Highlights
-
•
Disease progression following anti-CD19 monoclonal antibody Tafasitamab treatment should not preclude subsequent anti-CD19 CART-cell therapy.
-
•
Previous studies have demonstrated sequential anti-CD19 to be effective.
-
•
Larger studies among R/R DLBCL patients across anti-CD19 modalities are needed to guide sequencing of therapies following initial anti-CD19 therapy.
Keywords: Diffuse large B-cell lymphoma, Tafasitamab, Chimeric antigen receptor T-cell therapy, CD19
Abstract
Tafasitamab (MOR208) is an Fc-enhanced, humanized, monoclonal antibody that targets CD19. The L-MIND (NCT02399085) trial, an open-label, single-arm, phase II study of Tafasitamab (TAFA) plus lenalidomide (LEN), reported progression-free survival of 16 months in R/R DLBCL patients ineligible for autologous stem cell transplantation. Despite recent advances in anti-CD19 therapy, no clinical evidence exists to direct the sequencing of CAR T cell therapy following relapse after prior anti-CD19 therapy. We present the first published case of TAFA/LEN treatment followed by CAR T therapy with sustained remission. Disease progression following treatment with Tafasitamab may not preclude patients from CAR T cell therapy.
Abbreviations
- Relapsed/Refractory
R/R
- Diffuse Large B-cell Lymphoma
DLBCL
- Non-Hodgkin's Lymphoma
NHL
- Acute Lymphoblastic Leukemia
ALL
- Autologous Stem Cell Transplantation
ASCT
- Chimeric Antigen Receptor T-Cell
CAR T-cell
- Tafasitamab
TAFA
- Lenalidomide
LEN
- Complete remission
CR
- Fluorodeoxyglucose
FDG
- Fluorescence in situ hybridization
FISH
- Immunohistochemistry
IHC
- Dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin
DA-R-EPOCH
- Rituximab, ifosfamide, carboplatin, etoposide
R-ICE
- Rituximab, gemcitabine, oxaliplatin
R-GemOx
- Antibody drug conjugates
ADC
- Bispecfic T-cell engagers
BiTE
1. Introduction
Relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) accounts for approximately one-third of patients with DLBCL and remains a major cause of morbidity and mortality. The standard second-line treatment for this group of fit patients is salvage intensive chemoimmunotherapy followed by consolidative autologous stem cell transplantation (ASCT). However, about half of patients are ineligible for this treatment strategy due to ineffective salvage treatment or suboptimal fitness, while the other half will relapse after ASCT.
CD19-targeted chimeric antigen receptor T-cell (CAR T-cell) therapy has shown promising results for patients with R/R DLBCL after failure of ≥ 2 lines of systemic therapy, but the long-term durability remains unclear [1], [2]. Tafasitamab (TAFA, MOR208) is an Fc-enhanced, humanized, monoclonal antibody targeting CD19 that, in combination with lenalidomide (LEN), has been shown to achieve an objective response rate of 60% and durable remissions in patients with R/R DLBCL ineligible for ASCT [3].
With the recent advances in anti-CD19 therapy for R/R DLBCL, there is little clinical evidence available to direct the sequencing of these therapies. We present the first published case of a patient who received anti-CD19 monoclonal antibody treatment with TAFA/LEN as part of the L-MIND clinical trial followed by anti-CD19 CAR T-cell therapy.
2. Case description
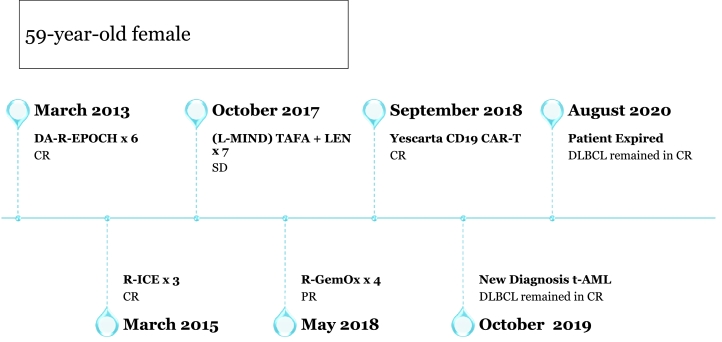
Our patient is a 59-year-old female who initially presented in March of 2013 with night sweats, fevers, and an 8 cm mesenteric mass. Biopsy confirmed germinal center B-cell like phenotype DLBCL with Ki-67 of 80% and immunohistochemistry (IHC) positive for CD20, PAX5, CD10, BCL-2, and BCL-6. Fluorescence in situ hybridization (FISH) analysis noted positive IGH/BCL-2 fusion gene rearrangement and negative BCL-6 and MYC rearrangements. PET/CT identified an intensely fluorodeoxyglucose (FDG) avid ~8 cm mass in the central abdomen and submandibular lymph node. Bone marrow biopsy was negative for lymphoma involvement. The patient received 6 cycles of dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin (DA-R-EPOCH) and achieved a complete remission. In February 2014, PET/CT noted new low level FDG uptake in a left mesenteric mass with obvious progression on imaging in January 2015. A laparoscopic small bowel resection with excision of the suspicious mass revealed DLBCL with grade 3A follicular lymphoma.
She was treated with 3 cycles of rituximab, ifosfamide, carboplatin, etoposide (R-ICE) salvage therapy and achieved a complete remission. She declined ASCT at the time and remained in remission until November 2016 when PET/CT reported a new mild FDG focus of a small mesenteric lymph node that was enlarged on follow up PET/CT in July 2017. Biopsy demonstrated a recurrence of germinal center B cell–like type DLBCL.
She was enrolled in the L-MIND trial in October 2017 and received TAFA plus LEN for 6 cycles. PET/CT following cycle 4 showed a partial response with interval decrease in multiple mesenteric nodules and lymph nodes. In April 2018, she progressed and was taken off trial. After 4 cycles of salvage chemotherapy with rituximab, gemcitabine, oxaliplatin (R-GemOx), PET/CT in July 2018 showed persistent intense FDG uptake in the dominant mesenteric mass. She underwent CAR T-cell therapy with axicabtagene ciloleucel in September 2018, and PET/CT scan in November 2018 showed a complete response.
Unfortunately, in October 2019, she was hospitalized with fever, night sweats, and pancytopenia. PET/CT showed diffuse homogeneous uptake in bone marrow. Bone marrow biopsy revealed a hypercellular bone marrow (> 90%) with multilineage dysplasia, marrow fibrosis, and ring sideroblasts, consistent with therapy-related myelodysplasia. Hematologic malignancy sequencing panel showed mutations in TP53 (allele frequency 40.9%), KMT2A (allele frequency 5.2%), and JAK2 (allele frequency 62.9%) with cytogenetics notable for a complex karyotype, including del5q, del7q, and trisomy 8. She was not a candidate for allogeneic hematopoietic stem cell transplantation and so was placed on a trial of azacitidine and magrolimab in May 2020. After 2 cycles, her bone marrow biopsy revealed acute erythroblastic leukemia, and she ultimately passed from her disease in August 2020.
3. Methods
This report is a retrospective chart review of 1 patient with R/R DLBCL treated with TAFA/LEN and anti-CD19 CAR T-cell therapy. Informed consent was not required per the University of California Los Angeles Institutional Review Board.
4. Discussion
Many different therapeutic modalities are currently being explored to target CD19 antigen expression in B-cell non-Hodgkin's lymphoma (NHL). While CAR T-cell therapy has been the most well studied to date in DLBCL, monoclonal antibodies, antibody drug conjugates (ADC), and bispecific T-cell engagers (BiTE) are also being evaluated [3], [4], [5]. An important question to address in the era of targeted cellular therapy is: can the same tumor antigen be targeted with a different cancer immune therapy after disease progression following a previous therapy targeting the same antigen? In such a clinical scenario, there is concern for sustained antigen blockade from the prior line of therapy or antigen loss, both potentially rendering future therapies that target the same antigen ineffective.
In B-cell acute lymphoblastic leukemia (ALL), CD19 loss in relapsed disease has been demonstrated after progression following anti-CD19 CAR T-cell therapy and BiTe therapy with blinatumomab [6], [7]. The data in R/R DLBCL is less clear. Chow et al. published a report of 3 R/R DLBCL patients who received axicabtagene ciloeucel after previous treatment with tisagenlecleucel or an investigational CD19 CAR T-cell therapy with 4–1BB costimulation [8]. Two patients progressed after treatment with axicabtagene ciloleucel, but their disease remained positive for CD19 expression [8].
Another study by Bezerra et al. investigated the use of repeat infusions of CAR T-cells in 44 patients total, including 19 with NHL. Sixteen patients (36%) responded to second CAR T-cell infusion, suggesting that sequential anti-CD19 therapy can still be effective. The authors found predictors of response after second CAR T-cell infusion to include high-intensity lymphodepletion prior to first CAR T-cell infusion and increasing the CAR T-cell dose at the time of second CAR T-cell infusion [9].
Thapa et al. evaluated anti-CD19 CAR T-cell therapy in 14 R/R DLBCL patients previously treated with an antibody drug conjugate loncastiuximab tersirine, an ADC composed of a humanized anti-CD19 monoclonal antibody conjugated to a pyrrolobenzodiazepine dimer toxin. After failure of loncastuximab, ten patients were positive for CD19 expression. Following CAR T-cell therapy, 6 patients had CR, 5 of whom had ongoing CR at 6 months. Notably, the 4 patients with unknown CD19 expression status following ADC therapy achieved a CR with CAR T-cell therapy [10].
Our patient received TAFA/LEN 5 months prior to CAR T-cell therapy. Given the half-life of TAFA is approximately 16 days, it was likely eliminated prior to subsequent anti-CD19 therapy. Although a biopsy was not done upon progression after TAFA, we assume that CD19 antigen escape did not account for the relapse, since the patient achieved sustained remission for nearly 1 year with anti-CD19 CAR T-cell therapy.
5. Conclusion
The above studies, as well as the report of our patient, delineate that even in patients with unmeasured CD19 expression, subsequent therapies targeting CD19 remain effective. Disease progression following treatment with the anti-CD19 monoclonal antibody Tafasitamab should not preclude patients from anti-CD19 CAR T-cell therapy. It would although be ideal to establish the persistence of CD19 expression prior to initiating subsequent CD19-targeted therapy. Larger scale studies among R/R DLBCL patients across anti-CD19 modalities are needed to guide sequencing of therapies following initial anti-CD19 therapy. (Fig. 1).
Fig. 1.
Schematic diagram of R/R DLBCL patient treated with TAFA/LEN followed by anti-CD19 CAR T-cell therapy.
CRediT authorship contribution statement
Nadeem Tabbara: Writing – review & editing. Daria Gaut: Writing – review & editing. Caspian Oliai: Writing – review & editing. Tara Lewis: Writing – review & editing. Sven de Vos: Writing – review & editing.
Declaration of Competing Interest
None, This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
None.
Contributor Information
Nadeem Tabbara, Email: ntabbara@mednet.ucla.edu.
Daria Gaut, Email: dgaut@mednet.ucla.edu.
Caspian Oliai, Email: coliai@mednet.ucla.edu.
Tara Lewis, Email: tllewis@mednet.ucla.edu.
Sven de Vos, Email: deVos@mednet.ucla.edu.
References
- 1.Neelapu S.S., Locke F.L., Bartlett N.L. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;37(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster S.J., Svoboda J., Chong E.A. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 2017;377(26):2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salles G., Duell J., González Barca E. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978–988. doi: 10.1016/S1470-2045(20)30225-4. [DOI] [PubMed] [Google Scholar]
- 4.Zammarchi F., Corbett S., Adams L. ADCT-402, a PBD dimer–containing antibody drug conjugate targeting CD19-expressing malignancies. Blood. 2018;131(10):1094–1105. doi: 10.1182/blood-2017-10-813493. [DOI] [PubMed] [Google Scholar]
- 5.Goebeler M.E., Knop S., Viardot A. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J. Clin. Oncol. 2016;34(10):1104–1111. doi: 10.1200/JCO.2014.59.1586. [DOI] [PubMed] [Google Scholar]
- 6.Lee D.W., Stetler-Stevenson M., Yuan C.M. Long-term outcomes following CD19 CAR T cell therapy for B-ALL are superior in patients receiving fludarabine/cyclophosphamide preparative regimen and post-CAR hematopoietic stem cell transplantation. Blood. 2016;128(22):218. doi: 10.1182/blood.V128.22.218.218. [DOI] [Google Scholar]
- 7.Topp M.S., Gökbuget N., Zugmaier G. Phase II trial of the anti-CD19 bispecific T cell–engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J. Clin. Oncol. 2014;32(36):4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 8.Chow V.A., Gopal A.K., Gauthier J. Axicabtagene ciloleucel for relapsed or refractory lymphoma after prior treatment with a different CD19-directed CAR T-cell therapy. Blood Adv. 2020;4(19):4869–4872. doi: 10.1182/bloodadvances.2020002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bezerra E.D., Gauthier J., Hirayama A.V. Factors associated with response, CAR-T cell in vivo expansion, and progression-free survival after repeat infusions of CD19 CAR-T cells. Blood. 2019;134(Supplement_1):201. doi: 10.1182/blood-2019-123807. [DOI] [Google Scholar]
- 10.Thapa B., Caimi P.F., Ardeshna K.M. CD19 antibody-drug conjugate therapy in DLBCL does not preclude subsequent responses to CD19-directed CAR T-cell therapy. Blood Adv. 2020;4(16):3850–3852. doi: 10.1182/bloodadvances.2020002587. [DOI] [PMC free article] [PubMed] [Google Scholar]