Abstract
Heavy metal (HM) contamination of water bodies is a serious global environmental problem. Because they are not biodegradable, they can accumulate in food chains, causing various signs of toxicity to exposed organisms, including humans. Due to its effectiveness, low cost, and ecological aspect, phycoremediation, or the use of microalgae's ecological functions in the treatment of HMs contaminated wastewater, is one of the most recommended processes. This study aims to examine in depth the mechanisms involved in the phycoremediation of HMs by microalgae, it also provides an overview of the prospects for improving the productivity, selectivity, and cost-effectiveness of this bioprocess through physicochemical and genetic engineering applications. Firstly, this review proposes a detailed examination of the biosorption interactions between cell wall functional groups and HMs, and their complexation with extracellular polymeric substances released by microalgae in the extracellular environment under stress conditions. Subsequently, the metal transporters involved in the intracellular bioaccumulation of HMs as well as the main intracellular mechanisms including compartmentalization in cell organelles, enzymatic biotransformation, or photoreduction of HMs were also extensively reviewed. In the last section, future perspectives of physicochemical and genetic approaches that could be used to improve the phytoremediation process in terms of removal efficiency, selectivity for a targeted metal, or reduction of treatment time and cost are discussed, which paves the way for large-scale application of phytoremediation processes.
Keywords: Microalgae, Heavy metal, Phycoremediation, Mechanisms, Bioengineering
Microalgae; Heavy metal; Phycoremediation; Mechanisms; Bioengineering.
1. Introduction
Except for a small amount that appeared through geologic time at Earth's impact events, metallic elements as they are known today, have been present on Earth since its formation. These elements have been naturally recycled between the environmental compartments, through different biotic and abiotic processes of the biogeochemical cycles (Garrett, 2000). As they are not biodegradable, metal elements are present at natural background levels in water, soil, sediment and living organisms (Dung et al., 2013). They are considered a contaminant when a particular metallic element is present in an inappropriate biotope at a concentration that exceeds the tolerance of the organisms that compose this ecosystem (Gałuszka, 2007). Indeed, inorganic pollution can be the result of a natural phenomenon (Erosion, infiltration, thermal springs activity, and volcanoes...), as well as of anthropogenic sources, such as the large-scale mining operations, irrational use of fertilizers in agriculture, non-hazardous waste management (Tchounwou et al., 2012). In order to distinguish the metallic elements necessary for the functioning of biological processes from those that cause signs of toxicity, a new terminology "Heavy Metal (HM)" was introduced. Among all the natural elements, 53 are considered as HMs, they represent the transition elements, the elements of actinides and lanthanides as well as some elements of the p block of the periodic table (Rahman and Singh, 2019). According to Wang and Chen (2009), HMs can also be classified into three categories: (i) toxic HMs, such as Hg, Cr, Pb, Zn, Cu, Ni, Cd, As, Co, Sn. (ii) precious metals mainly including Ag, Au, Ru, Pt and Pd, and (iii) radionuclides HMs such as Am, Ra, Th, and U. On the other hand, its commonly recognized that non-essential HMs have varying degrees of toxicity towards microorganisms, animals, plants, and humans even at very low concentrations (Ali et al., 2019). Thus, the treatment of HMs-contaminated water is a global issue that has piqued the attention of scientists, environmentalists, and legislators. Hence, to protect the environment and subsequently the public health, such contaminants should be removed from industrial wastewater and aquatic ecosystems. Various techniques have been applied to remove HMs, including hydroxide and sulfide precipitation, adsorption, nanofiltration, ultrafiltration and reverse osmosis, electrochemical treatment technologies and biological treatment approaches (Fu and Wang, 2011; Kumar et al., 2015). Indeed, because they are effective, environmentally friendly and inexpensive the use of bioremediation methods appear to be competitive and even advantageous compared to some conventional physicochemical techniques (Chibueze et al., 2016). Phycoremediation is a bioremediation subcategory proposed by John (2003), it refers to the use of algae in the removal or the mitigation of harmful pollutants. The phycoremediation could be mediated by different mechanisms, depending on the HM and its speciation, the used microalgae strain; living or non-living, and the operational condition (Gonzalez-dkila, 1995). The ability of living microalgae to remove and detoxify HMs is the result of several adaptive mechanisms developed over centuries of evolution (Monteiro et al., 2012). Harnessing this natural power in the treatment of HMs contaminated water seems to be a promising strategy. This review provides an in-depth understanding of the mechanisms involved by the living cells of green microalgae for the removal and mitigation of HMs toxicity. It also provides a future outlook for the physicochemical and the genetic modifications that can be applied with a view to improving the phycoremediation capacity, selectivity, and cost-effectiveness of this bioprocess.
2. Mechanisms of HMs phycoremediation using living microalgae
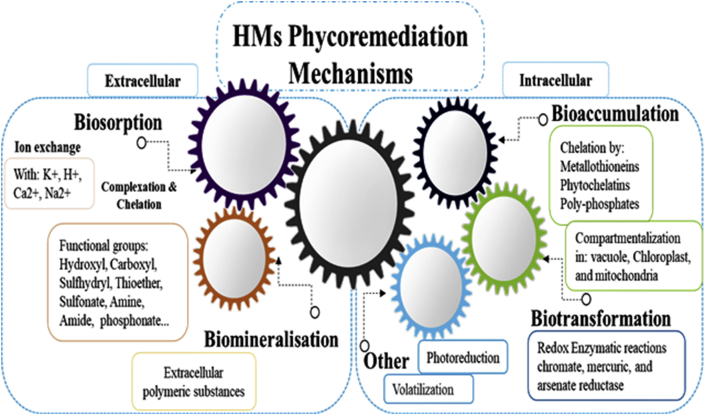
The application of living microalgae for the phycoremediation of HMs can include both extracellular and/or intracellular bioremediation strategies, Figure 1 summarizes the main pathways involved in the bioremediation and mitigation of HMs.
Figure 1.
HMs-phycoremediation mechanisms (modified from (García-García et al., 2016; Kumar et al., 2015)).
2.1. Extracellular HMs-bioremoval with living microalgae
The extracellular uptake of HMs by living cells of microalgae may occur by biosorption in the cell wall or in the extracellular polymeric substances (EPS) formed by microalgae in response to stress conditions. In the first case, HMs-biosorption refers to a physicochemical property of the microalgae cell surface that binds to HMs ions from the solution independently to the cellular metabolism. However, HMs-biosorption into the EPS is a metabolism-dependent process. In response to metallic stress, microalgae cells can not only regulate EPS synthesis, but also they can change the properties of these biopolymers as required (Naveed et al., 2019; Ubando et al., 2021).
2.1.1. Cell walls composition and its role in HMs-biosorption
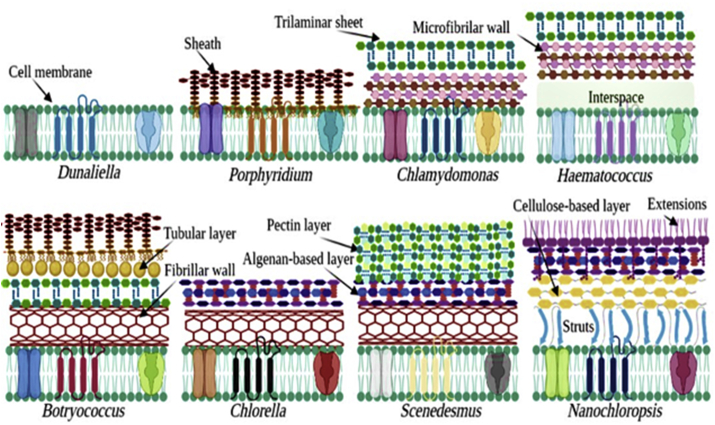
The cell wall is the interface between the intracellular compartment and the external environment (Macfie and Welbourn, 2000). It contains multifunctional macromolecules such as lipids, proteins and carbohydrates, which offer on its surface different negatively charged functional groups such as amino, hydroxyl, carboxyl, sulfhydryl, sulfate, phosphate, phenol...etc. (Javanbakht et al., 2014). These negatively charged groups permit the binding of ions from the surrounding environment, making the outer layer of the cell wall as the first participator in the removal of HMs (Leong and Chang, 2020; Saavedra et al., 2018; Singh et al., 2021). Therefore, understanding the structure, composition, and properties of the cell wall is essential when studying biosorption mechanisms (Podder and Majumder, 2017). In addition, this non-metabolic mechanism depends closely on the operating conditions, the influence of the physicochemical conditions including pH, temperature, presence of other ions and the ratio of adsorbate adsorbent must be controlled (Zeraatkar et al., 2016). In recent years, the most frequently used microalgae strains in the phycoremediation belong to the Chlorophyta phylum, particularly species of genera Chlorella and Scenedesmus (Spain et al., 2021). Nevertheless, even under similar operating conditions, the sensitivity and the biosorption efficiency vary depending on genus and species of microalgae (Kumar et al., 2015). For instance, the growth C. sorokiniana and S. obliquus in media contaminated with Pb(II), Cd(II), Cu(II) and Cr(VI) was significantly different (Danouche et al., 2020). This can be attributed to the physiology of the strain, in particular the cell walls composition and structure. Depending on the species and the growth stage, different structures and compositions of the call wall have been reported. Within this phylum, it is ranging from a simple cell membrane as of Dunaliella and Isochrysis species, which consists of a lipid bilayer with integrated and peripheral proteins and sometimes a cap of glycoproteins and glycolipids envelops the outer of the cells surface. To complex multilayer structures with additional intracellular material in vesicles like that of Dinoflagellates strains, or that of euglenophytes and cryptophytes species that characterized by both intracellular and extracellular material associated to the cell membranes (D'Hondt et al., 2017). Figure 2 illustrates the structure of the cell wall of some microalgae species. Furthermore, species of the same genus may exhibit also variations in the composition of their cell walls. For example, C. vulgaris has an inner layer (Rashidi and Trindade, 2018), while, C. homosphaera and C. zofingiensis both have an inner and an outer layer, and also have the trilaminar version of the outer layer (Rodrigues and Pinto, 2011). In the case of C. trilaminar, the outermost layer is composed by sporopollenin, the middle layer is principally composed by mannose and chitin-like polysaccharides and the innermost layer is a phospholipid bilayer (Dixon and Wilken, 2018).
Figure 2.
Schematic view of cell wall structures of some microalgae species (modified from (Baudelet et al., 2017; Carvalho et al., 2020), For better interpretation of the color figure, the reader could refer the web version of this article).
2.1.2. Physicochemical interactions of HMs and cell surface
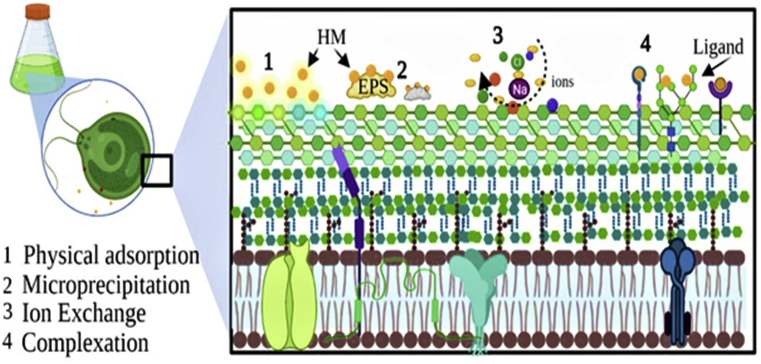
Understanding the interaction of HMs ions and cell surface of microalgae are challenging, due to the complexity of the cell surface. Several chemical and physical interactions have been reported. The chelation and the complexation of HMs with active groups of the cell wall were the main involved mechanisms. Furthermore, the cell surface of microalgae incorporates ions such as Na+, K+, Ca2+ and Mg2+, which can be reversibly substituted by other toxic HMs ions in solution, via a process called ion exchange. The physical forces principally van der Waals and electrostatic interactions, can in turn managed the physical adsorption mechanism of metal binding into the cell surface. On the other hand, the microprecipitation is a process that can be associated with both passive and active pathways of metal uptake (Navakoudis and Ververidis, 2018).
2.1.3. Extracellular polymeric substances interactions with HMs
EPS are high molecular weight extracellular biopolymers that can be produced by a diversity of microorganisms including microalgae species. They include nucleic acids, proteins, lipids, sugar, humic substances and other extracellular inorganic components that can bind to carbohydrates (Xiao and Zheng, 2016). Thus, we can divide the EPS of microalgae into three broad categories, soluble EPS in growth media (SL-EPS), attached EPS to the cells wall or Loosely Bound EPS (LB-EPS), as well as Tightly Bound EPS (TB-EPS), which are gel-like coatings of cells wall (Naveed et al., 2019). Microalgae are usually endangered in aquatic ecosystems by the presence of hazardous materials like toxic metals. One of the adaptive mechanisms developed by such organisms as a self-defense mechanism is the production of EPS. Generally, the EPS production increase under the metals stimulation. According to Yu et al. (2019), LB-EPS of Chlamydomonas reinhardtii had increased significantly after Cd-exposure. Recently, Li et al. (2021) indicted also an increase in EPS production by C. reinhardtii under Pb(II) and Cd(II) stresses. Similarly, Zhang et al. (2015) reported that the increase in EPS yields in Cu-enriched Chlorella sp. cultures indicates that Cu-uptake are carried out by EPS rather than intracellular chelation. Comparing EPS-free and EPS-covered cells of C. pyrenoidosa, Zhang et al. (2020) show that EPS improve adsorption capacity, reduce intracellular accumulation and increase the tolerance against As ions. EPS appear to be capable of forming an extracellular protective layer on the surface of the cell wall, preventing the harmful effect of HMs in intracellular environment. Therefore, their excretion is a survival mechanism, allowing a maintenance of cellular integrity (Hou et al., 2016; Naveed et al., 2019). Additionally, EPS have abundant charged hydrophobic groups, which are appropriate for an active binding to HMs (Zhang et al., 2020). According to Li et al. (2016), metal biosorption into EPS can be related to cell surface properties and functional groups. Figure 3 summarizes the interactions that can manage the biosorption mechanism of HMs into cells of microalgae.
Figure 3.
Extracellular bio-removal mechanisms of HMs using living cells of microalgae, modified from (Navakoudis and Ververidis, 2018).
2.2. HMs-bioaccumulation mechanisms in microalgae
In contrast to the biosorption process, bioaccumulation is a depending metabolic pathway. It refers to the intracellular accumulation of HMs through the cell membranes of living microalgae based on passive and/or active transport pathways (Chojnacka, 2010). It's characterized by two successive stages: first, a rapid, passive and non-specific absorption of metal ions on the cell wall. Followed by an active and/or passive transport across the cell wall and plasma membrane to the cytoplasm (Kumar et al., 2015). Indeed, Pérez-Rama et al. (2002) reported that the Cd(II)-uptake using Tetraselmis suecica was a biphasic process, assisted in the first phase by an adsorption to proteins or polysaccharides, followed by an energy-dependent accumulation to the cytosol. Furthermore, when the concentration of metal in the extracellular environment is significantly higher than the intracellular concentration, cations can be transported by the negative charged groups of the cell surface to reach the intracellular compartment via active transport across the plasma membrane after binding to thiols molecules primarily cysteine (Monteiro et al., 2012). The other amino acids such as histidine, glutamate and proline can play also a crucial role in metal chelation and detoxification (Hayat et al., 2012; Kumar et al., 2015; Leszczyszyn et al., 2010). As most of the HMs are hydrophilic, their transport across the plasma membrane (lipophilic) is mediated mainly through a specific protein (Metal transporters). Thereafter, several detoxification pathways can take place in intracellular compartments (Leong and Chang, 2020; Monteiro et al., 2011).
2.2.1. Metal transporters in microalgae cell membrane
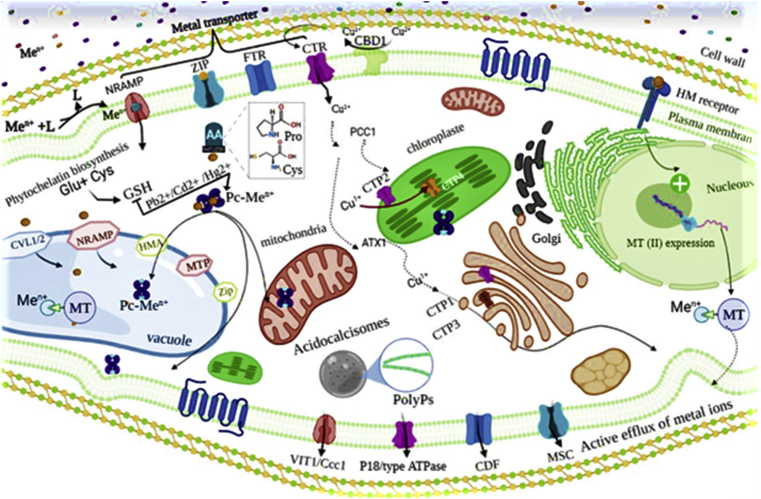
Metal transporters play an important role in the interaction of algae with their surroundings. They represent the first line of osmotic balances control, as well as they manage the intracellular concentration of essential ions for micronutrient homeostasis, in order to cope with the deleterious effects of nonessential HMs (Hanikenne et al., 2005). The involvement of membrane transporters have been characterized in various microalgae species (García-garcía et al., 2012; Rosakis and Köster, 2004). According to Blaby-haas and Merchant (2012), two transporter groups are responsible for the influx and efflux of each metal ions in the algal model C. reinhardtii. Indeed, the transporters group (A) ensures the traffic of HMs from the extracellular environment to the cytosol, including Natural Resistance-Associated Macrophage Proteins (NRAMP), Zrt-Irt-like proteins (ZIP), Fe-Transporter (FTR) and Cu-transporter (CTR) families. This group of transporters has been identified also in the vacuole membrane and has the same function as the assimilative transporters. The second group (B) includes members from the cation diffusion facilitator (CDF), P1B-type ATPases, FerroPortiN (FPN) and the families Ca (II)-sensitive cross-complementer1/Vacuolar iron transporter1 (Ccc1/VIT1) (Figure 4), they decrease the metal concentration in the cytoplasm through active mechanism of efflux of metal ions into the extracellular surroundings, This occurs when the metal concentration exceeds cellular requirements, or if the metal peptide complex starts to affect cellular metabolism (Tripathi et al., 2019). Very few studies have described the role of membrane transporters in HMs accumulation in microalgae. Thus, future research on this topic is crucial for a deeper understanding of the pathways of metal accumulation in cell of microalgae.
Figure 4.
General scheme of intracellular detoxification of HMs in microalgae cells adapted from (Blaby-Haas and Merchant, 2012; Torres et al., 2008).
2.2.2. Pathways of intracellular HMs detoxification
Various strategies are used by microalgae to maintain intracellular ion concentrations at the optimal level and protect the cell from non-essential metals (Torres et al., 2008). These strategies include the modification of the permeability of plasma membrane and function of the cell wall, the activation of phytochelatins synthase, the formation of HMs-metallothioneins, HMs-poly-phosphates complexes, the compartmentalization into organelles, as well as the activation of metal efflux systems (García-García et al., 2016; Navakoudis and Ververidis, 2018).
2.2.2.1. Chelation by metallothioneins
MTs are small peptides that can be divided into two groups, gene-encoded proteins (MTs class I and II) and enzymatically synthesized polypeptides (MTs class III) commonly named phytochelatins (PCs) (Cobbett and Goldsbrough, 2002). The MTs class II are a super-rich cysteine family located in the cytosol, characterized by low molecular weight (6–7 kDa). These metal-binding proteins are primarily involved in the control of intracellular concentrations of metals at regular levels (Gaur and Rai, 2001). MTs-class II are little studied in microalgae compared to other organisms. The most known MTs from microalgae are of Chlorella Aureococcus, Symbiodinium, Nannochloropsis, Thalassiosira, and Ostreococcus genera (Balzano et al., 2020). Since microalgae can survive in habitats contaminated with HM, they have the potential for new forms of MTs. Therefore, future research should focus on the discovery of such new MTs using in silico experiment and experimental researches.
2.2.2.2. Chelation by phytochelatins
In response to metal exposure, microalgae like other organisms synthesize enzymatically PCs rather than MTs that are genetically encoded. The PCs are also thiol-containing peptides consisting of three amino acids: glutamate (Glu), cysteine (Cys) and glycine (Gly), with general structure (γ -Glu-Cys)n-Gly, the sulfhydryl group in the cysteine molecule is responsible for metal binding. Their biosynthetic pathway starts with the formation of γ–Glu–Cys by γ–glutamylcysteine synthetase (γ-GCS). Afterwards, the glutathione synthetase (GS) catalyzes the production of glutathione (GSH). Next, the transfer of γ-Glu-Cys from GSH to another GSH molecule is followed by the formation of (γ-Glu-Cys) 2-Gly (Hirata et al., 2005). Indeed, GSH has been reported to be the key ligand when intracellular metal concentrations are low, while metal detoxification is ensured by PCs when metal concentrations are high (Gaur and Rai, 2001). The synthesis of MTs class III in microalgae strains has been documented in several studies, it was first identified in C. fusca after exposure to Cd(II) ions (Gekeler et al., 1988). Subsequently, numerous researches were aiming to clarify PCs biosynthesis under HMs exposure such as Gómez-Jacinto et al. (2015) who identified Hg–PCs in C. sorokiniana under Hg-exposure. also in S. bijugatus Cu(II)-treated (Nagalakshmi and Prasad, 2001), and Pb (II)-treated Stichococcus bacillaris (Pawlik-skowron, 2002). Furthermore, it has been reported that Cd(II) was the most effective stimulator of PCs synthase of Chlamydomonas species, while other metals have shown less effectiveness in various degrees (Abboud and Wilkinson, 2013; Kobayashi et al., 2006; Li et al., 2021; Suárez et al., 2010). Nevertheless, PC synthesis was most strongly induced by Zn in Dunaliella species (Hirata et al., 2001; Tsuji et al., 2003). Recently, Wang et al. (2017) reported that GSH was the main non-protein sulfhydryl compound in D. salina. Its exposure to As(III) and As(V) has induced PCs synthesis indicating therefore their involvement in As-detoxification. The HMs-bound to PCs, they can be stored into organelles of microalgae cells as organometallic complexes.
2.2.2.3. Chelation by poly-phosphates
In nature, orthophosphate polymers (polyp) are abounding in both eukaryotic and prokaryotic organisms. Numerous algal research report the accumulation of polyp body in acidocalcisomes founded mainly in granules of specific vacuoles in the trans-Golgi that show diverse variants according to their disposition, composition, and consistency (Goodenough et al., 2019; Tsednee et al., 2019). However, polyp can also be localized in other cellular compartments including the cell wall, cytoplasm, mitochondria, and endoplasmic reticulum and nucleus (Sanz-luque et al., 2020; Werner et al., 2007). For instance, the metabolism of polyp in C. reinhardtii can be regulate via enzymatic reaction of exopolyphosphatase, or through compartmentalization mechanisms, mainly by the acidocalcisome membrane transporters (Ruiz et al., 2001). HMs sequestration and detoxification are among the various functions and cellular responses where polyp are implicated including cycling phosphorus (P) in oceans, P reservoir, acclimatation to nutrient deprivation as well as in response to osmotic and heat stress conditions (Sanz-luque et al., 2020). It has been reported that polyp formation facilitates the accumulation of HMs and storage (Wang and Dei, 2006). In fact, the crucial function of acidocalcisomes and polyps in maintaining cellular homeostasis of essential ions underlines the hypothesis that this role could be extended to the regulation of levels of toxic HMs in the intracellular compartment bioaccumulation via chelation and compartmentalization (García-García et al., 2016).
2.2.3. HMs compartmentalization in the vacuole, chloroplast and mitochondria
As previously mentioned, the sequestration of the MTs-HMs complex in particular cell organelles, especially vacuoles, chloroplasts, and mitochondria has prompted researchers to develop hypotheses about metal bioaccumulation pathways, and the tolerance mechanisms associated. Various biophysical techniques can be used for studding the intracellular localization and the analysis of HMs or their complex (Polyp-HMs, MT-HMs PCs-HMs), mainly by using transmission electron microscope (TEM) with added techniques and accessories, such as energy-dispersive X-ray spectroscopy (EDS), electron energy loss spectroscopy (EELS), electron spectroscopic imaging (ESI), and atomic force microscopy (AFM) (Tripathi et al., 2019). In plant species, vacuolar compartmentalization has been reported as an indispensable component of HMs detoxification (Sharma et al., 2016). In contrast, metal sequestration has been identified in different cell organelles. For example, Shanab et al. (2012) found by means of TEM analysis, an electron-dense dark spherical bodies accumulated in the vacuoles of Pseudochlorococcum typicum exposed to Pb ions. Volland et al. (2012) located also the accumulation of Cr (IV) using TEM coupled with EELS and ESI inside cells of Micrasterias denticulata as chromium-iron-oxygen compound and increased vacuolation. In contrast, Hanikenne et al. (2009), described that the main storage location of PCs–Cd(II) complexes in C. reinhardtii was in the chloroplast. Likewise, Mendoza-co and Moreno-sa, (2005) found that more than 60% of the accumulated Cd(II) resides inside the chloroplast of Euglena gracilis. Similarly, Soldo et al. (2005) reported that the intracellular Cu(II) was detected in the thylakoids and the pyrenoid of O. nephrocytioides cells. In addition, Mendoza-co et al. (2005) demonstrated the accumulation of Cd(II) and Cd(II)-Mt (III) complexes in chloroplast and mitochondria of E. gracilis.
2.3. Biotransformation and mitigation of HMs by microalgae
The broad sense of biotransformation refers to the pathway by which xenobiotic or endobiotic chemicals are metabolized into products, which vary in their activity (activation vs deactivation), excretability (hydrophobic vs hydrophilic), and toxicity (detoxification vs toxication) (Rourke and Sinal, 2014). In the context of HMs-phycoremediation, the term "biotransformation" may apply to a variety of detoxification pathways, primarily enzymatic and biochemical transformation of toxic HMs.
2.3.1. Enzymatic biotransformation
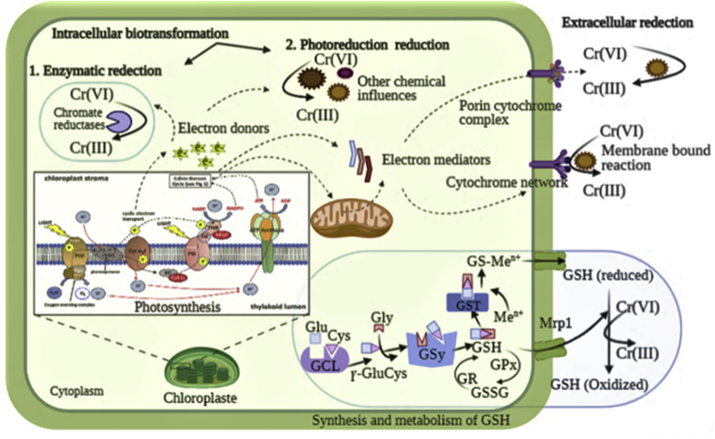
The enzymatic biotransformation of HMs can be defined as chemical transformation from a high toxic form to a less harmful through oxidation reduction reactions. In fact, HMs cannot be degraded, but they can be transformed from one oxidation state to another an inorganic complex form, in order to mitigate their toxic impact. Only few studies have emphasized the role of oxidoreductase enzymes in the detoxification of HMs by microalgae. The main redox enzymes reported in microalgae are chromate reductases, mercuric reductase and arsenate reductase (Leong and Chang, 2020). It has been reported by Lee et al. (2017) and Yen et al. (2017) that C. vulgaris strains have the potential to convert Cr(VI) to Cr(III) through an enzymatic reaction catalyzed by the chromate reductase. Also, Kelly et al. (2007) reported that the microalgae strains of Selenastrum minutum, C. fusca and Galdiera sulphuraria have the ability to catalyze the bio-transformation of Hg2+ into elemental Hg0 and metacinnabar (β-HgS) through mercuric reductase. The arsenate reductase has also been found in the green microalgae C. reinhardtii (Yin et al., 2011). Figure 5 depicts the intracellular and extracellular mitigation pathways for Cr (VI) by microalgae.
Figure 5.
Proposed biotransformation pathways of Cr(VI) developed from the finding of (Deng et al., 2006; Lee et al., 2017; Rahman and Thomas, 2021; Yen et al., 2017).
2.3.2. Biochemical transformations of HMs
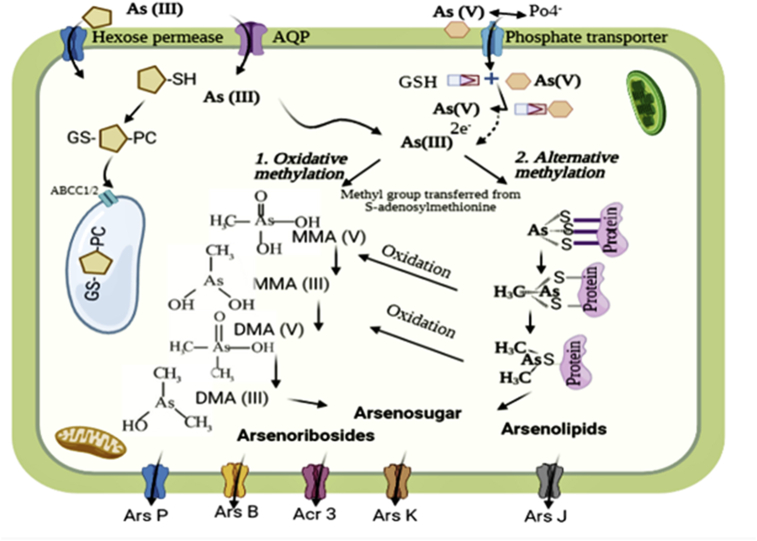
During the phytoremediation process, cells of microalgae may use biochemical mechanisms for HMs-mitigation. For example, the reaction of chromium reduction from the hexavalent oxidation state to the trivalent form is catalyzed by the transfer of electrons to the reduced form of GSH (Yen et al., 2017) (Figure 5). Moreover, the mitigation of the toxicity of inorganic arsenic can be the result of several other detoxification mechanisms (Hasegawa et al., 2019; Karadjova et al., 2008; Levy et al., 2005; Wang et al., 2015b). In fact, a variety of microalgae species seem to be able to reduce As(V) to As(III). For example, Karadjova et al. (2008) indicated that after 72 h of exposure of C. salina to As(V), 32% of total intracellular concentration of As(V) was converted to As (III). According to Hasegawa et al. (2001), the initial concentration of As(V) in the medium was firstly converted to As(III) by C. aciculare, and reached a peak concentration during the exponential growth. Generally, As-species are distributed among different cellular fractions of cells of microalgae including the lipid, cytosolic, cell membranes and debris fractions. The detoxification pathway begins with the reduction of As(V) into As(III) form, and then methylation to monomethylarsonate (MMA(V)) via oxidase and S-adenosylmethionine (SAM). The produced MMA is converted to dimethylarsinate (DMA(V)), that is further reduced to DMA(III), next its conversion to a range of organoarsenicals such as, arsenolipids, arsenosugars, arsenobetaine, and arsenoribosides (Arora et al., 2018; Wang et al., 2015b). Figure 6 shows the pathways of biotransformation of As(V and III) by microalgae.
Figure 6.
Proposed biotransformation pathways of As (V and III) developed from (Arora et al., 2018; Garbinski et al., 2019; Wang et al., 2015b).
2.3.3. Other biotransformation mechanisms of HMs
In addition to the intracellular HMs-biotransformation mechanism previously described, the biosynthesis of metal nanoparticles can occur both extracellularly and intracellularly, depending on the location of NP biosynthesis and the reductive agents (Hamida et al., 2020). It has been reported that microalgae have the potential to remodel toxic HMs into malleable forms, such as via their combination with protein, lipids, carbohydrate, pigments, and other antioxidants molecules which can reduce the charge of the metal ion to a zero-valence state (Chaudhary et al., 2020). Also in the extracellular surroundings, the charged functional groups of the cells surface as well as the EPS, the binding sites, organic ligands may contribute to speciation changes of the toxic HMs via redox reactions (Naveed et al., 2019). Indeed, Priyadarshini et al. (2019) reported that the extracellular biosynthesis of metal nanoparticles appears to be simpler compared to the intracellular environment, where the algae extract contains polysaccharides, proteins and pigments that act as reducing agents that stabilize metal ions and metal nanoparticles. On the other hand, biologically and non-biologically volatilization of mercury can be used by E. gracilis as another biotransformation mechanism for the mitigation of the toxic effect of mercury (Devars et al., 2000). Besides, Deng et al. (2006) reported that the Cr(VI)-biotransformation with C. vulgaris was managed by a photoreduction pathway.
3. Future outlook for enhancing the phycoremediation process
Numerous research studies have demonstrated the feasibility of using green microalgae in metal remediation, both technically and economically. In addition to optimizing the factors that influence the phycoremediation process, various physicochemical and biotechnological approaches can be used to (i) improve the extracellular uptake and selectivity for a target metal (Precious elements or rare earths), to (ii) increase the intracellular bioaccumulation capacity, or to (iii) enhance the biotransformation and mitigation capacity.
3.1. Cell manipulation for enhancing the biosorption capacity and selectivity
As detailed above, the biosorption of HMs occurs mainly in the cell surface. Thus, modifying their composition and their physicochemical properties can improve the interaction with ions of HMs, increasing thereby the biosorption performance.
3.1.1. Physicochemical approaches
3.1.1.1. Cell surface functionalization
According to Pearson acid-base concept, the affinity of the cell surface towards species of HMs considerably depends on the functional groups. For instance, the biosorption capacity of Pb(II), Cd(II), Zn(II), Cu(II), and Ni(II) into Neochloris minuta and N. alveolaris was related to the HSAB theory in terms of their biomass compositions and the type of hard or soft metal acid based (Giarikos et al., 2021). Indeed, changing the nature and density of functional groups at the cell surface, regulated the affinity and sorption behavior for specific metal species. The functionalization of the cell surface can be achieved by introduction of active functional groups (binding sites) using chemical reaction, or by inhibiting functional groups that have a negative impact on the target metal's biosorption (Yang et al., 2015).
3.1.1.2. Chemical pretreatment
Many pretreatment reagents can be used to change the physicochemical properties of the cell wall, and remove impurities on the surface, as well as to expose metal binding sites by eliminating blocking ions. These reagents include acid, alkali, organic solvents (alcohol, acetone and toluene), and inorganic salts (NaCl and Na2CO3) (Nagase et al., 2005). Besides the use of acid that may dissolve polysaccharides, an alkali washing can get rid of the liposome on the cell membrane. Both pretreatment processes can effectively remove the impurities and expose the binding sites by eliminating the blocking ions, thus improving the sorption capacity (Mehta et al., 2002).
3.1.1.3. Magnetic modification
The magnetically modification of microalgae with magnetic nano- and micro particles have been used in a variety of algae biotechnology research areas, including the removal of HMs ions (Safarik et al., 2020). According to Lalhmunsiama et al. (2017), cells of C. vulgaris coated with magnetic iron oxide NPs has successfully removed ions of Cd(II) and Pb(II) from aqueous solutions. Thus, their simultaneous biosorption was completed by distinguished binding sites. Pb(II) ions were chemically bound to amino groups, while Cd(II) ions were bound to dissociated hydroxyl or carboxyl groups through weak electrostatic forces. Likewise, Ferraro et al. (2018) reported that cells of native Chlorella sp. flocculated with polyethylenimine-coated magnetic have exhibited a high removal efficiency of Zn (II) ions. Although physicochemical modification can improve the binding performance of cellular metals, it can also present some drawbacks. Firstly, they can lead to a decrease in selectivity towards the targeted metal species. Secondly, washing the cells could lead to a loss of biomass, and finally, chemical and magnetic modifications are costly for large-scale applications (Cheng et al., 2019).
3.1.2. Bioengineering of cell-surface approaches
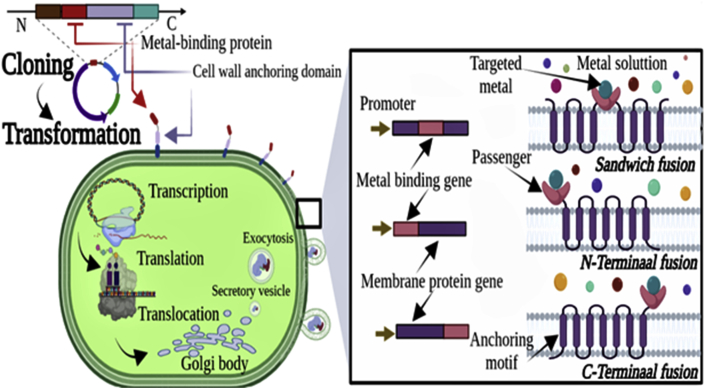
Adsorption-based processes have a high potential for HMs absorption, but their common weakness is a lack of selectivity for the absorption of target metal from heterogeneous metal ions solutions (Shen et al., 2017). The advancement of bioengineering of living microorganisms has recently enabled the construction of new biosorbents by adapting the metallosorption properties of the cell surfaces, in order to increase the adsorption selectivity for target metal species (Wang et al., 2021). This molecular technique is called cell surface engineering or cell surface display. It consists of expressing proteins of interest (passenger protein) such metal-binding proteins (MTs or PCs) on the cell surface by a translational fusion with anchoring motif (carrier protein), which allows the passenger protein to be exported across the cell envelope and anchors it to the cell surface (Kuroda and Ueda, 2011; Yang et al., 2015). Artificial proteins with new activities and/or imitative roles such as high metal-binding and pre-programmed properties for the removal of HMs in theory that can be located in any specific cellular compartment may be created through genetic and protein engineering (Agapakis and Silver, 2009). Figure 7 illustrates the cell surface engineering procedure for a target metal biosorption. First, the coding DNA of the target metal-binding peptide or protein previously was obtained via whole sequence synthesis or PCR amplification from genomic or plasmid DNA. Next it can be transformed to the host in the form of fusion of a protein under specific induction. The metal-binding protein/peptide can be displayed in the form of fusion of an anchor protein after transcription, translation and translocation. The secretory vesicles encompassing the passenger and carrier proteins pass through the cell membrane and anchor the passenger proteins to the surface of cell wall (Kuroda and Ueda, 2011; Li and Tao, 2013; Wang et al., 2021). There are considerable published works on genetically-engineered biosorbents for removal of HMs using bacteria, yeast and fungi (Wang et al., 2021; Yang et al., 2015). However, to our knowledge, there are not yet studies using microalgae as host cells for the surface display of target metal binding. He et al. (2011) showed that the removal capacity of Hg (II) using a transgenic C. reinhardtii (2AMT-2) designed to express a membrane-anchored MT polymer, was at high levels that could be released into the aqueous phase by sonication over a wide pH range of 2–9. Based on these preliminary studies, it's essential to emphasize the importance of future work on the bioengineering of the microalgae cell surface for the biosorption of HMs. Indeed, several benefits can be derived from overexpression of metal-binding-proteins into the cell surface of green microalgae. Firstly, due to the increase of the ligand on the surface of the cell, the processing time will be greatly shortened. Second, such a modification would increase the selectivity for a target metal. This adsorbed metal can thereby be easily recovered with mild pickling reagent, instead of breaking the cell wall to recover the metals inside the cell. This makes such biosorbent recyclable and economical. Finally, the surface adsorption is independent of metabolism and the dead biomass can therefore be used.
Figure 7.
Procedure of cell surface engineering for a target metal biosorption.
3.2. Microalgae genetically engineered for intracellular recovery of HMs
Application of genetic engineering strategy for the enhancement of the intra-cellular uptake of HMs encompass the genetic modification of genes encoding for: metals membrane transporters, high-affinity HM binding proteins such as genetically encoded chelators, enzymes that catalyze the reduction of toxic metals via redox transformations, and enzymes that scavenge reactive oxygen species (Rajamani et al., 2007). In fact, the genetic manipulation of microalgae for enhancing the bioaccumulation capacity typically comprises two techniques: gene overexpression and the construction of transgenic algae by introducing exogenous DNA into cells of microalgae (Cheng et al., 2019).
3.2.1. Metal-transporters transition in microalgae
Identification of microalgae genes encoding metalloregulatory proteins is critical for genetic engineering research for phytoremediation purposes. As detailed in the previous section (2.1.1), metal transporters play an important role in the interaction of microalgae with their surroundings. They constitute the second line of protection after the cell wall, via the control of the perturbations in cellular and subcellular homeostasis of metal (Blaby-Haas and Merchant, 2012). Despite the fact that this approach may allow both increased accumulation of metals in the cell and increased tolerance to metals, such as by transferring a toxic metal out of the cytosol and into an internal compartment, genetic manipulation of metal transporters towards HMs phycoremediation is still quite limited. Ibuot et al. (2017) reported that the overexpression of metal tolerance protein (MTP) in C. reinhardtii led to a significant increase in resistance to Cd(II) toxicity as well as bioaccumulation efficiency. This was proposed to be due to increased transfer and storage of Cd(II) into vacuoles. According to Ibuot et al. (2020), the expression of a plant Cd and Zn transporter (AtHMA4) known to bind metals in C. reinhardtii, either as a full-length protein or only as the C-terminal tail, had led to increase the bioaccumulation and internalization of both Cd(II) and Zn(II) ions, with expression of either the FL protein or the CT domain. This suggests that the enhancement of metal bioaccumulation was primarily the result of increased metal binding rather than metal transport. Ramírez-rodríguez et al. (2019) evaluated the As-bioremoval capability of Acr3-modified C. reinhardtii strain produced by transforming the wild-type strain with Agrobacterium tumefaciens using the construct pARR1 including a synthetic, optimized acr3 gene from Pteris vittata, reported to have two homologs proteins PvACR3;1 and PvACR3 localized in the membrane of vacuolar possible ensure the sequestration of As(III) by effluxing into the vacuole (Indriolo et al., 2010). The result of this study indicates that the acr3-modified strain removed 1.5 to 3 times more arsenic compared to the wild-type.
3.2.1. Molecular manipulation for AA and PCs biosynthesis
As previously mentioned in the section (2.2.2), the metal chelator is primarily responsible for the intracellular bioaccumulation of HMs. Genetic manipulation of the biosynthesis and metabolism of certain AA, and PCs seems to be an optimal strategy to increase the capacity of HMs bioaccumulation into microalgal cultures. However, despite the great potential for involvement of AA and the PCs biosynthesis in the HMs-tolerance and detoxification, the number of microalgae mutants developed for this purpose is still limited. Zheng et al. (2013) reported that overexpressing of HISN3 gene (Coding for phosphoribosylformimino-5-aminoimidazole carboxamide ribonucleotide isomerase) in C. reinhardtii induces a high tolerance towards Ni, with moderate increase of histidine accumulation in comparison to the wild type. Furthermore, Siripornadulsil et al. (2002) suggest that the expression of the P5CS (mothbean Δ 1 -pyrroline-5-carboxylate synthetase) gene in C. reinhardtii induces proline buildup compared to wild type, and increases their tolerance against Cd(II) ions. Similarly, the expression of the HAL2 gene in C. reinhardtii, which regulates cysteine synthesis increased the Cd-carrying capacity of transgenic cells compared to wild-type cells (Rajamani et al., 2007). Regarding the transgenic manipulation for the transformation of C. reinhardtii with a foreign MTs(II), it was firstly documented by Cai et al. (1999) that the cell density of C. reinhardtii cells expressing a foreign MTs(II) was higher compared to the wild type when exposed to Cd(II) ions.
3.2.3. Genetic transformation for HMs enzyme biocatalysts
The mitigation of toxic HMs by living microorganisms can also be improved using genetically engineered enzymes (Diep et al., 2018). For instance, the transformation of Chlorella spp. DT, by mercuric reductase (merA) gene from Bacillus megaterium, improved reduction of bioremoval Hg(II) up to twofold compared to the wild type (Huang et al., 2006). As previously noted in the section (2.2.2), the biosynthesis of GSH occurs via an enzymatic reaction catalyzed by γ -GCS. Recently, Piña-olavide et al. (2020) reported that the transformation of C. reinhardtii with a synthetic gene (gshA) encoding for γ-GCS had significantly increased Cd (II) bioaccumulation. Furthermore, Wang et al. (2015a) studied the transformation of C. reinhardtii with CrGNAT gene encoding for acetyltransferase, they reported also that such modification regulates excess Cu(II) bioaccumulation and tolerance. The acetyltransferase can be involved in the production of cysteine, glutathione and PCs under Cd(II) stress (Howarth et al., 2003).
4. Conclusion
Based on the above considerations, we can infer that the strains of green microalgae had multifaceted mechanisms for removing toxic HMs, including extracellular biosorption, EPS-complexation, intracellular bioaccumulation and compartmentalization, enzymatic reduction, bio-methylation, and volatilization. Although the process of phycoremediation of HMs is still on the lab scale, the analysis of the involved mechanisms provides insights for improving efficiency, selectivity, and reduces processing costs. Recently, physicochemical and molecular modifications of the microbial cell surface have demonstrated their applicability for the enhancement of bioremediation performance. In fact, several microorganisms with innovative cell surface properties have been bioengineered. In contrast, there are still limited examples of genetic manipulation of microalgae for HMs-removal purposes. Further research in the fields of genetic engineering, pre-treatment, immobilization techniques and a combination of other physicochemical strategies will allow phycoremediation processes to be used on a large scale.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Moroccan Foundation for Advanced Science, Innovation and Research (MAScIR), and Regional University Centre of Interface (CURI), Sidi Mohamed Ben Abdellah University.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Mohammed Danouche, Email: mohammed.danouche@usmba.ac.ma.
Naïma El Ghachtouli, Email: naima.elghachtouli@usmba.ac.
References
- Abboud P., Wilkinson K.J. Role of metal mixtures (Ca, Cu, and Pb) on Cd bioaccumulation and phytochelatin production by Chlamydomonas reinhardtii. Environ. Pollut. 2013;179:33–38. doi: 10.1016/j.envpol.2013.03.047. [DOI] [PubMed] [Google Scholar]
- Agapakis C.M., Silver P.A. Molecular BioSystems the design of novel biological networks w. Mol. Biosyst. 2009;5:704–713. doi: 10.1039/b901484e. [DOI] [PubMed] [Google Scholar]
- Ali H., Khan E., Ilahi I. Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019;2019 [Google Scholar]
- Arora N., Gulati K., Tripathi S., Pruthi V. Mechanisms of Arsenic Toxicity and Tolerance in Plants. 2018. Algae as a budding tool for mitigation of arsenic from aquatic systems; pp. 269–297. [Google Scholar]
- Balzano S., Sardo A., Blasio M., Chahine T.B., Anno F.D., Sansone C., Brunet C. Microalgal metallothioneins and phytochelatins and their potential use in bioremediation. Front. Microbiol. 2020;11:1–16. doi: 10.3389/fmicb.2020.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudelet P.H., Ricochon G., Linder M., Muniglia L. A new insight into cell walls of Chlorophyta. Algal Res. 2017;25:333–371. [Google Scholar]
- Blaby-Haas C.E., Merchant S.S. The ins and outs of algal metal transport. Biochim. Biophys. Acta. 2012;1823(9):1531–1552. doi: 10.1016/j.bbamcr.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Brown C., Adhiya J., Traina S.J., Sayre R.T. Growth and heavy metal binding properties of transgenic Chlamydomonas expressing a foreign metallothionein gene growth and heavy metal binding properties of transgenic Chlamydomonas expressing a foreign. Int. J. Phytoremediation. 1999;1:37–41. [Google Scholar]
- Carvalho J.C. De, Irineudo A.M., Jr., Vinicius G., Pereira D.M., Bianchi A., Medeiros P., Sydney E.B., Rodrigues C., Tatiana D., Aulestia M., Porto L., Vandenberghe D.S., Soccol V.T., Soccol C.R. Microalgal biomass pretreatment for integrated processing into biofuels, food, and feed. Bioresour. Technol. 2020;300:122719. doi: 10.1016/j.biortech.2019.122719. [DOI] [PubMed] [Google Scholar]
- Chaudhary R., Nawaz K., K.A. K., Hano C., Abbasi B.H., Anjum S. An overview of the algae-mediated biosynthesis of nanoparticles and their biomedical applications. Biomolecules. 2020;10:1497. doi: 10.3390/biom10111498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.Y., Show P., Lau F., Chang J., Ling T.C. Review new prospects for modified algae in heavy metal adsorption. Trends Biotechnol. 2019;37:1255–1268. doi: 10.1016/j.tibtech.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Chibueze C., Chioma A., Chikere B. Bioremediation techniques – classification based on site of application : principles , advantages , limitations and prospects. World J. Microbiol. Biotechnol. 2016;32:1–18. doi: 10.1007/s11274-016-2137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacka K. Biosorption and bioaccumulation–the prospects for practical applications. Environ. Int. 2010;36(3):299–307. doi: 10.1016/j.envint.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Cobbett C., Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- D’Hondt E., Martín-Juárez J., Bolado S., Kasperoviciene J., Koreiviene J., Sulcius S., Elst K., Bastiaens L. Microalgae-Based Biofuels and Bioproducts: from Feedstock Cultivation to End-Products. 2017. Cell disruption technologies; pp. 133–154. [Google Scholar]
- Danouche M., El Ghachtouli N., El Baouchi A., El Arroussi H. Heavy metals phycoremediation using tolerant green microalgae : enzymatic and non-enzymatic antioxidant systems for the management of oxidative stress. J. Environ. Chem. Eng. 2020;8:104460. [Google Scholar]
- Deng L., Wang H., Deng N. Photoreduction of chromium (VI) in the presence of algae Chlorella vulgaris. J. Hazard Mater. 2006;138(2):288–292. doi: 10.1016/j.jhazmat.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Devars S., Avilés C., Cervantes C., Moreno-sánchez R. Mercury uptake and removal by Euglena gracilis. Arch. Microbiol. 2000;174:175–180. doi: 10.1007/s002030000193. [DOI] [PubMed] [Google Scholar]
- Diep P., Mahadevan R., Yakunin A.F. Heavy metal removal by bioaccumulation using genetically engineered microorganisms. Front. Bioeng. Biotechnol. 2018;6 doi: 10.3389/fbioe.2018.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C., Wilken L.R. Green microalgae biomolecule separations and recovery. Bioresour. Bioprocess. 2018;5(1):1–24. [Google Scholar]
- Dung T.T.T., Cappuyns V., Swennen R., Phung N.K. From geochemical background determination to pollution assessment of heavy metals in sediments and soils. Rev. Environ. Sci. Biotechnol. 2013;12:335–353. [Google Scholar]
- Ferraro G., Toranzo R.M., Castiglioni M., Lima E., Vasquez M., Fellenz N.A., Zysler R.D., Pasquevich D.M., Bagnato C. Zinc removal by Chlorella sp. biomass and harvesting with low cost magnetic particles. Algal Res. 2018;33:266–276. [Google Scholar]
- Fu F., Wang Q. Removal of heavy metal ions from wastewaters: a review. J. Environ. Manag. 2011;92:407–418. doi: 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Gałuszka A. A review of geochemical background concepts and an example using data from Poland. Environ. Geol. 2007:861–870. [Google Scholar]
- Garbinski L.D., Rosen B.P., Chen J. Pathways of arsenic uptake and efflux. Environ. Int. 2019;126:585–597. doi: 10.1016/j.envint.2019.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-garcía J.D., Olin-sandoval V., Saavedra E., Girard L., Hernández G., Moreno-sánchez R. Sulfate uptake in photosynthetic Euglena gracilis. Mechanisms of regulation and contribution to cysteine homeostasis. BBA - Gen. Subj. 2012;1820:1567–1575. doi: 10.1016/j.bbagen.2012.05.002. [DOI] [PubMed] [Google Scholar]
- García-García J.D., Sánchez-Thomas R., Moreno-Sánchez R. Bio-recovery of non-essential heavy metals by intra- and extracellular mechanisms in free-living microorganisms. Biotechnol. Adv. 2016;34:859–873. doi: 10.1016/j.biotechadv.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Garrett R.G. Natural sources of metals to the environment. Hum. Ecol. Risk Assess. 2000;6:945–963. [Google Scholar]
- Gaur J.P., Rai L.C. Algal Adaptation to Environmental Stresses. 2001. Heavy metal tolerance in algae; pp. 363–388. [Google Scholar]
- Gekeler W., Grill E., Winnacker E.L., Zenk M.H. Algae sequester heavy metals via synthesis of phytochelatin complexes. Arch. Microbiol. 1988;150(2):197–202. [Google Scholar]
- Giarikos D.G., Brown J., Razeghifard R., Vo D., Castillo A., Nagabandi N. Effects of nitrogen depletion on the biosorption capacities of Neochloris minuta and Neochloris alveolaris for five heavy metals. Appl. Water Sci. 2021;11:1–15. [Google Scholar]
- Gómez-Jacinto V., García-Barrera T., Gómez-Ariza J.L., Garbayo-Nores I., Vílchez-Lobato C. Elucidation of the defence mechanism in microalgae Chlorella sorokiniana under mercury exposure. Identification of Hg-phytochelatins. Chem. Biol. Interact. 2015;238:82–90. doi: 10.1016/j.cbi.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Gonzalez-dkila M. The role of phytoplankton cells on the control of heavy metal concentration in seawater. Mar. Chem. 1995;48:215–236. [Google Scholar]
- Goodenough U., Heiss A.A., Roth R., Rusch J., Lee J. Acidocalcisomes : ultrastructure, biogenesis, and distribution in microbial eukaryotes. Protist. 2019;170:287–313. doi: 10.1016/j.protis.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Hamida R.S., Ali M.A., Redhwan A., Bin-Meferij M.M. Cyanobacteria–a promising platform in green nanotechnology: a review on nanoparticles fabrication and their prospective applications. Int. J. Nanomed. 2020;15:6033. doi: 10.2147/IJN.S256134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanikenne M., Krämer U., Demoulin V., Baurain D. Comparative inventory of metal transporters in the green alga Chlamydomonas reinhardtii and the red alga Cyanidioschizon merolae. Plant Physiol. 2005;137:428–446. doi: 10.1104/pp.104.054189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanikenne M., Merchant S.S., Hamel P. The Chlamydomonas Sourcebook. 2009. Transition metal Nutrition : a balance between deficiency and toxicity; pp. 333–399. [Google Scholar]
- Hasegawa H., Papry R.I., Ikeda E., Omori Y., Mashio A.S., Maki T., Rahman M.A. Freshwater phytoplankton: biotransformation of inorganic arsenic to methylarsenic and organoarsenic. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-48477-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Sohrin Y., Seki K., Sato M., Norisuye K., Naito K., Matsui M. Biosynthesis and release of methylarsenic compounds during the growth of freshwater algae. Chemosphere. 2001;43:265–272. doi: 10.1016/s0045-6535(00)00137-5. [DOI] [PubMed] [Google Scholar]
- Hayat S., Hayat Q., Alyemeni M.N., Wani A.S., Pichtel J., Ahmad A. Role of proline under changing environments: a review. Plant Signal. Behav. 2012;7:1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Siripornadulsil S., Sayre R.T., Traina S.J., Weavers L.K. Removal of mercury from sediment by ultrasound combined with biomass (transgenic Chlamydomonas reinhardtii ) Chemosphere. 2011;83:1249–1254. doi: 10.1016/j.chemosphere.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Hirata K., Tsuji N., Miyamoto K. Biosynthetic regulation of phytochelatins, peptides, heavy metal-binding. J. Biosci. Bioeng. 2005;100:593–599. doi: 10.1263/jbb.100.593. [DOI] [PubMed] [Google Scholar]
- Hirata K., Tsujimoto Y., Namba T., Ohta T., Hirayanagi N., Miyasaka H., Meinhart H., Miyasaka H., Miyamoto K. Strong induction of phytochelatin synthesis by zinc in marine green alga, Dunaliella tertiolecta. J. Biosci. Bioeng. 2001;92:24–29. doi: 10.1263/jbb.92.24. [DOI] [PubMed] [Google Scholar]
- Hou J., Yang Y., Wang P., Wang C., Miao L. Effects of CeO2 , CuO , and ZnO nanoparticles on physiological features of Microcystis aeruginosa and the production and composition of extracellular polymeric substances. Environ. Sci. Pollut. Res. 2016;24(1):226–235. doi: 10.1007/s11356-016-7387-5. [DOI] [PubMed] [Google Scholar]
- Howarth J.R., Dom R., Guti G., Wray J.L., Romero L.C., Gotor C. The serine acetyltransferase gene family in Arabidopsis thaliana and the regulation of its expression by cadmium. Plant Mol. Biol. 2003;51:589–598. doi: 10.1023/a:1022349623951. [DOI] [PubMed] [Google Scholar]
- Huang C.C., Chen M.W., Hsieh J.L., Lin W.H., Chen P.C., Chien L.F. Expression of mercuric reductase from Bacillus megaterium MB1 in eukaryotic microalga Chlorella sp. DT: an approach for mercury phytoremediation. Appl. Microbiol. Biotechnol. 2006;72:197–205. doi: 10.1007/s00253-005-0250-0. [DOI] [PubMed] [Google Scholar]
- Ibuot A., Dean A.P., McIntosh O.A., Pittman J.K. Metal bioremediation by CrMTP4 over-expressing Chlamydomonas reinhardtii in comparison to natural wastewater-tolerant microalgae strains. Algal Res. 2017;24:89–96. [Google Scholar]
- Ibuot A., Webster R.E., Williams L.E., Pittman J.K. Increased metal tolerance and bioaccumulation of zinc and cadmium in Chlamydomonas reinhardtii expressing a AtHMA4 C - terminal domain protein. Biotechnol. Bioeng. 2020:2996–3005. doi: 10.1002/bit.27476. [DOI] [PubMed] [Google Scholar]
- Indriolo E., Na G., Ellis D., Salt D.E., Banks J.A. A vacuolar arsenite transporter necessary for arsenic tolerance in the arsenic hyperaccumulating fern Pteris vittata is missing in flowering plants. Plant Cell. 2010;22:2045–2057. doi: 10.1105/tpc.109.069773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanbakht V., Alavi S.A., Zilouei H. Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci. Technol. 2014;69:1775–1787. doi: 10.2166/wst.2013.718. [DOI] [PubMed] [Google Scholar]
- John J. Modern Trends in Applied Aquatic Ecology. Springer; Boston, MA: 2003. Phycoremediation: algae as tools for remediation of mine-void wetlands; pp. 133–147. [Google Scholar]
- Karadjova I.B., Slaveykova V.I., Tsalev D.L. The biouptake and toxicity of arsenic species on the green microalga Chlorella salina in seawater. Aquat. Toxicol. 2008;87:264–271. doi: 10.1016/j.aquatox.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Kelly D.J.A., Budd K., Lefebvre D.D. Biotransformation of mercury in pH-stat cultures of eukaryotic freshwater algae. Arch. Microbiol. 2007:45–53. doi: 10.1007/s00203-006-0170-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Fujiwara S., Saegusa H., Inouhe M., Matsumoto H., Tsuzuki M. Relief of arsenate toxicity by Cd-stimulated phytochelatin synthesis in the green alga Chlamydomonas reinhardtii. Mar. Biotechnol. 2006;8:94–101. doi: 10.1007/s10126-005-5092-3. [DOI] [PubMed] [Google Scholar]
- Kumar K.S., Dahms H.U., Won E.J., Lee J.S., Shin K.H. Microalgae - a promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015;113:329–352. doi: 10.1016/j.ecoenv.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Kuroda K., Ueda M. Molecular design of the microbial cell surface toward the recovery of metal ions. Curr. Opin. Biotechnol. 2011;22:427–433. doi: 10.1016/j.copbio.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Lalhmunsiama, Gupta Prabuddha L., Hyunhoon J., Diwakar T., Sung-Ho K., Seung-Mok L. Insight into the mechanism of Cd ( II ) and Pb (II) removal by sustainable magnetic biosorbent precursor to Chlorella vulgaris. J. Taiwan Inst. Chem. Eng. 2017;71:206–213. [Google Scholar]
- Lee L., Hsu C.Y., Yen H.W. The effects of hydraulic retention time (HRT) on chromium(VI) reduction using autotrophic cultivation of Chlorella vulgaris. Bioproc. Biosyst. Eng. 2017;40:1725–1731. doi: 10.1007/s00449-017-1827-6. [DOI] [PubMed] [Google Scholar]
- Leong Y.K., Chang J.S. Bioremediation of heavy metals using microalgae: recent advances and mechanisms. Bioresour. Technol. 2020;303:122886. doi: 10.1016/j.biortech.2020.122886. [DOI] [PubMed] [Google Scholar]
- Leszczyszyn O.I., White C.R.J., Blindauer C.A. The isolated Cys 2 His 2 site in E C metallothionein mediates metal-specific protein folding. Mol. Biosyst. 2010;6:1592–1603. doi: 10.1039/c002348e. [DOI] [PubMed] [Google Scholar]
- Levy J.L., Stauber J.L., Adams M.S., Maher W.A., Kirby J.K., Jolley D.F. Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum) Environ. Toxicol. Chem. 2005;24:2630–2639. doi: 10.1897/04-580r.1. [DOI] [PubMed] [Google Scholar]
- Li C., Zheng C., Fu H., Zhai S., Hu F., Naveed S., Zhang C., Ge Y. Contrasting detoxification mechanisms of Chlamydomonas reinhardtii under Cd and Pb stress. Chemosphere. 2021;274:129771. doi: 10.1016/j.chemosphere.2021.129771. [DOI] [PubMed] [Google Scholar]
- Li N., Wei Dong, Wang Shaotong, Hu Lihua, Xu Weiying, Du Bin, Wei Qin, Wei D., Wang S., Hu L., Xu W., Du B., Wei Q. Comparative study of the role of extracellular polymeric substances in biosorption of Ni ( II ) onto aerobic/anaerobic granular sludge. J. Colloid Interface Sci. 2016;490:754–761. doi: 10.1016/j.jcis.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Li P., Tao H. Cell surface engineering of microorganisms towards adsorption of heavy metals. Crit. Rev. Microbiol. 2013;41:140–149. doi: 10.3109/1040841X.2013.813898. [DOI] [PubMed] [Google Scholar]
- Macfie S.M., Welbourn P.M. The cell wall as a barrier to uptake of metal ions in the unicellular green alga Chlamydomonas reinhardtii (Chlorophyceae) Arch. Environ. Contam. Toxicol. 2000;39:413–419. doi: 10.1007/s002440010122. [DOI] [PubMed] [Google Scholar]
- Mehta S.K., Tripathi B.N., Gaur J.P. Enhanced sorption of Cu 2+ and Ni 2+ by acid-pretreated. J. Appl. Phycol. 2002;14:267–274. [Google Scholar]
- Mendoza-co D., Loza-tavera H., Herna A., Moreno-sa R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast , protists and plants. FEMS Microbiol. Rev. 2005;29:653–671. doi: 10.1016/j.femsre.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Mendoza-co D.G., Moreno-sa R. Cd2+ transport and storage in the chloroplast of Euglena gracilis. Biochim. Biophys. Acta. 2005;1706:88–97. doi: 10.1016/j.bbabio.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Monteiro C.M., Castro P.M.L., Malcata F.X. Biomanagement of Metal-Contaminated Soils. Springer; Dordrecht: 2011. Microalga-mediated bioremediation of heavy metal–contaminated surface waters; pp. 365–385. [Google Scholar]
- Monteiro C.M., Castro P.M.L., Malcata F.X., Instituto I., Carlos A., Campos O., Maia C., Pedro P.--A.S. Metal uptake by Microalgae : underlying mechanisms and practical applications. Biotechnol. Prog. 2012;28(2):299–311. doi: 10.1002/btpr.1504. [DOI] [PubMed] [Google Scholar]
- Nagalakshmi N., Prasad M.N.V. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci. 2001;160:291–299. doi: 10.1016/s0168-9452(00)00392-7. [DOI] [PubMed] [Google Scholar]
- Nagase H., Inthorn D., Oda A., Nishimura J.U.N., Kajiwara Y., Park M., Hirata K. Improvement of selective removal of heavy metals in cyanobacteria by NaOH treatment. J. Biosci. Bioeng. 2005;99:372–377. doi: 10.1263/jbb.99.372. [DOI] [PubMed] [Google Scholar]
- Navakoudis A.M.E., Ververidis K.P.F. Microalgae: a potential tool for remediating aquatic environments from toxic metals. Int. J. Environ. Sci. Technol. 2018;15(8):1815–1830. [Google Scholar]
- Naveed S., Li C., Lu X., Chen S., Yin B., Zhang C., Ge Y. Microalgal extracellular polymeric substances and their interactions with metal(loid)s: a review. Crit. Rev. Environ. Sci. Technol. 2019;49:1769–1802. [Google Scholar]
- Pawlik-skowron B. Correlations between toxic Pb effects and production of Pb-induced thiol peptides in the microalga Stichococcus bacillaris. Environ. Pollut. 2002;119:119–127. doi: 10.1016/s0269-7491(01)00280-9. [DOI] [PubMed] [Google Scholar]
- Pérez-Rama M., Abalde Alonso J., Herrero López C., Torres Vaamonde E. Cadmium removal by living cells of the marine microalga Tetraselmis suecica. Bioresour. Technol. 2002;84:265–270. doi: 10.1016/s0960-8524(02)00045-7. [DOI] [PubMed] [Google Scholar]
- Piña-olavide R., Paz-maldonado L.M.T., Torre M.C.A. La, García-soto M.J., Ramírez-rodríguez A.E., Rosales S., Bañuelos-hernández B., De R.F.G., Bañuelos-hernández B., Fernando R., Increased G. De. Increased removal of cadmium by Chlamydomonas reinhardtii modified with a synthetic gene for γ - glutamylcysteine synthetase. Int. J. Phytoremediation. 2020:1–9. doi: 10.1080/15226514.2020.1765138. [DOI] [PubMed] [Google Scholar]
- Podder M.S., Majumder C.B. Prediction of phycoremediation of As(III) and As(V) from synthetic wastewater by Chlorella pyrenoidosa using artificial neural network. Appl. Water Sci. 2017;7:3949–3971. [Google Scholar]
- Priyadarshini E., Priyadarshini S.S., Pradhan N. Heavy metal resistance in algae and its application for metal nanoparticle synthesis. Appl. Microbiol. Biotechnol. 2019;103(8):3297–3316. doi: 10.1007/s00253-019-09685-3. [DOI] [PubMed] [Google Scholar]
- Rahman Z., Singh V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ. Monit. Assess. 2019;191 doi: 10.1007/s10661-019-7528-7. [DOI] [PubMed] [Google Scholar]
- Rahman Z., Thomas L. Chemical-assisted microbially mediated chromium (Cr) (VI) reduction under the influence of various electron donors , redox mediators , and other Additives : an outlook on enhanced Cr(VI) removal. Front. Microbiol. 2021;11:1–19. doi: 10.3389/fmicb.2020.619766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamani S., Siripornadulsil S., Falcao V., Torres M., Colepicolo P., Sayre R. Transgenic Microalgae as Green Cell Factories. 2007. Phycoremediation of heavy metals; pp. 99–109. [DOI] [PubMed] [Google Scholar]
- Ramírez-rodríguez A.E., Bañuelos-hernández B., Mariano J., Govea-alonso D.G., Rosales-mendoza S., Catalina M., Torre A. De, Monreal-escalante E., Paz-maldonado L.M.T., Bañuelos-hernández B., Monreal-escalante E., Arsenic L.M.T.P. Arsenic removal using Chlamydomonas reinhardtii modified with the gene acr3 and enhancement of its performance by decreasing phosphate in the growing media. Int. J. Phytoremediation. 2019:1–7. doi: 10.1080/15226514.2018.1546274. [DOI] [PubMed] [Google Scholar]
- Rashidi B., Trindade L.M. Detailed biochemical and morphologic characteristics of the green microalga Neochloris oleoabundans cell wall. Algal Res. 2018;35:152–159. [Google Scholar]
- Rodrigues A., Pinto E. Evaluation of Chlorella ( Chlorophyta ) as source of fermentable sugars via cell wall enzymatic hydrolysis. Enzym. Res. 2011;2011 doi: 10.4061/2011/405603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosakis A., Köster W. Transition metal transport in the green microalga Chlamydomonas reinhardtii genomic sequence analysis. Res. Microbiol. 2004;155:201–210. doi: 10.1016/j.resmic.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Rourke J.L., Sinal C.J. third ed. Vol. 2. Elsevier; 2014. Biotransformation/metabolism; pp. 289–294. (Encyclopedia of Toxicology). [Google Scholar]
- Ruiz F.A., Marchesini N., Seufferheld M., Docampo R. The polyphosphate bodies of Chlamydomonas reinhardtii possess a proton-pumping pyrophosphatase and are similar to acidocalcisomes. J. Biol. Chem. 2001;276(49):46196–46203. doi: 10.1074/jbc.M105268200. [DOI] [PubMed] [Google Scholar]
- Saavedra R., Muñoz R., Taboada M.E., Vega M., Bolado S. Comparative uptake study of arsenic, boron, copper, manganese and zinc from water by different green microalgae. Bioresour. Technol. 2018;263:49–57. doi: 10.1016/j.biortech.2018.04.101. [DOI] [PubMed] [Google Scholar]
- Safarik I., Baldikova E., Prochazkova J., Pospiskova K. Magnetic particles in algae biotechnology : recent updates. J. Appl. Phycol. 2020 [Google Scholar]
- Sanz-luque E., Bhaya D., Grossman A.R. Polyphosphate : a multifunctional metabolite in cyanobacteria and algae. Front. Microbiol. 2020;11:1–21. doi: 10.3389/fpls.2020.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanab S., Essa A., Shalaby E. Bioremoval capacity of three heavy metals by some microalgae species (Egyptian isolates) Plant Signal. Behav. 2012;7 doi: 10.4161/psb.19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.S., Dietz K., Mimura T. 2016. Vacuolar Compartmentalization as Indispensable Component of Heavy Metal Detoxification in Plants; pp. 1112–1126. [DOI] [PubMed] [Google Scholar]
- Shen N., Birungi Z.S., Chirwa E.M.N. Selective biosorption of precious metals by cell-surface engineered selective biosorption of precious metals by cell-surface engineered microalgae. Chem. Eng. Trans. 2017;61 [Google Scholar]
- Singh D.V., Bhat R.A., Upadhyay A.K., Singh R., Singh D.P. Microalgae in aquatic environs: a sustainable approach for remediation of heavy metals and emerging contaminants. Environ. Technol. Innov. 2021;21:101340. [Google Scholar]
- Siripornadulsil S., Traina S., Verma D.P.S., Sayre R.T. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell. 2002;14:2837–2847. doi: 10.1105/tpc.004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldo D., Hari R., Sigg L., Behra R. Tolerance of Oocystis nephrocytioides to copper : intracellular distribution and extracellular complexation of copper. Aquat. Toxicol. 2005;71:307–317. doi: 10.1016/j.aquatox.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Spain O., Plöhn M., Funk C. The cell wall of green microalgae and its role in heavy metal removal. Physiol. Plantarum. 2021;1–10 doi: 10.1111/ppl.13405. [DOI] [PubMed] [Google Scholar]
- Suárez C., Torres E., Pérez-Rama M., Herrero C., Abalde J. Cadmium toxicity on the freshwater microalga Chlamydomonas moewusii Gerloff: biosynthesis of thiol compounds. Environ. Toxicol. Chem. 2010;29:2009–2015. doi: 10.1002/etc.242. [DOI] [PubMed] [Google Scholar]
- Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy metals toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M.A., Barros M.P., Campos S.C.G., Pinto E., Rajamani S., Sayre R.T., Colepicolo P. Biochemical biomarkers in algae and marine pollution : a review. Ecotoxicol. Environ. Saf. 2008;71:1–15. doi: 10.1016/j.ecoenv.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Tripathi S., Arora N., Gupta P., Pruthi P.A., Poluri K.M., Pruthi V. Elsevier Ltd; 2019. Microalgae: an Emerging Source for Mitigation of Heavy Metals and Their Potential Implications for Biodiesel Production, Advanced Biofuels: Applications, Technologies and Environmental Sustainability; pp. 97–128. [Google Scholar]
- Tsednee M., Castruita M., Salomé X.P.A., Sharma A., Lewis X.B.E., Schmollinger S.R., Strenkert D., Holbrook K., Otegui X.M.S., Khatua K., Das S., Datta A., Chen X.S., Ramon C., Ralle M., Weber P.K., Stemmler T.L., Pett-ridge J., Hoffman B.M., Merchant S.S. Manganese co-localizes with calcium and phosphorus in Chlamydomonas acidocalcisomes and is mobilized in manganese-deficient conditions. J. Biol. Chem. 2019;294:17626–17641. doi: 10.1074/jbc.RA119.009130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji N., Hirayanagi N., Iwabe O., Namba T., Tagawa M. Regulation of phytochelatin synthesis by zinc and cadmium in marine green alga. Dunaliella tertiolecta. 2003;62:453–459. doi: 10.1016/s0031-9422(02)00559-9. [DOI] [PubMed] [Google Scholar]
- Ubando A.T., Africa A.D.M., Maniquiz-Redillas M.C., Culaba A.B., Chen W.H., Chang J.S. Microalgal biosorption of heavy metals: a comprehensive bibliometric review. J. Hazard Mater. 2021;402:123431. doi: 10.1016/j.jhazmat.2020.123431. [DOI] [PubMed] [Google Scholar]
- Volland S., Lütz C., Michalke B., Lütz-meindl U. Intracellular chromium localization and cell physiological response in the unicellular alga Micrasterias. Aquat. Toxicol. 2012;109:59–69. doi: 10.1016/j.aquatox.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chen C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009;27:195–226. doi: 10.1016/j.biotechadv.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Wang W., Dei R.C.H. Metal stoichiometry in predicting Cd and Cu toxicity to a freshwater green alga Chlamydomonas reinhardtii. Environ. Pollut. 2006;142:303–312. doi: 10.1016/j.envpol.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Wang Ye, Cheng Z.Z., Chen X., Zheng Q., Yang Z.M. CrGNAT gene regulates excess copper accumulation and tolerance in Chlamydomonas reinhardtii. Plant Sci. 2015;240:120–129. doi: 10.1016/j.plantsci.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Wang Y., Selvamani V., Yoo I., Kim T.W., Hong S.H. A novel strategy for the microbial removal of heavy Metals : cell-surface display of peptides A novel strategy for the microbial removal of heavy Metals : cell-surface display of peptides. Biotechnol. Bioproc. Eng. 2021 [Google Scholar]
- Wang Y., Zhang C., Zheng Y., Ge Y. Phytochelatin synthesis in Dunaliella salina induced by arsenite and arsenate under various phosphate regimes. Ecotoxicol. Environ. Saf. 2017;136:150–160. doi: 10.1016/j.ecoenv.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Wang Ya, Wang S., Xu P., Liu C., Liu M., Wang Yulan, Wang C. Review of arsenic speciation , toxicity and metabolism in microalgae. Rev. Environ. Sci. Bio/Technol. 2015;14:427–451. [Google Scholar]
- Werner T.P., Amrhein N., Freimoser F.M. Inorganic polyphosphate occurs in the cell wall of Chlamydomonas reinhardtii and accumulates during cytokinesis. BMC Plant Biol. 2007;11:1–11. doi: 10.1186/1471-2229-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R., Zheng Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016;34(7):1225–1244. doi: 10.1016/j.biotechadv.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Yang T., Chen M., Wang J. Genetic and chemical modification of cells for selective separation and analysis of heavy metals of biological or environmental significance. Trends Anal. Chem. 2015;66:90–102. [Google Scholar]
- Yen H., Chen P., Hsu C., Lee L. The use of autotrophic Chlorella vulgaris in chromium (VI) reduction under different reduction conditions. J. Taiwan Inst. Chem. Eng. 2017;74:1–6. [Google Scholar]
- Yin X., Wang L., Duan G., Sun G. Characterization of arsenate transformation and identification of arsenate reductase in a green alga Chlamydomonas reinhardtii. J. Environ. Sci. 2011;23:1186–1193. doi: 10.1016/s1001-0742(10)60492-5. [DOI] [PubMed] [Google Scholar]
- Yu Z., Zhang T., Hao R., Zhu Y. Sensitivity of Chlamydomonas reinhardtii to cadmium stress is associated with phototaxis. Environ. Sci. Process. Impacts. 2019;21:1011–1020. doi: 10.1039/c9em00013e. [DOI] [PubMed] [Google Scholar]
- Zeraatkar A.K., Ahmadzadeh H., Talebi A.F., Moheimani N.R., McHenry M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016;181:817–831. doi: 10.1016/j.jenvman.2016.06.059. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhou F., Liu Y., Huang F., Zhang C. Effect of extracellular polymeric substances on arsenic accumulation in Chlorella pyrenoidosa. Sci. Total Environ. 2020;704:135368. doi: 10.1016/j.scitotenv.2019.135368. [DOI] [PubMed] [Google Scholar]
- Zhang W., Tan N.G.J., Fu B., Li S.F.Y. Metallomics and NMR-based metabolomics of Chlorella sp. reveal the synergistic role of copper and cadmium in multi-metal toxicity and oxidative stress. Metallomics. 2015;7:426–438. doi: 10.1039/c4mt00253a. [DOI] [PubMed] [Google Scholar]
- Zheng Q., Cheng Z.Z., Yang Z.M. HISN3 mediates adaptive response of Chlamydomonas reinhardtii to excess nickel. Plant Cell Physiol. 2013;54:1951–1962. doi: 10.1093/pcp/pct130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.