Graphical abstract
Keywords: Pasteurization, Microfiltration, Ultrasonication, Shelf life, Proteomics, Skim milk
Highlights
-
•
Ultrasonication was synergistically combined with microfiltration for milk processing.
-
•
Ultrasonication further extended the shelf life of microfiltered skim milk.
-
•
Ultrasonication above 1296 J/mL changed the proteome of milk serum.
-
•
Microfiltration combined with ultrasonication retains ˃80% bioactive proteins.
Abstract
To extend the shelf life and retain bioactive proteins in milk, this study utilized microfiltration (MF) combined with ultrasonication to treat skim milk and investigated its efficiency in removing bacteria and retaining bioactive proteins compared with HTST pasteurization and microfiltration alone. Results showed that microfiltration combined with ultrasonication at 1296 J/mL could completely remove the bacteria in skim milk. Ultrasonication further extended the shelf life (4 °C) of microfiltered skim milk, which could reach at least 40 days when MF was combined with ˃1296 J/mL ultrasonication. In addition, ELISA showed that HTST pasteurization significantly decreased the levels of IgG by ~30%, IgA by ~ 50%, IgM by ~60%, and lactoferrin by ~40%, whereas the activity of the enzymes lactoperoxidase and xanthine oxidase were also decreased by ~ 20%. Compared with HTST, MF alone or combined with ultrasonication retained these bioactive proteins to a larger degree. On the other hand, proteomics indicated both damage to casein micelle and fat globule structures in milk when ultrasonication at >1296 J/mL was applied, as shown by increases in caseins and milk fat globular proteins. Simultaneously, this ultrasound intensity also decreased levels of bioactive proteins, such as complement factors. Taken together, this study provided new insights that may help to implement this novel combination of non-thermal technologies for the dairy industry aimed at improving milk quality and functionality.
1. Introduction
Bovine milk is an important source of nutrients for human beings. In addition to nutrition, bioactive proteins in milk, including xanthine oxidase (XO), immunoglobulins (Igs), lactoperoxidase (LPO), and lactoferrin (LTF), play an important role in regulation, transport, catalysis, fatty acid binding, antibacterial activity, and other biological functions [1], [2]. Recently, Abbring, Xiong, Diks, Baars, Garssen, Hettinga and van Esch [3], for example, reported the allergy-protective capacity and immunologically activity of raw milk whey proteins.
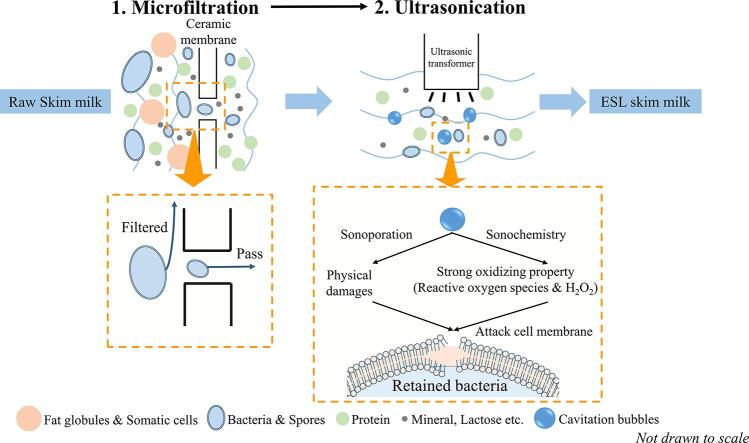
Thermal treatments, such as spray-drying, ESL, UHT, are widely used to realize the microbial safety, and thereby aim to extend the shelf life of milk products. However, more and more studies showed that many bio-active components are damaged during such heat treatments, although HTST pasteurization would retain relatively more bioactive components than more intense heat treatments [4], [5]. In dairy industry, based on the microbial quality of the raw milk, its shelf life could be only extended to approximately 7–14 days by HTST pasteurization (72–75 °C for 12–15 s), which limits the development of pasteurized milk products in Asian and other developing regions, where logistics and sales would require dairy products to have a longer shelf life. In the past few decades, prolonging the shelf life of pasteurized milk has been extensively studied, for example by the use of microfiltration alone or combined with thermal treatments [6], [7]. Until now, non-thermal treatments are still quite new for milk processing. Among non-thermal techniques, UV-C, ultrasonication, and high pressure processing are the most promising alternatives, and recently several studies have reported the ability of these non-thermal treatments to sufficiently kill relevant bacteria [8], [9].
Microfiltration (MF), a pore size of 1.4 µm, has been widely applied in dairy industry, since it can remove bacteria, somatic cells and some spores from skim milk without damaging other components [10], [11]. The efficiency of MF in removing bacteria relies on the applied pore size, and a pore size of 1.4 µm is able to realize an optimal balance between removal of bacteria and membrane flux, with little effects on the level of proteins, mineral and lactose [7].
Even though MF membranes are efficient in removal of bacteria (achieving a 3–6 log reduction) and spores, MF cannot make sure a 100% removal of pathogens [12]. Thus, MF was usually used as pretreatment before pasteurization or another thermal treatment, to ensure the safety of dairy products. There have been a lot of reports on the shelf life of milk processed by the combination of microfiltration and heat treatment, however, few studies focused on the combination of MF with other non-thermal treatments, which could have great potential for both extending shelf life and retaining bioactive protein. In our previous study, a MF system combined with UV-C was developed and showed a satisfactory result in killing bacteria and retaining bio-active proteins [13]. Ultrasonication, as a new technique in milk processing, would kill bacteria or viruses by generating strong acoustic cavitation and shearing [14] and may therefore be useful in combination with microfiltration. However, the efficiency of MF combined with ultrasonication in improving microbial and protein quality has not been reported yet. To guarantee microbial safety, generally a higher ultrasonication power or prolonged treatment time is needed, which might damage the heat-sensitive proteins and may bring off-flavors in milk [15], [16]. Therefore, studies on how to ensure the microbial inactivation while avoiding adverse effects on bioactive proteins are needed. This study therefore aimed to improve the quality of skim milk by combining microfiltration with different intensity of ultrasonication treatments and investigated the changes of bioactive protein and microbial shelf life. A proteomics approach based LC-MS/MS was used to analyze the changes of whey proteins as caused by different processes applied. This study could provide new ideas for extending the shelf life of skim milk as well as retaining the bioactive proteins, thereby facilitating the understanding on the application of ultrasonication in dairy processing.
2. Material and methods
2.1. Skim milk preparation and experimental design
Fifty Liters of fresh raw milk was sampled from a local dairy factory in a sterilized plastic container. The whole milk was skimmed by a disc stack centrifuger (CLARA 20, Alfa Laval, Sweden) at room temperature until the fat content was below 0.1% (fat content was determined according to AOAC method 989.05 [17]). After that, the skim milk was kept in a stainless steel tank below 6 °C until subsequent processing. Skim milk was then treated with HTST pasteurization, microfiltration, or microfiltration combined with ultrasonication treatment. Samples were collected into sterile plastic bottles after each process and cooled to approximately 6 °C on ice immediately. All the treatments were completed within 10 h.
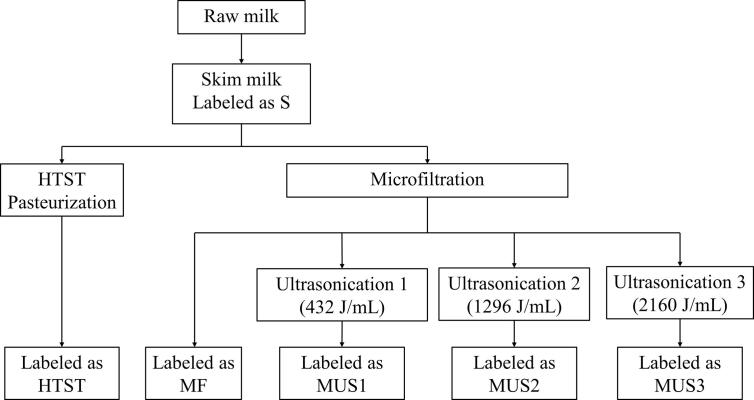
Part of the skim milk was used for the protein analyses, while another part was put into 15 mL sterile bottles (polypropylene) under high-hygiene conditions and stored at 4 °C for the shelf-life study. On first day of treatments, 3 bottles were randomly selected from each treatment group for the determination of bacterial count, coliform count, and spore count. During the shelf life, 3 bottles were randomly selected to determine bacteria count every 4 days, whereas IgG and LTF were quantified every 8 days. The workflow of the experiments is shown in Fig. 1.
Fig. 1.
Workflow and schematic for skim milk processes.
2.2. Skim milk processes
2.2.1. HTST pasteurization
The 10 L skim milk was HTST (72 °C-15 s) treated using a UHT/HTST heat exchanger system (Power Point International, Toda-Shi, Japan), and aseptically filled into sterilized bottles.
2.2.2. Microfiltration
For MF treatment, a laboratory scale microfiltration unit (GCM-C-03, Guochu Technology, Fujian, China) equipped with three tubular ceramic membranes (TAMI Industries, Nyons, France) was used. The pore size and length of membranes were 1.4 µm and 500 mm respectively, and the total membrane area was 0.312 m2. During the microfiltration process, the temperature of the skim milk was kept between 45 and 50 °C, the cross-flow velocity and the transmembrane pressure (TMB) were 7.0 m/s and 75 kPa respectively. After each run, the membrane was immediately rinsed with deionized water at 60 °C for 20 min, then rinsed with 2% NaOH (w/v) at 80 °C for 30 min, and then neutralized with deionized water at room temperature. After that, 1% HNO3 (v/v) was used to rinse at 50 °C for 30 min, and the whole microfiltration system was neutralized again with deionized water.
2.2.3. Ultrasonication
Ultrasonication was performed using an ultrasonic processor (JY99-IIDN, Ningbo Scientz Biotechnology Co., Ltd., China) with a probe of 20 mm (diameter), and the probe was kept 3 cm below the milk surface. A sample of 300 mL skim milk after the microfiltration process was placed in a 400 mL glass beaker, which was incubated in a 1 L double jacketed beaker filled with circulating water of 40 °C [9], [18]. The treatments were performed in intermittent pulse mode, with an output power of 720 W, pulse time of 5 s, intermittent time of 5 s, and a total ultrasonic time of 3 min, 9 min, or 15 min. The temperature of the samples was monitored and did not exceed 60 °C during ultrasonication. The energy density was used to calculate the ultrasonication intensity according to the following formula [19]:
where D represents energy density (J/mL), Pd represents output power (W), t represents treating time (s), and V represents sample volume (mL). The energy densities of the ultrasonication used in this research were 432, 1296, and 2160 J/mL, corresponding to a treatment time of 3, 9, and 15 min, respectively.
2.3. Microbiological analysis
After each process, total bacteria count (TBC) and coliform count of samples were determined as described by Zhang, Liu, Li, Xu and Zhou [13]. The bacterial count was recorded in Mean ± SD. Spores count was determined as described by Fritsch and Moraru [20]. All the vegetative bacteria in skim milk were inactivated by heating at 80 °C for 12 min, then determined using the same method as for TBC as described above.
2.4. Determination of native milk serum and skim milk proteins
Caseins and denatured serum proteins were precipitated by adjusting the pH of skim milk to 4.6 with 1 M HCl [21], then samples were placed at 4 °C for an hour. After that, milk samples were ultracentrifuged (Optima L-80, Beckman Coulter, USA) at 33100 rpm for 90 min at room temperature to obtain milk serum samples. Based on the required sample amount and availability of samples (the amount of milk serum was limited while skim milk was available in large volumes), the protein concentration in skim milk and milk serum was determined by Kjeldahl apparatus (K1302, Sonnen Automated Analysis Instrument Co., Ltd., Shanghai, China) and Coomassie (Bradford) method, respectively.
2.5. SDS-PAGE of native milk serum protein
Milk serum was diluted five times with Milli-Q water to reach approximately 1 mg/mL and mixed with loading buffer containing 5% of β-mercaptoethanol. After heating at 100 °C for 3 min, 10 μL of the diluted sample was loaded on an SDS-PAGE kit gel (Cat. No. P0012A, Beyotime, China). The current setting, the staining and destaining methods were referred to the methods of Zhang, Liu, Li, Xu and Zhou [13].
2.6. Protein digestion for LC-MS/MS analysis
Proteolysis was carried out according to the method of Zhang, Liu, Li, Xu and Zhou [13]. Fifty microgram of milk protein was placed into low-binding Eppendorf tubes and mixed with 50 mM NH4HCO3 to a total volume of 100 µL. Then samples were reduced and alkylated by dithiothreitol and iodoacetamide, respectively. Next, 30 µg alkylated sample was pipetted on a Millipore Microcon centrifugal filter unit (YM-10) and centrifuge at 13,200 rpm for 30 min. After adjusting the pH to 8.0 with 50 mM NH4HCO3, trypsin was added to the samples at a ratio of 1:100 and the samples was incubated at 37 °C overnight. After digestion, the obtained peptides were desalted with an C18 tip (prepared in-house), and then evaporated with a vacuum centrifuger. The dry peptides were dissolved with 0.1% formic acid and 2% acetonitrile and centrifuged at 13,200 rpm at 4 °C for 10 min. The supernatant was finally transferred to new low-binding tubes for further analysis.
2.7. Protein identification by Nano-LC-MS/MS
An Easy-nLC1000 system (Thermo Fisher Scientific, USA) coupled with a Q Exactive™ Hybrid Quadrupole-Orbitrap™ Mass Spectrometer (Thermo Fisher Scientific, USA) with an ESI nanospray source was used to analyze the samples. The digested peptides (5 µL) were injected into a nano column (100 μm × 10 cm in-house made column packed with a reversed-phase) and then eluted using the ReproSil-Pur C18-AQ resin (3 μm, 120 Å, Dr. Maisch GmbH, Germany). The mobile phase A and B were 0.1% formic acid in water and acetonitrile respectively. Gradient elution with 4% to 10% B for 5 min, 10% to 22% B for 80 min, 22% to 40% B for 25 min, 40% to 95% B for 5 min, and 95% to 95% B for 5 min was used. For the mass spectrometry, the spray voltage was 2.2 kV and the capillary temperature was 270 °C. The MS resolution was 70,000 at 400 m/z and MS precursor m/z range was 300–1400; The MS/MS ion scan range was started from m/z 100, and the MS/MS scan range were set from 200 to 2000 m/z.
MaxQuant (1.6.2.10) was used to analyze the raw data files and the proteins were identified based on a bovine protein database (source: Uniprot UP000009136). The detailed parameters were listed below: fixed modifications were carbamidomethyl (C), variable modifications were oxidation (M) and acetylation (Protein N-term). The enzyme was set as trypsin, the maximum missed cleavages was set as 2, and the precursor ion mass tolerance and MS/MS tolerance were both set to 20 ppm. The length of peptides was set ˃7 amino acids. Proteins were identified as highly confident based on at least two distinct peptides: at least one unique and one unmodified. The proteomic data was further processed by Perseus 1.6.12. The label free quantification (LFQ) intensity was used to quantify the identified proteins. Differences with a p value < 0.05 were regarded as significantly different, using Permutation-based FDR correction of the p value.
2.8. Immunoglobulins and lactoferrin quantification
Igs (IgG, IgA, IgM) and LTF were determined by ELISA kits (Cat. No. E10- 118, 131, 101, and 126, Bethyl Laboratories, USA) based on a provided protocol and a previous study [9]. Each sample was determined in triplicates.
2.9. Lactoperoxidase and xanthine oxidase activity
LPO activity was determined according to a fluorescence method [22]. Each milliliter LPO assay reagent contained 23.1 μL dye solution, 4.6 μL KSCN solution and 972.3 μL 0.1 M PBS. After that, the 80-fold diluted milk sample and the LPO assay reagent were mixed at the ratio of 2:13 in volume, and then 225 μL mixture was added into a 96-well microplate. The microplate was incubated at 37 °C for 20 min in a microplate reader (CYTATION5, BioTek, America), after which 50 μL H2O2 was added to activate the reaction. The fluorescence intensity was measured under an excitation wavelength of 544 nm and an emission wavelength of 590 nm. Different concentrations of H2O2 were prepared as standard curve.
XO activity was also determined according to a fluorescence method [23]. The XO reaction reagent was prepared with 10 μL 10 mM AR, 4 μL 200 U/mL HRP, 40 μL 10 mM hypoxanthine, and 946 μL 0.1 M phosphate buffer (pH 7.4). Then, 50 μL of diluted milk samples was mixed with 50 μL of XO reaction reagent in a 96-well microplate, after which the fluorescence intensity was measured at 544/590 nm (excitation/emission) every 30 s for 30 min. Different concentrations of H2O2 were prepared as standard curve. The blank reagent was prepared by replacing hypoxanthine with distilled water.
2.10. Data analysis and visualization
Except for the proteomics data mentioned above, for the other data the average and standard deviation were calculated using Excel, one-way ANOVA was performed with SPSS 16.0, and differences with a p value < 0.05 was regarded as significant difference. Cluster analysis and visualization were performed with MeV 4.9.0 and GraphPad 8.0.
3. Results and discussion
3.1. Shelf life of skim milk
Table 1 shows the total protein content and microbial quality of skim milk after different treatments. Skim milk after different treatments all met the standards of FDA and China (GB) for pasteurized milk (Total bacterial count < 2 × 104 CFU/mL and < 5 × 104 CFU/mL, respectively; Coliform < 10 CFU/mL and < 5 CFU/mL, respectively). Larger reduction of total bacteria and spores were found in skim milk after microfiltration, compared with HTST. Similarly, previous studies reported that microfiltration could realize a reduction of vegetative bacteria from 3 to 6 log CFU/mL, and effectively remove spores [6], [20]. MF relies on physical filtration to remove microorganisms from milk, so the initial microbial load and morphological characteristics have a great impact on the filtration efficiency, which makes that MF can’t achieve complete removal of all bacteria and spores [12]. As shown in Table 1, there were 2.46 log CFU/mL of bacteria left in milk after MF. These retained bacteria were subsequently inactivated by ultrasonication (Tab.1). Fig. 2 displays the microbial quality during storage at 4 °C. This further reduction of bacteria by ultrasonication after MF in turn extended the shelf life of skim milk in a dose-dependent manner. Different from MF, the mechanical effects and reactive oxide species (ROS) resulting from ultrasound-induced acoustic cavitation had strong physicochemical (e.g. sonoporation and sonochemical) effects on the cell membrane structure and intracellular substances of most bacteria [24], being responsible for inactivating the bacteria, thereby extending the shelf-life of skim milk. Otherwise, applying ultrasound alone has been shown to require a relative long treatment time to inactivate desired numbers of bacteria [25], which may lead to damages to the bioactive milk proteins [9]. Therefore, the combination of MF and ultrasound appear to outperform either of these techniques alone.
Table 1.
Protein content and microbial quality of skim milk subjected to different processing methods.
| Protein concentration (mg/mL) | Bacteria count (log CFU/mL) | Coliform (log CFU/mL) | Spores (log CFU/mL) | |
|---|---|---|---|---|
| S | 36.0 ± 0.4a | 6.16 ± 0.02d | 2.42 ± 0.16 | 3.69 ± 0.01a |
| HTST | 35.7 ± 0.2a | 4.02 ± 0.09c | ND | 3.64 ± 0.01a |
| MF | 35.2 ± 0.1a | 2.46 ± 0.02b | ND | ND |
| MUS1 | 34.2 ± 0.3a | 1.73 ± 0.04a | ND | ND |
| MUS2 | 34.0 ± 1.0a | ND | ND | ND |
| MUS3 | 33.8 ± 0.9a | ND | ND | ND |
S: skim milk, HTST: HTST pasteurization, MF: microfiltration, MUS1, MUS2 and MUS3 represent microfiltration combination with ultrasonication (with energy density of 432, 1296 and 2160 J/mL) respectively. Different letters in the same column indicate significant difference (p < 0.05).
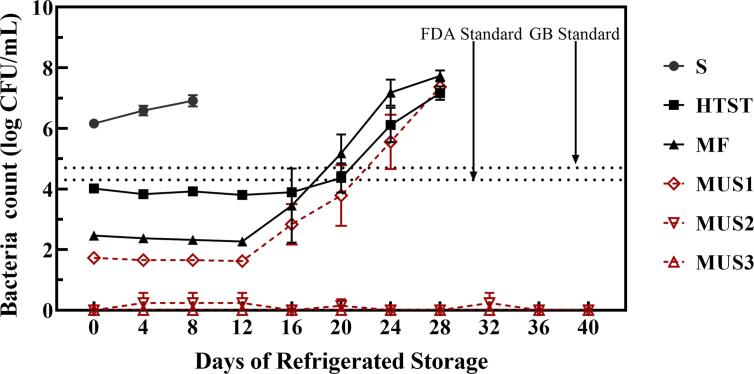
Fig. 2.
Bacteria count in skim milk after different treatments during shelf life. The black dashed lines indicate the China national standard (GB) and FDA standard for pasteurized milk. S: Skim milk; HTST: HTST pasteurization; MF: Microfiltration; MUS1, MUS2 and MUS3: Microfiltration combined with ultrasonication with different energy densities at 432, 1296 and 2160 J/mL, respectively.
During the shelf life, the bacteria count of untreated skim milk increased rapidly and exceeded the limit of raw milk (2 × 106 CFU/mL for GB standard) after 4 days. Although a lower microbial load was found in MF skim milk compared to HTST skim milk, the shelf life of MF skim milk was not significantly longer. However, compared with microfiltration alone, microfiltration combined with ultrasonication did extend the shelf life of skim milk, especially after ultrasonication at a higher intensity (MUS2 and MUS3). Shelf life of MUS1 treated milk was extended to ~ 20 days, and no noteworthy bacterial growth was found in MUS2 and MUS3 treated skim milk even after 40 days of storage.
It is worth noting that the microorganisms in HTST, MF and MUS1 skim milk were not completely inactivated, and they all grew rapidly during shelf life. Although the initial microbial load of MF milk was lower, the microbial load of MF milk increased much faster than HTST milk, which resulted in a similar shelf-life. This could be the result of the variety of bacteria that remained in skim milk after those treatments. There are lots of non‐pathogenic organisms in pasteurized milk, including bacterial spores, some spore‐formers such as Paenibacillus and some thermoduric non‐spore‐formers such as Coryneforms, micrococci, and some streptococci [26]. Due to the character of filtering microorganisms based on pore size, microfiltration therefore, may remove more thermoduric bacteria, including non‐spore‐former and spores, but can’t remove all types of organisms. Schmidt, Kaufmann, Kulozik, Scherer and Wenning [27] reported several major bacteria in microfiltrated milk, including heat-resistant high G + C (base pairs of gene) Gram-positive bacteria, obligate aerobic Gram-negative bacteria and lactic acid bacteria. This could be the main reason for the rapid growth of bacteria in MF and MUS1 treated milk during shelf life.
3.2. Concentration of native milk serum protein and SDS-PAGE
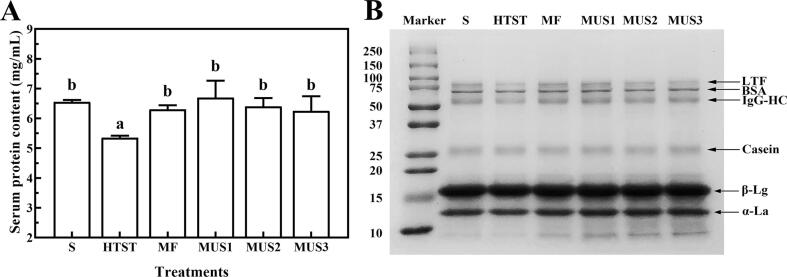
Fig. 3A displays the concentration and protein patterns of native milk serum proteins after different treatments. As shown in Fig. 3A, the concentration of native serum protein was decreased in HTST treated skim milk, while no significant change was found in MF and ultrasonication treated skim milks. As shown in Fig. 3B, β-Lg, α-La, BSA, LTF, and IgG-heavy chain (IgG-HC) were the major proteins in milk serum. In contrast to raw milk, the band intensity of BSA, β-Lg, and α-La of HTST treated skim milk remained the same as raw milk, however, the band intensity of IgG-HC and LTF were obviously decreased. During thermal treatment, LTF and IgG-HC easily aggregate with themselves, or with other whey proteins and caseins through covalent interactions, such as thiol-disulfide reactions, and were then removed during acid precipitation and ultracentrifugation as used during sample preparation [13]. MF, as a non-thermal processing method, didn’t decrease the major serum proteins as shown by SDS-PAGE. Moreover, the bands of LTF and IgG in MF combined with ultrasonication did not show an obvious decrease, which suggests that these proteins were not damaged by either MF or ultrasonication, as HTST did. The results of SDS-PAGE were in accordance with the concentration of native serum proteins. Similar results were found by Liu, Xiong, Kontopodi, Boeren, Zhang, Zhou and Hettinga [9], they which showed that whole milk treated with ultrasonication had no significant differences in the major serum proteins as observed by SDS-PAGE.
Fig. 3.
Protein concentration (A) and SDS-PAGE patterns (B) of native milk serum proteins after different treatments. S: Skim milk; HTST: HTST pasteurization; MF: Microfiltration; MUS1, MUS2 and MUS3: Microfiltration combined with ultrasonication with different energy densities at 432, 1296 and 2160 J/mL, respectively. Different letters on top of columns suggest significant differences (p < 0.05).
3.3. Identification and analysis of milk serum proteome
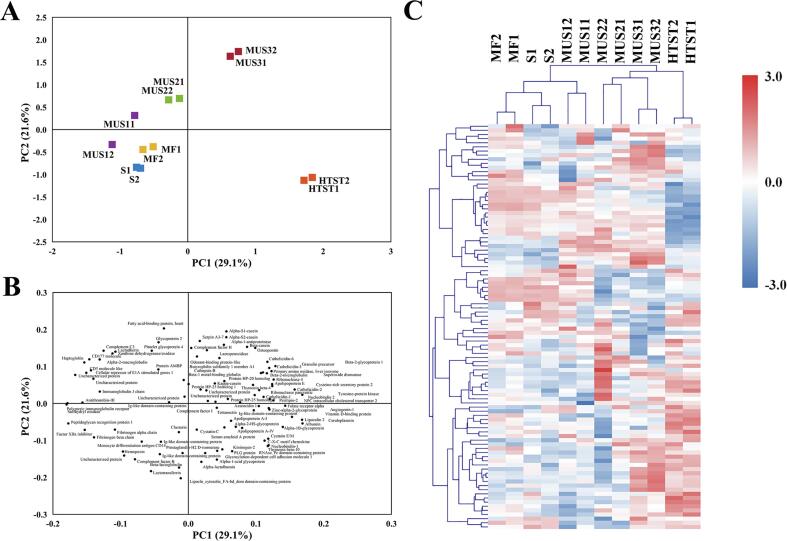
In this study, a total of 283 proteins were identified. Among them, 98 proteins commonly shared in all samples were used for further analysis. Fig. 4 shows the results of a PCA and cluster analysis of the shared quantified proteins after different treatments. The first two principal components (PCs) explained 50.7% of the total variance. As shown in the score plot (Fig. 4A), milk samples were clustered separately depending on their treatments. Among them, MF, MUS1 could not be distinguished from the S group by the first two PCs. HTST milk is separated from the raw milk in the direction of PC1, and MUS2 and MUS3 treated milks were separated in the direction of PC2. The loading plot is shown in Fig. 4B, and each black dot represents a quantified protein. According to this loading plot, protein with a high contribution to PC1 are mainly antithrombin-III (SERPINC1), folate receptor alpha (FOLR1), perilipin-2 (PLIN2), polymeric immunoglobulin receptor (PIGR), Immunoglobulin J chain (JCHAIN), Sulfhydryl oxidase (QSOX1), fibrinogen beta chain (FGA), while for PC2 this are mainly serpin A3-7 (SERPINA3-7), caseins, lactoferrin (LTF), Monocyte differentiation antigen CD14 (CD14), alpha-lactalbumin (LALBA). Among them, PIGR, JCHAIN, FGA, CD14, LTF, and QSOX1 can act as carrier proteins and immune effectors and were generally recognized as heat-labile [28]. Hierarchical clustering was used to further analyze the quantified proteins, and Fig. 4C shows the resulting heatmap. This shows that the samples are divided into two major clusters according to their protein profiles. MF, S, and MUS1 treated samples form one cluster; MUS2, and MUS3 form a second cluster, while HTST is on its own, suggesting that HTST is different from all other samples. These results also demonstrate that MF, alone or combined with low-intensity ultrasonication treatments, would not influence the low-abundant whey proteins, while MF combined with ultrasonication at higher intensity would to a limited extent influence these proteins. As mentioned above, the influence of MUS2 and MUS3 treatments on low-abundant whey proteins still differed from HTST. The abundances of some proteins in MUS2 and MUS3 treated milk are higher than those in HTST milk, including e.g. osteopontin (SPP1), CD177 molecule (CD177), αs1-casein (CSN1S1), αs2-casein (CSN1S2), β-casein (CSN2), and milk fat derived proteins such as butyrophilin subfamily 1 member A1 (BTN1A1), and lactadherin (MFGE8). Ultrasonication may break both the native MFGM and the casein micelle structures, resulting in the increase of these proteins in milk serum [29], [30]. The clustering results were generally consistent with the PCA results shown in Fig. 4A.
Fig. 4.
PCA analysis (Scores (A); Loadings (B)) and heatmap (C) of the quantified milk serum proteome after different treatments. S: Skim milk; HTST: HTST pasteurization; MF: Microfiltration, MUS1, MUS2 and MUS3; Microfiltration combined with ultrasonication with different energy densities at 432, 1296 and 2160 J/mL, respectively.
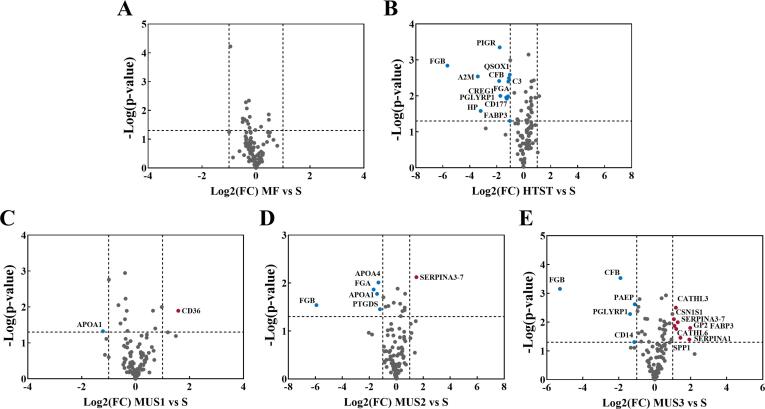
To further analyze these significantly different proteins, Fig. 5 demonstrates the volcano plot analysis between the treatment groups and untreated skim milk. The significantly different proteins (|Log2 FC|˃1, p < 0.05) are marked with their gene names, and the corresponding protein names and more information are shown in the Supplementary material. As shown in Fig. 5A, MF had no significant effect on the abundance of milk serum proteins. However, 12 serum proteins were significantly reduced in HTST treated milk compared to the untreated skim milk (Fig. 5B), which is in agreement with previous reports [18]. Most of these reduced proteins are related to cellular processes, metabolic processes, binding, catalytic activity, such as polymeric immunoglobulin receptor (PIGR), alpha-2-macroglobulin (A2M), complement C3 (C3), and haptoglobin (HP). PIGR is involved in the transport and secretion of Igs, transferring IgA and IgM from epithelial cells to mucosal secretion [31]. A2M could inhibit protease activities and interact with cytokines to participate in the immune regulation [32]. The complement system, similar to immunoglobulin, is part of the innate human immune system [33], and HP plays an important role in inflammatory response [34]. These heat-sensitive proteins are generally aggregated by disulfide bonds with other serum proteins or caseins during heating, leading to a loss of their bioactivities [3], [5].
Fig. 5.
Volcano plots of the quantified milk serum proteome after different treatments. S: Skim milk; HTST: HTST pasteurization; MF: Microfiltration; MUS1, MUS2 and MUS3: Microfiltration combined with ultrasonication with different energy densities at 432, 1296 and 2160 J/mL, respectively.
Fig. 5C-E show that the number of significantly different proteins after ultrasonication gradually increased along with the increase of ultrasonication intensity. MUS1 did not show major significant differences, while MUS2 and MUS3 did cause minor changes of the milk serum proteome. A total of 9 proteins showed significant increases in abundance in MUS treated milk, and 9 proteins significantly decreased.
Most of these significantly decreased proteins are related to immune or antibacterial functions, including complement factor B (CFB), peptidoglycan recognition protein 1 (PGLYRP1), monocyte differentiation antigen CD14 (CD14). Liu, Xiong, Kontopodi, Boeren, Zhang, Zhou and Hettinga [9] also found that a variety of bioactive serum proteins were significantly decreased in whole milk after ultrasonication. Ultrasound could produce local high temperatures (2000–5000 K), high pressure, and strong shear, which all could lead to the unfolding of milk serum protein, resulting in the formation of aggregates through hydrophobic interactions and disulfide bonds [14].
Among all the significantly increased proteins, milk fat globule membrane (MFGM) proteins and caseins were the major ones, such as platelet glycoprotein 4 (CD36), fatty acid-binding protein (FABP3), αs1-casein, and osteopontin (SPP1). Submicron fat globules (100–400 nm) or lipid-protein complexes (<500 nm) can be formed during milk processing, such as shear homogenization, and be eventually retained in skim milk [35], [36]. The MFGM proteins bound to these lipid particles can be released into the milk serum phase by the homogenization effect of ultrasonication [36] and then be identified. In addition, it was reported that ultrasonication would affect the structure of milk proteins, especially casein micelles [30]. The collision and interaction between protein aggregates would be increased by the strong shearing effect of ultrasonication. Proteins bound by weak hydrophobic interactions or Van der Waals forces would partly collapse because of the collision, resulting in decreasing particle size [14]. Ultrasonication could loosen the structure of casein micelles, after which a part of the caseins from the micelles could diffuse into the milk serum phase. SPP1 is a kind of acid phosphorylated protein with calcium binding sites that allows it to bind to casein micelles [37]. Due to the mechanical effect of ultrasonication, some SPP1 bound to casein micelles may have been released which may explained its increased level in the milk serum fraction.
3.4. Immunoglobulins, and lactoferrin
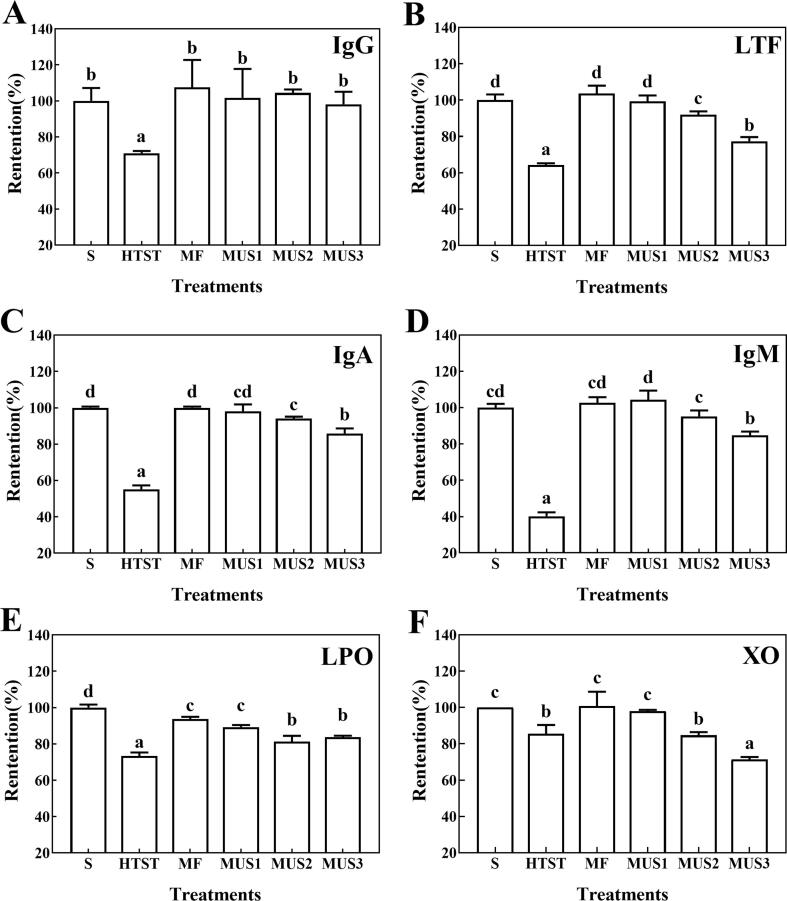
To determine the bioactive proteins in skim milk after processing, ELISA was used to determine the content of Igs and LTF. Fig. 6 shows the retention of the concentration of bioactive proteins (IgG, LTF, IgA and IgM). After HTST pasteurization, IgG, LTF, IgA and IgM significantly decreased by 30%, 35%, 45%, and 60%, respectively, which is in agreement with a previous report [4]. Comparing the different processes, MF and MUS1 didn’t reduce the concentration of those bioactive proteins. MUS treatments did not decrease the content of IgG compared with raw milk, while LTF, IgA and IgM concentration showed a slight but significant decrease in the MUS2 and MUS3 treatments, which was in a dose-dependent manner. Generally speaking, ultrasonication treatments at higher intensity (MUS2 and MUS3) caused a limited effect on bioactive proteins such as LTF; however, they performed better than the HTST treatment. These findings were in agreement with the report of Liu, Xiong, Kontopodi, Boeren, Zhang, Zhou and Hettinga [9], who also found that thermo-ultrasonication reduced the abundance of whey proteins to a limited extent. Retention of these bioactive proteins was closely related with ultrasonication intensity. Although ultrasonication with higher energy density (>1296 J/mL) showed a better inactivation capacity (Table 1), owing to physical effects (liquid shear force, shock wave, micro-jets, etc.), and local high temperature and pressure caused by acoustic cavitation [24], this would simultaneously result in the damage of bioactive proteins. In summary, MF combined with ultrasonication applied in this study realized a better preservation of bioactive proteins in skim milk compared with HTST, while achieving a better bacterial inactivation and a longer shelf life.
Fig. 6.
Retention of IgG (A), LTF (B), IgA (C), IgM (D) concentrations, and LPO (E) and XO activity (F) after different treatments. S: Skim milk; HTST: HTST pasteurization; MF: microfiltration, MUS1, MUS2 and MUS3: microfiltration combined with ultrasonication with different energy densities at 432, 1296 and 2160 J/mL, respectively. Different letters indicate significant difference (p < 0.05).
3.5. LPO and XO activity
LPO and XO are two important natural antibacterial enzymes in milk which interact with each other to form the XO-LPO system, which may promote the intestinal immune system of humans, especially for newborns [38]. The XO in skimmed milk only accounts for 5–10% of the total XO activity. It was reported that part of XO on the MFGM could be released into skim milk during transportation and homogenization [39], [40]. The XO activity measured in this research only represents the part of its activity in skim milk. The retention of LPO and XO activities was demonstrated in Fig. 6E and F. As expected, HTST significantly reduced the activities of LPO and XO by ~ 30% and 20%, respectively. The changes in LPO activity was in agreement with the findings of Griffiths [41], who found that ~ 70% of LPO was retained after heating at 72 °C for 15 s. The retention of LPO and XO activities gradually decreased with the increase of ultrasonication intensity in a dose-dependent manner, especially for XO activity. Villamiel and de Jong [42] studied the effect of ultrasound on several enzymes in whole milk, and found that LPO showed a certain resistance to ultrasound, and the subsequent reduction of LPO activity also occurred in a dose-dependent manner. Zhang, Liu, Li, Xu and Zhou [13] reported that HTST (72 °C for 15 s) could reduce XO activity by 20% in skim milk, which was in accordance with our results. Another research reported that the XO activity in whole milk was reduced by 60% and 55% after heating at 63 °C for 30 min and 73 °C for 15 s, respectively [43]. These differences in XO activity may be affected by the variables in milk fat content, thermal treatment systems and parameters, or determination methods [44]. The partial inactivation of LPO and XO in milk treated with ultrasonication may not only relate to protein denaturation caused by local high temperature and high shear force, but also relate to the structural changes of the enzyme resulting from the free radicals formed during the decomposition of H2O molecules due to ultrasonication [44]. Even though MF combined with ultrasonication would slightly reduce the bioactive proteins in skim milk, however, ultrasonication with the highest intensity still shows a better retention for most of these proteins compared with HTST treatment.
In addition, to check the stability and retention status of these bioactive components in milk. We also measured the content of LTF and IgG during shelf life. Fig. S1 shows the changes of IgG and LTF content in skim milk during shelf life. It could be observed that the concentration of IgG and LTF after different treatments remained stable during the whole shelf life.
4. Conclusion
This study compared the effects of HTST pasteurization and microfiltration, alone or combined with ultrasonication at different intensity, on the shelf life and bioactive proteins of skim milk. The results showed that the shelf life of skim milk stored at 4 °C was extended up to 40 days, depending on the ultrasonication intensity, without a major decrease of the bioactive components in milk. The results thus show that MF combined with ultrasonication has a better performance in preserving bioactive proteins than the commonly used HTST pasteurization. These findings could give inspirations to produce extended shelf-life milk with high levels of bioactive proteins, and consequently improve the current dairy processing. However, further studies such as milk flavor evaluations and development of large-scale ultrasonication equipment are needed to realize the industrial application of ultrasonication.
CRediT authorship contribution statement
Wenjin Zhang: Conceptualization, Methodology, Formal analysis, Writing - original draft. Yaowei Liu: Conceptualization, Supervision, Software, Validation, Writing - review & editing. Zhibin Li: Methodology, Validation, Writing - review & editing. Shu Xu: Methodology, Investigation, Writing - review & editing. Jie Zhang: Methodology, Validation, Writing - review & editing. Kasper Hettinga: Visualization, Software, Writing - review & editing. Peng Zhou: Supervision, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFD0400600), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_1815), and China Scholarship Council (CSC) Program (No. 201906790028).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105668.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Madureira A.R., Pereira C.I., Gomes A.M.P., Pintado M.E., Xavier Malcata F. Bovine whey proteins – Overview on their main biological properties. Food Res. Int. 2007;40:1197–1211. [Google Scholar]
- 2.Mehra R., Marnila P., Korhonen H. Milk immunoglobulins for health promotion. Int. Dairy J. 2006;16:1262–1271. doi: 10.1016/j.idairyj.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbring S., Xiong L., Diks M.A.P., Baars T., Garssen J., Hettinga K., van Esch B. Loss of allergy-protective capacity of raw cow's milk after heat treatment coincides with loss of immunologically active whey proteins. Food Function. 2020;11:4982–4993. doi: 10.1039/d0fo01175d. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y., Zhang W., Zhang L., Hettinga K., Zhou P. Characterizing the changes of bovine milk serum proteins after simulated industrial processing. LWT. 2020;133 [Google Scholar]
- 5.Brick T., Ege M., Boeren S., Bock A., von Mutius E., Vervoort J., Hettinga K. Effect of Processing Intensity on Immunologically Active Bovine Milk Serum Proteins. Nutrients. 2017;9:963. doi: 10.3390/nu9090963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elwell M.W., Barbano D.M. Use of microfiltration to improve fluid milk quality. J. Dairy Sci. 2006;89:E20–E30. doi: 10.3168/jds.S0022-0302(06)72361-X. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez Garcia L., Riera Rodriguez F.A. Combination of microfiltration and heat treatment for ESL milk production: Impact on shelf life. J. Food Eng. 2014;128:1–9. [Google Scholar]
- 8.Atik A., Gumus T. The effect of different doses of UV-C treatment on microbiological quality of bovine milk. LWT. 2021;136 [Google Scholar]
- 9.Liu Y., Xiong L., Kontopodi E., Boeren S., Zhang L., Zhou P., Hettinga K. Changes in the milk serum proteome after thermal and non-thermal treatment. Innovative Food Sci. Emerg. Technol. 2020;66:102544. doi: 10.1016/j.ifset.2020.102544. [DOI] [Google Scholar]
- 10.Pafylias I., Cheryan M., Mehaia M.A., Saglam N. Microfiltration of milk with ceramic membranes. Food Res. Int. 1996;29 [Google Scholar]
- 11.Saboyainsta L., Maubois J.L. Current Developments of Microfiltration Technology in the Dairy Industry. Lait. 2000;80:541–553. [Google Scholar]
- 12.Fernández García L., Álvarez Blanco S., Riera Rodríguez F.A. Microfiltration applied to dairy streams: Removal of bacteria. J. Sci. Food Agric. 2013;93(2):187–196. doi: 10.1002/jsfa.5935. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W., Liu Y., Li Z., Xu S., Hettinga K., Zhou P. Retaining Bioactive Proteins and Extending Shelf Life of Skim Milk by Microfiltration Combined with Ultraviolet-C Treatment. LWT-Food Sci. Technol. 2021;141:110945. doi: 10.1016/j.lwt.2021.110945. [DOI] [Google Scholar]
- 14.Munir M., Nadeem M., Qureshi T., Leong T., Gamlath C., Martin G., Ashokkumar M. Effects of high pressure, microwave and ultrasound processing on proteins and enzyme activity in dairy systems — A review. Innovative Food Sci. Emerg. Technol. 2019;57 [Google Scholar]
- 15.Riener J., Noci F., Cronin D.A., Morgan D.J., Lyng J.G. Characterisation of volatile compounds generated in milk by high intensity ultrasound. Int. Dairy J. 2009;19:269–272. [Google Scholar]
- 16.Juliano P., Torkamani A.E., Leong T., Kolb V., Watkins P., Ajlouni S., Singh T.K. Lipid oxidation volatiles absent in milk after selected ultrasound processing. Ultrason. Sonochem. 2014;21:2165–2175. doi: 10.1016/j.ultsonch.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 17.AOAC, Official Methods of Analysis, 17th ed, Association of Official Analytical Chemists International, Arlington, VA, 2000.
- 18.Czank C., Simmer K., Hartmann P.E. Simultaneous pasteurization and homogenization of human milk by combining heat and ultrasound: effect on milk quality. J. Dairy Res. 2010;77(2):183–189. doi: 10.1017/S0022029909990483. [DOI] [PubMed] [Google Scholar]
- 19.Chandrapala J., Martin G., Kentish S., Ashokkumar M. Dissolution and reconstitution of casein micelle containing dairy powders by high shear using ultrasonic and physical methods. Ultrason. Sonochem. 2014;21:1658–1665. doi: 10.1016/j.ultsonch.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Fritsch J., Moraru C.I. Development and optimization of a carbon dioxide-aided cold microfiltration process for the physical removal of microorganisms and somatic cells from skim milk. J. Dairy Sci. 2008;91:3744–3760. doi: 10.3168/jds.2007-0899. [DOI] [PubMed] [Google Scholar]
- 21.Law A., Leaver J. Effect of pH on the Thermal Denaturation of Whey Proteins in Milk. J. Agric. Food. Chem. 2000;48:672–679. doi: 10.1021/jf981302b. [DOI] [PubMed] [Google Scholar]
- 22.Zou Z., Bauland J., Hewavitharana A., Alshehri S., Duley J., Cowley D., Koorts P., Shaw P., Bansal N. A sensitive, high-throughput fluorescent method for the determination of lactoperoxidase activities in milk and comparison in human, bovine, goat and camel milk. Food Chem. 2021;339 doi: 10.1016/j.foodchem.2020.128090. [DOI] [PubMed] [Google Scholar]
- 23.Zou Z., Pury C., Hewavitharana A., Alshehri S., Duley J., Cowley D., Koorts P., Shaw P., Bansal N. A sensitive and high-throughput fluorescent method for determination of oxidase activities in human, bovine, goat and camel milk. Food Chem. 2020;336 doi: 10.1016/j.foodchem.2020.127689. [DOI] [PubMed] [Google Scholar]
- 24.Dai J., Bai M., Li C., Cui H., Lin L. Advances in the mechanism of different antibacterial strategies based on ultrasound technique for controlling bacterial contamination in food industry. Trends Food Sci. Technol. 2020;105:211–222. [Google Scholar]
- 25.Gera N., Doores S. Kinetics and Mechanism of Bacterial Inactivation by Ultrasound Waves and Sonoprotective Effect of Milk Components. J. Food Sci. 2011;76:111–119. doi: 10.1111/j.1750-3841.2010.02007.x. [DOI] [PubMed] [Google Scholar]
- 26.H. Deeth, M. Lewis, Heat Treatments of Milk – Thermisation and Pasteurisation, New York, 2017, 15-39.
- 27.Schmidt V., Kaufmann V., Kulozik U., Scherer S., Wenning M. Microbial biodiversity, quality and shelf life of microfiltered and pasteurized extended shelf life (ESL) milk from Germany, Austria and Switzerland. Int. J. Food Microiol. 2012;154:1–9. doi: 10.1016/j.ijfoodmicro.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Benabdelkamel H., Masood A., Alanazi I.O., Alzahrani D.A., Alrabiah D.K., AlYahya S.A., Alfadda A.A. Proteomic Profiling Comparing the Effects of Different Heat Treatments on Camel (Camelus dromedarius) Milk Whey Proteins. Int. J. Mol. Sci. 2017;18:721. doi: 10.3390/ijms18040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Boeren S., Zhang L., Zhou P., Hettinga K. Ultrasonication retains more milk fat globule membrane proteins compared to equivalent shear-homogenization. Innovative Food Sci. Emerg. Technol. 2021;70 [Google Scholar]
- 30.Shanmugam A., Chandrapala J., Ashokkumar M. The effect of ultrasound on the physical and functional properties of skim milk. Innovative Food Sci. Emerg. Technol. 2012;16:251–258. [Google Scholar]
- 31.Johansen B. Brandtzaeg, Role of J Chain in Secretory Immunoglobulin Formation. Scand. J. Immunol. 2010;52:240–248. doi: 10.1046/j.1365-3083.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- 32.Peslova G., Petrak J., Kuzelova K., Hrdy I., Halada P., Kuchel P., Soe-Lin S., Ponka P., Sutak R., Becker E., Huang M., Suryo Rahmanto Y., Richardson D., Vyoral D. Hepcidin, the hormone of iron metabolism, is bound specifically to -2-macroglobulin in blood. Blood. 2009;113:6225–6236. doi: 10.1182/blood-2009-01-201590. [DOI] [PubMed] [Google Scholar]
- 33.Korhonen H., Marnila P., Gill H.S. Milk immunoglobulins and complement factors. Br. J. Nutr. 2000;84(S1):75–80. doi: 10.1017/s0007114500002282. [DOI] [PubMed] [Google Scholar]
- 34.Günther J., Koczan D., Yang W., Nürnberg G., Repsilber D., Schuberth H.-J., Park Z., Maqbool N., Molenaar A., Seyfert H.-M. Assessment of the immune capacity of mammary epithelial cells: Comparison with mammary tissue after challenge with Escherichia coli. Vet. Res. 2009;40:31. doi: 10.1051/vetres/2009014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michalski M.-C., Geneste C. Appearance of submicronic particles in the milk fat globule size distribution upon mechanical treatments. Lait. 2002;82:193–208. [Google Scholar]
- 36.Michalski M.-C., Januel C. Does homogenization affect the human health properties of cow's milk? Trends Food Sci. Technol. 2006;17:423–437. [Google Scholar]
- 37.H.P.S. Arpitkumar Hareshbhai Patel, Advanced dairy chemistry. Volume 1A. Proteins: basic aspects, 4th edition, 2013.
- 38.Shin K., Tomita M., Lonnerdal B. Identification of lactoperoxidase in mature human milk. J. Nutr. Biochem. 2000;11:94–102. doi: 10.1016/s0955-2863(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 39.Briley M.S., Eisenthal R. Association of xanthine oxidase with the bovine milk-fat-globule membrane. Catalytic properties of the free and membrane-bound enzyme. Biochem. J. 1974;143(1):149–157. doi: 10.1042/bj1430149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma R., Kaur S., Rajput Y., Kumar R. Activity and thermal stability of indigenous enzymes in cow, buffalo and goat milk. Milchwissenschaft. 2009;64:173–175. [Google Scholar]
- 41.Griffiths M. Use of Milk Enzymes as Indices of Heat Treatment. J. Food Prot. 1986;49:696–705. doi: 10.4315/0362-028X-49.9.696. [DOI] [PubMed] [Google Scholar]
- 42.Villamiel M., de Jong P. Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk (vol 48, pg 472. J. Agric. Food. Chem. 2000;48(2000):3068. doi: 10.1021/jf0006224. 3068. [DOI] [PubMed] [Google Scholar]
- 43.Sharma P., Oey I., Bremer P., Everett D. Reduction of bacterial counts and inactivation of enzymes in bovine whole milk using pulsed electric fields. Int. Dairy J. 2014;39:146–156. [Google Scholar]
- 44.O'Donnell C., Brijesh kumar T., Bourke P., Cullen P.J. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010;21:358–367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.