Abstract
Chondral defects of the glenohumeral joint are common but still remain a diagnostic and management challenge. Whereas arthroplasty is a reasonable treatment option in the elderly and low-demand population, joint preservation should be aimed for the remaining patients. For larger defects the current gold standard of treatment is autologous chondrocyte implantation. However, disadvantages such as high cost, the restriction in availability of specialized laboratories, and the 2-stage surgical design need to be accounted for if choosing this option. Showing first good clinical results for the knee joint, minced cartilage implantation is moreover a cost-effective procedure bringing autologous cartilage chips harvested from the defect walls and bringing them into the area of damage in a single-step open or arthroscopic approach. We describe an arthroscopic strategy of this technique to treat chondral defects at the glenohumeral joint.
Technique Video
Dry arthroscopy of a left shoulder with the patient in beach-chair position. 00:13 minutes: Diagnostic arthroscopy through the standard posterior portal in a dry manner. 00:39 minutes: Suggestion of established working portals. 00:52 minutes: Collecting healthy cartilage with a 3-mm shaving device. 01:06: Creating a vertical rim of the chondral defect using a ringed curette. 01:37 minutes: Final measurement of the defect using a probing hook and final drying of the lesion side. 01:47 minutes: Mincing of the collected cartilage fragments on the back table using the 3-mm shaving device. 02:03 minutes: Disconnecting the graft net device and transferring the minced cartilage into a metal bowl. 02:32 minutes: Mixing the cartilage with ACP. 02:43 minutes: Implantation of the ACP-cartilage mixture onto the lesion side. 03:03 minutes: Applying thrombin for initial stability. 03:19 minutes: Adding a mixture of thrombin and ACP for final sealing. (ACP, autologous conditioned plasma)
Chondral defects of the glenohumeral joint still remain a diagnostic and management challenge. Causes include acute injuries, rotator cuff tears, degenerative processes, and physical labor, as well as overhead sports.1, 2, 3 The incidence varies between diverse groups of patients and falls between 13% to 17%.4,5 Whereas there is agreement among the literature that total joint arthroplasty is a reasonable option for elderly and low-demand patients with symptomatic cartilage lesions,6 it is still a matter of debate what treatment is most suitable for the younger and more active cohort because of the risk of hardware loosening and the higher demand of the shoulder.7,8 Research is therefore focusing on joint-preserving methods such as arthroscopic debridement,9 microfracture,10 osteochondral autograft transfer11 or allograft transplantation,12 partial shoulder resurfacing,13, 14, 15 and autologous chondrocyte implantation (ACI).16 Minced cartilage implantation17 is a technique that was first described in the early 1980s18 but is now gaining more and more interest, especially in the field of knee surgery where first clinical trials support good outcomes.19 It is a cost-effective procedure that brings autologous cartilage chips harvested from the defect itself, the surrounding defect wall, lose bodies, or nonweightbearing areas and brings them into the area of damage in a single-step open or arthroscopic approach.
Surgical Technique
Preoperative Procedure
Next to a detailed patient history report, a complete clinical examination is undertaken including inspection of the shoulder, passive and active range of motion (ROM), measurement of strength of the rotator cuff, as well as testing for impingement signs to evaluate concomitant pathologies. Preoperative radiography and magnetic resonance imaging (MRI) are conducted to determine the position and the severity of the chondral defect on the humeral head. With regard to the use of ACI, we recommend applying this method for isolated chronic or acute, symptomatic International Cartilage Repair Society grade 3 or 4 lesions (Video 1).
Positioning and Diagnostic Arthroscopy
Preoperative single-shot antibiotics are recommended. In the case of platelet-rich plasma (PRP) usage, such collection can be performed before the start of anesthesia and antibiotics. The patient is placed into the beach-chair position, and the index arm is fixed within an adjustable arm holder. Anatomic bony landmarks are drawn onto the shoulder. To identify the position of the chondral defect and evaluate accessibility, as well as to address concomitant injuries, there is first a diagnostic arthroscopy performed through the standard posterior portal. During the whole procedure, arthroscopy can be performed in a dry manner. If starting wet, it is necessary to drain the arthroscopic fluid with the shaver suction and dry the lesion carefully before the chondral chips are implanted.
Chondral Defect Preparation
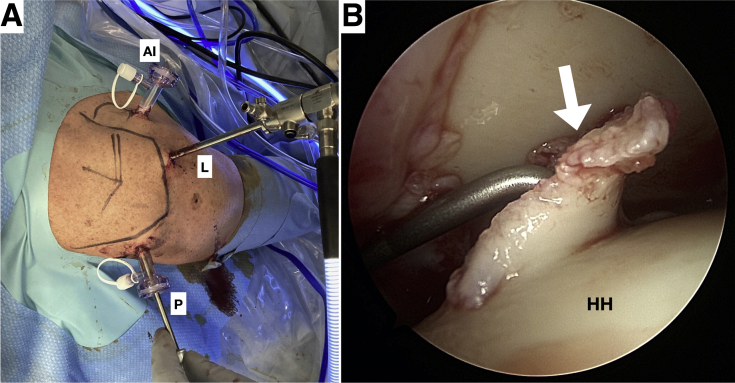
If the indication for treatment is confirmed by the arthroscopic findings, the defect is measured using a probing hook. Setting up a lateral and anterior-inferior portal and adding transparent cannulas into the anterior-inferior and posterior portal and switching the camera into the lateral portal could give a good view on the chondral defect; however, the setting is dependent on the side of the lesion (Fig 1 A and B).
Fig 1.
Arthroscopy in a left shoulder with the patient in the beach-chair position. The index arm is fixed within an adjustable arm holder, and anatomic bony landmarks are drawn onto the shoulder (A). Setting up a lateral and anterior-inferior portal with adding transparent cannulas into the anterior-inferior and posterior portal and switching the camera into the lateral portal should give a good view on the chondral defect (B). During the whole procedure, a dry arthroscopy can be performed. However, starting off wet and switching to a dry procedure just before the chondral chips are about to be implanted represents a second option (AI, anterior-inferior portal; L, lateral portal; P, posterior portal; HH, humerus head; white arrow, chondral defect.)
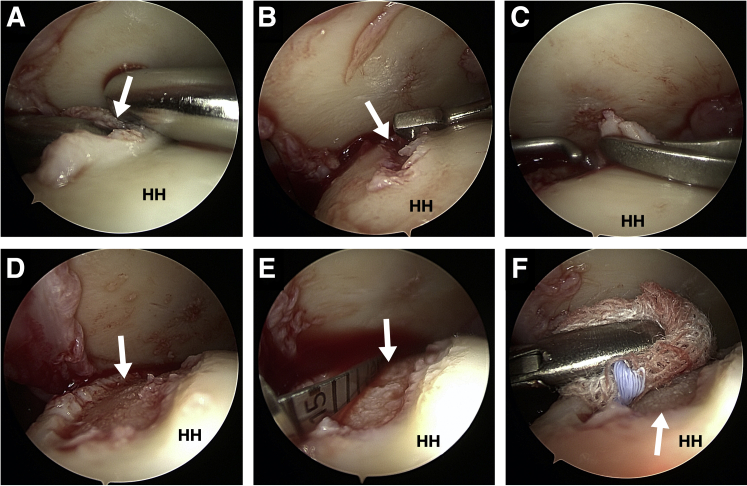
First the defect should be debrided and prepared in standard fashion to remove all defective cartilage. The subchondral bone should not be violated, but the calcifying layer is removed. Significant bony defects should be repaired via cancellous bone plasty before implantation of the chips. Hereafter, cartilage can be collected from the chondral walls of the defect edge in a circumferential manner. For this, 2 techniques are available: first a designated shaver device (Shaver blade, model Sabre with 3-mm shaver blades connected to a GraftNet autologous tissue collector, Arthrex) can be used (Fig 2A). Alternatively, one can collect viable cartilage with a spoon or ringed curette (Fig 2B) and, if needed, grasp the fragments with forceps (Fig 2C) particulate such at the back table using a 10 or 15 blade or precut the cartilage and then finally mince it by using a 3.0 mm shaver blade (Fig 3A). If a sufficient amount of cartilage is removed from the edges of the defect in a circumferential manner until there is vertical rim bordering the defect (Fig 2D), the lesion is measured again using a probing hook (Fig 2E). Consequently, the lesion itself will be only marginally enlarged after defect preparation.
Fig 2.
Arthroscopic visualization from the lateral portal in a left shoulder with the patient in the beach-chair position. To harvest healthy cartilage from the chondral defect, a 3-mm shaver connect to a harvesting device can be used (A). Using the curette, cartilage can be collected from the chondral walls of the defect edges in a circumferential manner until there is vertical rim bordering the defect (B-D). These can be pulled out of the portal while just sticking to the curette. Alternatively, a grasping forceps can be used. The lesion is measured using a probe (E) and then dried with a swap (F). (HH, humerus head.)
Fig 3.
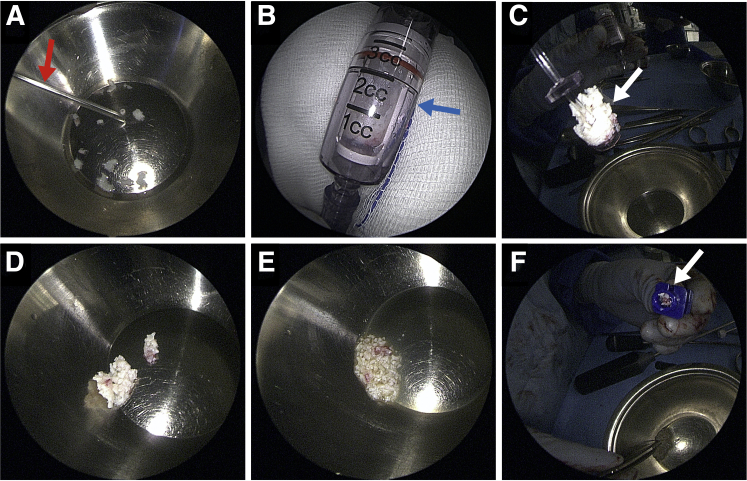
Chondral fragment preparation of the harvested chips. The fragments that were collected using the curette were transferred into a metal bowl. To cut gain the optimal size, they can be processed on the back table with a 3-mm shaver (red arrow) connected to a harvesting device (blue arrow) (A and B). Afterward the suction needs to be disconnected and the basket containing the chondral chips can be pulled out carefully (white arrow = chondral chips) (C). The chondral fragments already have the suggested optimal size of 1 × 1 × 1 mm and can be mixed with autologous conditioned plasma (ACP) in an approximate ratio of 3:1 until they show a paste-like appearance (E). This paste can be transferred into the application cannula (white arrow) with a surgical forceps (F).
Chondral Fragment Preparation
If harvested by using the shaver blade and GraftNet system, the chondral chips already have the recommended size and can be transferred from the collector into an empty 1 mL syringe or into a metal basin (Fig 3D).
Using the curette, the chips must be transferred into a sterile metal basin first. There, the cartilage can be precut using a 10 or 15 blade and then minced using a shaver as described above (Fig 3A).
Because studies in animal models show that there is significant loss of viability and further cellular performance if the cartilage is separated by blunt methods, care must be taken during particulation.20,21 This is recommended to finally gain a paste-like appearance of the chips (Fig 3D). Particulation processes and intraoperative chondral chip storage should always be performed under liquid (water or PRP) conditions. Size of the fragments should be 1 × 1 × 1 mm or smaller, whereas approximately 150 chips can be placed per 1 cm2 of cartilage lesion (in a defect depth greater than 2 mm).22
Insertion of the Chondral Fragments Into the Lesion Side
For fixation of the fragments, a purely autologous approach is used. To gain autologous PRP, which serves as the biological agent, it is advised that venous blood be drawn from the patient (e.g., cubital veins) under strictly sterile conditions before the start of anesthesia. This avoids adverse effects of anesthetics and antibiotics on the PRP. At least 10 to 15 mL of pure PRP should be collected, which then can be further processed during arthroscopy. To produce the autologous thrombin solution, 3 mL of the ACP is poured into a so-called Thrombinator System (Arthrex, GmbH, Munich, Germany). It uses the blood clotting cascade to create a gel that forms a binding agent to better handle and fix the cartilage chips. The gel is generated according to the manufacture’s description.
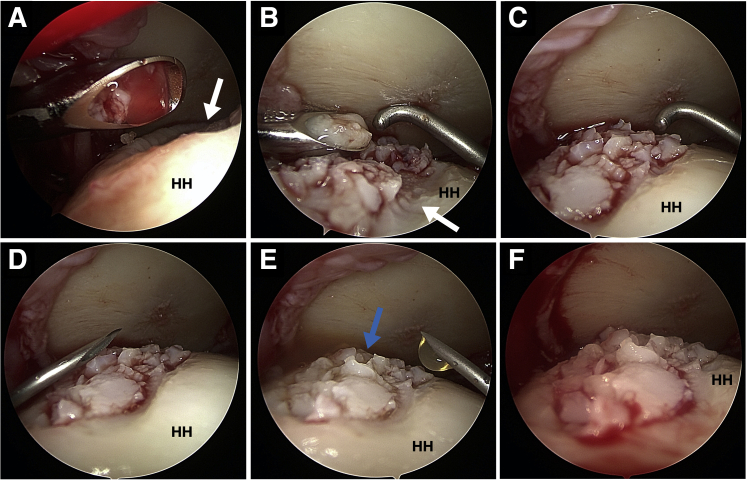
Using a shaver blade and a connected collective device (e.g., GraftNet; Arthrex) a “female-to-female” adapter can be attached to the syringe containing the cartilage chips. Connecting a syringe with ACP to the opposite side enables mixing of these components in a 3:1 ratio until a pasty mass has formed. The syringe with the fragments can now be plugged on the application cannula, and the chips are pushed into the cannula (using a trocar) until seen at the tip. Alternatively, the chips can be mixed with the ACP in a metal basin (Fig 3D). By harvesting the cartilage chips using the curette, fragments can be mixed with ACP in an approximate ratio of 3:1 on the basin (Fig 3E). This mixture is then transferred into the application cannula, with the chips pushed to the tip of the canula using a trocar (Fig 3F). Now the lesion side needs to be dried as much as possible using a swab (Fig 2F). The cannula containing the cartilage chips is inserted toward the lesion side, and the mixture is carefully distributed into the defect (Fig 4A-C). Here, it is sufficient to reach 80% to 90% filling. The back of the cannular or of a probe is used to create evenly allocated chips. The resulting fragment-paste is then covered with the before prepared thrombin solution (Fig 4D). The fibrinogen in the paste and the thrombin now form a stable clot of fibrin. To completely seal the lesion, thrombin and ACP are mixed in a 1:1 ratio at the back table and is quickly applied drop by drop on the now filled defect (Fig 4E and F). After 2 minutes the arthroscopic instruments can be removed. The portals are closed by stitches and covered with a sterile compression bandage.
Fig 4.
Arthroscopic visualization from the lateral portal in a left shoulder with the patient in the beach-chair position. Through the posterior portal, the cannula was inserted toward the lesion side (A), and the mixture of chips and autologous conditioned plasma (ACP) was carefully distributed into the defect (B). The tip of the measure-probe was used to create evenly allocated chips (C). After that, the fragment-paste was covered with the previously prepared thrombin solution using a syringe (D). To seal the lesion, thrombin and ACP were mixed in a 1:1 ratio and quickly applied drop by drop on the now-filled defect with a new syringe (E). The fragments should now be evenly distributed in the lesion side (F). (white arrow, chondral defect; blue arrow, implanted chordal chips; HH, humerus head.)
Postoperative Procedure
The shoulder is immobilized in a brace in internal rotation position for 24 hours. The brace is applied for 4 weeks. From day 2 the shoulder is mobilized by guided passive motion exercises for a total period of 4 weeks. Active motion exercises without limitations are initiated after 6 weeks.
Discussion
Cartilage defects of the glenohumeral joint still remain a diagnostic and management challenge. Although surgeons agree to use total joint arthroplasty as a reasonable option for elderly and low-demand patients,6 it is still a subject of discussion regarding what joint-preserving treatment to apply for the younger and more active cohort.
The technique described here is an alternative joint-preserving approach to treat symptomatic chondral lesions and possibly also osteochondral of the glenohumeral joint through a 1-stage arthroscopic approach using minced cartilage.
Previously used joint-preserving methods such as arthroscopic debridement results in pain relief but lacks preventing the development of early-onset arthritis.23 Microfracture is also considered a treatment option for patients with articular cartilage damage less than 2.5 cm. However, in radiographic findings these patients showed a progression of osteoarthritis as well.10
Osteochondral autograft transfer is a second-line option for small defects where cylindrical grafts are harvested from nonweightbearing areas such as the intercondylar notch and are transplanted into the area of osteochondral damage. As an unusual but serious complication, donor site morbidity after arthroscopically intervention needs to be considered.24
Allograft transplantation is reserved for salvage cases and is not standard of care in Europe. However, short-term results showed improved clinical outcomes in the field of knee surgery.25
Partial shoulder resurfacing is a procedure that only replaces a segment of the affected joint. This leaves more options for a potentially later necessary total arthroplasty. Recently published results suggest promising clinical outcomes during a 5-year follow-up.26 However, there are risks caused by hardware implantation (e.g., infection, loosening), as well as severe glenoid lesions that are presented as a contraindication for this procedure.
ACI is a commonly used treatment in hip and knee surgery but is barely tested for glenohumeral chondral defects. First studies showed promising results,16,27 but disadvantages such as high cost, chondral dedifferentiation and senescence, an unsuccessful growth of chondrocytes, the restriction in availability of specialized laboratories, and the 2-stage surgical design need to be taken into account. In addition, a very recent study showed that a delay from time of biopsy to implantation can lead to defect expansion.28
In 1983, Albrecht et al.18 described a method using cartilage cut into small pieces and reimplanted directly into the knees of rabbits. Later, Lu et al.29 showed in their study from 2006 that autologous chondrocyte implantation can be achieved without the need for growing the cells under laboratory conditions beforehand. They strictly used cartilage tissue (in contrast to morselized osteochondral grafts, which consist of a sparse amount of cartilage tissue and quite large bone contribution30 and delivered the fragments into a nonbleeding chondral defect, which results in a repair mechanism more likely promoted by the chondrocyte itself instead of other cells (i.e., mesenchymal stem cells), which can show osteogenic potential.
Also, animal studies indicate promising results for this method; there was a proven potential that implanted chondrocytes proliferate and can form a functionable cartilage that is superior to microfracture and comparable to the current gold standard ACI.31, 32, 33
Recent studies investigating the proliferation and migration of chondrocytes in an atelocollagen gel using ACI compared to minced cartilage show beneficial outcomes for the implantation of minced cartilage. Additionally it was demonstrated that there was improved production of the cartilage matrix.34 Another point of concern using ACI for osteochondral damages in joints is the implantation of chondrocytes lacking their surrounding microenvironment, the pericellular matrix. Among others consisting of type VI collagen,35 it forms a functional unit with the enclosed chondrocyte and is then called the “chondron.” It was shown before that the pericellular matrix is crucial for behavior and survival of the enclosed chondrocyte, which is consistent with the findings from Rothdiener et al.35 who revealed that cultured chondrons outperform chondrocytes regarding cell number, survival, collagen type II synthesis, the mRNA expression of phenotype-relevant genes, and the expression of enzymatically active GAPDH protein. Using minced cartilage, viable chondrocytes are transferred into the defect site at a high number, where outgrowth is promoted of these embedded cells by increasing the surface area resulting from fragmentation.36
As a source for harvesting the needed cartilage, it was shown that samples collected from the center of a cartilage lesion appeared to have lower qualities containing chondrocytes less viable and with inferior ability to form cartilage as compared to the closely flanking peripheral areas.37 If not enough material is available, cartilage can be taken from nonweightbearing areas as shown in the knee, for example, from the intercondylar notch.38 However Aurich et al.39 demonstrated an inferior redifferentiation potential of chondrocytes harvested from these areas when compared to the cells from the margins of the defects.
There are 2 ways to approach mincing the collected cartilage: first is to chop it with a sharp, frequently renewed scalpel on a stable, clean ground. Second is to use custom-made devices that automatically produce fragments in the recommended size. The latter is an efficient, time-saving way to finely cut the cartilage; however, both techniques show similar outgrowth potential and matrix deposition under laboratory conditions.40
For fixation of the minced cartilage, several methods containing the use of hydrogels with or without membrane coverage and potentially augmented by PRP/platelet-poor plasma are described in literature so far.41, 42, 43 Since it was shown that chondrocytes function best in the absence of any primary fixation methods and disturbances after implantation,44 a purely autologous technique was preferred with PRP used as the biological agent. The fibrinogen and a finally autologous-produced thrombin solution eventually form a stable clot. It was recently shown in a cadaver knee model that this method leads to a robust construct as long as the fibrin level is not applied too liberally.45 Domínguez Pérez et al.33 demonstrated in a sheep model that the minced cartilage particles showed good repair with platelet-poor plasma and PRP with mature chondrons and a matrix containing collagen fibers in a similar distribution and intensity as the flanking healthy cartilage.
Clinical trials of the minced cartilage procedure are still rare and restricted to the knee joint so far. The few published studies show good clinical outcomes and evaluate it as a safe and effective method that results in good cartilage repair.19,46,47
In 2011, Cole et al.47 compared the 2-year outcome of a scaffold-based minced cartilage procedure with microfracture in a randomized controlled trial. They reported a similar outcome of the 2 groups with the MRI data and standardized outcomes assessment tools indicating that this minced cartilage procedure is a safe and feasible method to treat medium-sized, focal cartilage defects in the knee.
Christensen et al.46 studied the outcome of the use of combined autologous bone and cartilage chips for treating osteochondritis dissecans lesions, where the defects were filled with bone and covered with minced cartilage from the intercondylar notch embedded in fibrin glue. Analysis of the MRI and computed tomography scans and the clinical scores 1 year after surgery showed very good subchondral bone restoration and good cartilage repair. Also patient outcome showed significant improvements suggesting this method is a promising, low-cost treatment option for osteochondral injuries.46
More frequently used in the United States, particulated juvenile articular cartilage represents another single-step procedure for chondral joint lesions using juvenile allograft chondral chips, which may have increased proliferative and restorative potential.48 Research undertaken so far shows good results; however, reports are scarce and thus lack a high level of evidence.
The most recent study from Massen et al.19 prospectively evaluated the 2-year clinical and radiological outcomes after the treatment of chondral and osteochondral knee joint lesions by a single-step autologous minced cartilage procedure. They reported positive results in knee function, pain, and satisfaction.19
Schneider et al.49 recently introduced this method as third-generation minced cartilage implantation via an all-arthroscopic approach in the field of knee surgery. However, further clinical data, especially with regard to long-term follow-up, are still required.
To provide a more distinctive view on the benefits of this procedure, especially in comparison to the available alternatives for glenohumeral chondral defects, studies with long-term follow-up and larger cohorts will be needed. All the pearls and pitfalls of this technique are listed in Table 1, and the advantages and disadvantages are listed in Table 2.
Table 1.
Pearls and Pitfalls of the Minced Cartilage Procedure
| Pearls |
| Entirely homologous approach |
| Impromptu procedure possible |
| Rapid procedure |
| No crucial change of tissue |
| Pitfalls |
| Success partly depended on ACP quality |
| Comorbidities need to be addressed |
| Consider contraindications of ACP usage |
ACP, Autologous conditioned plasma.
Table 2.
Advantages and Disadvantages of the Minced Cartilage Procedure
| Advantages |
| Fast and one step procedure |
| Economically appealing |
| Entire arthroscopic approach |
| Chondrocyte and extracellular matrix transplantation |
| Disadvantages |
| Potentially restricted to defect size |
| Up to now no long-term follow-up available |
Footnotes
The authors report the following potential conflicts of interest or sources of funding: G.M.S. reports personal fees from Arthrex and Smith & Nephew; M.S. reports personal fees from Arthrex. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Dry arthroscopy of a left shoulder with the patient in beach-chair position. 00:13 minutes: Diagnostic arthroscopy through the standard posterior portal in a dry manner. 00:39 minutes: Suggestion of established working portals. 00:52 minutes: Collecting healthy cartilage with a 3-mm shaving device. 01:06: Creating a vertical rim of the chondral defect using a ringed curette. 01:37 minutes: Final measurement of the defect using a probing hook and final drying of the lesion side. 01:47 minutes: Mincing of the collected cartilage fragments on the back table using the 3-mm shaving device. 02:03 minutes: Disconnecting the graft net device and transferring the minced cartilage into a metal bowl. 02:32 minutes: Mixing the cartilage with ACP. 02:43 minutes: Implantation of the ACP-cartilage mixture onto the lesion side. 03:03 minutes: Applying thrombin for initial stability. 03:19 minutes: Adding a mixture of thrombin and ACP for final sealing. (ACP, autologous conditioned plasma)
References
- 1.Paley K.J., Jobe F.W., Pink M.M., Kvitne R.S., ElAttrache N.S. Arthroscopic findings in the overhand throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16:35–40. doi: 10.1016/s0749-8063(00)90125-7. [DOI] [PubMed] [Google Scholar]
- 2.Ruckstuhl H., de Bruin E.D., Stussi E., Vanwanseele B. Post-traumatic glenohumeral cartilage lesions: a systematic review. BMC Musculoskelet Disord. 2008;9:107. doi: 10.1186/1471-2474-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross C.E., Chalmers P.N., Chahal J., et al. Operative treatment of chondral defects in the glenohumeral joint. Arthroscopy. 2012;28:1889–1901. doi: 10.1016/j.arthro.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Guntern D.V., Pfirrmann C.W., Schmid M.R., et al. Articular cartilage lesions of the glenohumeral joint: diagnostic effectiveness of MR arthrography and prevalence in patients with subacromial impingement syndrome. Radiology. 2003;226:165–170. doi: 10.1148/radiol.2261012090. [DOI] [PubMed] [Google Scholar]
- 5.Gartsman G.M., Taverna E. The incidence of glenohumeral joint abnormalities associated with full-thickness, reparable rotator cuff tears. Arthroscopy. 1997;13:450–455. doi: 10.1016/s0749-8063(97)90123-7. [DOI] [PubMed] [Google Scholar]
- 6.Sperling J.W., Antuna S.A., Sanchez-Sotelo J., Schleck C., Cofield R.H. Shoulder arthroplasty for arthritis after instability surgery. J Bone Joint Surg Am. 2002;84:1775–1781. doi: 10.2106/00004623-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Elser F., Braun S., Dewing C.B., Millett P.J. Glenohumeral joint preservation: current options for managing articular cartilage lesions in young, active patients. Arthroscopy. 2010;26:685–696. doi: 10.1016/j.arthro.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Denard P.J., Wirth M.A., Orfaly R.M. Management of glenohumeral arthritis in the young adult. J Bone Joint Surg Am. 2011;93:885–892. doi: 10.2106/JBJS.J.00960. [DOI] [PubMed] [Google Scholar]
- 9.Richards D.P., Burkhart S.S. Arthroscopic debridement and capsular release for glenohumeral osteoarthritis. Arthroscopy. 2007;23:1019–1022. doi: 10.1016/j.arthro.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Frank J.K., Heuberer P.R., Laky B., Anderl W., Pauzenberger L. Glenohumeral Microfracturing of Contained Glenohumeral Defects: Mid- to Long-term Outcome. Arthrosc Sports Med Rehabil. 2020;2:e341–e346. doi: 10.1016/j.asmr.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheibel M., Bartl C., Magosch P., Lichtenberg S., Habermeyer P. Osteochondral autologous transplantation for the treatment of full-thickness articular cartilage defects of the shoulder. J Bone Joint Surg Br. 2004;86:991–997. doi: 10.1302/0301-620x.86b7.14941. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell J.J., Vap A.R., Sanchez G., et al. Concomitant reverse Hill-Sachs lesion and posterior humeral avulsion of the glenohumeral ligament: treatment with fresh talus osteochondral allograft and arthroscopic posterior humeral avulsion of the glenohumeral ligament and labrum repair. Arthrosc Tech. 2017;6:e987–e995. doi: 10.1016/j.eats.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holschen M, Berg D, Schulte T, Bockmann MB, Witt KA, Steinbeck J. Arthroscopic and open partial arthroplasty for the treatment of focal grade IV cartilage defects of the humeral head [published online July 26, 2020]. Arch Orthop Trauma Surg. https://doi.org/10.1007/s00402-020-03552-x. [DOI] [PubMed]
- 14.Scalise J.J., Miniaci A., Iannotti J.P. Resurfacing arthroplasty of the humerus: indications, surgical technique, and clinical results. Tech Shoulder Elbow Surg. 2007;8:152–160. [Google Scholar]
- 15.Pauzenberger L., Heuberer P., Anderl W. Partieller Oberflächenersatz beim Knorpelschaden der Schulter. Obere Extremität. 2017;12:159–164. [Google Scholar]
- 16.Boehm E., Minkus M., Scheibel M. Autologous chondrocyte implantation for treatment of focal articular cartilage defects of the humeral head. J Shoulder Elbow Surg. 2020;29:2–11. doi: 10.1016/j.jse.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Salzmann GM, Ossendorff R, Gilat R, Cole BJ. Autologous Minced Cartilage Implantation for Treatment of Chondral and Osteochondral Lesions in the Knee Joint: An Overview [published online July 25, 2020]. Cartilage. https://doi.org/10.1177/1947603520942952. [DOI] [PMC free article] [PubMed]
- 18.Albrecht F.H. [Closure of joint cartilage defects using cartilage fragments and fibrin glue] Fortschr Med. 1983;101:1650–1652. [PubMed] [Google Scholar]
- 19.Massen F.K., Inauen C.R., Harder L.P., Runer A., Preiss S., Salzmann G.M. One-step autologous minced cartilage procedure for the treatment of knee joint chondral and osteochondral lesions: a series of 27 patients with 2-year follow-up. Orthop J Sports Med. 2019;7 doi: 10.1177/2325967119853773. 2325967119853773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tew S.R., Kwan A.P., Hann A., Thomson B.M., Archer C.W. The reactions of articular cartilage to experimental wounding: role of apoptosis. Arthritis Rheum. 2000;43:215–225. doi: 10.1002/1529-0131(200001)43:1<215::AID-ANR26>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 21.Redman S.N., Dowthwaite G.P., Thomson B.M., Archer C.W. The cellular responses of articular cartilage to sharp and blunt trauma. Osteoarthritis Cartilage. 2004;12:106–116. doi: 10.1016/j.joca.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Hunziker E.B., Quinn T.M., Hauselmann H.J. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage. 2002;10:564–572. doi: 10.1053/joca.2002.0814. [DOI] [PubMed] [Google Scholar]
- 23.Millett P.J., Horan M.P., Pennock A.T., Rios D. Comprehensive arthroscopic management (CAM) procedure: clinical results of a joint-preserving arthroscopic treatment for young, active patients with advanced shoulder osteoarthritis. Arthroscopy. 2013;29:440–448. doi: 10.1016/j.arthro.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Depalma A.A., Gruson K.I. Management of cartilage defects in the shoulder. Curr Rev Musculoskelet Med. 2012;5:254–262. doi: 10.1007/s12178-012-9131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams B., Southworth T., Naveen N., et al. Clinically significant outcome achievement after osteochondral allograft surgery. Arthroscopy. 2021;37:e47–e48. [Google Scholar]
- 26.Pauzenberger L., Heuberer P., Laky B., Kriegleder B. Five-year outcome of arthroscopic partial humeral head resurfacing. Arthroscopy. 2019;35:e22. [Google Scholar]
- 27.Niethammer T.R., Altmann D., Holzgruber M., et al. Patient-reported and magnetic resonance imaging outcomes of third-generation autologous chondrocyte implantation after 10 years. Arthroscopy. 2020;36:1928–1938. doi: 10.1016/j.arthro.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Pettit R., Everhart J., Dibartola A., et al. Factors predicting lesion expansion in two-stage chondrocyte implantation procedures. Arthroscopy. 2021;37:e16. [Google Scholar]
- 29.Lu Y., Dhanaraj S., Wang Z., et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24:1261–1270. doi: 10.1002/jor.20135. [DOI] [PubMed] [Google Scholar]
- 30.Stone K., Walgenbach A. Surgical technique for articular cartilage transplantation to traumatic and arthritic defects in the knee joint 1701. Med Sci Sports Exercise. 1997;29:299. [Google Scholar]
- 31.Christensen B.B., Olesen M.L., Lind M., Foldager C.B. Autologous cartilage chip transplantation improves repair tissue composition compared with marrow stimulation. Am J Sports Med. 2017;45:1490–1496. doi: 10.1177/0363546517694617. [DOI] [PubMed] [Google Scholar]
- 32.Matsushita R., Nakasa T., Ishikawa M., et al. Repair of an osteochondral defect with minced cartilage embedded in atelocollagen gel: a rabbit model. Am J Sports Med. 2019;47:2216–2224. doi: 10.1177/0363546519854372. [DOI] [PubMed] [Google Scholar]
- 33.Domínguez Pérez J.M., Fernández-Sarmiento J.A., Aguilar García D., et al. Cartilage regeneration using a novel autologous growth factors-based matrix for full-thickness defects in sheep. Knee Surg Sports Traumatol Arthrosc. 2019;27:950–961. doi: 10.1007/s00167-018-5107-z. [DOI] [PubMed] [Google Scholar]
- 34.Tsuyuguchi Y., Nakasa T., Ishikawa M., et al. The benefit of minced cartilage over isolated chondrocytes in atelocollagen gel on chondrocyte proliferation and migration. Cartilage. 2021;12:93–101. doi: 10.1177/1947603518805205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothdiener M., Uynuk-Ool T., Sudkamp N., et al. Human osteoarthritic chondrons outnumber patient- and joint-matched chondrocytes in hydrogel culture-Future application in autologous cell-based OA cartilage repair? J Tissue Eng Regen Med. 2018;12:e1206–e1220. doi: 10.1002/term.2516. [DOI] [PubMed] [Google Scholar]
- 36.Salzmann G.M., Nuernberger B., Schmitz P., et al. Physicobiochemical synergism through gene therapy and functional tissue engineering for in vitro chondrogenesis. Tissue Eng Part A. 2009;15:2513–2524. doi: 10.1089/ten.tea.2008.0479. [DOI] [PubMed] [Google Scholar]
- 37.Acevedo L., Iselin L., Berkelaar M.H.M., et al. Comparison of human articular cartilage tissue and chondrocytes isolated from peripheral versus central regions of traumatic lesions. Cartilage. 2020 doi: 10.1177/1947603520958154. 1947603520958154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niemeyer P., Pestka J.M., Kreuz P.C., et al. Standardized cartilage biopsies from the intercondylar notch for autologous chondrocyte implantation (ACI) Knee Surg Sports Traumatol Arthrosc. 2010;18:1122–1127. doi: 10.1007/s00167-009-1033-4. [DOI] [PubMed] [Google Scholar]
- 39.Aurich M., Hofmann G.O., Best N., Rolauffs B. Induced redifferentiation of human chondrocytes from articular cartilage lesion in alginate bead culture after monolayer dedifferentiation: an alternative cell source for cell-based therapies? Tissue Eng Part A. 2018;24:275–286. doi: 10.1089/ten.TEA.2016.0505. [DOI] [PubMed] [Google Scholar]
- 40.Levinson C., Cavalli E., Sindi D.M., et al. Chondrocytes from device-minced articular cartilage show potent outgrowth into fibrin and collagen hydrogels. Orthop J Sports Med. 2019;7(9) doi: 10.1177/2325967119867618. 2325967119867618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christensen B.B., Foldager C.B., Olesen M.L., Hede K.C., Lind M. Implantation of autologous cartilage chips improves cartilage repair tissue quality in osteochondral defects: a study in Gottingen minipigs. Am J Sports Med. 2016;44:1597–1604. doi: 10.1177/0363546516630977. [DOI] [PubMed] [Google Scholar]
- 42.Salzmann G.M., Calek A.K., Preiss S. Second-generation autologous minced cartilage repair technique. Arthrosc Tech. 2017;6:e127–e131. doi: 10.1016/j.eats.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 44.Walter S.G., Ossendorff R., Schildberg F.A. Articular cartilage regeneration and tissue engineering models: a systematic review. Arch Orthop Trauma Surg. 2019;139:305–316. doi: 10.1007/s00402-018-3057-z. [DOI] [PubMed] [Google Scholar]
- 45.Bogunovic L., Wetters N.G., Jain A., Cole B.J., Yanke A.B. In vitro analysis of micronized cartilage stability in the knee: effect of fibrin level, defect size, and defect location. Arthroscopy. 2019;35:1212–1218. doi: 10.1016/j.arthro.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 46.Christensen B.B., Foldager C.B., Jensen J., Lind M. Autologous dual-tissue transplantation for osteochondral repair: early clinical and radiological results. Cartilage. 2015;6:166–173. doi: 10.1177/1947603515580983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole B.J., Farr J., Winalski C.S., et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39:1170–1179. doi: 10.1177/0363546511399382. [DOI] [PubMed] [Google Scholar]
- 48.Riboh J.C., Cole B.J., Farr J. Particulated articular cartilage for symptomatic chondral defects of the knee. Curr Rev Musculoskelet Med. 2015;8:429–435. doi: 10.1007/s12178-015-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider S., Ossendorff R., Holz J., Salzmann G.M. Arthroscopic minced cartilage implantation (MCI): a technical note. Arthrosc Tech. 2021;10:e97–e101. doi: 10.1016/j.eats.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dry arthroscopy of a left shoulder with the patient in beach-chair position. 00:13 minutes: Diagnostic arthroscopy through the standard posterior portal in a dry manner. 00:39 minutes: Suggestion of established working portals. 00:52 minutes: Collecting healthy cartilage with a 3-mm shaving device. 01:06: Creating a vertical rim of the chondral defect using a ringed curette. 01:37 minutes: Final measurement of the defect using a probing hook and final drying of the lesion side. 01:47 minutes: Mincing of the collected cartilage fragments on the back table using the 3-mm shaving device. 02:03 minutes: Disconnecting the graft net device and transferring the minced cartilage into a metal bowl. 02:32 minutes: Mixing the cartilage with ACP. 02:43 minutes: Implantation of the ACP-cartilage mixture onto the lesion side. 03:03 minutes: Applying thrombin for initial stability. 03:19 minutes: Adding a mixture of thrombin and ACP for final sealing. (ACP, autologous conditioned plasma)
Dry arthroscopy of a left shoulder with the patient in beach-chair position. 00:13 minutes: Diagnostic arthroscopy through the standard posterior portal in a dry manner. 00:39 minutes: Suggestion of established working portals. 00:52 minutes: Collecting healthy cartilage with a 3-mm shaving device. 01:06: Creating a vertical rim of the chondral defect using a ringed curette. 01:37 minutes: Final measurement of the defect using a probing hook and final drying of the lesion side. 01:47 minutes: Mincing of the collected cartilage fragments on the back table using the 3-mm shaving device. 02:03 minutes: Disconnecting the graft net device and transferring the minced cartilage into a metal bowl. 02:32 minutes: Mixing the cartilage with ACP. 02:43 minutes: Implantation of the ACP-cartilage mixture onto the lesion side. 03:03 minutes: Applying thrombin for initial stability. 03:19 minutes: Adding a mixture of thrombin and ACP for final sealing. (ACP, autologous conditioned plasma)