Abstract
Background
Interpretation of incidental findings on term neonatal MRI brain imaging can be challenging as there is a paucity of published normative data on asymptomatic term neonates. Reporting radiologists and clinicians need to be familiar with these incidental findings to avoid over-investigation and misinterpretation particularly in relation to neurodevelopmental outcome. This study aimed to determine the prevalence of incidental findings in a large group of asymptomatic term neonates participating in the Developing Human Connectome Project (dHCP) who were invited for neurodevelopmental assessment at 18 months.
Methods
We retrospectively reviewed MRI brain scans performed on 500 term neonates enrolled in the dHCP study between 2015 and 2019 with normal clinical examination. We reviewed the results of the Bayley Scales of Infant and Toddler Development (Bayley III) applied to participants who attended for neurodevelopmental follow-up at 18 months. Scores considered “delayed” if <70 on language, cognitive or motor scales.
Findings
Incidental findings were observed in 47% of term infants. Acute cerebral infarcts were incidentally noted in five neonates (1%). More common incidental findings included punctate white matter lesions (PWMLs) (12%) and caudothalamic subependymal cysts (10%). The most frequent incidental finding was intracranial haemorrhage (25%), particularly subdural haemorrhage (SDH). SDH and PWMLs were more common in infants delivered with ventouse-assistance versus other delivery methods.
Neurodevelopmental results were available on 386/500 (77%). 14 infants had a language score < 70 (2 SD below the mean). Of the 386 infants with neurodevelopmental follow up at 18 months, group differences in motor and language scores between infants with and without incidental findings were not significant (p = 0·17 and p = 0·97 respectively). Group differences in cognitive scores at 18 months between infants with (median (interquartile range) -100 (95–105)) and without (100 (95–110)) incidental findings were of small effect size to suggest clinical significance (Cliff's d = 0·15; p<0·05).
Interpretation
Incidental findings are relatively common on brain MRI in asymptomatic term neonates, majority are clinically insignificant with normal neurodevelopment at 18 months.
Funding
This work was supported by the European Research Council under the European Union's Seventh Framework Programme (FP7/20072013/ERC grant agreement no. [319456] dHCP project), by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London and/or the NIHR Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Research in context.
Evidence before this study
PubMed was used to search for articles published between 01/01/2000 and 01/11/2020 using the following search terms: “incidental”, “findings”, “brain”, “MRI”, “MR imaging”, “term”, “neonates”, “asymptomatic”, “healthy”. There was one study on incidental findings in brain MRI of asymptomatic neonates (from The FinnBrain Birth Cohort Study), limited to 175 neonates (born 37–42 weeks gestation with two exceptions born at 36 weeks and two with missing information) scanned 2–5 weeks post-delivery with an overall prevalence of incidental findings at 7·4%. Intracranial haemorrhage was present in 6·9% of their participants, subdural haemorrhage had the highest prevalence at 5·7%, and ventouse assistance was noted to be a risk factor for subdural haemorrhage. No imaging findings were detected in the section-delivered group.
Added value of this study
This study reported a higher overall prevalence of incidental findings on MR brain imaging (47%) in the largest cohort of asymptomatic term neonates to date (n = 500) with majority scanned within one week of age in contrast to the previous study.
Neurodevelopmental results were available in 386 participants (77%) who attended for follow-up at 18 months.
Intracranial haemorrhage (particularly SDH), punctate white matter lesions and caudothalamic subependymal cysts were the most common incidental findings evident in this study; incidence of SDH and PWMLs was highest within the ventouse delivered group. In contrast to the reported absence of SDH in caesarean deliveries on the previous study, SDH was evident in four neonates delivered by emergency caesarean section but there was no apparent SDH in those delivered by elective CS.
The presence of cerebral infarction in 1 percent of the participants is notable given the absence of symptoms and signs prior to the scan.
Implications of all the available evidence
Incidental findings are relatively common on term neonatal MR brain imaging and should be anticipated in research and clinical settings but the majority are clinically insignificant with normal neurodevelopment at 18 months. It is not possible to determine the risk of subsequent neurodevelopmental sequelae in later childhood associated with rare incidental findings such as cerebral infarction but it is important to raise awareness of clinically silent infarcts in term neonates.
The potential occurrence of intracranial haemorrhage on term neonatal brain imaging is not an adequate indication for modifying delivery practices.
Routine brain MR imaging is not advocated in term neonates if no risk factors for injury and asymptomatic.
The information provided on incidental findings will ensure appropriate use of health services, inform consent in research settings and assist with design of incidental findings management protocols.
Alt-text: Unlabelled box
1. Introduction
Magnetic resonance imaging (MRI) is an ideal and safe technique for imaging the term neonatal brain in both clinical and research environments. Incidental findings are common particularly with the improved quality and increasing use of brain MRI. Incidental findings have been defined as observations of potential clinical significance unexpectedly discovered in healthy subjects or patients recruited to brain imaging research studies and unrelated to the purpose or variables of the study [1]. Interpretation of the clinical significance of incidental findings detected on neonatal MRI brain can be challenging as there is a relative lack of published normative data on asymptomatic term neonates. A previous study of incidental MRI brain findings was limited to 175 asymptomatic neonates and reported an overall prevalence of 7·4% with intracranial haemorrhage accounting for the highest prevalence at 6·9%, particularly subdural haemorrhage at 5·7% [2]. Reporting radiologists and clinicians need to be familiar with common incidental findings demonstrated on MR neuroimaging in asymptomatic term neonates to avoid over-investigation and misinterpretation particularly in relation to neurodevelopmental outcome. The aim of this study was to determine the nature and prevalence of incidental findings in a large group of asymptomatic term neonates participating in the Developing Human Connectome Project (dHCP) with neurodevelopmental assessment at 18 months.
2. Method
2.1. Study participants and imaging technique
This was a retrospective study of 500 term neonates (≥37 weeks gestation) consecutively recruited into the Developing Human Connectome Project (dCHP) during a four-year period (February 2015 to February 2019) who underwent brain MR imaging. We excluded 27 neonates admitted to neonatal intensive care, one neonate with antenatally diagnosed vermian hypoplasia, one neonate who later died from congenital heart disease, and nine neonates with incomplete or abandoned scans. The final cohort of 500 term neonates had normal findings on clinical examination by a qualified neonatologist prior to the MRI brain scan. The majority were scanned within one week of delivery. Delivery information was collected for all participants. The Developing Human Connectome Project (dHCP) aims to create a dynamic map of human brain connectivity from 20 to 44 weeks post-conceptional age, which will link together imaging, clinical, behavioural, and genetic information. The objectives and methods of the dHCP study are described in detail elsewhere (http://www.developingconnectome.org) [3].
A dedicated neonatal brain imaging system was used as described by Hughes and colleagues [4]. Neonates were imaged in natural sleep using a 3T Philips Achieva TX with a dedicated 32-channel neonatal head coil (RAPID Biomedical) and subject handling system. Axial and sagittal T2-TSE and sagittal 3D T1-MPRAGE were acquired for each subject (see Table 1). This structural information was gathered as part of a broader study where volumetric structural, resting-state functional and diffusion weighted imaging were also performed to study brain development within the Developing Human Connectome Project.
Table 1.
Imaging Parameters.
| Parameter | Axial and sagittal T2 TSE | Sagittal 3D T1-MPRAGE |
|---|---|---|
| TR (ms) | 12,000 | 11 |
| TE (ms) | 156 | 4·6 |
| TI (ms) | – | 713 |
| Voxel size (mm) | 0·8 × 0·8 × 1·6 | 0·8 × 0·8 × 0·8 |
| Slice thickness (mm) | 1.6 | 0.8 |
| Slice gap (mm) | −0·8 | 0 |
| Number of slices | 125 (axial); 145 (sagittal) | 135 |
| SENSE factor | 2·1 | 1·2 |
| TA (mins) | 3·12 | 4·35 |
TSE, turbo spin echo; 3D, three-dimensional; MPRAGE, magnetization prepared rapid acquisition gradient echo; TR, repetition time; TE, echo time; TI, inversion time; SENSE, sensitivity encoding; TA, acquisition time.
The T1- and T2- weighted imaging was reviewed by two experienced neuroradiologists with consensus reached on all image findings. The prevalence of each incidental brain finding was calculated. There was no statistical hypothesis tested. Neonates with incidental findings requiring additional clinical workup were referred to the relevant specialist.
2.2. Neurodevelopmental assessment
Infants enrolled in the dHCP study were invited for neurodevelopmental assessment at 18 months using the Bayley Scales of Infant and Toddler Development – Bayley III [5]. It provides 3 norm-referenced Composite Scores: Cognitive, Language and Motor (Mean = 100; standard deviation (SD) = 15). A composite score between 85 – 115 identifies typically developing children. Scores of our participants were considered abnormal or “delayed” if below 70 on language, cognitive or motor scales.
2.3. Statistical analysis
Where statistical testing was performed, normality of the data was assessed using Shapiro-Wilk test. To test for group differences, we used independent t-test if the data were normally distributed and Mann-Whitney U test if the data were not normally distributed. The effect size of observed significant group difference was quantified using Cohen's d (for t-test) or Cliff's delta (Cliff's d; for Mann-Whitney), the latter ranging from −1 to 1, with 0 indicating stochastic equality of the two groups.
2.4. Ethical approval
All study procedures were reviewed and approved by the Health Research Authority. Written informed consent was obtained for all participants prior to scanning by someone with parental responsibility.
2.5. Role of funding
This work was supported by the European Research Council under the European Union's Seventh Framework Programme (FP7/20072013/ERC grant agreement no. [319456] dHCP project), by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
3. Results
We studied 500 asymptomatic term neonates described in Table 2. All had normal findings on clinical examination performed prior to the scan.
Table 2.
Characteristics of the study population.
| Term neonates (n = 500) | |
|---|---|
| Gestational age at birth (weeks) Median (IQR) |
40·14 (39·14 – 40·86) |
| Postmenstrual age at scan (weeks) Median (IQR) |
41·29 (40·14 – 42·57) |
| Postnatal age (days) Median (IQR) |
4 (2 – 16) |
| Female, No. (%) | 224 (45%) |
| Mode of delivery, No. (%) Spontaneous vaginal delivery, No. (%) Emergency Caesarean Section, No. (%) Elective Caesarean Section, No. (%) Ventouse, No. (%) Forceps, No. (%) |
207 (41%) 114 (23%) 70 (14%) 46 (9%) 63 (13%) |
| Birth weight (kg) Mean (SD) |
3.41 (0.49) |
| Birth head circumference (cm) Median (IQR) |
35 (33·5 – 35·5) |
| Apgar score (1 min) Median (IQR) |
9 (9 – 9) |
| Apgar score (5 min) Median (IQR) |
10 (9 – 10) |
| Punctate white matter lesions, No. (%) | 61 (12%) |
| Caudothalamic subependymal cysts, No. (%) | 50 (10%) |
| Subdural Haemorrhage, No. (%) | 115 (23%) |
| Intracranial Haemorrhage, No. (%) | 123 (25%) |
| Incidental Findings, No. (%) | 235 (47%) |
| Bayley III Scale, No. (%) Cognitive score, Median (IQR) Motor score, Median (IQR) Language score, Median (IQR) |
386 (77%) 100 (95 – 110) 103 (97 – 110) 97 (89 – 109) |
*Median and interquartile range (IQR) are used unless other specified. Missing data: birth weight 1; birth head circumference 27; Apgar score at 1 min – 34, at 5 min – 38. Bayley III results – 114.
3.1. Incidental findings
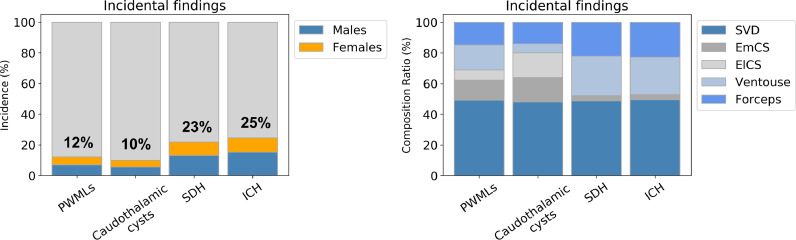
The overall prevalence of incidental findings was 47%, none of which were clinically suspected prior to imaging apart from three neonates with known mild ventriculomegaly. Table 3 shows the prevalence of each incidental finding. Intracranial haemorrhage (ICH), punctate white matter lesions (PWMLs) and caudothalamic subependymal cysts were the most common incidental findings. Subdural haemorrhage (SDH) and PWMLs were more frequent in infants delivered with ventouse assistance (Table 4). There was no apparent difference in the incidence of these findings between male and female term neonates (Fig. 1).
Table 3.
Incidental findings.
| Incidental Findings on 500 Term Neonatal MRI brain scans | |
|---|---|
| Finding | No. (%) |
| Intracranial Haemorrhage Extradural Subdural Subarachnoid Germinal matrix Intraventricular Cerebellar haemorrhage |
123 (25) 2 (0·4) 115 (23) 1 (0·2) 4 (0·8) 3 (0·6) 11 (2·2) |
| Extracranial Haemorrhage Cephalohaematoma |
25 (5) |
| Punctate white matter lesions | 61 (12) |
| Caudothalamic Subependymal cysts Frontal horn/connatal cysts |
50 (10) 3 (0·6) |
| Asymmetric lateral ventricles/mild lateral ventriculomegaly Lateral ventricle asymmetry Mild lateral ventriculomegaly |
14 (2·8) 9 (1·8) 5 (1) |
| Prominent cisterna magna | 11 (2·0) |
| Neonatal cerebral infarction Deep medullary vein thrombosis/engorgement |
5 (1·0) 7 (1·4) |
| Other findings Arachnoid cyst Choroid fissure cyst Temporal horn cyst Developmental venous anomaly (DVA) Ectopic posterior pituitary Pars intermedia cyst Subependymal heterotopia Dacrocystocele Tongue lesion Hypothalamic hamartoma |
3 (0·6) 1 (0·2) 2 (0·4) 4 (0·8) 2 (0·4) 1 (0·2) 1 (0·2) 3 (0·6) 2 (0·4) 1 (0·2) |
Table 4.
Main Incidental findings and mode of delivery:.
| Infants (n = 500) | Punctate white matter lesions (n = 61) | Caudothalamic subependymal cysts (n = 50) | Subdural Haemorrhage (n = 115) | |
|---|---|---|---|---|
| SVD | 207 (41%) | 30 (14%) | 24 (12%) | 56 (27%) |
| Emergency CS | 114 (23%) | 8 (7%) | 8 (7%) | 4 (4%) |
| Elective CS | 70 (14%) | 4 (6%) | 8 (11%) | – |
| Ventouse | 46 (9%) | 10 (22%) | 3 (7%) | 29 (63%) |
| Forceps | 63 (13%) | 9 (14%) | 7 (11%) | 26 (41%) |
SVD, spontaneous vaginal delivery; CS, caesarean section.
Fig. 1.
Main incidental findings versus sex and mode of delivery
PWMLs, punctate white matter lesions; SDH, subdural haemorrhage; ICH, intracranial haemorrhage (includes SDH); SVD, spontaneous vaginal delivery; EmCS, emergency caesarean section; ElCS, elective caesarean section.
3.2. Cerebral infarction
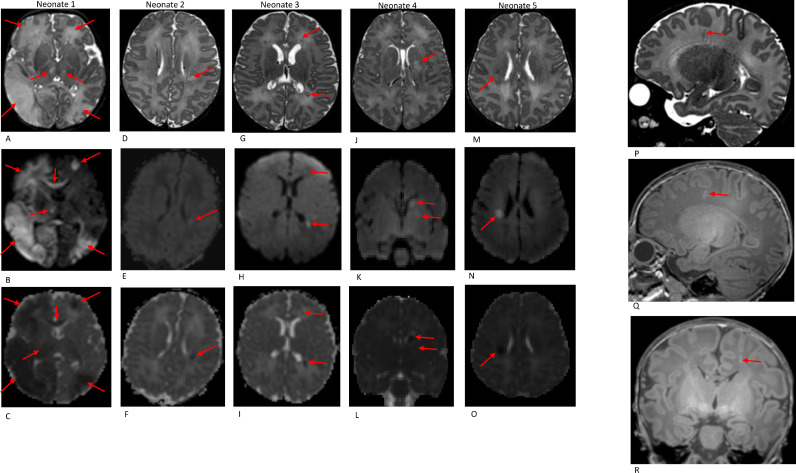
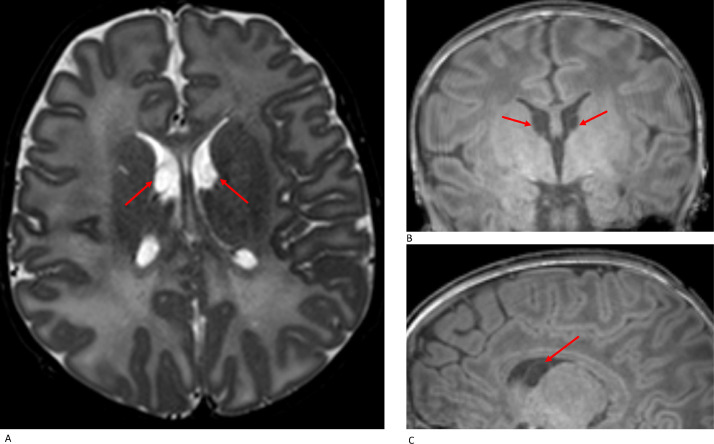
Acute cerebral infarcts were incidentally noted in five neonates (1%) (see Table 5 and Fig. 2). All had normal antenatal scans and were born in good condition with good Apgar scores. Clinical examination prior to the scan was normal in all cases but the neonate with the most extensive bilateral infarction developed seizures following the scan (Fig. 2; neonate 1). Septic screen and general coagulation screen were negative. One of two neonates with isolated corona radiata infarction had a history of maternal Group B streptococcus colonisation (Fig. 2; neonate 5). Another neonate with left basal ganglia infarction, bilateral caudothalamic subependymal cysts and bifrontal pseudocysts was later discovered to have a history of maternal cannabis use during pregnancy (Fig. 2, neonate 4). All 5 neonates were referred to a Paediatric Neurologist for follow-up; two have hemiplegia and three were discharged as developmentally appropriate (Table 5).
Table 5.
Term neonates with cerebral infarction.
| Sex | GA(wks) | PMA at scan (wks) | Delivery | Apgar score (1 min) | Apgar score (5 min) | Findings | Cognitive score | Motor score | Language score | Clinic outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| M | 41·57 | 41·71 | Forceps | 9 | 10 | Bilateral cerebral arterial infarction, more extensive on the right, involving the right fronto-temporo-parietal-occipital lobes. Watershed arterial infarcts in the left cerebral hemisphere involving the left frontal region and left temporo-parieto-occipital region. Involvement of the bilateral perirolandic cortex, corpus callosum, internal capsules, thalami and left basal ganglia also noted. (see Fig. 2, neonate 1) |
100 | 79 | 100 | Hemiplegia |
| F | 39·71 | 40·57 | Ventouse | 9 | 10 | Small left corona radiata infarct (MCA territory) (see Fig. 2, neonate 2) |
115 | 82 | 100 | Discharged at 18 mths as developmentally appropriate (slightly late walker) |
| F | 41·29 | 42·14 | SVD | 9 | 10 | Bilateral small infarcts involving the frontal and parietal deep white matter (see Fig. 2, neonate 3) |
.. | .. | .. | Discharged at 18 mths as developmentally appropriate |
| F | 40·14 | 41·71 | SVD | 9 | 10 | Left basal ganglia infarcts involving left caudate and globus pallidus (MCA lenticulostriate territory) Additional image findings of a few bilateral parietal punctate white matter lesions, bilateral caudothalamic subependymal cysts and bifrontal periventricular pseudocysts (see Fig. 2, neonate 4) |
95 | 103 | 97 | Hemiplegia |
| F | 41·57 | 42·0 | SVD | 10 | 10 | Right corona radiata infarct (MCA territory) (see Fig. 2, neonate 5) |
100 | 112 | 91 | Discharged at 15 mths as developmentally appropriate |
GA, gestational age at birth; PMA, postmenstrual age; SVD, spontaneous vaginal delivery; MCA, middle cerebral artery.
Fig. 2.
Acute infarction: Axial T2W (A-M), DWI (B-N) and ADC (C—O) imaging in 5 term neonates (see details in table 5)
Deep medullary vein thrombosis: Sagittal T2W (P), sagittal and coronal T1W (Q + R) imaging in a term neonate showing a left frontal white matter T2 hypointense/T1 hyperintense radial linear lesion suggestive of deep medullary vein engorgement/thrombosis.
Seven neonates had MR imaging appearances suggestive of deep medullary vein engorgement/thrombosis represented by radial linear T2 hypointense/T1 hyperintense lesions in the territory of distribution of the deep medullary veins (Fig. 2). All were unilateral and 4/7 involved the posterior periventricular/peritrigonal white matter with adjacent focal white matter changes in 2 cases suggestive of secondary deep medullary vein infarcts.
3.3. Punctate white matter lesions (PWMLs)
PWMLs were demonstrated in 61 neonates (12%), evident in unmyelinated white matter as hyperintense on T1- and hypointense on T2-weighted imaging (Fig. 3). Unilateral PWMLs (36/61 = 59%) were more common than bilateral (25/61 = 41%). 27/61 (44%) had an isolated PWML and 34/61 (56%) had multiple PWMLs. Specifically, 38 neonates had less than 3 PWMLs, 10 had 3–6, and 13 had greater than 6 PWMLs. The majority of PWMLs were evident in the posterior periventricular white matter/peritrigonal regions followed by the frontal white matter and centrum semiovale. Multiple lesions tended to occur in clusters. It was not possible to distinguish haemorrhagic from non-haemorrhagic PWML due to the absence of susceptibility-weighted imaging (SWI) in our protocol.
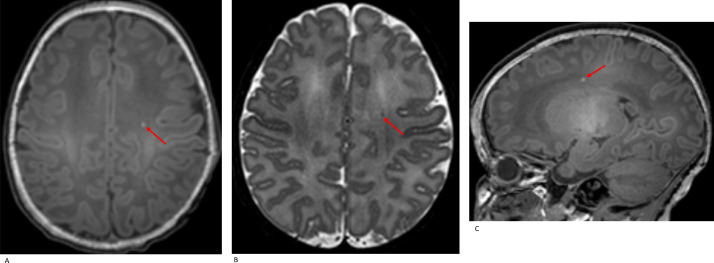
Fig. 3.
Punctate white matter lesion (PWML): axial T1W (A), axial T2W (B) and sagittal T1W (C) imaging demonstrate an isolated PWML left centrum semiovale in a term neonate (SVD, GA 40•14 w, PMA at scan 41 w).
No group differences were observed in GA at birth, birth weight and HC between infants with and without PWMLs (GA at birth, p = 0·23; birth weight, p = 0·28; HC, p = 0.08). Almost half of participants with PWMLs were delivered by SVD (30/61) and almost a third were instrumental deliveries (19/61). The incidence of PWML varied with the mode of delivery, highest in the ventouse-assisted group (22%).
Of the 61 neonates with PWMLs, 23 (38%) also had evidence of ICH, most commonly SDH (20 SDH, 4 cerebellar haemorrhage and 3 GMH).
3.4. Haemorrhage
3.4.1. Intracranial haemorrhage (ICH)
ICH was evident in 123 (25%) of the participants. Representative images of different types of ICH are shown in Fig. 4. The most common subtype was SDH, present in 115 (23%) neonates and found mainly in the posterior fossa (79/115 = 69%). 28 neonates had supratentorial and infratentorial SDH while eight neonates had supratentorial SDH only. All supratentorial SDHs were predominantly in the posterior half of the cranium.
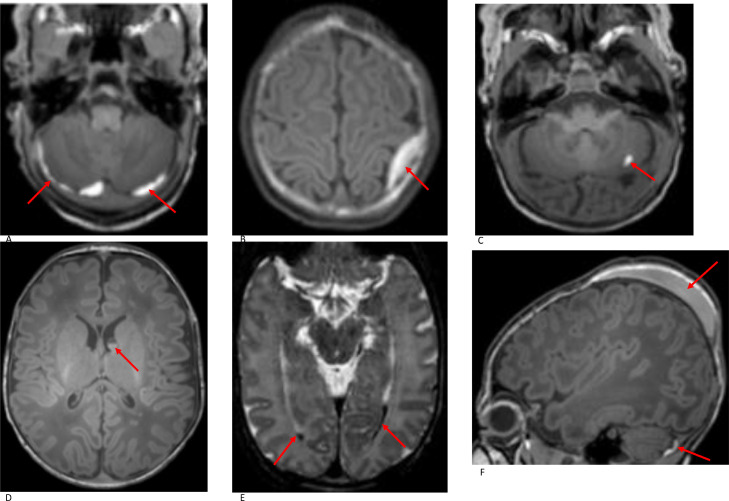
Fig. 4.
Haemorrhage: A. Subdural Haemorrhage (SDH). Axial T1W imaging in a term neonate (ventouse delivery, GA 38+5 w, PMA at imaging 40+5 w) shows SDH in the posterior fossa; B. Extradural Haemorrhage. Axial T1W imaging in a term neonate (ventouse delivery, GA 41+1 w, PMA at imaging 42 w) shows a small extradural haemorrhage overlying the left superior parietal lobe; C. Cerebellar haemorrhage. Axial T1W imaging in a term neonate (SVD, GA 39+2 w, PMA at imaging 40+4 w) shows a small focal haemorrhage in the left cerebellar hemisphere; D. Germinal matrix haemorrhage. Axial T1W imaging in a term neonate (SVD, GA 39+0 w, PMA at imaging 41+0 w) shows bilateral germinal matrix haemorrhage, larger on the left side with a fluid level. PWMLs are also noted in the anterior and posterior deep periventricular white matter; E Intraventricular haemorrhage. Axial T2W imaging in a term neonate (forceps-assisted delivery, GA 40+6 w, PMA at imaging 41+0 w) shows small volume of intraventricular haemorrhage in the occipital horns of both lateral ventricles; F. Cephalohaematoma. Sagittal T1W MRI imaging in a term neonate (ventouse-assisted delivery, GA 38+5 w, PMA at imaging 40+5 w) shows a left parietal cephalohaematoma. A small subdural haemorrhage is also noted in the posterior fossa.
Cerebellar haemorrhages were noted in 11 neonates (ten SVD and one forceps-assisted). The majority of these haemorrhages (8/11 = 73%) were focal, small (< 10 mm) and unilateral. Two neonates had bilateral cerebellar haemorrhage. Of neonates with cerebellar haemorrhage, four had PWMLs and three had caudothalamic subependymal cysts.
Less common subtypes of ICH included germinal matrix haemorrhage in four neonates (two spontaneous vaginal delivery (SVD), one ventouse and one forceps-assisted), intraventricular haemorrhage (IVH) in three neonates (two SVD and one forceps-assisted), subarachnoid haemorrhage (SAH) in one neonate (SVD) and extradural haemorrhage (EDH) in two neonates (one SVD and one ventouse-assisted).
Nine neonates had haemorrhage in two locations including five neonates with SDH and cerebellar haemorrhage (SVD), one neonate with SDH and IVH (forceps-assisted), one neonate with SDH and GMH (SVD) and two neonates with SDH and EDH (one SVD and one ventouse-assisted). Two neonates had haemorrhage in three locations; one neonate with SDH, SAH and IVH who was born in good condition by SVD at 40+5 w gestational age (GA) and another neonate with SDH, cerebellar haemorrhage and IVH who was born in good condition by SVD at 37+0 w GA with a maternal history of pre-eclampsia.
Infants with ICH were born on average heavier (mean (SD) - 3.5 (0.45) kg) than infants without ICH (3.39 (0.5) kg; Cohen's d = 0.28, p < 0.05) but there were no differences in GA at birth (p = 0·85) and HC (p = 0·096).
The rate of SDH was highest in instrumental deliveries, particularly ventouse-assisted (29/46 = 63%). SDH was less frequent in the Caesarean section group, present in only 4 neonates delivered by emergency CS (EmCS) and absent in elective CS (ElCS).
3.4.2. Extracranial haemorrhage
Cephalohaematomas were incidentally noted in 26 neonates (5%), predominantly parietal in location. 11/26 (44%) also had SDH. The majority of cephalohaematomas (16/26, 62%) were associated with instrumental deliveries, slightly higher incidence in ventouse (17%) versus forceps-assisted group (13%).
3.5. Periventricular pseudocysts
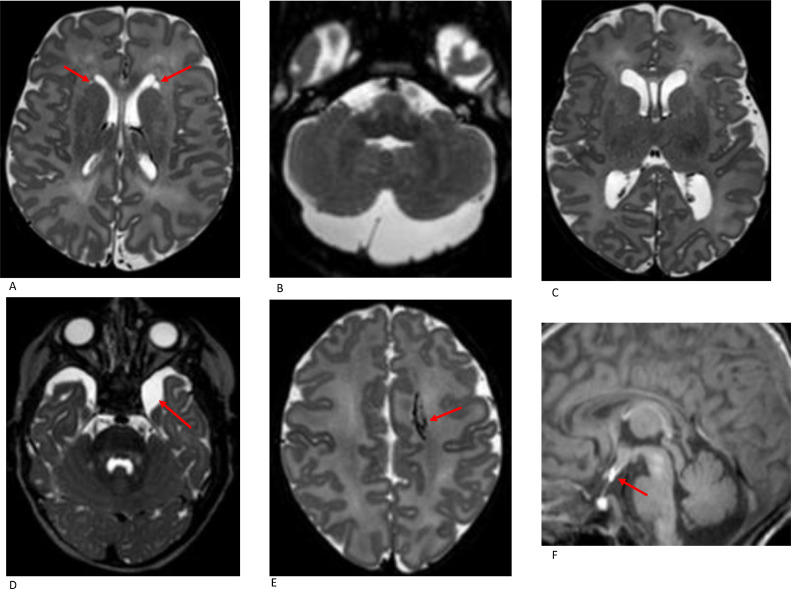
Periventricular pseudocysts (PVPC) are defined as cystic cavities that lack the ependymal lining found in true cysts [6]. They have been divided into connatal cysts (frontal horn cysts) and subependymal pseudocysts (SEPC) [7]. Connatal cysts occur anterior to the foramina of Monro at level of the superolateral angles of the lateral ventricles whereas subependymal cysts are located below the angles, posterior to the foramina of Monro and at the caudothalamic groove. Three term neonates (0·6%) had connatal cysts. Caudothalamic subependymal cysts were more common (Fig. 5), evident in 50 term neonates (10%) of which 26 (26/50 = 52%) had bilateral and 24 (24/50 = 48%) had unilateral cysts. There were no differences in GA at birth, birth weight and head circumference (HC) between infants with and without caudothalamic cysts (GA at birth, p = 0·35; birth weight, p = 0·92; HC, p = 0.69).
Fig. 5.
Caudothalamic subependymal cysts: axial T2W (A), coronal T1W (B) and sagittal T1W (C) imaging demonstrate bilateral caudothalamic subependymal cysts in a term neonate (EmCS, GA 39·71 w, PMA at scan 43·57 w).
Less common intracranial cysts included temporal horn cysts in two neonates and small arachnoid cysts in three neonates (one left middle cranial fossa and two posterior fossa). Other intracranial cysts included choroidal fissure cyst and pars intermedia cyst.
3.6. Mild ventriculomegaly
Five term neonates had mild ventriculomegaly (lateral ventricular diameter 10 – 12 mm), three unilateral and two bilateral (diagnosed antenatally in three neonates). An additional finding of enlarged cisterna magna was noted in two of the cases with mild ventriculomegaly.
3.7. Other incidental findings
Other incidental findings included developmental venous anomalies (DVA) (n = 4) and ectopic posterior pituitary (n = 2) (Fig. 6). Both neonates with ectopic posterior pituitary were referred to a Paediatric Endocrinologist and pituitary function tests were normal.
Fig. 6.
Rare incidental findings A. Axial T2W imaging in a term neonate (SVD, GA 40+6 w, PMA at imaging 42+0 w) shows bilateral pseudocysts adjacent to the frontal horns. B. Axial T2W imaging shows a mega cisterna magna in a term neonate (SVD, GA 38+2 w, PMA at imaging 43+2 w); C. Axial T2-weighted imaging shows mild lateral ventricle dilatation in a term neonate (SVD, GA 38+0 w, PMA at imaging 39+0 w); D. Axial T2W imaging in a term neonate (GA 40+4 w, PMA at imaging 40+6 w) shows a small arachnoid cyst in the left middle cranial fossa; E Axial T2W imaging in a term neonate (SVD, GA 40+0 w, PMA at imaging 41+5 w) shows a left frontal developmental venous anomaly (DVA). F. Sagittal T1W imaging in a term neonate (EmCS, GA 38+1 w, PMA at imaging 39+0 w) shows an ectopic posterior pituitary as a hyperintense nodule adjacent to the hypothalamus.
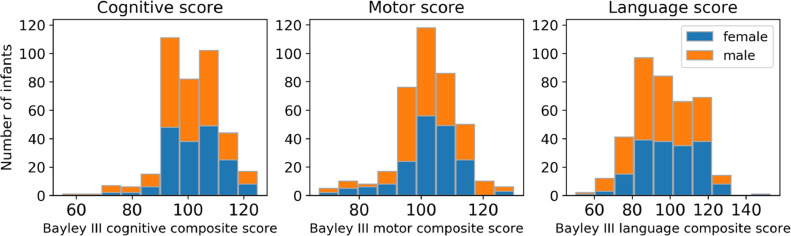
3.8. Neurodevelopmental outcome
Results of Bayley III applied at 18 months were available on 386/500 (77%) (Table 2 and Fig. 7). Perinatal and demographic characteristics of infants with and without neurodevelopmental follow-up are listed in Table 6. Fourteen infants had language score below 70 (2 SD below the mean) of whom nine were exposed to more than one language at home. 9/14 of these infants had incidental MRI brain findings including PWMLs (2), caudothalamic cysts (5), SDH (5), cerebellar haemorrhage (1) and IVH (1). One infant scored below 70 on all three scales. This infant was delivered by EmCS at 41+2 w gestation with Apgar score of 5 at one minute and 9 at 5 min.
Fig. 7.
Neurodevelopmental assessment at 18 months using the Bayley Scales of Infant and Toddler Development (Bayley –III).
Table 6.
Perinatal and demographic characteristics of infants with and without neurodevelopmental follow-up at 18 months.
| Infants with behavioural follow-up (n = 386) |
Infants without behavioural follow-up (n = 114) |
p-value | |
|---|---|---|---|
| Gestational age at birth (weeks) Median (IQR) |
40·14 (39·14 – 40·86) | 40·14 (39·18 – 40·86) | p = 0·88 |
| Postmenstrual age at scan (weeks) Median (IQR) |
41·14 (40 – 42·57) | 41·57 (40·47 – 42·57) | p = 0·17 |
| Postnatal age (days) Median (IQR) |
4 (2 –14·75) | 6·5 (2 –17) | p = 0·32 |
| Female n (%) | 178 (46%) | 46 (40%) | p = 0·33 |
| Mode of delivery, n (%) Spontaneous Vaginal Delivery Emergency Caesarean Section Elective Caesarean Section Ventouse Forceps |
158 (41%) 86 (22%) 55 (14%) 33 (9%) 54 (14%) |
49 (43%) 28 (25%) 15 (13%) 13 (11%) 9 (8%) |
p = 0·44 |
| Birth weight (kg) Mean (SD) |
3.4 (0.49) | 3.46 (0.5) | p = 0·3 |
| Birth head circumference (cm) Median (IQR) |
35 (33·5 – 35·5) | 34·5 (33·5 – 35·5) | p = 0·90 |
| Apgar score (1 min) Median (IQR) |
9 (9 – 9) | 9 (9 – 9) | p = 0·90 |
| Apgar score (5 min) Median (IQR) |
10 (9 – 10) | 10 (9 – 10) | p = 0·26 |
| Punctate white matter lesions, n (%) |
57 (15%) | 4 (4%) | p = 0·002 |
| Caudothalamic subependymal cysts, n (%) |
41 (11%) | 9 (8%) | p = 0·50 |
| Subdural Haemorrhage, n (%) |
83 (22%) | 29 (25%) | p = 0·49 |
| Intracranial Haemorrhage, n (%) |
93 (24%) | 30 (26%) | p = 0·72 |
| Incidental Findings, n (%) |
188 (49%) | 47 (41%) | p = 0·16 |
Of the 386 infants with neurodevelopmental follow up at 18 months, group differences in motor and language scores between infants with and without incidental findings were not significant (p = 0·17 and p = 0·97 respectively). Group differences in cognitive score at 18 months between infants with (median (interquartile range) - 100 (95–105)) and without (100 (95–110)) incidental findings were of small effect size to suggest clinical significance (Cliff's d = 0·15; p<0·05).
4. Discussion
We have reported the incidental findings detected on brain MRI in the largest cohort of asymptomatic term neonates to date. A recent comparative study of incidental findings in asymptomatic neonates from The FinnBrain Birth Cohort Study, was limited to 175 neonates (born 37–42 weeks gestation with two exceptions born at 36 weeks and two with missing information) scanned 2–5 weeks post delivery [2]. The main strengths of our study include the large sample size of 500 term neonates and uniform MRI brain protocol with majority scanned within one week of age and standardised neurodevelopmental follow-up at 18 months. We found a higher prevalence of incidental brain findings (47% vs 7.4% in prior study) of which ICH, PWMLs, and caudothalamic subependymal cysts were the most frequent incidental image findings. The incidence of ICH and PWMLs was highest within the ventouse delivered group. The majority of infants (59%) in our study were delivered via caesarean section (37%) or with instrumental assistance (22%) while 41% were SVD. This contrasts with the aforementioned study where the majority (71%) were SVD. The differing rates in mode of delivery may account for the higher prevalence of incidental findings in our group.
ICH particularly SDH without mass effect was the commonest incidental finding. There have been previous reports of SDH in asymptomatic neonates after delivery but there is a wide range of incidence (5·7 – 46%) [2,8,9,10]. Infratentorial/posterior fossa SDH was more common than supratentorial SDH in our cohort in keeping with most previous research studies [2,9,10]. The incidence of SDH in our study (23%) is higher than Kumpulainen (2020) who found SDH in 5·7% of asymptomatic infants (scanned mainly on weeks 3 and 4 after birth) but our result is less than a prospective study by Rooks et al. (2008) who reported an incidence of 46% in 101 asymptomatic term neonates (scanned within 3 days of life on a 1.5T MRI scanner) [2,8]. Our results are similar to Looney et al. (2007) who also used 3T MRI and found SDH in 26% of neonates delivered by SVD scanned between 1 and 5 weeks of age [10]. Similarly we found SDH in 27% of the neonates delivered by SVD confirming that SDH is a common finding after uncomplicated SVD.
Age at MRI examination is an important factor in determining the incidence of SDH in neonates as haemorrhage resorption can occur during the first days and weeks of life. Rooks et al. suggest most SDH present at birth are resolved by one month [8]. They completed follow-up imaging in 18/46 (39·1%) of their neonates with SDH and all demonstrated resolution by 3 months. The highest postnatal age in our neonates with SDH was 28 days. This is valuable information for radiologists required to comment on the aetiology of SDH in infants. A potential limitation of our study is the lack of follow-up imaging in neonates with SDH.
The risk of SDH and other haemorrhages varies with the mode of delivery [11]. The incidence of SDH was higher in infants delivered with instrumental assistance particularly ventouse (63%) compared with SVD (27%). Ventouse assistance was also noted to increase the risk for SDH in the study by Kumpulainen and colleagues [2]. Indeed vaginal delivery and vacuum-assistance were the only significant risk factors for ICH from a number of obstetric variables evaluated in their study. However, as pointed out by the authors, the potential occurrence of ICH is not an adequate indication for modifying delivery practices [2].
A notable finding in our study was the presence of SDH in four neonates delivered by EmCS (three with infratentorial and one with both supratentorial and infratentorial SDH) with no apparent SDH in those delivered by ElCS. This finding contrasts with the studies by Kumpulainen et al., Whitby et al. and Looney et al. who reported absence of SDH in caesarean deliveries [2,9,10]. However, our result is supported by research from Rooks et al. where 18% of neonates delivered by CS had SDH (supratentorial only) [8]. One of these neonates was born via ElCS, whereas 3 of 4 (75%) had failed a trial of oxytocin-augmented labour before caesarean delivery. The low rate of SDH in EmCS suggests it occurs with delivery of the head and not with fetal hypoxia or failure to progress.
Local mechanical trauma, head squeezing, and overlapping sutures during labour and delivery leading to venous compression and rupturing of bridging veins and capillaries have been proposed in the literature as possible mechanisms for SDH [8,12,13,14]. The observation that neonatal SDH is particularly common over the dural folds bearing the venous sinuses supports the role of the venous plexuses in SDH [15]. Increased birth weight has also been suggested as an additional risk factor for ICH [8]. The neonates with ICH in this study were, on average, heavier than those without ICH but with negligible effect size.
Scheimberg and colleagues (2013) reported an association between hypoxia and SDH [16]. Kelly et al. (2014) reviewed the incidence of SDH in infants with congenital heart disease (CHD) and found no demonstrable relationship between SDH and hypoxia [17]. However, in a recent study of neuroimaging findings in neonates with CHD prior to surgery, SDH occurred 5 times more frequently than reported in healthy normal vaginal delivery and 10 times more frequently in ventouse delivery [18]. The overall incidence of SDH was higher (33% versus 23% in our cohort).
Our study identified other less common subtypes of ICH in asymptomatic term neonates including small cerebellar haemorrhages in 11 infants. Additional SDH was evident in six of these neonates. This contrasts with Kumpulainen et al. where only two infants with SDH had additional parenchymal involvement [2]. The present study also confirms that neonatal ICH can occur in multiple locations in asymptomatic term neonates. Our study did not include susceptibility weighted imaging (SWI) which would likely have detected further microhaemorrhages.
PWMLs were the second most common incidental finding with prevalence of 12%. Similar to SDH, PWMLs were more frequent in those delivered with ventouse assistance (22%) versus other modes of delivery. PWML are a known common finding in preterm infants [19]. There have been few reports in term infants with research mainly focused on full-term infants with congenital heart defects undergoing surgery in the neonatal period [20]. Interestingly there were no reported PWMLs in the recent study of incidental MRI findings where the majority were born via SVD [2]. An earlier study by Hayman and colleagues (2019) reviewed 42 (near) term infants with PWMLs on MRI performed in the first 28 postnatal days [21]. The lesion load was high (>6) in 71% (versus 13/61 = 21% with >6 PWMLs in our results) but, unlike our study, their study was not a cohort of low risk neonates. The major clinical association reported was perinatal asphyxia, present in 45%.
The exact aetiology of PWML has yet to be established and may have a heterogenous pathology. According to Rutherford and colleagues (2010), some lesions may be related to venous congestion representing small venous infarction with haemorrhage (suggested by increased prevalence in ventouse deliveries in our study), whereas others may be related to regions of infarction or increased cellularity resulting in gliosis [22]. SWI helps in distinguishing haemorrhagic from non-haemorrhagic PWML [23]. SWI was not part of our standard protocol, a recognised limitation of our study. Nevertheless, our findings show that PWML can occur in asymptomatic term neonates with normal neurodevelopmental outcome at 18 months.
Periventricular pseudocysts (PVPC), particularly subependymal cysts (SEPC) were the third most frequent incidental finding (10%). Connatal or frontal horn cysts were rarer (<1%). Caudothalamic cysts were less frequent in the study by Kumpulainen et al., present in only 3/175 neonates. 2 However PVPC are found in 0·5–5% of healthy term neonates on transcranial ultrasound in first few days of life [24]. Two types of reported pathogenesis: germinal matrix haemorrhage and germinolysis; associated with congenital infections, metabolic disorders, chromosomal aberrations, and maternal cocaine consumption [25], [26], [27], [28]. Interestingly one neonate with bilateral pseudocysts in our study was later discovered to have maternal history of cocaine consumption. PVPC have been reported as isolated findings in otherwise healthy newborns. Makhoul et al. (2001) concluded in a meta-analysis of literature on congenital subependymal pseudocysts in infants that, in the absence of additional factors, including IUGR, fetal infections, malformations and chromosomal aberrations or persistence of PVPC, a favourable outcome is expected [29]. Neurodevelopmental outcome on prenatally diagnosed isolated PVPC also reported normal [30]. Our results support the current literature on PVPC.
The presence of cerebral infarction in 1 percent of our cohort is a notable incidental finding. It highlights the occurrence of clinically silent infarcts in term neonates with potential risk of long term morbidity, such as cerebral palsy or epilepsy. A recognised strength of our study is that participants were invited for formal neurodevelopmental assessment at 18 months. However it is not possible to determine the risk of subsequent neurodevelopmental sequelae in later childhood including cognitive and behavioural impairments.
In conclusion, there is a relatively high prevalence of incidental findings on brain MR imaging of asymptomatic term neonates. Subdural haemorrhage, punctate white matter lesions and caudothalamic subependymal cysts are the most frequent incidental findings as evidenced in this large cohort. The majority of incidental findings are clinically insignificant with normal short term neurodevelopmental follow-up. These findings should be anticipated in research and clinical settings. We do not advocate the use of routine brain MR imaging in term neonates if no risk factors for injury and asymptomatic. The results of this study will aid in the interpretation of incidental findings on MR imaging of the term neonatal brain and avoid unnecessary investigations. Information on these incidental findings will also inform consent in research settings and assist with design of incidental findings management protocols.
Declaration of Competing Interest
Professors David Edwards, Joseph Hajnal and Daniel Rueckert have received a Synergy research grant from the European Research Council (ERC). Professor Joseph Hajnal has received support from Philips Healthcare in the form of research access to software on their MRI systems and expert advice on their technology. Professor Daniel Rueckert has received additional research grant funding from The Engineering and Physical Sciences Research Council (ESPRC), Wellcome Trust, AvH, InnovateUK and British Heart Foundation; royalties/licenses (IXICO) and consultancies (IXICO and Heartflow). The remaining co-authors declare that they have no conflict of interest.
Acknowledgments
Fundings
This work was supported by the European Research Council under the European Union's Seventh Framework Programme (FP7/20072013/ERC grant agreement no. [319456] dHCP project), by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London and/or the NIHR Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Acknowledgment
We would like to thank the families who participated in the study and all the team involved in the Developing Human Connectome Project.
References
- 1.Illes J., Kirschen M.P., Edwards E. Ethics. Incidental findings in brain imaging research. Science. 2006;311(5762):783–784. doi: 10.1126/science.1124665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumpulainen V., Lehtola S.J., Tuulari J.J. Prevalence and risk factors of incidental findings in brain MRIs of healthy neonates–The FinnBrain Birth Cohort Study. Front Neurol. 2020;10:1347. doi: 10.3389/fneur.2019.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makropoulos A, Robinson EC, Schuh A, et al. The developing human connectome project: A minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage 2018;173:88-112. [DOI] [PMC free article] [PubMed]
- 4.Hughes E.J., Winchman T., Padormo F. A dedicated neonatal brain imaging system. Magn Reson Imag. 2017;78(2):409–418. doi: 10.1002/mrm.26462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayley N. 3rd Ed. Harcourt Assessment Inc; San Antonio, TX: 2006. Bayley scales of infant and toddler development. [Google Scholar]
- 6.Larroche J.C. Sub-ependymal pseudo-cysts in the newborn. Biol Neonate. 1972;21(3):170–183. doi: 10.1159/000240506. [DOI] [PubMed] [Google Scholar]
- 7.Epelman M., Daneman A., Blaser S.I. Differential diagnosis of intracranial cystic lesions at head US; correlation with CT and MR imaging. Radiographics. 2006;26(1):173–196. doi: 10.1148/rg.261055033. [DOI] [PubMed] [Google Scholar]
- 8.Rooks V.J., Eaton J.P., Ruess L., Petermann G.W., Keck-Wherley J., Pedersen R.C. Prevalence and evolution of intracranial haemorrhage in asymptomatic term neonates. AJNR. 2008;29(6):1082–1089. doi: 10.3174/ajnr.A1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitby E.H., Griffiths P.D., Rutter S. Frequency and natural history of subdural haemorrhages in babies and relation to obstetric factors. Lancet. 2004;363(9412):846–851. doi: 10.1016/S0140-6736(04)15730-9. [DOI] [PubMed] [Google Scholar]
- 10.Looney C.B., Smith J.K., Merck L.H. Intracranial haemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology. 2007;242(2):535–541. doi: 10.1148/radiol.2422060133. [DOI] [PubMed] [Google Scholar]
- 11.Towner D., Castro M.A., Eby-Wilkens E. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341(23):1709–1714. doi: 10.1056/NEJM199912023412301. [DOI] [PubMed] [Google Scholar]
- 12.Brouwer A.J., Groenendaal F., Koopman C., Nievelstein R.J., Han S.K., De Vries L.S. Intracranial haemorrhage in full-term newborns: a hospital-based cohort study. Neuroradiology. 2010;52(6):567–576. doi: 10.1007/s00234-010-0698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menezes A.H., Smith D.E., Bell W.E. Posterior fossa haemorrhage in the term neonate. Neurosurgery. 1983;13(4):452–456. doi: 10.1227/00006123-198310000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Huang A.H., Robertson R.L. Spontaneous superficial parenchymal and leptomeningeal haemorrhage in term neonates. AJNR Am J Neuroradio. 2004;25(3):469–475. [PMC free article] [PubMed] [Google Scholar]
- 15.Squier W., Mack J. The neuropathology of infant subdural haemorrhage. Forensic Sci Int. 2009;187(1–3):6–13. doi: 10.1016/j.forsciint.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Scheimberg I., Cohen M.C., Zapata-Vazquez R.E. Nontraumatic intradural and subdural haemorrhage and hypoxic ischaemic encephalopathy in fetuses, infants, and children up to three years of age: analysis of two audits of 636 cases from two referral centers in the United Kingdom. Pediatr Dev Pathol. 2013;16(3):149–159. doi: 10.2350/12-08-1232-OA.1. [DOI] [PubMed] [Google Scholar]
- 17.Kelly P., Hayman R., Shekerdemian L.S. Subdural haemorrhage and hypoxia in infants with congenital heart disease. Paediatrics. 2014;134(3):e773–e781. doi: 10.1542/peds.2013-3903. [DOI] [PubMed] [Google Scholar]
- 18.Kelly C.J., Arulkumaran S., Tristão Pereira C. Neuroimaging findings in newborns with congenital heart disease prior to surgery: an observational study. Arch Dis Child. 2019;104(11):1042–1048. doi: 10.1136/archdischild-2018-314822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyet L.E., Kennea N., Counsell S.J. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118(2):536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 20.Miller S.P., McQuillen P.S., Hamrick S. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357(19):1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 21.Hayman M., van Wezel–Meijler G., van Straaten H., Brilstra E., Groenendaal F., De Vries L.S. Punctate white matter lesions in the full-term newborn: underlying aetiology and outcome. Eur J Paediatr Neurol. 2019;23(2):280–287. doi: 10.1016/j.ejpn.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Rutherford M.A., Supramaniam V., Ederies A. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology. 2010;52(6):505–521. doi: 10.1007/s00234-010-0700-y. [DOI] [PubMed] [Google Scholar]
- 23.Niwa T., de Vries L., Benders M.J.N.L., Takahara T., Nikkels P.G.J., Groenendaal F. Punctate white matter lesions in infants: new insights using susceptibility-weighted imaging. Neuroradiology. 2011;53(9):669–679. doi: 10.1007/s00234-011-0872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heibel M., Heber R., Bechinger D., Kornhuber HH. Early diagnosis of perinatal cerebral lesions in apparently normal full-term newborns by ultrasound of the brain. Neuroradiology. 1993;35(2):85–91. doi: 10.1007/BF00593960. [DOI] [PubMed] [Google Scholar]
- 25.Rademaker K.J., De Vries L.S., Barth P.G. Subpendymal pseudocysts: ultrasound diagnosis and findings at follow-up. Acta Paediatr. 1993;82(4):394–399. doi: 10.1111/j.1651-2227.1993.tb12705.x. [DOI] [PubMed] [Google Scholar]
- 26.Shackelford G.D., Fulling K.H., Glasier C.M. Cysts of the subependymal germinal matrix: sonographic demonstration with pathologic correlation. Radiology. 1983;149(1):117–121. doi: 10.1148/radiology.149.1.6310678. [DOI] [PubMed] [Google Scholar]
- 27.Russel I.M., Van Sonderen L., Van Straaten H.L., Barth P.G. Subependymal germinolytic cysts in Zellweger syndrome. Pediatr Radiol. 1995;25(4):254–255. doi: 10.1007/BF02011090. [DOI] [PubMed] [Google Scholar]
- 28.Cohen H.L., Sloves J.H., Laungani S., Glass L., DeMarinis P. Neurosonographic findings in full-term infants born to maternal cocaine abusers: visualisation of subependymal and periventricular cysts. J Clin Ultrasound. 1994;22(5):327–333. doi: 10.1002/jcu.1870220507. [DOI] [PubMed] [Google Scholar]
- 29.Makhoul I.R., Zmora O., Tamir A., Shahar E., Sujov P. Congenital subependymal pseudocysts: own data and meta-analysis of the literature. Isr Med Assoc J. 2001;3(3):178–183. [PubMed] [Google Scholar]
- 30.Cooper S., Bar-Yosef O., Berkenstadt M., Hoffmann C., Achiron R., Katorza E. Prenatal evaluation, imaging features, and neurodevelopmental outcome of prenatally diagnosed periventricular pseudocysts. AJNR. 2016;37(12):2382–2388. doi: 10.3174/ajnr.A4916. [DOI] [PMC free article] [PubMed] [Google Scholar]