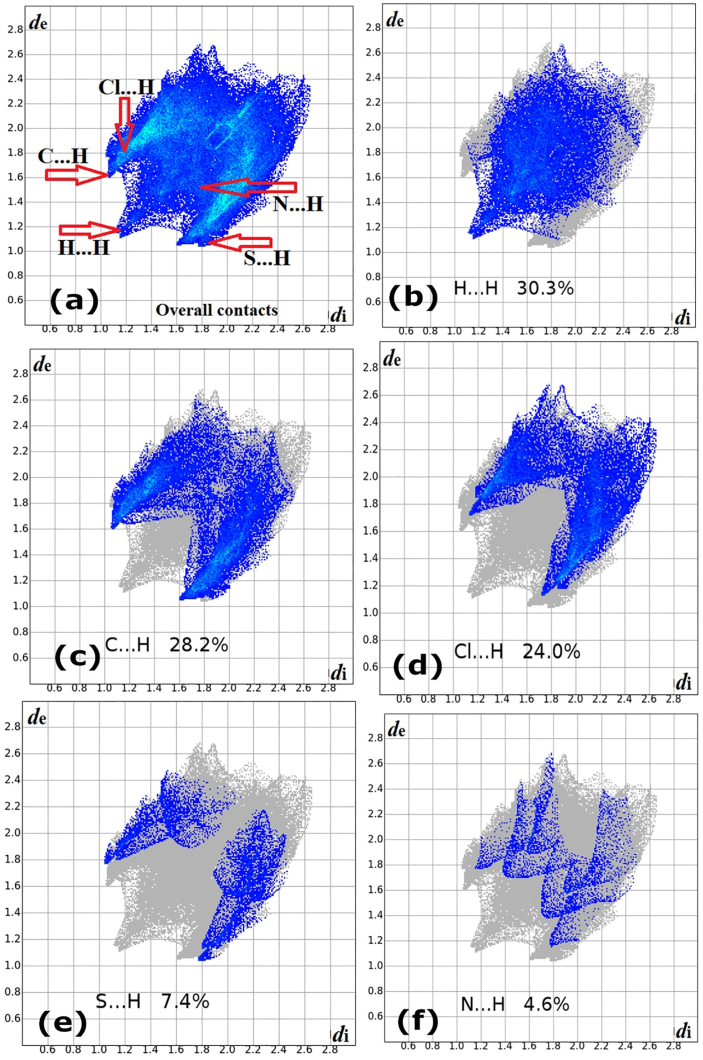
With CrystalExplorer 17.5, the 3D d norm was mapped on Hirshfeld surface (HS) with colour scale in between -0.583 au (blue) to 1.359 au (red). The calculated volume of the Hirshfeld surface is 451.41 Å3 with an area of 402.38 Å2. The 2D fingerprint plots were generated at 0.6–2.8 Å, with di and de distance scales.
In the dnorm (Figure 8a), colour codes indicate the different molecular interactions; red with negative dnorm, white with zero dnorm, and blue with positive dnorm indicates the short contacts, intermolecular distances equal to van der Waals radii, and longer contacts [38, 39], respectively. Figure 9 shows the expanded 2D fingerprint plots [40] with the de and di distance scales. The finger print plot reveals the contribution of overall contacts (Figure 9a) including each individual intermolecular contact to the total molecular HS. The contributions of various interactions; H–H (30.3%) [Figure 9b], C–H (28.2%) [Figure 9c], Cl–H (24.0%) [Figure 9d], S–H (7.4%) [Figure 9e], N–H (4.6%) [Figure 9f], C–Cl (1.9%), S–Cl (1.0%), Cl–Cl (0.9%), C–S (0.6%) and C–N (0.1%) were quantified through 2D fingerprint plots. The contribution of H⋯H contacts will be of 30.3% to the total Hirshfeld surface. The shape index, curvedness of HS were generated (Figure 8b and c) to analyze the π-stacking interactions [41, 42, 43].
Figure 8.
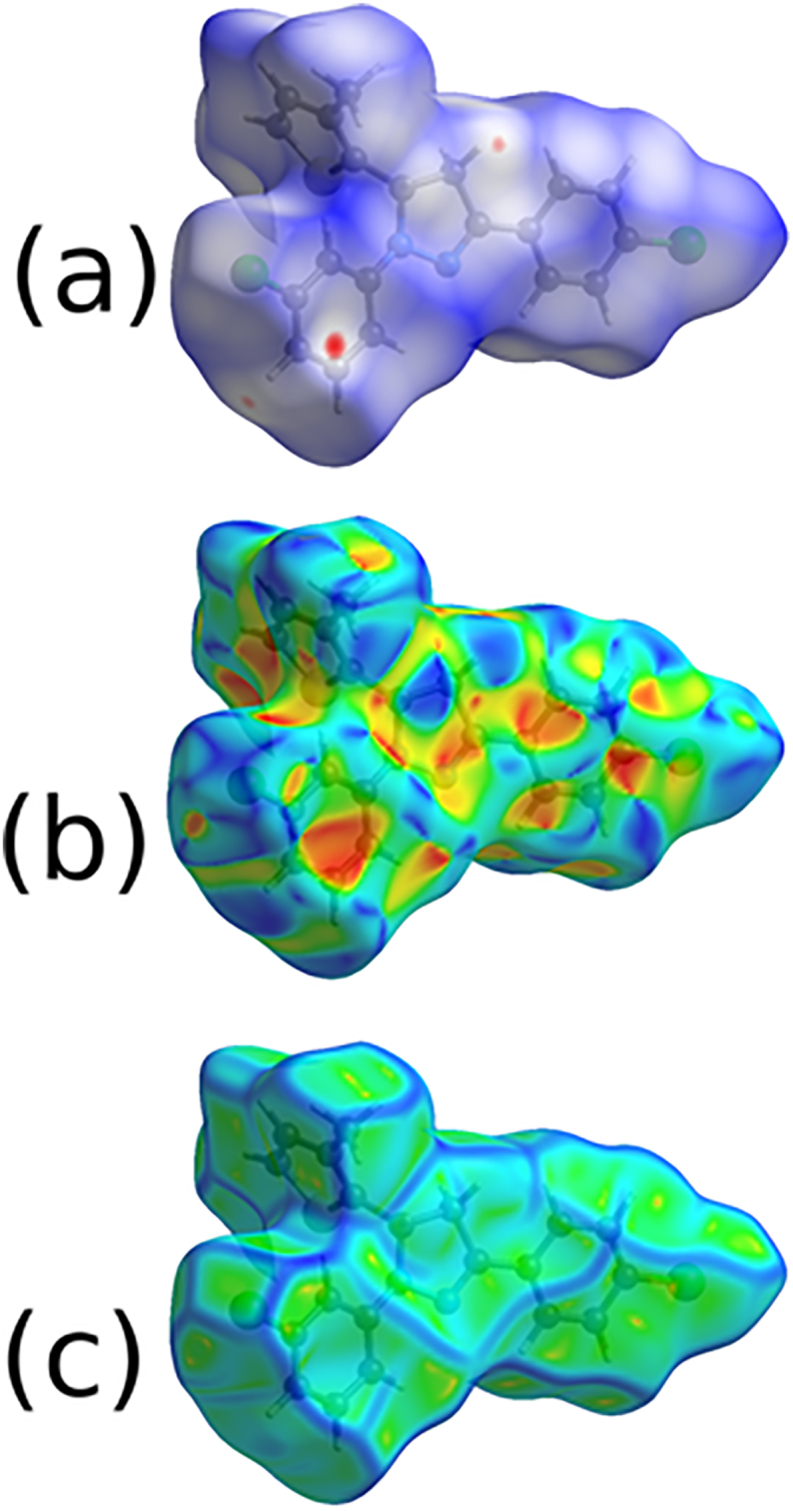
(a) dnorm; (b) shape index and (c) curvedness mapped on Hirshfeld surface of the molecule 5d.
Figure 9.
2D fingerprint plot of molecule 5d displaying the intermolecular (a) Overall contact, (b) H⋯H, (c) C⋯H, (d) Cl⋯H, (e) S⋯H, and (f) N⋯H interactions.
3.5. Density functional theory (DFT) calculations
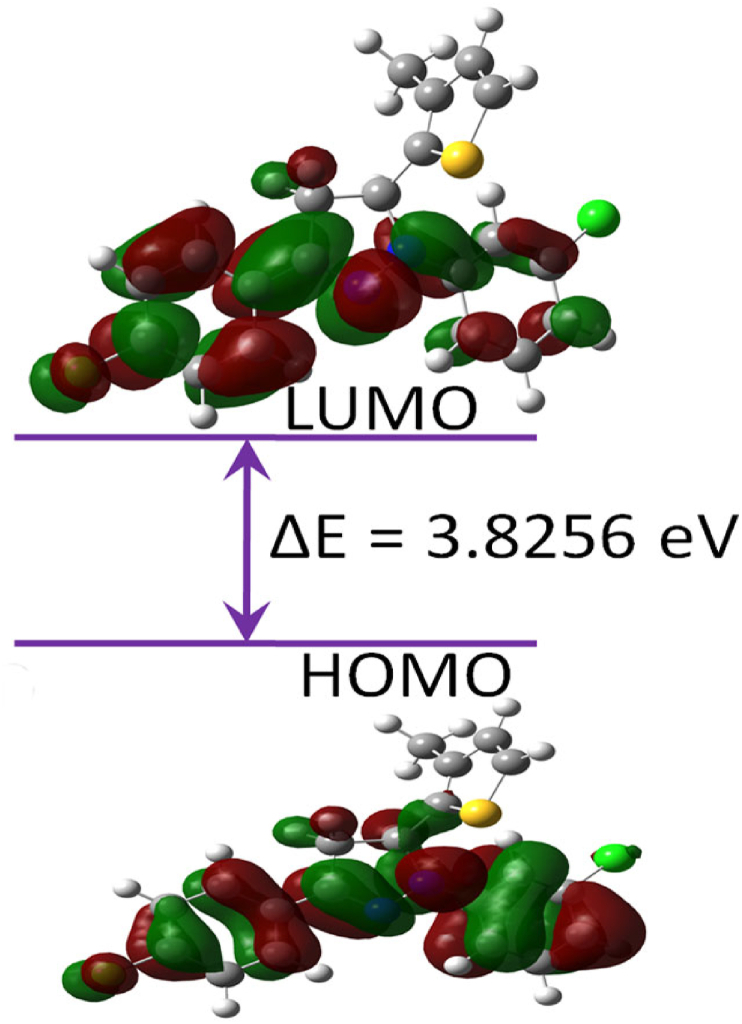
The electronic and chemical active regions of a compounds were identified with the quantum chemical calculations, FMO energies, and MEP surface analysis. The structural coordinates are optimized (Figure 10) in gas phase using DFT method with B3LYP hybrid functional and 6–311++G(d,p) basis set. The calculated structural parameters were compared with experimental results. The optimized structure substantiates the experimental findings with the correlation coefficient values; bond lengths (0.9929), bond angles (0.9954) and torsion angles (0.9995) (Tables 3, 4 and 5). Further, the FMO energies (EHOMO, ELUMO and Eg) and chemical reactive descriptors [44, 45, 46, 47] were calculated (Table 6).
Figure 10.
The optimized structure of molecule 5d.
Table 6.
FMO energies and chemical reactive descriptors of molecule 5d.
| Parameters | Value [B3LYP/6–311++G(d,p)] (eV) |
|---|---|
| EHOMO | -5.3005 |
| ELUMO | -1.4749 |
| Egap | 3.8256 |
| Ionization potential (I) | 5.3005 |
| Electron affinity (A) | 1.4749 |
| Electronegativity (χ) | 3.3877 |
| Chemical hardness (η) | 1.9128 |
| Global softness (σ) | 0.5228 |
| Electrophilicity (ω) | 2.9999 |
| Chemical potential (μ) | -3.3877 |
| Dipole moment (D) | 2.9618 |
Where, χ = (I + A)/2, η = (I-A)/2, σ = 1/η and ω = μ2/2η.
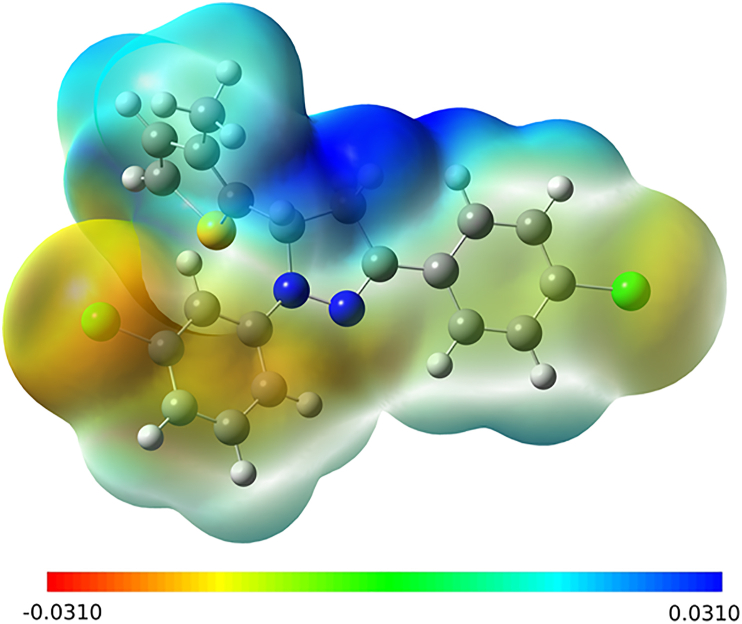
The polarizability and chemical reactivity of a compound was assessed on the band gap energy. The FMO HOMO-LUMO energies with the calculated energy gap of Egap = 3.8256 eV (Figure 11). The red and green colors on the molecular surface indicates the positive and negative phases of the wave functions. The MEP map drawn in the range of -3.100e−2 au (red) to +3.100 e−2 au (blue) (Figure 12). The positive (blue), and negative (green) regions of MEP represent an electrophilic and nucleophilic reactivity. The red regions are related to the electrophilic attack. The red and light blue colors are spread over chlorine and nitrogen, hydrogens [48], respectively.
Figure 11.
FM orbitals (HOMO-LUMO) with energy gap of a moleucle 5d.
Figure 12.
Molecular electrostatic potential map of a molecule 5d.
3.6. Radical scavenging activity
The radicals are highly reactive and least stable species, formed during various metabolic processes that cause cellular damages in living cells, such as capable to break DNA and cause strand breakage [49]. In recent times, antioxidants display radical scavenging activity acts as anticancer, anti-inflammatory and antiaging agents [50]. Therefore, the antioxidant ability of new compounds 5(a-i) was assessed by DPPH and Hydroxyl radical assay using ascorbic acid (AA) and Butylated hydroxyanisole (BHA), as control treatment. The experiments were performed in triplicates with varied concentrations. The IC50 values were computed by using graphpad Prism using equation Y=Bottom + (Top-Bottom)/(1 + 10ˆ((LogIC50-X)∗HillSlope)) from the observed percentage inhibition of the compounds 5(a-i) (Table 7).
Table 7.
DPPH and hydroxyl radical scavenging activity of the compounds, 5(a-i).
| Compounds | DPPH radical assay |
Hydroxyl radical assay |
|---|---|---|
| IC50 (μM)a | IC50 (μM)b | |
| 5a | 0.807 ± 0.01 | 3.534 ± 0.01 |
| 5b | 0.488 ± 0.02 | 1.439 ± 0.01 |
| 5c | 0.529 ± 0.02 | 1.813 ± 0.01 |
| 5d | 0.673 ± 0.01 | 2.641 ± 0.02 |
| 5e | 0.616 ± 0.03 | 1.965 ± 0.04 |
| 5f | 0.694 ± 0.04 | 2.649 ± 0.02 |
| 5g | 0.245 ± 0.01 | 0.905 ± 0.01 |
| 5h | 0.284 ± 0.02 | 0.892 ± 0.01 |
| 5i | 0.491 ± 0.01 | 1.913 ± 0.04 |
| AA∗ | 0.483 ± 0.01 | -- |
| BHA∗∗ | -- | 1.739 ± 0.01 |
∗Ascorbic acid; ∗∗Butylated hydroxyanisole were used as controls; a,bvalues represent mean ± SEM (n = 3).
The preliminary assessment results indicated that each compound of series 5(a-i) exhibit modest to good radical scavenging activities as compared with respective standards AA and BHA. Of the nine tested pyrazoles, five shown moderate to potent abilities in both the assays, indicating their reducing abilities.
The plausible mechanism involves the transfer of acidic H-atom by a compound to DPPH to form DPPH-H. The most active compounds were 5g, and 5h (IC50 = 0.245 ± 0.01, and 0.284 ± 0.02 μM, respectively). These have potent RSA than ascorbic acid (IC50 = 0.483 ± 0.01μM), indicating that, the electron donating methyl and methoxy substituents in 3-substituted aromatic ring increases the antioxidant activity of pyrazoles. The two molecules 5b (IC50 = 0.488 ± 0.02μM) and 5i (IC50 = 0.491 ± 0.01μM) shows good RSA, but lower than ascorbic acid activity. The rest of the compounds 5c (IC50 = 0.488 ± 0.02μM), 5e (IC50 = 0.488 ± 0.02μM), 5d (IC50 = 0.488 ± 0.02μM), 5f (IC50 = 0.488 ± 0.02μM), and 5a (IC50 = 0.488 ± 0.02μM), displayed moderate activities. Interestingly, all of these compounds showed the better antioxidant properties than the structurally related compounds, 5-(3,4-dichlorophenyl)-3′-naphthalen-2-yl-1′-phenyl-3,4-dihydro-1′H-[3,4′]bi pyrazoles reported, which showed DPPH radical scavenging activities in the range of IC50 = 9.66 ± 0.34 to 12.02 ± 0.63μM [51].
In the Hydroxyl radical scavenging assay; the most active molecules were 5g (IC50 = 0.905 ± 0.01μM), and 5h (IC50 = 0.892 ± 0.01μM). They exhibited potent RSA than BHA (IC50 = 1.739 ± 0.01μM). The compounds 5b (IC50 = 1.439 ± 0.01μM), and 5i (IC50 = 1.913 ± 0.04μM) shows good RSA, but lower than BHA. The rest of the compounds 5c (IC50 = 1.813 ± 0.01μM), 5e (IC50 = 1.965 ± 0.04μM), 5d (IC50 = 2.641 ± 0.02μM), 5f (IC50 = 2.649 ± 0.02μM), and 5a (IC50 = 3.534 ± 0.01μM), displayed moderate activities. However, all new compounds show better activities compared with the reported similar pyrazoles; like 1,3-diaryl-4-(aryl-propenonyl)-pyrazoles (IC50 = 13.5–13.5μM) [52], 3-(2-bromophenyl)-4-(4,5-diphenyl-1H-imidazole-2yl)-1-(p-tolyl)-1H-pyrazole (IC50 = 10.2μM) [53], N,N-bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)thiazol-2-amine (IC50 = 14.76μM) [54], suggesting that the new compounds of the present work could act as potent antioxidants. The presence of substituents on the two phenyl rings at 1,3 positions of pyrazole nucleus influences the RSA. The molecule 5a of the series, with two unsubstituted benzene rings at 1 and 3 positions of pyrazole exhibited least activity in both assays. It was observed that the presence of the electron donating 4-methyl, 4-methoxy, and 3,4-dimethoxy groups on C-3 substituted aromatic ring enhanced the DPPH and OH radical scavenging activities.
3.7. Molecular docking and ADME/Tox predictions
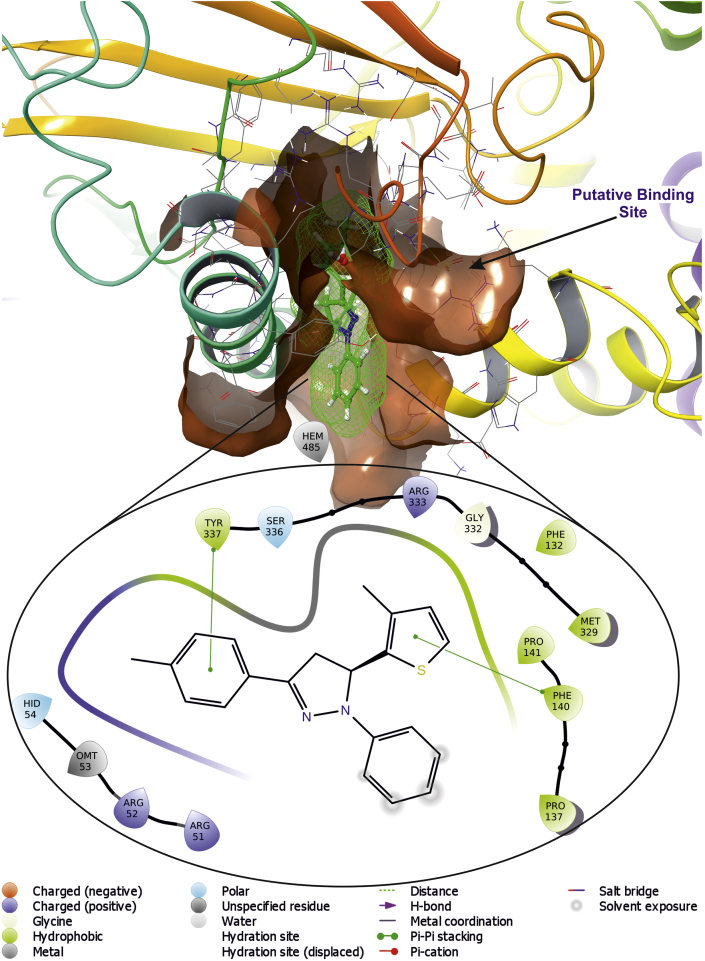
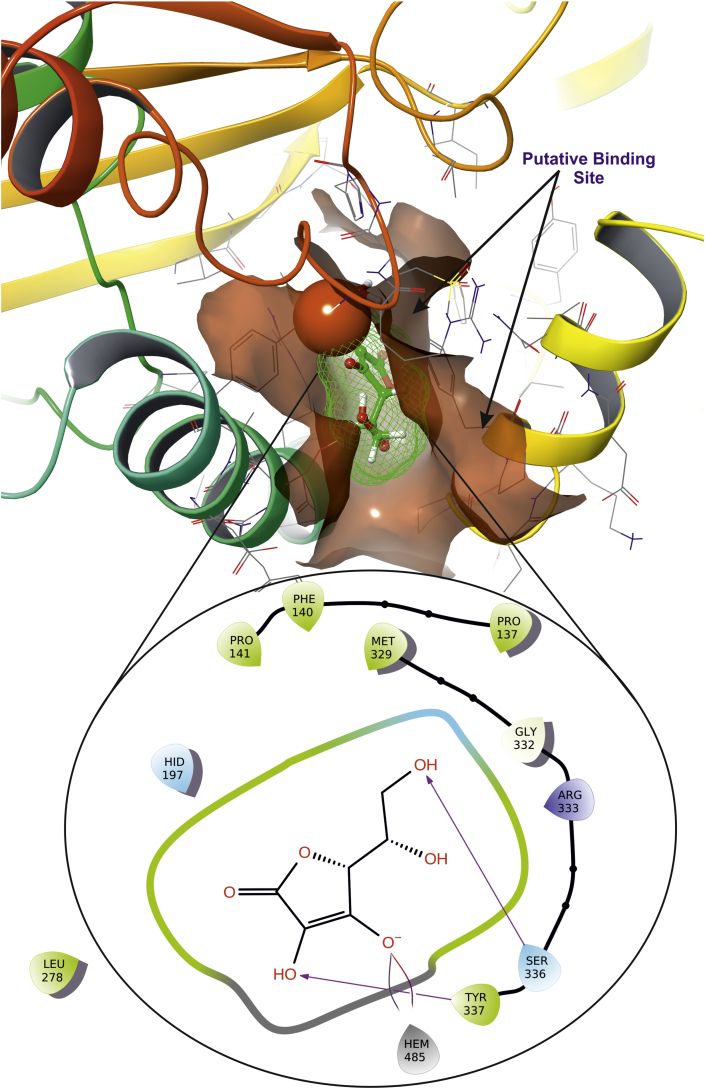
Molecular docking studies for newly synthesized compounds suggest that the ligand 5h and 5g comparatively possess better antioxidant activity among 5(a-i), as compared with the standard antioxidant molecule such as ascorbic acid. Antioxidants play an important role in various patho-physiological disease conditions such as, cancer, chronic inflammation, diabetics, arthritis, neuro-degenerative disorder. Hence decrease in free radicals in these diseases’ conditions, enhance the patho-physiological condition towards the normal [31]. The ligand 5g forms π-π stacking with Catalase via Try337 and Phe140 (Figure 13) which are closely resided at active site, whereas ascorbic acid forms salt bridge with Arg333 and with Protoporphyrin IX containing Fe (Figure 14). Three-dimensional view of 5g and ascorbic acid suggest that it is deeply embedded into active site surrounded with active site amino acids which is very important in aiding catalysis with dock score of -5.41 and -9.25 (kcal/mol) (Table 8).
Figure 13.
The putative binding pose of compound 5g with Catalase (PDB ID: 2CAG); the ligand 5g is represented as green wire mesh deeply embedded into active site.
Figure 14.
The putative binding pose of standard ascorbic acid with Catalase (PDB ID: 2CAG); Ascorbic acid is represented as green wire mesh deeply embedded into active site.
Table 8.
Docking scores of the newly synthesized compound 5(a-i) against Catalase and CuZn superoxide dismutase. Docking scores and Glide energies (kcal/mol) as obtained through Glide docking.
| Protein |
Catalase (PDB id: 2CAG) |
CuZn superoxide dismutase (PDB id: 1CB4) |
||||||
|---|---|---|---|---|---|---|---|---|
| Ligand | Docking Score (kcal/mol) | GEnergy (kcal/mol) | GEmodel (kcal/mol) | XP Hbond (kcal/mol) | Docking Score (kcal/mol) | GEnergy (kcal/mol) | GEmodel (kcal/mol) | XP Hbond (kcal/mol) |
| 5a | -3.26 | -31.73 | -42.14 | -0.27 | 1.93 | -30.29 | -39.26 | 0.00 |
| 5b | -3.74 | -33.95 | -42.02 | -0.70 | -1.20 | -33.28 | -42.85 | -0.05 |
| 5c | 0.07 | -39.67 | -44.00 | 0.00 | -1.08 | -32.85 | -41.16 | -0.21 |
| 5d | -3.90 | -36.88 | -48.85 | -0.70 | 1.85 | -32.80 | -41.69 | 0.00 |
| 5e | -2.00 | -30.77 | -45.65 | -0.13 | 0.16 | -32.89 | -39.33 | 0.00 |
| 5f | -2.31 | -32.28 | -46.86 | -0.48 | 1.88 | -34.93 | -45.06 | -0.08 |
| 5g | -5.41 | -39.09 | -60.37 | -0.57 | -1.23 | -33.78 | -43.78 | -0.20 |
| 5h | -4.40 | -41.81 | -49.69 | -0.01 | 1.56 | -33.04 | -44.76 | -0.35 |
| 5i | -4.06 | -47.53 | -61.02 | -0.02 | 1.85 | -34.92 | -48.88 | -0.38 |
| Ascorbic acid | -9.25 | -23.53 | -37.25 | -2.53 | -4.24 | -25.72 | -26.66 | -2.11 |
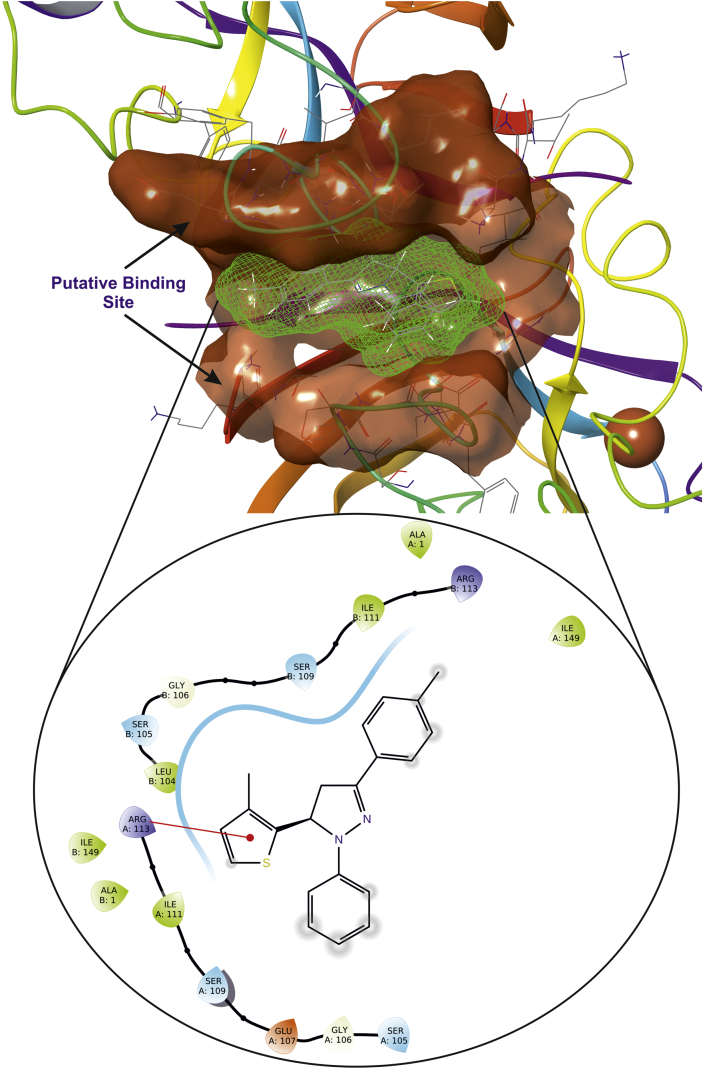
Superoxide dismutase (SOD) is another very important enzyme which acts as antioxidant defense against oxidative stress in the body. The ligand 5g form π-cation with Arg113 (Figure 15); while ascorbic acid form hydrogen bond with Ile111 and Gly106. The compound binds at Cu–Zn domain of SOD led to increase in an antioxidant activity and decrease in oxidative stress [52]. ADME/Tox properties depict that the ligands possess druggable properties, with 95% of available drugs which are in market. The results molecular docking (Table 8) and ADME studies/Tox (Table 9) prove that, ligands 5h and 5g are potent with druggable properties without violation of the Lipinski's rule.
Figure 15.
Putative binding pose of compound 5g with CuZn superoxide dismutase (PDB ID: 1CB4); the ligand is represented as green wire mesh deeply embedded into active site.
Table 9.
In silico ADME/Tox screening of the new compounds 5(a-i).
| Ligand | a∗ | b∗ | c∗ | d∗ | e∗ | f∗ | g∗ | h∗ | i∗ | j∗ | k∗ | l∗ | m∗ | n∗ | o∗ | p∗ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 40.35 | 11.48 | 14.01 | 5.31 | 6.28 | -7.18 | -6.56 | -6.35 | 8246.1 | 0.56 | 7264.66 | -0.07 | 1.41 | 1.00 | 100.0 | 1.00 |
| 5b | 40.60 | 11.06 | 14.38 | 5.10 | 6.51 | -7.54 | -6.94 | -6.23 | 8186.7 | 0.67 | 10000.00 | -0.21 | 1.45 | 1.00 | 100.0 | 1.00 |
| 5c | 44.58 | 12.62 | 16.03 | 5.35 | 7.24 | -9.30 | -7.67 | -6.88 | 4473.6 | 0.54 | 10000.00 | -0.79 | 1.76 | 1.00 | 100.0 | 1.00 |
| 5d | 42.97 | 12.64 | 15.52 | 4.83 | 7.28 | -8.66 | -8.02 | -6.18 | 8254.0 | 0.90 | 10000.00 | -0.40 | 1.66 | 1.00 | 100.0 | 1.00 |
| 5e | 42.20 | 11.81 | 14.91 | 5.55 | 6.34 | -7.33 | -6.85 | -6.29 | 8288.3 | 0.50 | 7327.00 | -0.16 | 1.38 | 1.00 | 100.0 | 1.00 |
| 5f | 43.64 | 12.44 | 15.82 | 5.34 | 6.87 | -8.12 | -7.58 | -6.25 | 8340.7 | 0.68 | 10000.00 | -0.31 | 1.52 | 1.00 | 100.0 | 1.00 |
| 5g | 42.41 | 11.70 | 14.62 | 5.01 | 6.65 | -7.77 | -6.86 | -6.25 | 8570.2 | 0.58 | 7661.63 | -0.22 | 1.60 | 1.00 | 100.0 | 1.00 |
| 5h | 44.23 | 12.19 | 15.86 | 5.81 | 6.43 | -7.53 | -7.14 | -6.25 | 8335.3 | 0.44 | 7336.01 | -0.24 | 1.37 | 1.00 | 100.0 | 1.00 |
| 5i | 45.50 | 12.76 | 16.60 | 5.56 | 6.92 | -8.24 | -7.87 | -6.13 | 8306.8 | 0.61 | 10000.00 | -0.41 | 1.50 | 1.00 | 100.0 | 1.00 |
| Ascorbic acid | 11.83 | 6.17 | 14.89 | 14.6 | -1.85 | -0.64 | -0.76 | -2.83 | 36.24 | -1.81 | 13.71 | -5.64 | -0.95 | 2.00 | 44.01 | 0.00 |
Note: a∗-QPpolrz; b∗-QPlogPC16; c∗-QPlogPoct; d∗-QPlogPw; e∗-QPlogPo/w; f∗-QPlogS; g∗-CIQPlogS; h∗-QPlogHERG; i∗-QPPCaco; j∗-QPlogBB; k∗-QPPMDCK; l∗-QPlogKp; m∗-QPlogKhsa; n∗-Human Oral Absorption; o∗-Percent Human Oral Absorption; p∗-Rule of Five.
4. Conclusion
In summary, a novel thiophene-pyrazole pharmacophores 5(a-i) were synthesized using a re-useable Amberlyst-15 catalyst, and assessed for their in vitro DPPH and Hydroxyl radical scavenging abilities. The fine structure of the compound 5d is confirmed by crystallographic studies. The analysis revealed the presence of C–H⋯N type H-bond and stabilized by C–H...π and π---π interactions. The chemically reactive regions were identified by HSA, and DFT studies. All the new compounds showed promising DPPH and Hydroxyl radical scavenging activities, However, two compounds 5g and 5h showed excellent activities comparable with standards ascorbic acid and BHA, respectively; which was substantiated by docking and ADMET analysis. Therefore, the compounds 5g and 5h can be used as lead antioxidants for further investigations.
Declarations
Author contribution statement
Karthik Kumara: Analyzed and interpreted the data; Wrote the paper.
Malledevarapura Gurumurthy Prabhudeva, Channa Basappa Vagish: Performed the experiments.
Hamse Kameshwar Vivek: Contributed reagents, materials, analysis tools or data.
Kuriya Madavu Lokanatha Rai: Analyzed and interpreted the data.
Neratur Krishnappagowda Lokanath: Conceived and designed the experiments.
Kariyappa Ajay Kumar: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data associated with this study has been deposited at http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax:(+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk under the accession number CCDC 1838509.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thanks to the IOE Instrumentation Facility, Vijnana Bhavana, University of Mysore, for recording spectra and X-ray diffraction studies.
Contributor Information
Neratur Krishnappagowda Lokanath, Email: lokanath@physics.uni-mysore.ac.in.
Kariyappa Ajay Kumar, Email: ajaykumar@ycm.uni-mysore.ac.in.
References
- 1.Farbstein D., Kozak-Blickstein A., Levy A.P. Antioxidant vitamins and their use in preventing cardiovascular disease. Molecules. 2010;15(11):8098–8110. doi: 10.3390/molecules15118098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naveen S., Kumara Karthik, Dileep Kumar A., Ajay Kumar K., Zarrouk A., Warad I., Lokanath N.K. Synthesis, characterization, crystal structure, Hirshfeld surface analysis, antioxidant properties and DFT calculations of a novel pyrazole derivative: ethyl 1-(2,4-dimethylphenyl)-3-methyl-5-phenyl-1H-pyrazole-4-carboxylate. J. Mol. Struct. 2021;1226(A):129350. [Google Scholar]
- 3.Jeyaveeran J.C., Praveen C., Arun Y., Prince A.A.M., Perumal P.T. Cycloisomerization of acetylenic oximes and hydrazones under gold catalysis: synthesis and cytotoxic evaluation of isoxazoles and pyrazoles. J. Chem. Sci. 2016;128:73–83. [Google Scholar]
- 4.Zhang H., Wei Q., Zhu G., Qu J., Wang B. A facile and expeditious approach to substituted 1H-pyrazoles catalyzed by iodine. Tetrahedron Lett. 2016;57:2633–2637. [Google Scholar]
- 5.Shaabani A., Sepahvand H., Nejad M.K. A re-engineering approach: synthesis of pyrazolo[1,2-a]pyrazoles and pyrano[2,3-c]pyrazoles via an isocyanide-based four-component reaction under solvent-free conditions. Tetrahedron Lett. 2016;57:1435–1437. [Google Scholar]
- 6.Dileep Kumar A., Vagish C.B., Lokeshwari D.M., Sowmya R., Ajay Kumar K. Design, synthesis, characterization, evaluation for anticancer and citotoxic properties of new pyrazole carbothioamides. Asian J. Org. & Med. Chem. 2021;6(1):53–58. [Google Scholar]
- 7.Fricero P., Bialy L., Brown A.W., Czechtizky W., Mendez M., Harrity J.P.A., J P A Synthesis and modular reactivity of pyrazole 5-trifluoroborates: intermediates for the preparation of fully functionalized pyrazoles. J. Org. Chem. 2017;82:1688–1696. doi: 10.1021/acs.joc.6b02847. [DOI] [PubMed] [Google Scholar]
- 8.Lokeshwari D.M., Kameshwar V.H., Kariyappa A.K. Synthesis of furan tethered 2-pyrazolines via 1,3-dipolar cycloaddition reactions: In vitro evaluation for their antioxidant and antimicrobial activities, molecular docking and ADMET studies. Biointerface Res. App. Chem. 2017;7(5):2158–2165. [Google Scholar]
- 9.Achutha D.K., Vagish C.B., Renuka N., Lokeshwari D.M., Kariyappa A.K. Green synthesis of novel pyrazoline carbothioamides: a potent antimicrobial and antioxidant agents. Chem. Data Coll. 2020;28:100445. [Google Scholar]
- 10.Vishnu W.K., Abeesh P., Guruvayoorappan C. Pyrazole (1, 2-diazole) induce apoptosis in lymphoma cells by targeting BCL-2 and BCL-XL genes and mitigate murine solid tumour development by regulating cyclin-D1 and Ki-67 expression. Toxicol. Appl. Pharmacol. 2021;418:115491. doi: 10.1016/j.taap.2021.115491. [DOI] [PubMed] [Google Scholar]
- 11.Bansal G., Singh S., Monga V., Thanikachalam P.V., Chawla P. Synthesis and biological evaluation of thiazolidine-2,4-dione-pyrazole conjugates as antidiabetic, anti-inflammatory and antioxidant agents. Bioorg. Chem. 2019;92:103271. doi: 10.1016/j.bioorg.2019.103271. [DOI] [PubMed] [Google Scholar]
- 12.Prabhudeva M.G., Vivek H.K., Ajay Kumar K. Synthesis of novel pyrazole carboxamides using reusable catalyst as antimicrobial agents and molecular docking studies. Chem. Data Coll. 2019;20:100193. [Google Scholar]
- 13.Shaikh S., Dhavan P., Pavale G., Ramana M.M.V., Jadhav B.L. Design, synthesis and evaluation of pyrazole bearing α-aminophosphonate derivatives as potential acetylcholinesterase inhibitors against Alzheimer's disease. Bioorg. Chem. 2020;96:103589. doi: 10.1016/j.bioorg.2020.103589. [DOI] [PubMed] [Google Scholar]
- 14.Zare H. Effects of Salvia Officinalis extract on the breast cancer cell line. Sci. Med. J. 2019;1(1):25–29. [Google Scholar]
- 15.Chzhu O.P., Araviashvili D.E., Danilova I.G. Studying properties of prospective biologically active extracts from Marine Hydrobionts. Emerg. Sci. J. 2020;4(1):37–43. [Google Scholar]
- 16.Tasneem A., Rai G.P., Reyaz S., Bairagya H.R. The possible molecular mechanism of SARS-CoV-2 Main Protease: new structural insights from computational methods. Sci. Med. J. 2020;2:108–126. (Sp. Iss. "COVID -19") [Google Scholar]
- 17.Vagish C.B., Kumar A.D., Kumara K., Vivek H.K., Renuka N., Lokanath N.K., Kumar K.A. Environmentally benign synthesis of substituted pyrazoles as potent antioxidant agents, characterization and docking studies. J. Iran. Chem. Soc. 2021;18:479–493. [Google Scholar]
- 18.Rana M., Arif R., Khan F.I., Maurya V., Singh R., Faizan M.I., Yasmeen S., Dar S.H., Alam R., Sahu A., Ahmad T. Rahisuddin, Pyrazoline analogs as potential anticancer agents and their apoptosis, molecular docking, MD simulation, DNA binding and antioxidant studies. Bioorg. Chem. 2021;108:104665. doi: 10.1016/j.bioorg.2021.104665. [DOI] [PubMed] [Google Scholar]
- 19.Alharthy R.D. Design and synthesis of novel pyrazolo[3,4-d]Pyrimidines: in vitro cytotoxic evaluation and free radical scavenging activity studies. Pharm. Chem. J. 2020;54:273–278. [Google Scholar]
- 20.Liao L., Shi J., Jiang C., Zhang L., Feng L., Liu J., Zhang J. Activation of anti-oxidant of curcumin pyrazole derivatives through preservation of mitochondria function and Nrf2 signaling pathway. Neurochem. Int. 2019;125:82–90. doi: 10.1016/j.neuint.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Rizk H.F., El-Borai M.A., Ragab A., Ibrahim S.A. Design, synthesis, biological evaluation and molecular docking study based on novel fused pyrazolothiazole scaffold. J. Iran. Chem. Soc. 2020;17:2493–2505. [Google Scholar]
- 22.Pogaku V., Krishnan R., Basavoju S. Synthesis and biological evaluation of new benzo[d][1,2,3]triazol-1-yl-pyrazole-based dihydro-[1,2,4]triazolo[4,3-a]pyrimidines as potent antidiabetic, anticancer and antioxidant agents. Res. Chem. Intermed. 2021;47:551–571. [Google Scholar]
- 23.Joshi G., Sharma M., Kalra S., Gavande N.S., Singh S., Kumar R. Design, synthesis, biological evaluation of 3,5-diaryl-4,5-dihydro-1H-pyrazole carbaldehydes as non-purine xanthine oxidase inhibitors: tracing the anticancer mechanism via xanthine oxidase inhibition. Bioorg. Chem. 2021;107:104620. doi: 10.1016/j.bioorg.2020.104620. [DOI] [PubMed] [Google Scholar]
- 24.Rigaku . 2011. Crystal Clear. [Google Scholar]
- 25.Sheldrick G.M. Shelxt - integrated space-group and crystal-structure determination. Acta Crystallogr. 2015;C71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheldrick G.M. Phase annealing in SHELX-90: direct methods for larger structures. Acta Crystallogr. 1990;A46:467–473. [Google Scholar]
- 27.Spek A.L. PLATON, an integrated tool for the analysis of the results of a single crystal structure determination. Acta Crystallogr. 1990;A46:34. [Google Scholar]
- 28.Macrae C.F., Bruno I.J., Chisholm J.A., Edgington P.R., McCabe P., Pidcock E., Rodriguez L.M., Taylor R., van de Streek J., Wood P.A. Mercury CSD 2.0-new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008;41:466–470. [Google Scholar]
- 29.Kumar G.V., Govindaraju M., Renuka N., Khatoon B.B.A., Mylarappa B.N., Kumar K.A. Synthesis of 1,3,5-triaryl-4,6-dioxo-pyrrolo[3,4-d]-7,8-dihydropyrzoles and their antimicrobial and antioxidant activity. Rasayan J. Chem. 2012;5(3):338–342. [Google Scholar]
- 30.Raghavendra K.R., Renuka N., Kumar K.A., Shashikanth S. An accessible route for the synthesis of novel lignan derivatives and their biological evaluation. Pharmaceut. Chem. J. 2017;51:661–669. [Google Scholar]
- 31.Gurunanjappa P., Kameshwar V.H., Kariyappa A.K. Bioactive formylpyrazole analogues: synthesis, antimicrobial, antioxidant and molecular docking studies. Asian J. Chem. 2017;29:1549–1554. [Google Scholar]
- 32.Sastry G.M., Adzhigirey M., Day T., Annabhimoju R., Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- 33.Dileep Kumar A., Naveen S., Vivek H.K., Prabhuswamy M., Lokanath N.K., Ajay Kumar K. Synthesis, crystal and molecular structure of ethyl 2-(4-chlorobenzylidene)-3-oxobutanoate: studies on antioxidant, antimicrobial activities and molecular docking. Chem. Data Coll. 2016;5(6):36–45. [Google Scholar]
- 34.Prabhudeva M.G., Kumara A.D., Kumar M.B., Ningappa N.K., Lokanath K., Kumar Ajay. Amberlyst-15 catalyzed synthesis of novel thiophene–pyrazoline derivatives: spectral and crystallographic characterization and anti-inflammatory and antimicrobial evaluation. Res. Chem. Intermed. 2018;44:6453–6468. [Google Scholar]
- 35.Kumar A.D., Prabhudeva M.G., Bharath S., Kumara K., Lokanath N.K., Kumar K.A. Design and Amberlyst-15 mediated synthesis of novel thienyl-pyrazole carboxamides that potently inhibit Phospholipase A2 by binding to an allosteric site on the enzyme. Bioorg. Chem. 2018;80:444–452. doi: 10.1016/j.bioorg.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A.D., Vivek H.K., Srinivasan B., Naveen S., Kumara K., Lokanath N.K., Byrappa K., Kumar K.A. Design, synthesis, characterization, crystal structure, Hirshfeld surface analysis, DFT calculations, anticancer, angiogenic properties of new pyrazole carboxamide derivatives. J. Mol. Struct. 2021;1235:130271. [Google Scholar]
- 37.Kumara K., Dileep Kumar A., Ajay Kumar K., Lokanath N.K. Synthesis of ethyl 5-(4-chlorophenyl)-3-methyl-1-phenyl-1H-pyrazole-4-carboxylate by an unusual protocol: crystal and molecular structure, Hirshfeld surface analysis. Chem. Data Coll. 2017;9–10:89–97. [Google Scholar]
- 38.Seth S.K. Structural elucidation and contribution of intermolecular interactions in O-hydroxy acyl aromatics: insights from X-ray and Hirshfeld surface analysis. J. Mol. Struct. 2014;1064:70–75. [Google Scholar]
- 39.Spackman M.A., McKinnon J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm. 2002;4(66):378–392. [Google Scholar]
- 40.McKinnon J.J., Jayatilaka D., Spackman M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. (J. Chem. Soc. Sect. D) 2007;37:3814–3816. doi: 10.1039/b704980c. [DOI] [PubMed] [Google Scholar]
- 41.Seth S.K. Tuning the formation of MOFs by pH influence: X-ray structural variations and Hirshfeld surface analyses of 2-amino-5-nitropyridine with cadmium chloride. CrystEngComm. 2013;15:1772–1781. [Google Scholar]
- 42.Luo Y.H., Wu G.G., Mao S.L., Sun B.W. Complexation of different metals with a novel N-donor bridging receptor and Hirshfeld surfaces analysis. Inorg. Chim. Acta. 2013;397:1–9. [Google Scholar]
- 43.Bernstein J., Davis R.E., Shimoni L., Chang N.-L. Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem., Int. Ed. Engl. 1995;34:1555–1573. [Google Scholar]
- 44.Jyothi K.L., Kumara K., Hema M.K., Mahesha, Gautam R., Guru Row T.N., Lokanath N.K. Structural elucidation, theoretical insights and thermal properties of three novel multicomponent molecular forms of gallic acid with hydroxypyridines. J. Mol. Struct. 2020;1207:127828. [Google Scholar]
- 45.Kumara K., Harish K.P., Shivalingegowda N., Tandon H.C., Mohana K.N., Lokanath N.K. Crystal structure studies, Hirshfeld surface analysis and DFT calculations of novel 1-[5-(4-methoxy-phenyl)-[1,3,4]oxadiazol-2-yl]-piperazine derivatives. Chem. Data Coll. 2017;11–12:40–58. [Google Scholar]
- 46.Kumara K., Kumar A.D., Kumar K.A., Lokanath N.K. Synthesis, spectral and X-ray crystal structure of 3-(3-methoxyphenyl)-5-(3-methylthiophen-2-yl)-4,5-dihydro-1H-pyrazole-1-carboxamide: Hirshfeld surface, DFT calculations and thermo-optical studies. Chem. Data Coll. 2018;13–14:40–59. [Google Scholar]
- 47.Kumara K., Kumar A.D., Naveen S., Kumar K.A., Lokanath N.K. Synthesis, spectral characterization and X-ray crystal structure studies of 3-(benzo[d][1,3]dioxol-5-yl)-5-(3-methylthiophen-2-yl)-4,5-dihydro-1H-pyrazole-1-carboxamide: Hirshfeld surface, DFT and thermal analysis. J. Mol. Struct. 2018;1161:285–298. [Google Scholar]
- 48.Xavier S., Periandy S., Ramalingam S. NBO, conformational, NLO, HOMO–LUMO, NMR and electronic spectral study on 1-phenyl-1-propanol by quantum computational methods, Spectrochim. Acta Part A: Mol. Biomol. Spect. 2015;137:306–320. doi: 10.1016/j.saa.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 49.Shalini V.K., Srinivas L. Lipid peroxide induced DNA damage: protection by turmeric (Curcuma longa) Mol. Cell. Biochem. 1987;77:3–10. doi: 10.1007/BF00230145. 1987. [DOI] [PubMed] [Google Scholar]
- 50.Kontogiorgis C., Litinas K.E., Makri A., Nicolaides D.N., Vronteli A., Hadjipavlou-Litina D.J., Pontiki E., Siohou A. Synthesis and biological evaluation of novel angular fused pyrrolocoumarins. J. Enzym. Inhib. Med. Chem. 2008;23:43–49. doi: 10.1080/14756360701400801. [DOI] [PubMed] [Google Scholar]
- 51.Ali S.A., Awad S.M., Said A.M., Mahgoub S., Taha H., Ahmed N.M. Design, synthesis, molecular modelling and biological evaluation of novel 3-(2-naphthyl)-1-phenyl-1H-pyrazole derivatives as potent antioxidants and 15-Lipoxygenase inhibitors. J. Enzym. Inhib. Med. Chem. 2020;35:847–863. doi: 10.1080/14756366.2020.1742116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bandgar B.P., Gawande S.S., Bodade R.G., Gawande N.M., Khobragade C.N. Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agents. Bioorg. Med. Chem. 2009;17:8168–8173. doi: 10.1016/j.bmc.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 53.Brahmbhatt H., Molnar M., Pavic V. Pyrazole nucleus fused tri-substituted imidazole derivatives as antioxidant and antibacterial agents. Karbala Int. J. Modern Sci. 2018;4:200–206. [Google Scholar]
- 54.Kaddouri Y., Abrigach F., Yousfi El.B., El Kodadi M., Touzani R. New thiazole, pyridine and pyrazole derivatives as antioxidant candidates: synthesis, DFT calculations and molecular docking study. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax:(+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk under the accession number CCDC 1838509.