Abstract
The antioxidant function of the phospholipid hydroperoxide glutathione peroxidase (GPx4) is vital for the homeostasis of many cell types, from neoplastic cells to normal erythroid precursors. However, some functional proteins in erythroid precursors are lost during the development of red blood cells (RBCs); whether GPx4 is maintained as an active enzyme in mature RBCs has remained unclear. Our meta-analyses of existing RBC proteomics and metabolomics studies revealed the abundance of GPx4 to be correlated with lipid-anchored proteins. In addition, GPx4 anti-correlated with lyso-phospholipids and complement system proteins, further supporting the presence of active GPx4 in mature RBCs. To test the potential biological relevance of GPx4 in mature RBCs, we correlated the rate of hemolysis of human RBCs during storage with the abundance of GPx4 and other heritable RBC proteins. Of the molecules that anti-correlated with the rate of hemolysis of RBCs, proteins that mediate the cellular response to hydroperoxides, including GPx4, have the greatest enrichment. Western blotting further confirmed the presence of GPx4 antigenic protein in RBCs. Using an assay optimized to measure the activity of GPx4 in RBCs, we found GPx4 to be an active enzyme in mature RBCs, suggesting that GPx4 protects RBCs from hemolysis during blood bank storage.
Keywords: Red blood cells, GPx4, Blood storage
Graphical abstract
Highlights
-
•
Red blood cells (RBCs) have active GPx4, phospholipid hydroperoxide glutathione peroxidase.
-
•
The abundance of GPx4 correlates with lipid-anchored proteins.
-
•
The rate of hemolysis of RBCs during storage anti-correlates with proteins that mediate the response to hydroperoxides.
-
•
The abundance of GPx4 in RBCs is 75% heritable and varies at least 4-fold across subjects.
-
•
Multi-omic meta-analysis of heritable biomolecules links GPx4 to RBC storage hemolysis.
1. Introduction
Over 100 million units of blood are collected worldwide each year [1]. In blood banks, blood products, such as red blood cells (RBCs), are typically stored for days to weeks, but units of RBCs degrade and hemolyze during storage at variable and unpredictable rates [2,3]. The ability to predict and reduce rates of hemolysis during storage of RBCs could improve transfusion therapy. Transfusions would be more efficacious because: more functional RBCs would enter recipient circulation; there would be a lower burden of cellular debris and its potential adverse effects; the condition of RBCs delivered to remote areas during disaster responses would be enhanced; neonates receiving multiple transfusions from a single blood unit over time would receive more effective transfusions with reduced donor exposures; reduced risk of alloimmunization; and reduced risk of transfusion reactions.
Methods for predicting the rate of hemolysis during storage of RBCs are lacking in part because the molecular underpinnings of hemolysis during storage of RBCs are poorly understood. We hypothesized that genetic variation in donors leads to variability in select biochemical pathways that impact the rate of hemolysis during storage of RBCs.
In a classic twin study, analyses of RBCs collected from a set of monozygotic and dizygotic twin pairs revealed that the rate of hemolysis in RBCs during storage is a heritable trait [3]. Heritability estimates ranged from 28% to 54% across 14, 28, 42, and 56 days of storage [3]. However, the identity of genetic variants and downstream alterations in biochemical pathways underlying the heritability of storage-hemolysis have not been explored. Previous proteomic and metabolomic studies of RBCs from the twin study revealed a set of 119 heritable RBC proteins and 148 heritable RBC metabolites [[4], [5], [6]]. In the present study we interrogated our twin database for relationships between storage hemolysis and heritable molecule abundances.
In an orthogonal approach, a genome-wide association study (GWAS) of >11,000 donors conducted by the REDS-III RBC-Omics group [7] demonstrated that features such as ancestry and sex, which reflect differences in genetic background, are modifiers of the rate of hemolysis of RBCs [2]. Integration of hits from this GWAS with hits from our orthogonal twin study has the potential to identify high-confidence targets for further investigations of key biochemical pathways that impact RBC hemolysis.
Here, our integrative analysis reveals correlations between heritable RBC proteins, heritable RBC metabolites, and the rate of hemolysis during storage of RBCs. We used a subset of the resource generated by this work to reveal that the rate of hemolysis during storage of RBCs is altered by heritable differences in enzymes that mediate the cellular response to oxidative distress arising from exposure to hydroperoxides. We identified a central role in RBC homeostasis for glutathione peroxidase 4.
Glutathione peroxidase 4 (GPx4), alias, phospholipid hydroperoxide glutathione peroxidase, is a selenium-containing, membrane-associated peroxidase that reduces phospholipid hydroperoxides in lipid regions of cellular membranes to their corresponding phospholipid alcohol [8]. The removal of phospholipid hydroperoxides by GPx4 was established in the 1980s [8,9]. Beyond its role in the peroxide-removal systems of cells and tissues, GPx4 is essential for mammalian development; mice lacking Gpx4 die in utero [10]. GPx4 activity has recently been shown to have central roles in cancer biology, e.g. ferroptosis, and is a target for investigational small-molecule anti-cancer agents [[11], [12], [13], [14]]. Recent studies have shown that GPx4 prevents necroptosis in mouse erythroid precursor cells [15]. An in-depth quantitative analysis of the red blood cell (RBC) proteome revealed that GPx4 protein is present in RBCs [16]. These findings suggest that GPx4 may have a role in maintaining homeostasis in RBCs. However, it is known that during the maturation of sperm the biochemical function of GPx4 changes from a peroxidase enzyme to a structural protein without peroxidase activity [17]. This raises the question whether GPx4 is an active enzyme with peroxidase activity in mature RBCs.
Establishing that GPx4 as an active enzyme in mature RBCs will enhance our understanding of RBC biochemistry and biology. In clinical application, the presence of GPx4 in RBCs could affect their stability during blood bank storage. Units of blood are collected worldwide and stored for up to six weeks [1]. During storage, RBCs hemolyze and degrade. However, the rate of degradation of RBCs during storage is variable and unpredictable from donor to donor [2,3]. Developing the ability to predict and reduce rates of hemolysis during storage of RBCs could improve transfusion therapy.
We hypothesized that genetic variation in donors of RBCs leads to variability in select biochemical pathways that impact the rate of hemolysis during storage. We analyzed a subset of the twin study database to reveal that rates of hemolysis of RBCs during storage are altered by heritable differences in enzymes that mediate the cellular response to oxidative distress arising from exposure to hydroperoxides, including GPx4. Based on this, we adapted our GPx4 assay to determine if GPx4 is an active enzyme in human erythrocytes. We also provide tools that may lead to further development of methodologies that can be used to estimate the rate of deterioration of RBCs during storage.
2. Materials and methods
All aspects involving the use of human subjects in this study were approved by the Human Subjects Office of The University of Iowa.
2.1. Data set integration
The rates of hemolysis during storage for RBCs were integrated into a compiled RBC donor twin study data set containing the “day 0” RBC proteomics data set (mass spectrometry-based measurements of the abundances of 1280 RBC proteins at “day 0” of storage; raw data from Ref. [4]) and the “day 0” RBC metabolomics data set (mass spectrometry-based measurements of the abundances of 330 RBC metabolites at “day 0” of storage; raw data from Ref. [4]) using Microsoft Excel for Mac version 16.16.15. Available heritability estimates for RBC molecules from the twin study [4] were added to this compiled data set (Supplementary Data Set 1), which was used as the foundational data set for the subsequent analyses in this study.
2.2. Storage hemolysis vs. molecule abundance correlations
Correlation coefficients were calculated for all hemolysis-molecule abundance pairs using a python script based on SciPy's Spearman's ranked-ordered correlation package (https://docs.scipy.org/doc/scipy-0.14.0/reference/generated/scipy.stats.spearmanr.html) (Supplementary Data Set 2). The analysis did not assume linear relationships between the abundances of molecules and hemolysis rates and did not assume normal distributions. Spearman's rank correlation coefficients were therefore used instead of Pearson's coefficients. To focus analyses on correlations containing at least 20 data points and molecules with adequate data for heritability estimates, the correlation matrix was trimmed to include only molecules with valid heritability calculations, as described in Ref. [4], regardless of the magnitude of heritability estimates. The trimmed correlation matrix containing molecules with heritability estimates is depicted as a heatmap. Further detailed analyses of individual molecules correlated or anti-correlated with storage hemolysis focused on relationships in which the absolute value of the Spearman's rank correlation coefficient was >0.3. This cutoff of |ρ| > 0.3 approximated a significant (p < 0.05–0.10) correlation, which varied depending on the number of data points included in the individual hemolysis-molecule pairs. The number of data points included in each hemolysis-molecule pair varied because of the stochastic nature of mass spectrometry proteomics in which proteins are not always observed across all samples.
2.3. Gene ontology (GO) category analysis of proteins anti-correlated with storage hemolysis
GO analyses were performed using GoMiner [18], as accessed online at https://discover.nci.nih.gov/gominer 2020.07. The full set of proteins used in the analysis comprised all RBC proteins with estimated heritability (n = 385). The subset of proteins used in the analysis was comprised of proteins anti-correlated with storage hemolysis (n = 33), as defined by ρ < −0.3. UniProtKB limited to H. sapiens was used as the online data source, including all evidence code levels. Lookup settings included “Cross Reference” and “Synonym”. A p-value constraint of <0.05 was used as a cutoff for statistical reports. 100 randomizations were used. Categories with <5 members in the data set were excluded from the analysis. The root categories were limited to biological processes.
2.4. Calculation of rates of hemolysis of RBCs during storage
Standard red blood cell units were collected from volunteer twin subjects and stored in extended storage media (AS-3) under standard blood bank conditions and then sampled for hemolysis as previously described [5]. Measurements of RBC hemolysis (% hemolyzed RBCs compared to the total number of RBCs) at days 0, 14, 28, 42, and 56 of cold storage were available for 17 of the 18 twin pairs (n = 34 individuals) (raw data from Ref. [3]). These data (n = 170 hemolysis measurements) were used in subsequent analyses without removal of possible outliers because units with high hemolysis rates could reflect meaningful biology. Hemolysis rates (% hemolysis per day) across the 56 days of storage were determined by calculating the slope of the line of best fit for percent hemolysis across each of the five timepoints.
2.5. Validation of the GPx4 antibody for the use on RBC samples
Control HepG2 cells were cultured in MEM, containing 10% fetal bovine serum supplemented with 500 nM selenomethionine to maximize expression of GPx1 and GPx4 [19]. In addition to our previous work on validation [19], to extend our efforts to validate the anti-GPx4 antibody (Abcam [EPNCIR144] ab125066), proteins were extracted from RBCs and HepG2 cells using stock buffer of Tris-Base (0.10 M, pH 8.0) containing EDTA, NaN3 (2.0 mM, 1.5 mM), and Triton X-100 (0.1%) to solubilize membrane bound proteins.
RBCs from a single study subject were washed 1–5 times to determine if GPx4 protein was lost in washing. For these experiments, 10 μg of RBC protein and 3, 6, and 9 μg of protein from HepG2 control cells were loaded onto a 4–20% precast polyacrylamide gel (Mini-Protean TGX Precast Protein Gels, Bio-Rad). The proteins were electrophoresed for 70 min at 110 V and 2.0 A. Total protein mass was determined using the DC™ Protein Assay (Bio-Rad). Protein was electro transferred to polyvinylidene fluoride (PVDF) membranes for 1 h at 100 V and 2.0 A. After blocking in 5% non-fat milk in phosphate buffered saline with Tween 20® (TPBS) (1–2 h), the membranes were incubated with the primary antibody (overnight at 4 °C) followed by secondary antibodies conjugated with horseradish peroxidase (1:20,000, Millipore, Temecula, CA) (1–2 h at room temperature). Membranes were washed for 5–10 min 5 times and treated with SuperSignal West Pico Plus chemiluminescent substrate (ThermoFisher) for 5 min. X-ray film was then exposed to the membrane for 5–120 s to visualize bands on the film.
Because common loading controls (e.g., GAPDH, β-actin, and tubulin) were unreliable for western blots of RBCs, we used whole gel Coomassie stains to determine protein loading consistency. The gel was submerged in Coomassie Brilliant Blue R-250 Staining Solution #1610436 (Bio-Rad) for 2 h followed by washes in a solution containing 10% acetic acid, 50% methanol, and 40% H2O until the background was nearly clear. Coomassie-stained bands identified the location of residual proteins.
2.6. Optimization of the GPx1 and GPx4 combined activity assays for human RBCs
2.6.1. Combination assay overview
The enzymatic activities of GPx1 and GPx4 were determined using the combined assay for GPx1 and GPx4, which monitors oxidation of NADPH via UV–Vis spectroscopy [19]. The substrate for GPx4 activity was phosphatidylcholine hydroperoxide, as prepared by the detailed procedure of [19]. H2O2 was the substrate for GPx1. Whole blood collected in heparin tubes from 4 subjects was centrifuged (2000 g, 20 min), then a portion of packed RBCs was aspirated, avoiding the buffy coat. RBCs were washed and counted. As it is crucial to ensure the linearity of activity (i.e., more cells yield more observed activity), the suspension of RBCs was diluted to generate a set of samples consisting of (0.5–5) x 106 cells. The packed RBCs were resuspended in Tris-Base (0.10 M, pH 8.0) containing EDTA, NaN3 (2.0 mM, 1.5 mM), and 0.1% Triton X-100. Observed activities were normalized to protein content as determined by the DC™ Protein Assay (Bio-Rad, Hercules, CA, USA), which is based on the Lowry assay [20].
2.6.2. Enzymology sample preparations
Heparinized whole blood was collected and centrifuged (2000 g, 20 min). Plasma was removed and a portion of the packed RBCs was aspirated, avoiding the buffy coat. RBC samples were washed in PBS and counted using a Z2™ Coulter Counter® Cell and Particle Counter (Beckman-Coulter, Miami, FL). To reduce the interference of the Soret band, the RBCs (approximately (2–4) x108 cells) were suspended in 30 mM phosphate buffer and 4 mM magnesium sulfate to lyse the cells. To remove hemoglobin, the suspension was centrifuged at 13,200 g for 10–20 min, the supernatant was removed, and the process was repeated 5 times. When the supernatant appeared transparent, the resulting pellets were suspended in Tris-Base buffer containing Triton X-100 (0.1%). However, interference of the Soret band of residual contaminating hemoglobin led to invalid results in several RBC samples; see Fig. S4 for examples. Cells from human cell lines (HepG2 and H1299) and rat liver were homogenized in the same buffer.
HepG2 (human hepatocellular carcinoma) and H1299 (human lung carcinoma) cells were purchased from American Type Culture Collection (ATCC). The cell lines were cultured in media recommended by ATCC. As appropriate, confirmation of all cell lines was accomplished by IDEXX-RADIL. The genetic profiles of cell stocks were compared to the genetic profiles of cell lines available in the DSMZ STR database to ensure that they do not match any other reported profiles in the DSMZ database. Rat liver (male Sprague-Dawley) was flash frozen in liquid nitrogen directly after harvesting and stored at −80 °C until analyzed.
3. Results
3.1. Select RBC molecule abundances correlate with storage hemolysis
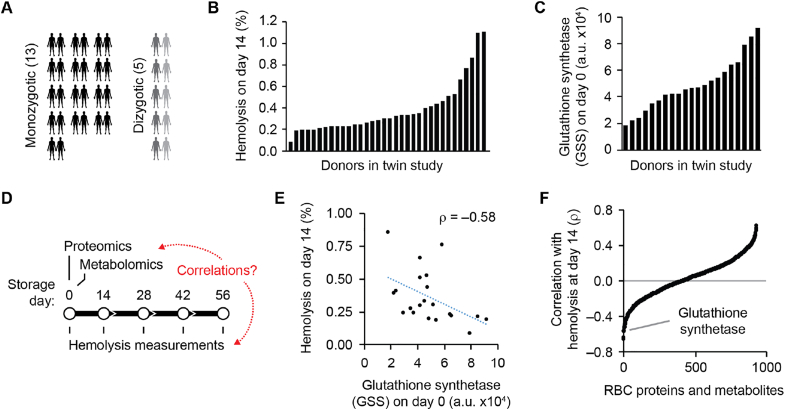
Our prior twin study [3,5,6] established an RBC donor cohort with variable RBC hemolysis during storage across five study time points (days 0, 14, 28, 42, and 56 of storage) (Fig. 1A and B, and Supplementary Fig. S1A) and donor-dependent variability in the abundances of RBC proteins and metabolites quantified at “day 0” of storage (e.g., glutathione synthetase (GSS), Fig. 1C) (Supplementary Data Set 1). Here, to explore the biochemical basis for the donor-dependent variability in RBC storage hemolysis, we investigated correlations between hemolysis at each of the five time points in this study and the abundances of RBC proteins and RBC metabolites at “day 0” of storage (Fig. 1D). For example, GSS was found to be anti-correlated with storage hemolysis at each of the five time points (Fig. 1E and Fig. S1B), suggesting that GSS plays a role in protecting RBCs from storage hemolysis. Similar pairwise comparisons were made between all RBC molecules and each time point in the data set (Fig. 1F and Supplementary Data Set 2). This analysis identified 205 RBC molecules anti-correlated with storage hemolysis (Fig. S1C).
Fig. 1.
Abundances of select RBC molecules anti-correlate with rates of hemolysis during storage.
(A) Schematic of the 13 monozygotic twin pairs and the 5 dizygotic twin pairs analyzed in this study.
(B) Red blood cell (RBC) hemolysis (percent of the total RBCs that were hemolyzed) at day 14 of cold storage across the individual donors in the twin study. Raw data from Ref. [3].
(C) Abundance of the protein glutathione synthetase (GSS; arbitrary units, a. u, based on mass spectrometry signal) in RBCs from donors in the twin study. Raw data from Ref. [4].
(D) Schematic depicting the time points at which samples of the stored RBC units were analyzed for hemolysis. Using samples isolated on the day of donation, RBC proteins and metabolites were quantified by mass spectrometry (“day 0” time point).
(E) Scatter plot of RBC hemolysis at day 14 of storage (%) versus the abundance of the protein GSS.
(F) Rank-ordered plot of RBC molecule correlations (ρ) with hemolysis at day 14, highlighting GSS. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Select RBC molecule abundances correlate with storage hemolysis
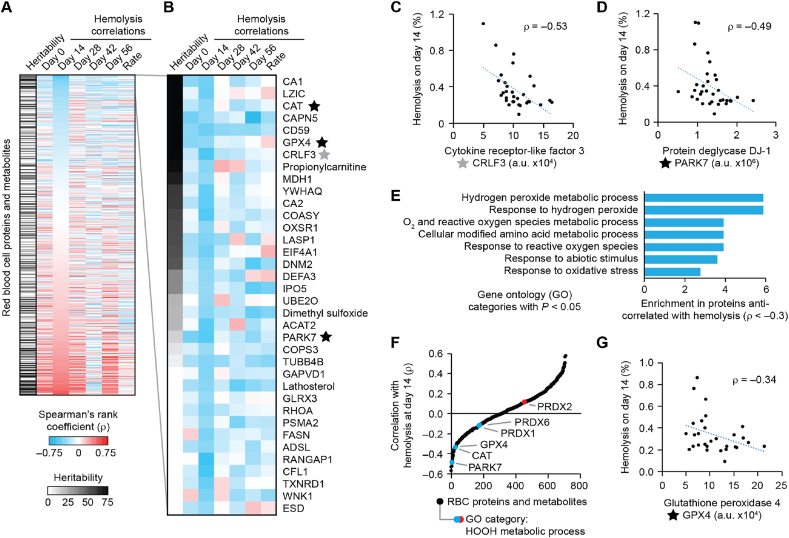
To explore heritable biochemical pathways associated with the rate of hemolysis during RBC storage, further analyses were conducted with a subset of data containing molecules for which sufficient data to calculate heritability estimates were available (Supplementary Data Set 3). In this trimmed data set, correlations and anti-correlations between storage hemolysis and molecule abundances were observed at each of the time points in the study (Fig. 2A). Many molecules were consistently correlated or anti-correlated at each time point. For example, the glycoprotein CD59, in which mutations can cause paroxysmal nocturnal hemoglobinuria from uncontrolled complement-mediated RBC hemolysis [21] was strongly anti-correlated with storage hemolysis at every time point (Fig. 2B). However, differences were observed across time points for some molecules, likely due to previously described varying biological conditions during storage [22].
Fig. 2.
Select heritable RBC molecule abundances anti-correlate with rates of hemolysis during storage.
(A) Heatmap of Spearman's rank correlation coefficients (ρ) between the abundances of individual RBC proteins or RBC metabolites (arrayed on the ordinate) (proteomics data from Ref. [4])and measures of RBC hemolysis during storage (arrayed on the abscissa) (hemolysis data from Ref. [3]), organized by correlations with hemolysis at day-14 of storage. The heritability of each protein or metabolite (as defined previously in Ref. [4]) is also indicated (black and white column on far left).
(B) Detailed view of the molecules most anti-correlated with hemolysis at day-14, as defined by ρ < −0.3 and sorted by heritability. Stars mark molecules highlighted in panels C–G.
(C) Scatter plot of RBC hemolysis at day-14 of storage (%) (hemolysis data from Ref. [3]) vs. the abundance of the protein CRLF3 (arbitrary units, a. u, based on mass spectrometry signal) (proteomics data from Ref. [4]).
(D) Scatter plot of RBC hemolysis at day-14 of storage (%) (hemolysis data from Ref. [3]) vs. the abundance of the protein PARK7 (proteomics data from Ref. [4]).
(E) Gene Ontology (GO) categories significantly (p < 0.05) enriched in proteins anti-correlated with hemolysis (n = 33), as defined by ρ < −0.3, compared to all RBC proteins with measured heritability (n = 385).
(F) Rank-ordered plot of RBC molecule correlations (ρ) with hemolysis at day-14, highlighting proteins observed from the GO category for “hydrogen peroxide metabolic process”.
(G) Scatter plot of RBC hemolysis at day-14 of storage (%) (hemolysis data from Ref. [3]) vs. the abundance of the protein GPx4 (proteomics data from Ref. [4]).
We focused the analysis on hemolysis at day-14 of storage because day-14 was the time point nearest the estimated median time of storage at which RBC units are transfused (20 days) [23]; also, day-14 is a defining storage time for fresh vs. older blood in clinical trials that examined the relationship between the age of RBC units and clinical outcomes [[24], [25], [26]]. Biochemically, day-14 of storage approximates an inflection point at which significant age-dependent biochemical alterations in RBCs (e.g., ATP depletion) have been previously observed [5,22,27]. Upon infusion of stored RBCs containing cell-free hemoglobin, nitric oxide homeostasis can be impaired leading to endothelial dysfunction, platelet activation, and vasculopathy [28]. We hypothesized that heritable differences in RBC proteins or metabolites are significantly associated with the rate of hemolysis of RBCs at this biochemically dynamic time point.
Analyses showed robust hemolysis-molecule abundance anti-correlations at storage-day-14 time point (Fig. S3A). Using a strict cutoff of Spearman's ρ < – 0.45, we observed eight molecule-hemolysis anti-correlations at storage-day-14 — more than at any of the other time points (Figs. S3B and S3C). These included CRLF3 (Fig. 2C), an erythropoietin receptor homolog [29], and PARK7 (Fig. 2D), a deglycase involved in cellular antioxidant responses [30]. An unbiased gene ontology analysis demonstrated that molecules anti-correlated with hemolysis at day-14 of storage were enriched for proteins that mediate the cellular response to hydroperoxides (Fig. 2E and F and Supplementary Data Set 4), suggesting that these proteins protect RBCs from early storage hemolysis. These anti-correlated proteins included PARK7 (Fig. 2D), catalase (CAT) (starred in Fig. 2B), and GPx4 (Fig. 2G). This suggests that GPx4 enzyme activity in mature RBCs may be part of a heritable biological program for protecting RBCs from oxidative damage.
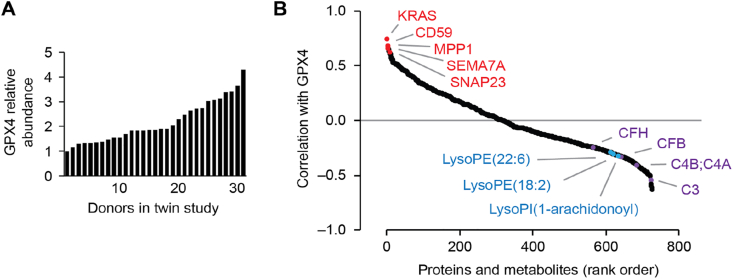
3.3. GPx4 protein is correlated with functionally related molecules in RBCs
We mined our existing RBC twin study data set for multi-omic evidence of GPx4 activity in RBCs. Specifically, we examined correlations between the abundance of GPx4 protein and the abundances of other biomolecules in our publicly available proteomics and metabolomics data set from an RBC donor twin study [4]. In this prior work, over a 4-fold variance in GPx4 abundance was observed across 31 RBC donors [4] (Fig. 3A). Within the proteomic data set, GPx4 abundance correlates with lipid-anchored signaling proteins (Fig. 3B and Fig. S1), suggesting that GPx4 activity supports lipid membrane-associated pathways. GPx4 abundance anti-correlates with several members of the complement system (Fig. 3B). This included an anti-correlation with complement factor H (CFH), which directly binds lipid peroxidation-generated malondialdehyde epitopes [31]. Low malondialdehyde levels in RBCs resulting from higher GPx4 activity could account for a lower RBC-associated CFH abundance, and vice versa.
Fig. 3.
RBCs have GPx4 and its abundance varies across RBC donors and correlates with select RBC molecules.
(A) Relative abundance of GPx4 protein in RBCs at “day 0” of cold storage across the individual donors in the twin study. Proteomics data from Ref. [4].
(B) Rank order plot of RBC molecules correlated or anti-correlated with GPx4, highlighting select lipid-anchored proteins correlated with GPx4 (red), complement proteins anti-correlated with GPx4 (purple), and lysophospholipids anti-correlated with GPx4 (blue). Proteomics and metabolomics data from Ref. [4]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
GPx4 is also anti-correlated with lyso-phospholipids (Fig. 3B). Lyso-phospholipids are generated by phospholipase A-catalyzed hydrolysis of phospholipid-hydroperoxides [32], which is an alternative phospholipid-hydroperoxide degradation pathway in the absence of GPx4 activity. Lack of GPx4 activity in tumor cells elevates levels of lyso-phospholipids [14]. Thus, higher levels of lyso-phospholipids in RBCs could result from lower GPx4 activity. The anti-correlations between GPx4 abundance and CFH and lyso-phospholipids also suggest that GPx4 is an active enzyme in human RBCs.
3.4. Validation of the antibody for Western blot analysis of GPx4 in RBCs
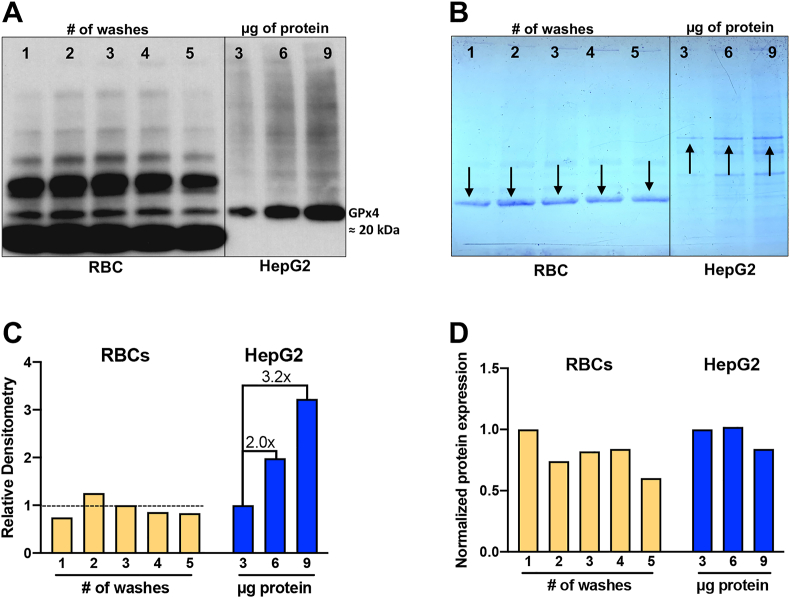
To examine if GPx4 is active as a peroxidase enzyme we employed western blotting as a probe for protein abundance in conjunction with an activity assay specific for GPx4. To validate the use of the anti-GPx4 antibody in samples of RBCs probed by western analysis, we compared results from RBC samples to the results from HepG2 cells. We have previously validated this antibody for use with samples from HepG2 cells grown and prepared with the same protocols [19]. We observed that loss of GPx4 protein due to washing of RBC samples is minimal, even after 5 washes (Fig. 4A). The bands of GPx4 appeared at approximately 20 kDa, consistent with the molecular weight of the cytosolic form of the enzyme (19.5 kDa) and information from the supplier of the antibody. In samples from HepG2 cells, there was an expected increase in the density of the GPx4-band as more protein as loaded (Fig. 4A). The bands for GPx4 from RBCs showed variation, suggesting that some protein was lost with repeated washing. Off-target bands were observed that could represent nonspecific binding of the antibody but are more likely due to one or more oxidases in the RBCs that activate the electro-chemiluminescence compound, generating the observed bands.
Fig. 4.
Validation of GPx4 antibody and loading control for Western blot analysis of RBCs.
(A) Western blot of samples from RBCs and HepG2 cells. Approximately 4 × 108 RBCs from one subject were washed 1–5 times and 10 μg of protein from each wash was loaded onto the gel. After 3 washes there may be a small loss of GPx4 protein. This is to be expected as GPx4 is non-covalently bound to membranes [33]. HepG2 lysate, as a positive control, shows an increase in GPx4 as more cellular protein is loaded.
(B) The Coomassie-stained gel shows that the intensity of bands corresponds to the amount of protein loaded. Appropriate bands were selected (marked by arrows) for the RBCs and HepG2 cells to perform densitometry measurements, panel C·
(C) Quantification of the Coomassie bands for RBCs shows that the amount of protein loaded in each of the 5 lanes is approximately the same. The Coomassie stain for the HepG2 samples shows the expected increase in band density with increasing amounts of protein loaded (i.e., 3, 6, and 9 μg of protein loaded would be expected to have relative intensities of 1x, 2x, and 3x, respectively). The observed intensities are as anticipated.
(D) The intensity of GPx4 bands (panel A) were normalized to the intensities of the Coomassie-stained bands. An apparent decrease of RBC GPx4 was observed with increasing numbers of washes. Because increasing amounts of protein were loaded for the HepG2 positive control samples, the relative GPx4 expression is similar for all three samples, as expected.
A Coomassie stain was used to visualize the amount of protein loaded on the gel (Fig. 4B). The Coomassie bands were used to determine if the amount of protein loaded correlated with the amount of protein observed in the gel. This is important because it was unclear if loading a certain mass of protein was the correct approach for Western blot analysis of RBCs.
Quantification of the Coomassie bands was performed using ImageJ (Fig. 4C). The densitometry reading of the protein bands was determined and normalized. For the RBCs, wash #3 was used to normalize the RBC bands. For HepG2 cells, the 3-μg lane was used for normalization. RBC samples equivalent to 10 μg of protein were loaded for all of the washes. This amount would be expected to generate equal Coomassie staining of the bands. As a positive control, an increasing amount of protein from the HepG2 cells was loaded (3, 6, and 9 μg) to generate expected relative band intensities of 1x, 2x, and 3x, respectively. The measured relative intensities were, 1.0x, 2.0x and 3.2x, closely reflecting the expected intensities. These results validated the approaches used to detect GPx4 antigenic protein in RBCs.
Ideal ratios of protein/loading control are 1, as shown for the HepG2 samples. This means that the band intensity of GPx4 increases parallel to the amount of protein loaded onto the gel. Upon normalization of the GPx4 band for RBCs in the Coomassie-stained gel, a decreased ratio was observed after wash #4 suggesting that a small amount of GPx4 protein may have been lost (Fig. 4D).
These data validate the use of the antibody for the detection of GPx4 antigenic protein in RBCs.
3.5. GPx4 is an active enzyme in mature red blood cells
To measure the activities of GPx1 and GPx4, a kinetic UV–Vis experimental setup is typically used. We used an assay that measures the activities of both GPx1 and GPx4 in a single sample [19]. The activity of GPx1, which is considerably greater in RBCs than GPx4, can be used as a control to determine the reliability of the determination of GPx4 activity. If GPx1 activity is not within the expected range, the GPx4 activity measurement is assumed to be invalid, probably due to a technical issue in sample preparation or running the assay.
The combined GPx activity assay relies on the oxidation of NADPH, which is the source for electrons needed to recycle GPxs. Recycling of GPxs is required after it reduces (phospholipid) hydroperoxides to their alcohol equivalent. NADPH is oxidized as GPx is recycled; its loss is observed in a UV–Vis spectrophotometer at 340 nm. To apply this methodology to RBCs, it was necessary to overcome the technical challenge of very high concentrations of hemoglobin (intracellular levels of ≈5 mM; 20 mM in heme), which interferes with spectrophotometry. Due to hemoglobin, the UV–Vis spectrum of RBC lysates has an intense absorbance at approximately 415 nm, referred to as the Soret band, ε415 = 5.2 × 105 M−1 cm−1. The which is considerably greater than the extinction coefficient of NADPH at its peak of 340 nm, ε340 = 6.3 × 103 M−1 cm−1. Because of its absorbance at 340 nm, hemoglobin must be minimized in samples to allow detection of NADPH at 340 nm. Optimization and validation of the combined GPx activity assay for RBCs was performed by determining linearity of activity vs. cell number (Supplemental Fig. S4).
We used the optimized assay to explore if the GPx4 antigenic protein found in human RBCs is functional, i.e. does this protein have the expected enzyme activity of GPx4? Activity of GPx4 is required to reduce phospholipid hydroperoxides (PLOOH) to their alcohol equivalents (PLOH) (Fig. 5A). Upon oxidation of the enzyme, two molecules of GSH supply the two electrons needed to recycle GPx4, forming GSSG. This reaction is essential to efficiently blunt the damaging chain reactions of lipid peroxidation.
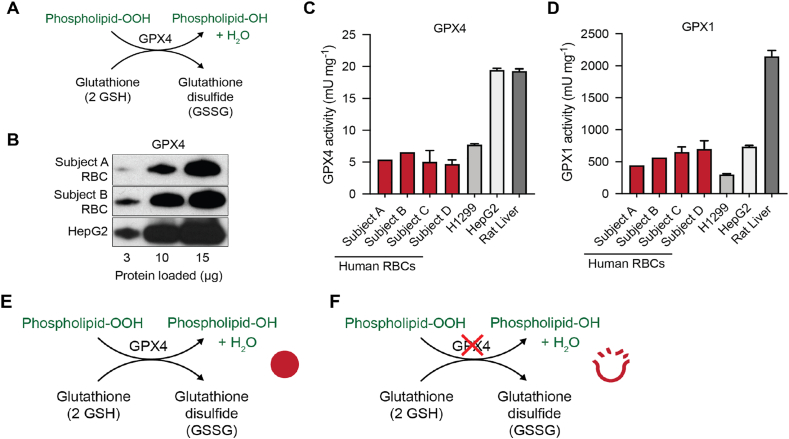
Fig. 5.
RBCs have GPx4 protein that has the classic peroxidase activity of GPx4.
(A) Schematic of the peroxidase activity of GPx4.
(B) GPx4 protein expression detected by Western blot analysis of RBCs and HepG2 cell lysates. The expected increase in intensity is observed with increasing amounts of protein loaded.
(C) RBCs have lower activity of GPx4 compared to human lung carcinoma (H1299), human hepatocellular carcinoma (HepG2) cell lines, and rat liver. Error bars (standard deviation) represent replicates of a single sample ran >3 times in our combined assay for GPx1 and GPx4 activities.
(D) GPx1 activity of RBCs was comparable to human carcinoma cell lines, but lower than rat liver. The observed activity of GPx4 is approximately 1% of that of GPx1 in each of the samples, although absolute comparison of measured activities of GPx4 and GPx1 is not possible due to different kinetics in the activity assays. Note the scales for the ordinates of panels C and D differ by a factor of 100. (See Supplementary Data for a discussion of the kinetics of the combination assay.) Error bars (standard deviation) represent replicates of a single sample ran >3 times in our combined assay for GPx1 and GPx4 activities. The subjects, A – D, in this figure are different from subjects #1 – #4 in Fig. S4.
(E) GPx4 may prevent potential oxidative damage of RBCs by removing phospholipid hydroperoxides.
(F) Reduced concentration and activity of GPx4 could lead to increased oxidative damage by the reactions of phospholipid-OOH, leading to increased rates of hemolysis during storage of RBCs.
The copious amount of hemoglobin in RBCs is a major interference for determining the presence of GPx4. RBCs were therefore lysed and centrifuged to remove hemoglobin. The resulting pellet representing the membrane fraction was used to determine the presence of GPx4 protein. To demonstrate the presence of GPx4, immunoblots were loaded with varying amounts of total protein from the membrane fraction (See Fig. 4 for validation of the antibody for western blotting for GPx4 in RBCs.). We observed a corresponding increase in the intensity of the band for GPx4 with increasing amounts of total protein loaded (Fig. 5B). A lysate of HepG2 cells was used as a positive control for Western blot detection of GPx4 protein. A similar increase in the intensity of the bands for GPx4 was observed with increasing amounts of total protein loaded (Fig. 5B). These observations strongly support the presence of GPx4 protein in RBCs.
Enzyme activity assays were performed to confirm the presence of functional GPx4 in RBCs. Results were compared to assays of human cell lines and rat liver (Fig. 5C). Samples from a total of four human subjects were analyzed to provide a survey of GPx4 activity in RBCs. As expected, the activity of GPx4 in RBCs is low compared to that of liver-derived cell lines and liver tissue. However, enzyme activity for GPx4 in RBCs was observed well above the limit of detection of the assay (Fig. 5C).
To confirm the comparatively low activity of GPx4, we measured the activity of GPx1 in RBCs, two human cell lines, and rat liver (Fig. 5D). As expected, considerably greater GPx1 activity was observed in liver tissue compared to our membrane preparations of RBCs. Although a precise, quantitative comparison of the enzyme activities of GPx1 and GPx4 is not possible due to differing enzyme kinetics, the observed activity of GPx4 is approximately 1% of that of GPx1 in each of the different samples. Furthermore, the removal of hemoglobin as described above will also reduce the amount of GPx1 available. GPx1 is mainly localized in the cytosol of cells of which a large fraction was removed. Therefore, our data may be an underestimation of the actual GPx1 activity. This hypothesis is consistent with the data of Bryk and Wisńiewski on the proteome of RBCs in which the copy number for GPx4 protein is about 5% of that of GPx1, each as monomers [16]. (See Supplementary Data for a discussion of the kinetics of the combination assay.) Together, these data indicate that the GPx4 antigenic protein we detected in mature RBCs is an active peroxidase (Fig. 5).
4. Discussion
RBCs maintain homeostasis despite constant exposure to reactive oxygen species both in vivo and during blood bank storage, but the mechanistic details of how RBCs protect themselves from oxidative damage are incompletely defined [34]. While many biomolecules in RBCs have the potential to be oxidatively damaged, lipid oxidation has recently been shown to be particularly detrimental to RBC homeostasis [35]. Our study indicates that functional GPx4 is present in RBCs and likely plays a role in blood bank storage by protecting RBCs from oxidative damage to lipids. The presence of GPx4 protein in mature RBCs has now been demonstrated by both immunoblot and mass spectrometry proteomics. Furthermore, our ex vivo enzymology experiments have shown that GPx4 is an active enzyme in mature RBCs, with the capacity to catalyze glutathione-dependent reduction of phospholipid hydroperoxides to their corresponding phospholipid alcohols (Fig. 2, Fig. 3). The antioxidant function of GPx4 in RBCs is further supported by multi-omic analyses that correlated or anti-correlated GPx4 with metabolically-related biomolecules such as complement factor H (CFH) and lysophospholipids. Importantly, the abundance of GPx4 is highly heritable and varies at least 4-fold across subjects; this variance in the abundance of GPx4 anti-correlates with the rate of hemolysis of RBC during storage.
GPx4 activity plays a key role in the biology of cancer cells by regulating ferroptosis, a type of cell death triggered by lipid oxidation [[11], [12], [13], [14]]. GPx4 is a focal point for ferroptosis because it catalyzes the reduction of lipid hydroperoxides into non-toxic lipid alcohols. Small molecule GPx4 inhibitors can cause cancer cell death; but, for unclear reasons, cancer cell lines exhibit variable sensitivity to GPx4 inhibition [36]. The variability can be partially attributed to differences in a recently described coenzyme Q (CoQ)-dependent pathway mediated by ferroptosis suppressor protein 1 (FSP1) that acts in parallel with GPx4 [37]. However, genetically-driven differences in GPx4 abundance, as occurs in RBCs, could also play a role in tumor sensitivity to the inhibition of GPx4. Our findings that abundance of GPx4 in RBCs is 75% heritable and varies at least 4-fold across RBCs from a small cohort of individuals suggest that similar person-to-person variability in GPx4 could be present in other tissues as well as neoplasms. These genetically-determined differences in GPx4 have the potential to modulate the effects of GPx4 inhibitors, including both on-target ferroptosis in tumor cells and off-target hemolysis in RBCs.
In addition to this focused investigation of GPx4 in RBCs, our study defines a list of heritable RBC proteins and metabolites correlated or anti-correlated with RBC storage hemolysis, adding to the growing resource provided by our twin study of RBC donors. Our results:
-
1.
Provide a set of new candidate biomarkers for predicting rates of storage hemolysis;
-
2.
Identify biochemical pathways that could be enhanced or inhibited to reduce storage hemolysis; and
-
3.
Advance our understanding of the biological mechanisms that underlie RBC storage hemolysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Nick Rose, Joseph Connor, John Weiss, Fatima Aldarweesh, and Eric Destrampe for their helpful comments. This work was supported by a MSTP physician-scientist preceptorship via NIH MSTP T32GM008692, 2UL 1TR000442, P01 CA217797, R01 CA169046, R01 GM073929, NIH P30 ES005605, and P42 ES013661. The ESR Facility at The University of Iowa provided invaluable support, supported in part by P30 CA086862.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.102073.
Contributor Information
Thomas J. Raife, Email: TRaife@uwhealth.org.
Garry R. Buettner, Email: Garry-Buettner@uiowa.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Carson J.L., Guyatt G., Heddle N.M., Grossman B.J., Cohn C.S., Fung M.K., Gernsheimer T., Holcomb J.B., Kaplan L.J., Katz L.M., Peterson N., Ramsey G., Rao S.V., Roback J.D., Shander A., Tobian A.A. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. J. Am. Med. Assoc. 2016;316:2025–2035. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 2.Kanias T., Lanteri M.C., Page G.P., Guo Y., Endres S.M., Stone M., Keating S., Mast A.E., Cable R.G., Triulzi D.J., Kiss J.E., Murphy E.L., Kleinman S., Busch M.P., Gladwin M.T. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv. 2017;1:1132–1141. doi: 10.1182/bloodadvances.2017004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van 't Erve T.J., Wagner B.A., Martin S.M., Knudson C.M., Blendowski R., Keaton M., Holt T., Hess J.R., Buettner G.R., Ryckman K.K., Darbro B.W., Murray J.C., Raife T.J. The heritability of hemolysis in stored human red blood cells. Transfusion. 2015;55:1178–1185. doi: 10.1111/trf.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisenhorn E.M., van ’t Erve T.J., Riley N.M., Hess J.R., Raife T.J., Coon J.J. Multi-omics evidence for inheritance of energy pathways in red blood cells. Mol. Cell. Proteomics. 2016;15:3614–3623. doi: 10.1074/mcp.M116.062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van 't Erve T.J., Wagner B.A., Martin S.M., Knudson C.M., Blendowski R., Keaton M., Holt T., Hess J.R., Buettner G.R., Ryckman K.K., Darbro B.W., Murray J.C., Raife T.J. The heritability of metabolite concentrations in stored human red blood cells. Transfusion. 2014;54:2055–2063. doi: 10.1111/trf.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van 't Erve T.J., Doskey C.M., Wagner B.A., Hess J.R., Darbro B.W., Ryckman K.K., Murray J.C., Raife T.J., Buettner G.R. Heritability of glutathione and related metabolites in stored red blood cells. Free Radic. Biol. Med. 2014;76:107–113. doi: 10.1016/j.freeradbiomed.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleinman S., Busch M.P., Murphy E.L., Shan H., Ness P., Glynn S.A. The national heart, lung, and blood Institute recipient epidemiology and donor evaluation study (REDS-III): a research program striving to improve blood donor and transfusion recipient outcomes. Transfusion. 2014;54:942–955. doi: 10.1111/trf.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ursini F., Maiorino M., Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim. Biophys. Acta Gen. Subj. 1985;839:62–70. doi: 10.1016/0304-4165(85)90182-5. [DOI] [PubMed] [Google Scholar]
- 9.Clemens M.R., Waller H.D. Lipid peroxidation in erythrocytes. Chem. Phys. Lipids. 1987;45:251–268. doi: 10.1016/0009-3084(87)90068-5. [DOI] [PubMed] [Google Scholar]
- 10.Yant L.J., Ran Q., Rao L., Van Remmen H., Shibatani T., Belter J.G., Motta L., Richardson A., Prolla T.A. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan V.S., Ryan M.J., Dhruv H.D., Gill S., Eichhoff O.M., Seashore-Ludlow B., Kaffenberger S.D., Eaton J.K., Shimada K., Aguirre A.J., Viswanathan S.R., Chattopadhyay S., Tamayo P., Yang W.S., Rees M.G., Chen S., Boskovic Z.V., Javaid S., Huang C., Wu X., Tseng Y.Y., Roider E.M., Gao D., Cleary J.M., Wolpin B.M., Mesirov J.P., Haber D.A., Engelman J.A., Boehm J.S., Kotz J.D., Hon C.S., Chen Y., Hahn W.C., Levesque M.P., Doench J.G., Berens M.E., Shamji A.F., Clemons P.A., Stockwell B.R., Schreiber S.L. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascon S., Hatzios S.K., Kagan V.E., Noel K., Jiang X., Linkermann A., Murphy M.E., Overholtzer M., Oyagi A., Pagnussat G.C., Park J., Ran Q., Rosenfeld C.S., Salnikow K., Tang D., Torti F.M., Torti S.V., Toyokuni S., Woerpel K.A., Zhang D.D. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H., Schreiber S.L., Stockwell B.R. Targeting dependency on the GPX4 lipid peroxide repair pathway for cancer therapy. Biochemistry. 2018;57:2059–2060. doi: 10.1021/acs.biochem.8b00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., Brown L.M., Girotti A.W., Cornish V.W., Schreiber S.L., Stockwell B.R. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canli O., Alankus Y.B., Grootjans S., Vegi N., Hultner L., Hoppe P.S., Schroeder T., Vandenabeele P., Bornkamm G.W., Greten F.R. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood. 2016;127:139–148. doi: 10.1182/blood-2015-06-654194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryk A.H., Wiśniewski J.R. Quantitative analysis of human red blood cell proteome. J. Proteome Res. 2017;16:2752–2761. doi: 10.1021/acs.jproteome.7b00025. [DOI] [PubMed] [Google Scholar]
- 17.Ursini F., Heim S., Kiess M., Maiorino M., Roveri A., Wissing J., Flohe L. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285:1393–1396. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- 18.Zeeberg B.R., Feng W., Wang G., Wang M.D., Fojo A.T., Sunshine M., Narasimhan S., Kane D.W., Reinhold W.C., Lababidi S., Bussey K.J., Riss J., Barrett J.C., Weinstein J.N. GoMiner: a resource for biological interpretation of genomic and proteomic data. Genome Biol. 2003;4:R28. doi: 10.1186/gb-2003-4-4-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stolwijk J.M., Falls-Hubert K.C., Searby C., Wagner B.A., Buettner G.R. Simultaneous detection of the enzyme activities of GPx1 and GPx4 guide optimization of selenium in cell biological experiments. Redox Biology. 2020;32:101518. doi: 10.1016/j.redox.2020.101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Brodsky R.A. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124:2804–2811. doi: 10.1182/blood-2014-02-522128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Alessandro A., Kriebardis A.G., Rinalducci S., Antonelou M.H., Hansen K.C., Papassideri I.S., Zolla L. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55:205–219. doi: 10.1111/trf.12804. [DOI] [PubMed] [Google Scholar]
- 23.Roback J.D. Perspectives on the impact of storage duration on blood quality and transfusion outcomes. Vox Sang. 2016;111:357–364. doi: 10.1111/vox.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heddle N.M., Cook R.J., Arnold D.M., Liu Y., Barty R., Crowther M.A., Devereaux P.J., Hirsh J., Warkentin T.E., Webert K.E., Roxby D., Sobieraj-Teague M., Kurz A., Sessler D.I., Figueroa P., Ellis M., Eikelboom J.W. Effect of short-term vs. Long-term blood storage on mortality after transfusion. N. Engl. J. Med. 2016;375:1937–1945. doi: 10.1056/NEJMoa1609014. [DOI] [PubMed] [Google Scholar]
- 25.Steiner M.E., Ness P.M., Assmann S.F., Triulzi D.J., Sloan S.R., Delaney M., Granger S., Bennett-Guerrero E., Blajchman M.A., Scavo V., Carson J.L., Levy J.H., Whitman G., D'Andrea P., Pulkrabek S., Ortel T.L., Bornikova L., Raife T., Puca K.E., Kaufman R.M., Nuttall G.A., Young P.P., Youssef S., Engelman R., Greilich P.E., Miles R., Josephson C.D., Bracey A., Cooke R., McCullough J., Hunsaker R., Uhl L., McFarland J.G., Park Y., Cushing M.M., Klodell C.T., Karanam R., Roberts P.R., Dyke C., Hod E.A., Stowell C.P. Effects of red-cell storage duration on patients undergoing cardiac surgery. N. Engl. J. Med. 2015;372:1419–1429. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacroix J., Hébert P.C., Fergusson D.A., Tinmouth A., Cook D.J., Marshall J.C., Clayton L., McIntyre L., Callum J., Turgeon A.F., Blajchman M.A., Walsh T.S., Stanworth S.J., Campbell H., Capellier G., Tiberghien P., Bardiaux L., van de Watering L., van der Meer N.J., Sabri E., Vo D. Age of transfused blood in critically ill adults. N. Engl. J. Med. 2015;372:1410–1418. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 27.Bordbar A., Johansson P.I., Paglia G., Harrison S.J., Wichuk K., Magnusdottir M., Valgeirsdottir S., Gybel-Brask M., Ostrowski S.R., Palsson S., Rolfsson O., Sigurjónsson O.E., Hansen M.B., Gudmundsson S., Palsson B.O. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion. 2016;56:852–862. doi: 10.1111/trf.13460. [DOI] [PubMed] [Google Scholar]
- 28.Donadee C., Raat N.J., Kanias T., Tejero J., Lee J.S., Kelley E.E., Zhao X., Liu C., Reynolds H., Azarov I., Frizzell S., Meyer E.M., Donnenberg A.D., Qu L., Triulzi D., Kim-Shapiro D.B., Gladwin M.T. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn N., Knorr D.Y., Liebig J., Wüstefeld L., Peters K., Büscher M., Bucher G., Ehrenreich H., Heinrich R. The insect ortholog of the human orphan cytokine receptor CRLF3 is a neuroprotective erythropoietin receptor. Front. Mol. Neurosci. 2017;10:223. doi: 10.3389/fnmol.2017.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taira T., Saito Y., Niki T., Iguchi-Ariga S.M., Takahashi K., Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weismann D., Hartvigsen K., Lauer N., Bennett K.L., Scholl H.P., Charbel Issa P., Cano M., Brandstätter H., Tsimikas S., Skerka C., Superti-Furga G., Handa J.T., Zipfel P.F., Witztum J.L., Binder C.J. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parthasarathy S., Steinbrecher U.P., Barnett J., Witztum J.L., Steinberg D. Essential role of phospholipase A2 activity in endothelial cell-induced modification of low density lipoprotein. Proc. Natl. Acad. Sci. U. S. A. 1985;82:3000–3004. doi: 10.1073/pnas.82.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cozza G., Rossetto M., Bosello-Travain V., Maiorino M., Roveri A., Toppo S., Zaccarin M., Zennaro L., Ursini F. Glutathione peroxidase 4-catalyzed reduction of lipid hydroperoxides in membranes: the polar head of membrane phospholipids binds the enzyme and addresses the fatty acid hydroperoxide group toward the redox center. Free Radic. Biol. Med. 2017;112:1–11. doi: 10.1016/j.freeradbiomed.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 34.D'Alessandro A., Hansen K.C., Eisenmesser E.Z., Zimring J.C. Protect, repair, destroy or sacrifice: a role of oxidative stress biology in inter-donor variability of blood storage? Blood Transfus. 2019;17:281–288. doi: 10.2450/2019.0072-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howie H.L., Hay A.M., de Wolski K., Waterman H., Lebedev J., Fu X., Culp-Hill R., D'Alessandro A., Gorham J.D., Ranson M.S., Roback J.D., Thomson P.C., Zimring J.C. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv. 2019;3:2272–2285. doi: 10.1182/bloodadvances.2019000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou Y., Palte M.J., Deik A.A., Li H., Eaton J.K., Wang W., Tseng Y.Y., Deasy R., Kost-Alimova M., Dancik V., Leshchiner E.S., Viswanathan V.S., Signoretti S., Choueiri T.K., Boehm J.S., Wagner B.K., Doench J.G., Clish C.B., Clemons P.A., Schreiber S.L. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 2019;10:1617. doi: 10.1038/s41467-019-09277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R., Bassik M.C., Nomura D.K., Dixon S.J., Olzmann J.A. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.