Abstract
Abiraterone acetate (AA) has been proven effective for metastatic castration-resistant prostate cancer (mCRPC), and it has been proposed that adaptive AA may reduce toxicity and prolong time to progression, when compared to continuous AA. We developed a simple quantitative model of prostate-specific antigen (PSA) dynamics to evaluate prostate cancer (PCa) stem cell enrichment as a plausible driver of AA treatment resistance. The model incorporated PCa stem cells, non-stem PCa cells and PSA dynamics during adaptive therapy. A leave-one-out analysis was used to calibrate and validate the model against longitudinal PSA data from 16 mCRPC patients receiving adaptive AA in a pilot clinical study. Early PSA treatment response dynamics were used to predict patient response to subsequent treatment. We extended the model to incorporate metastatic burden and also investigated the survival benefit of adding concurrent chemotherapy for patients predicted to become resistant. Model simulations demonstrated PCa stem cell self-renewal as a plausible driver of resistance to adaptive therapy. Evolutionary dynamics from individual treatment cycles combined with metastatic burden measurements predicted patient response with 81% accuracy (specificity=92%, sensitivity=50%). In those patients predicted to progress, simulations of the addition of concurrent chemotherapy suggest a benefit between 1% and 11% reduction in probability of progression when compared to adaptive AA alone. This study developed the first mCRPC patient-specific mathematical model to use early PSA treatment response dynamics to predict subsequent responses to adaptive AA, demonstrating the putative value of integrating mathematical modeling into clinical decision making.
Keywords: Prostate cancer, Metastatic, Mathematical, Modeling, Predictive, Adaptive therapy, Prostate-specific antigen
Introduction
Prostate cancer (PCa) is the most prevalent cancer in men in the US. About 1 in 9 American men will be diagnosed with PCa in his lifetime (>248,000 estimated in 2021) and 1 in 41 will die from it. Consequently, PCa remains the second leading cause of death in American men [2]. Following surgery or radiation treatment, androgen deprivation therapy (ADT) has been the mainstay treatment for hormone sensitive PCa for over 70 years [3]. ADT suppresses the production of testicular androgen, which both the normal and cancerous prostate cells depend on for survival and proliferation [4]. Despite new strategies in “precision medicine” in which the specific therapy is guided by molecular biomarkers for individual patients, treatment protocols are typically based on the conventional strategy of “maximum tolerated dose until progression”. This often results in the competitive release of the resistant phenotype, leading to early treatment progression [5]. In an effort to delay progression, intermittent ADT has been shown to be a promising alternative that reduces toxicity and delays progression [6], [7], [8]. Despite this advance, patients inevitably develop castration-resistant prostate cancer, which often progresses to metastatic castration-resistant prostate cancer (mCRPC).
Second-line hormone therapy with abiraterone acetate (AA), which inhibits the production of androgens from other cells by blocking the protein CYP179, has been shown to prolong overall survival when compared to prednisone or placebo [9], [10], [11], [12], [13]. In the COU-AA-302 trial evaluating AA with prednisone versus prednisone alone in mCRPC, AA was shown to improve median overall survival (35.3 with AA vs. 30.1 month without) and more than double median radiographic progression-free survival (16.5 vs. 8.2 months). While radiographic progression-free survival (rPFS) and overall survival (OS) were the primary endpoints, the study also evaluated prostate-specific antigen (PSA) progression. The PSA response rate (PSA decline 50%) to AA was 68%, compared to just 29% in the prednisone alone group, with a median time to PSA progression of 11.1 months versus 5.6 months with prednisone alone [13]. It has been proposed that adaptively administering AA in mCRPC, allowing for treatment holidays when a patient has sufficiently responded, may be able to increase overall response and delay progression [5]. However, maximizing the benefit of adaptive therapy requires understanding the dominant drivers of resistance, predicting individual patient responses, and identifying when and how to modulate treatment to maximize response time.
Many mathematical models have been developed to simulate the various biological mechanisms of clinical PCa treatment resistance [5,[14], [15], [16], [17], [18], [19]]. These studies have contributed significantly to our understanding of how the different mechanisms that may lead to resistance could be exploited for adaptive therapies, but most mathematical models have yet to be rigorously calibrated and validated with patient-specific disease dynamics, and to be evaluated for predictive power [20]. For predictive certainty, model complexity and uncertainty must be kept proportional to the available data. PSA has been used as a marker of PCa development and progression for many years, despite concerns regarding overdiagnosis and overtreatment. We have previously demonstrated how PSA dynamics, rather than measurements taken at single timepoints, can be used to predict individual patient responses to intermittent hormone therapy in castration-naïve PCa [1]. In more advanced PCa, such as in the case of mCRPC, alternative biomarkers such as cell-free DNA and circulating tumor DNA, have been shown to be correlated with PSA response rates [21], [22], [23], [24]. However, very few studies routinely collect such data, when compared to PSA. Here, we sought to investigate if PSA dynamics could be used to predict response to adaptive AA in mCRPC.
We extended our relatively simple model of PCa stem cell dynamics with previously demonstrated predictive power [1]. We calibrated and validated the model against longitudinal PSA data from 16 mCRPC patients receiving adaptive AA in a proof-of-principle prospective pilot trial (NCT02415621). We then used early treatment dynamics to predict how individual patient respond to subsequent treatment cycles. We further investigated how to integrate individual metastatic burden into the predictive modeling framework to increase patient-specific prediction accuracy. We also use the model to simulate the addition of concurrent chemotherapy in those patients predicted to progress during the second cycle of treatment.
Materials and Methods
Patient Data
Sixteen mCRPC patients received adaptive AA therapy as part of a pilot trial at Moffitt Cancer Center [5]. Prior to trial registration, patients received AA plus prednisone as standard of care. Patients who achieved a 50% or more decline of their PSA were eligible to enroll in the trial. Each patient's PSA immediately prior to beginning AA was considered as their baseline. Treatment with
AA was paused when PSA fell below 50% of the individual baseline PSA, and resumed when PSA rose above baseline levels. PSA was monitored every 4 to 6 weeks, with restaging bone scan, pelvic and abdominal CT scan performed every 12 weeks. Patients remained on the trial until radiographic progression based on PCWG2 criteria [25].
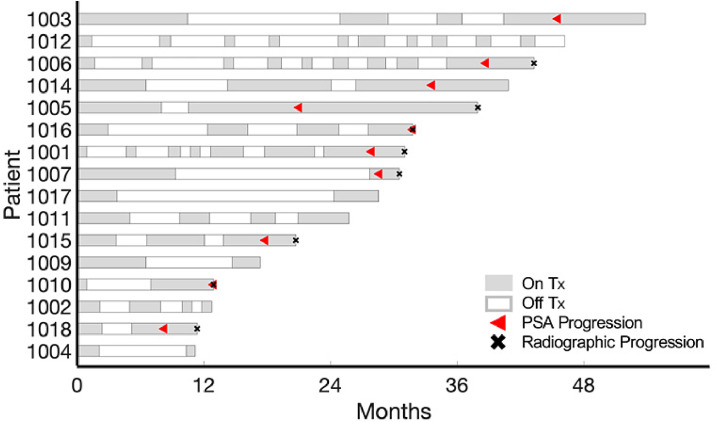
We analyzed longitudinal PSA data (collected every 4-6 weeks both whilst receiving AA and during AA holidays) for the patients enrolled in the trial (average number of data points per patient = 24, range = 9 – 41) to evaluate early PSA response dynamics as predictive biomarker of PSA progression. PSA progression is defined as PSA increasing and at least above the nadir, confirmed by a second value 3 or more weeks later. Eight of the 16 patients developed PSA progression within the first four cycles of treatment, and eight patients were still responding after four cycles (one patient developed resistance after 9 cycles of treatment) (Fig. 1).
Fig. 1.
Treatment times for patients included in the study. Sixteen patients were included in the model analysis. Gray and white denote when treatment was on and off, respectively. Red triangles and black x's denote when patient developed PSA and radiographic progression, respectively. (Color version of figure is available online)
Mathematical Model
The mathematical model simulates the dynamics of prostate cancer stem-like cells (PCaSCs) (), non-stem differentiated cells (), and PSA (). PCaSCs divide at rate to produce either a PCaSC and non-stem PCa cell with probability (asymmetric division) or two PCaSCs with probability (symmetric division), with negative feedback from the differentiated cells. Non-stem PCa cells die at rate in response to treatment, which is modulated by the parameter and , denoting when treatment is on and off, respectively. PSA is produced by the non-stem PCa cells at rate and decays at rate . The mathematical equations describing these interactions are given by
| (1) |
Model calibration and validation
Previous analysis has shown that the rate at which uninhibited PCaSCs divide can be approximated as once a day, i.e., [26]. Sensitivity and correlation analysis showed that and could be uniform among all patients and and be patient-specific without significantly changing the model results [1]. We deployed a Type 1b bootstrap internal validation leave-one-out analysis [27] to determine the uniform values for and while allowing and to be patient-specific. That is, for patient , we used nested optimization to find the uniform values for and and the patient-specific values for and for all patients , with , in the training set, over each patient's treatment course. We then validated the model using and to determine the patient-specific and for patient . This process was repeated for all patients.
Treatment response prediction
For patient , and were used to fit the model to each cycle individually for all patients in the training set. That is, finding the optimal value for and while allowing and to remain fixed for each individual cycle (Fig. 2A). Given and for cycle for patient , we used the relative changes in for all patients in our training set to generate the cumulative probability distribution of relative changes in from cycle to cycle (Fig. 2B). We sampled from this distribution to determine 100 values of for cycle As and are exponentially related [1], we sampled from the 95% confidence interval around the exponential curve relating and (Fig. 2C) to find 100 values for . We used these values to simulate the distribution of patient ’s responses in cycle .
Fig. 2.
Cycle by cycle parameter changes. (A) Parameter distributions of for Cycles 1 through 4. (B) Cumulative probability distribution of relative changes in from Cycle 1 to Cycle 2. (C) vs. fit (black curve) and 95% confidence interval (gray) for () pairs (black dots) for Cycle 2.
Each model simulation was determined to predict response or resistance based on the PSA progression criteria defined in the trial. That is, if PSA increased more than 25% and at least 2 above the nadir during treatment, then the simulation was classified as resistant. Of the 100 response simulations, we quantified the number of resistant simulations to derive a probability of resistance, . If was greater than a given threshold, , obtained from the training set for each predicted treatment cycle , then the prediction was considered resistant. Otherwise, it was classified as responsive. For each leave-one-out patient, an optimal cutoff value for cycle was determined to be the threshold that maximized the accuracy within the training set. Model predictions correctly classifying clinically observed responders as responders ( were denoted true negative, while correctly classified clinically resistors ( were denoted as true positive.
Statistical analysis
The two-sample t-test was used to calculate the statistical significance of the difference between parameter distributions.
Results
Model accurately describes clinical data
The model is able to simulate longitudinal PSA data from mCRPC patients (Fig. 3A-B). In line with previous results of intermittent ADT [1], the model fits to the data demonstrate that continuous responders have a slowly increasing PCaSC population (Fig. 3A), while patients who progress have a rapidly increasing PCaSC population (Fig. 3B). This shows that prostate cancer stem cell enrichment is a plausible driver of resistance to AA therapy. A comparison of the stem cell self-renewal rate between responsive and resistant patients showed that resistant patients tend to have a larger value, though not significant in this small cohort (Fig. 3C). No difference in the uniform values for and for each leave-one-out analysis were found (Fig. 3D).
Fig. 3.
Leave-one-out analysis results. Model fits to PSA data and corresponding stem cell dynamics for a (A) continuous responder and (B) a patient who developed resistance in his third cycle of treatment. Parameter distributions for (C) patient-specific parameters and and (D) uniform model parameters and determined via leave-one-out analysis.
Early treatment dynamics can predict subsequent response
Model predictions are classified as true positive, true negative, false positive and false negative in comparison to the clinical outcomes (Fig. 4A). Model predictions for a continuous responder and a patient who progressed in the third cycle are shown in Fig. 4B-C. The model was fit over the first treatment cycle using the and values obtained from the training set. Using the relative change in from cycle to cycle as well as the exponential relationship between and , we obtained 100 parameter pairs of () that were used to forecast the patient's response in the subsequent cycle. The model accurately predicted that Patient 1011 would continue to respond in both the second and third cycles (Fig. 4B). Patient 1014 progressed in his third cycle of treatment. Though in cycle 2, this is below the threshold and consequently the model correctly predicts that this patient will continue to respond. In cycle 3, which is larger than . This is a correct prediction as Patient 1014 develops resistance in cycle 3. Overall, the model is able to correctly predict patient response with 78% accuracy (specificity = 92%, sensitivity = 38%).
Fig. 4.
Model prediction results. (A) Prediction classifier table. Model predictions for (B) Patient 1011 who was a continuous responder and (C) Patient 1014 who progressed in the third cycle. The model correctly predicted that Patient 1011 would continue to respond in both the second and third cycles and that Patient 1014 would continue to respond in the second cycle and develop resistance in the third cycle. Prediction classifications are determined using cycle- and patient-specific κ values. If the P(Ω) > κ, then the patient is predicted to develop resistance. Model predictions were compared to actual data to determine accuracy.
Incorporating metastatic burden
Despite treatment, several patients continued to develop metastases during the trial. Though rising PSA has been shown to be correlated with metastatic burden, it is not predictive of the development of new metastatic growths [28]. We sought to investigate whether correlating metastatic growth with the stem cell self-renewal rate, , would improve the prediction accuracy of our model. That is, developing new metastatic growths during a cycle of treatment is likely the consequence of a larger number of anatomically distributed PCaSCs, whose dynamics are primarily driven by the self-renewal rate Thus, we propose a novel way to correlate new metastatic growths with .
As previously described, () pairs for cycle were obtained by uniformly sampling from the cumulative probability distribution of relative changes in from cycle to . Fig. 5A shows that in general, the relative change ranged between -75% and 150%, with about 30% of the patients experiencing larger relative changes in . To incorporate the metastatic burden, we used a skewed sampling to sample from the cumulative probability distribution, with the degree of the skew dependent on the number of new metastatic growths. This resulted in larger relative increases in for patients with larger metastatic burden As shown in Fig. 3C, patients with larger developed resistance to adaptive AA faster in comparison to those with smaller values. Consequently, the skewed sampling results in a larger fraction of simulations that predict resistance, thereby increasing the It should be noted that this also resulted in changing in response to larger for particular patients within the training cohort.
Fig. 5.
Model prediction results when considering metastatic burden.(A) Parameter sampling with and without metastases. When a patient's metastatic burden is not considered, a uniform sampling is performed to determine value in next cycle. If a patient develops a new metastatic growth, the sampling is skewed such that the sampled stem cell self-renewal value is more likely to be larger in the next cycle. (B) Model predictions for Patient 1010 with and without incorporating metastatic burden. Patient 1010 developed on new metastatic growth during his first off-treatment cycle. Using PSA dynamics alone, the model incorrectly predicts that Patient 1010 will continue to respond. Using a skewed sampling results in a correct prediction that Patient 1010 will progress in cycle 2.
A comparison between the model predictions using a uniform and skewed sampling for an individual patient are shown in Fig. 5B. Patient 1010 developed a new metastatic growth during his first cycle of treatment. With a uniform sampling, the model predicted that the As this was below the threshold , the model incorrectly predicted that this patient would continue to respond in the second cycle (false negative). Using a skewed sampling, the increased to 0.53, which was larger than the new threshold resulting in a correct resistant prediction (true positive). Overall, incorporating each patient's metastatic increased the accuracy to 81% (specificity = 92%, sensitivity = 50%).
Concurrent chemotherapy can improve time to progression
Concurrently administering the chemotherapeutic docetaxel with ADT has shown benefit in metastatic hormone-naïve prostate cancer patients [29], [30], [31]. In particular, the STAMPEDE trial conducted in 2016 showed a 10 month increase in overall survival in high-risk, locally advanced PCa patients receiving ADT with docetaxel compared to ADT alone [31]. Cabazitaxel is a second-line chemotherapeutic that has shown survival benefit after progression on docetaxel and is often given as standard of care after AA [32]. As previous studies have shown that chemotherapy provides more benefit when administered earlier in the disease course, we sought to investigate the effect of administering concurrent chemotherapy prior to progression on AA alone.
Unlike AA, chemotherapy can induce cell death in both PCaSCs and non-stem cells [33]. As such, we extended our model to include death terms () on both cell populations in response to chemotherapy (). That is,
| (2) |
Due to the patients included in our study being chemotherapy-naïve, we did not have clinical data to calibrate the model to obtain values for and Consequently, the values for these parameters were derived from literature, where it is said that approximately three times more non-stem cells die in response to chemotherapy than PCaSCs[33]. We chose and such that model simulations would show a decline in each population when chemotherapy was given.
For the patients predicted to progress during their second cycle of adaptive therapy, we simulated adding up to 10 cycles of concurrent chemotherapy during cycle two and compared the responses with and without chemotherapy (Fig. 6). While some patients received minimal benefit (3% decrease in Patient 1015) from concurrent therapy, others received as much as an 11% decrease in with concurrent treatment (Patient 1005).
Fig. 6.
Model simulation comparison between adaptive abiraterone with and without concurrent chemotherapy. (A) Without concurrent chemotherapy, Patient 1015 is predicted to have a 58% probability of developing resistance in his second cycle of treatment (left). Concurrent chemotherapy (right) provides a minimal reduction (3%) in the probability of developing resistance. (B) Patient 1005 has a 79% probability of progressing during cycle 2 without concurrent chemotherapy (left). This can be reduced by 11% if chemotherapy is given concurrently during cycle 2 (right).
Discussion
In this study, we have analyzed a simple mathematical model of PCaSC and PSA dynamics to predict individual mCRPC patient responses to adaptive AA therapy. The model has been calibrated and validated against clinical data from 16 mCRPC patients from a pilot trial. With just two patient-specific parameters, our results show that the model is able to accurately describe individual patient dynamics. Similar to previous analyses of PCa stem cell dynamics underlying resistance to intermittent ADT, we found that stem cell enrichment is a likely driver of treatment resistance, despite AA working through an alternative pathway than ADT [9]. As these patients are advanced metastatic PCa patients, their PSA dynamics are likely also driven by multiple factors that the model may not be able to account for. Despite that, the model was able to use early treatment evolutionary PSA dynamics to predict subsequent responses with 78% accuracy.
Incorporating the metastatic burden improved the overall accuracy to 82%, with a specificity of 92% and a sensitivity of 50%. The lower sensitivity is due to the few progression events within this data cohort. While patients respond on average for 3.3 treatment cycles, the majority of progression events occurred within the second cycle. This limitation made it difficult to determine the optimal threshold value for cycle predictions where few patients progressed, resulting in a higher proportion of false negative results. We are confident that model accuracy, as well as sensitivity and specificity, would increase with a larger patient cohort. Nevertheless, the patients who were incorrectly predicted to develop resistance during the second cycle instead progressed during the third cycle. As such, the model may serve as an early indicator of treatment resistance for a subset of patients.
We also investigated the potential benefit of concurrent chemotherapy with AA prior to progression on AA alone. Model simulations showed a 3% to 11% decrease in the probability of resistance when compared to AA alone. While 3% may be dismissed mathematically, it may mean a difference for individual patients based on their individual risk-benefit situation; as such the model predicted probabilities may provide invaluable information for the clinician to discuss with each patient when deciding if, when and how to adapt treatment. Using the presented model, the oncologist can discuss different treatment routes with associated risk and likelihood of success to guide patients decide what is the best course of action for their individual treatment expectations. As such, we believe that this is an option that should be considered for patients and warrants critical evaluation in a prospective clinical trial setting. We do note that the concurrent chemotherapy simulations used chemotherapy sensitivity parameters ( and ) that were not previously calibrated to clinical data. Future studies will include calibrating the model to clinical data of patients receiving concurrent chemotherapy to determine the values for these parameters that can be used in further model simulations.
As previously mentioned, alternative biomarkers such as ctDNA, cfDNA, and PCa stem cell antigen (PSCA) and other stem cell markers have been shown to be correlated with advanced PCa and metastasis [[21], [22], [23], [24],34]. However, due to the lack of availability of data, we were not able to design our model based on these dynamics. Collecting and analyzing such biomarkers are promising future avenues for clinical studies and mathematical modeling.
Conclusions
This validation study demonstrates the utility of mathematical modeling in clinical decision making. Using a simple dynamic model of PCaSC, non-stem cells, and PSA, we are able to use early treatment dynamics to predict who may or may not respond to subsequent treatment in mCRPC. Despite PSA notoriously being considered a poor biomarker for treatment response, both in early- and advanced-stage prostate cancer, we showed that our model could use PSA dynamics – rather than absolute PSA values – to predict response with an 81% accuracy. Our model is the first of its kind to correlate PSA dynamics with metastatic burden to understand how an individual patient's disease is evolving. As this model predicts PSA, and not radiographic progression, this may provide sufficient lead-in time for clinicians to intercede with alternative treatment options prior to radiographic progression, potentially minimizing adverse events and toxicity and maximizing survival. After prospective validation, such integrated approach could equip clinicians with an additional decision support tool to use when deciding how to effectively treat patients.
Author Contributions
R.B.N., J.Z., R.A.G. and H.E. conceptualized the study. R.B.N. and H.
E. performed the modeling and statistical analyses. R.B.N., J.Z., T.Z., A.Z.W., R.B., R.A.G. and
H.E. wrote the manuscript.
Acknowledgements
We thank the participants of the clinical trial. This work was supported by NIH/NCI R21CA234787 “Predicting patient-specific responses to personalize ADT for prostate cancer”, and in part by NIH/NCI U54CA143970-05 (Physical Science Oncology Network) “Cancer as a complex adaptive system”, the Ocala Royal Dames for Cancer Research, Inc., and The JAYNE KOSKINAS TED GIOVANIS FOUNDATION FOR HEALTH AND POLICY, a Maryland private foundation dedicated to effecting change in health care for the public good. The opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and not necessarily represent the official views of the National Institutes of Health, the Ocala Royal Dames for Cancer Research, or the JAYNE KOSKINAS TED GIOVANIS FOUNDATION FOR HEALTH AND POLICY, its directors, officers, or staff.
Footnotes
✩Conflict of Interest: The authors declare no potential conflicts of interest.
References
- 1.Brady-Nicholls R, Nagy JD, Gerke TA, Zhang T, Wang AZ, Zhang J. Prostate-specific antigen dynamics predict individual responses to intermittent androgen deprivation. Nat Commun. 2020;11:1750. doi: 10.1038/s41467-020-15424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Damodaran S, Kyriakopoulos CE, Jarrard DF. Newly diagnosed metastatic prostate cancer: has the paradigm changed? Urol Clin North Am. 2017;44:611–621. doi: 10.1016/j.ucl.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Cunningham JJ, Brown JS, Gatenby RA. Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat Commun. 2017;8:1816. doi: 10.1038/s41467-017-01968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruchovsky N, Klotz L, Crook J, Malone S, Ludgate C, Morris WJ. Final results of the Canadian prospective phase II trial of intermittent androgen suppression for men in biochemical recurrence after radiotherapy for locally advanced prostate cancer: clinical parameters. Cancer. 2006;107:389–395. doi: 10.1002/cncr.21989. [DOI] [PubMed] [Google Scholar]
- 7.Crook JM, O’Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain M, Tangem CM, Berry DL, Higano CS, Crawford ED, Liu G. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314–1325. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fizazi K, Tran N, Enrique Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY. LATITUDE: A phase III, double-blind, randomized trial of androgen deprivation therapy with abiraterone acetate plus prednisone or placebos in newly diagnosed high-risk metastatic hormone-naive prostate cancer. J Clin Oncol. 2017;35:LBA3. [Google Scholar]
- 12.Hoyle AP, Ali A, James ND, Cook A, Parker CC, de Bono JS. Abiraterone in “high-” and “low-risk” metastatic hormone-sensitive prostate cancer. Eur Urol. 2019;76:719–728. doi: 10.1016/j.eururo.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Rathkopf DE, Smith MR, de Bono JS, Logothetis CJ, Shore ND, de Souza P. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302) Eur Urol. 2014;66:815–825. doi: 10.1016/j.eururo.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baez J, Kuang Y. Mathematical models of androgen resistance in prostate cancer patients under intermittent androgen suppression therapy. Appl Sci. 2016;6:352. [Google Scholar]
- 15.Elishmereni M, Kheifetz Y, Shukrun I, Bevan GH, Nandy D, McKenzie KM. Predicting time to castration resistance in hormone sensitive prostate cancer by a personalization algorithm based on a mechanistic model integrating patient data. Prostate. 2016;76:48–57. doi: 10.1002/pros.23099. [DOI] [PubMed] [Google Scholar]
- 16.Hirata Y, Bruchovsky N, Aihara K. Development of a mathematical model that predicts the outcome of hormone therapy for prostate cancer. J Theor Biol. 2010;264:517–527. doi: 10.1016/j.jtbi.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Ideta AM, Tanaka G, Takeuchi T, Aihara Z. A mathematical model of intermittent androgen suppression for prostate cancer. J Nonlinear Sci. 2008;18:593–614. [Google Scholar]
- 18.Portz T, Kuang Y., Nagy J. A clinical data validated mathematical model of prostate cancer growth under intermittent androgen suppression therapy. AIP Advances. 2012;2:011002-1–011002-14. [Google Scholar]
- 19.Strobl MAR, West J, Viossat Y, Damaghi M, Robertson-Tessi M, Brown JS. Turnover modulates the need for a cost of resistance in adaptive therapy. Cancer Res. 2020:1135–1147. doi: 10.1158/0008-5472.CAN-20-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brady R, Enderling H. Mathematical models of cancer: when to predict novel therapies, and when not to. Bull Math Biol. 2019:3722–3731. doi: 10.1007/s11538-019-00640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azad AA, Volik SV, Wyatt AW, Haegert A, Le Bihan S, Bell RH. Androgen receptor gene aberrations in circulating cell-free dna: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res. 2015;21:2315–2324. doi: 10.1158/1078-0432.CCR-14-2666. [DOI] [PubMed] [Google Scholar]
- 22.Kohli M, Tan W, Zheng T, Wang A, Montesinos C, Wong C. Clinical and genomic insights into circulating tumor DNA-based alterations across the spectrum of metastatic hormone-sensitive and castrate-resistant prostate cancer. EBioMedicine. 2020;54 doi: 10.1016/j.ebiom.2020.102728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumiyoshi T, Mizuno K, Yamasaki T, Miyazaki Y, Makino Y, Okasho K. Clinical utility of androgen receptor gene aberrations in circulating cell-free DNA as a biomarker for treatment of castration-resistant prostate cancer. Sci Rep. 2019;9:4030. doi: 10.1038/s41598-019-40719-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyatt AW, Azad AA, Volik SV, Annala M, Beja K, McConeghy B. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol. 2016;2:1598–1606. doi: 10.1001/jamaoncol.2016.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scher HI, Morris MJ, Basch E, Heller G. End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. J Clin Oncol. 2011;29:3695–3704. doi: 10.1200/JCO.2011.35.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsu-Ura T, Dovzhenok A, Aihara E, Rood J, Le H, Ren Y. Intercellular coupling of the cell cycle and circadian clock in adult stem cell culture. Mol Cell. 2016;64:900–912. doi: 10.1016/j.molcel.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the tripod statement. ann intern med. 2015;162:55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 28.Thomsen FB, Westerberg M, Garmo H, Robinson D, Holmberg L, Olmert HD. Prediction of metastatic prostate cancer by prostate-specific antigen in combination with T stage and Gleason Grade: Nationwide, population-based register study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gravis G, Boher JM, Joly F, Soulie M, Albiges L, Priou F. Androgen Deprivation Therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 Trial. Eur Urol. 2016;70:256–262. doi: 10.1016/j.eururo.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahl A, Oudard S, Tombal B, Ozgüroglu M, Hansen S, Kocak I. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann Oncol. 2013;24:2402–2408. doi: 10.1093/annonc/mdt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaworska D, Szliszka E. Targeting apoptotic activity against prostate cancer stem cells. Int J Mol Sci. 2017;18:E1648. doi: 10.3390/ijms18081648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saeki N, Gu J, Yoshida T, Wu X. Prostate stem cell antigen: a Jekyll and Hyde molecule? Clin Cancer Res. 2010;16:3533–3538. doi: 10.1158/1078-0432.CCR-09-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]