Abstract
As an alternative to traditional hatching in the hatchery, fertilized incubated eggs can be placed in the rearing barn on embryonic d 18 for hatching to occur on-farm, omitting several hatchery procedures, and transport of day-old chicks. In addition, this practice further allows newly hatched chicks access to feed and water immediately post-hatch. The aim of the present study was to examine welfare implications of hatching slower-growing organic broilers on-farm (OF) using the One2Born system (One2Born, Uden, the Netherlands). Hatchery-hatched chicks (HC) transported to the farm were used as control. Six flocks of both treatments, each comprising approximately 3,600 mixed-sex Hubbard JA57 ColorYield broilers, housed with veranda and outdoor access were included in the study. Compared to HC, the hatchability was higher in OF chicks (95.3% vs. 94.8%; P = 0.0097), whereas the number of second grade chicks was lower (11.6% vs. 16.1%; P < 0.0001). The chick quality was lower for OF than HC (odds ratio: 1.79; P = 0.0009), but this was not reflected in the first week mortality (OF: 0.41%, HC: 0.99%; P < 0.0001) or total mortality (OF: 1.51%, HC: 2.20%; P < 0.0001). No difference was found between treatments for the live body weight at slaughter age (P = 0.73). Breast blisters were more common in HC males than in OF males and in females from both treatments (P = 0.038), whereas OF males and females from the 2 treatments did not differ (P = 0.91). There was no effect of treatment on litter quality, footpad dermatitis, gait, skin injuries, and rejection rates at slaughter (P ≥ 0.35). In conclusion, OF hatching appears to be a viable concept, resulting in reduced mortality and increased hatchability, though knowledge on the topic is sparse. Therefore, more research should be addressed to the welfare implications of hatching OF, specifically to impacts on litter quality, footpad dermatitis, and how chick quality impacts other animal welfare indicators.
Key words: broiler; mortality, on-farm hatching; organic; welfare
INTRODUCTION
Hatching in a hatchery is standard practice that involves day-old chicks undergoing procedures, which are not only stressors affecting the chick in the moment of processing, but also have been shown to affect layer chicks long-term (Hedlund et al., 2019). After hatching, the chicks are separated from the eggshells and unhatched eggs in the egg separator, and subsequently they go through transport on conveyor belts, quality control, photoelectric counting, and lastly, crating (Giersberg et al., 2020a). Depending on the type of production, manual sex-sorting and subcutaneous or spray vaccination may be included in the hatchery. Depending on the hatching window (i.e., the spread of hatch, typically 24–48 h), time needed for hatchery processing, and transportation duration, chicks can experience delays of feed and water intake of up to 72 h post-hatch (Willemsen et al., 2010).
Prolonged feed and water deprivation has been shown to have negative consequences for the growth, development of immune system, and stress response of chicks (Shira et al., 2005; Archer and Mench, 2014; Simon et al., 2015; Hollemans et al., 2018; Hedlund et al., 2019; Uni and Ferket, 2019). In addition, delayed feed intake post-hatch increases the risk of hypothermia (Willemsen et al., 2010) and has adverse effects on mortality and the development of intestines and muscles (Uni and Ferket, 2019). According to Careghi et al. (2005), feed deprivation causes both weight loss during holding time and depressed growth rate after chicks gain access to feed, with the level of depression depending on the time of hatch.
As an alternative to traditional hatching in hatcheries, fertilized and incubated eggs can be placed on the farm on embryonic d 18 (E18) for hatching to take place on-farm (OF), which ensures access to feed and water immediately after hatching. Thus, hatching OF offers a potential solution to the aforementioned issues, while also omitting a range of hatchery procedures and transport of day-old chicks that likewise can negatively affect chick welfare (Bergoug et al., 2013; Jacobs et al., 2016, 2017; Hollemans et al., 2018; Mancinelli et al., 2018; Hedlund et al., 2019; Giersberg et al., 2020b).
In support of the importance of nutritional access in the early postnatal period, previous studies of OF hatching report higher first week body weight in fast-growing broilers hatched OF (de Jong et al., 2019, 2020). Furthermore, a meta-analysis reported repressed development of duodenum, jejunum, and ileum in the first week of life when chicks have late access to feed and water (de Jong et al., 2017). What has also been found in fast-growing broilers hatched OF is a reduced prevalence of footpad dermatitis (de Jong et al., 2019, 2020; Giersberg et al., 2021), a painful condition that develops due to poor litter quality (de Jong et al., 2014). In agreement with this, de Jong et al. (2020) found the dry matter content of litter to be higher in the OF hatching system. However, neither de Jong et al. (2020) nor Giersberg et al. (2021) found differences between barns housing broilers from different hatching locations when visually assessing the litter quality. Improving the development of the digestive tract during the neonatal period by providing early access to feed and water may benefit excreta texture, such that the litter remains dry for longer and consequently, chickens develop fewer footpad lesions.
Previous studies have exposed one drawback to OF hatching; a decrease in chick quality. OF hatched chicks especially show more problems with their navels and hocks (de Jong et al., 2019). These issues are likely due to the microclimate around the eggs being harder to control in a barn environment than in the hatchery. Indeed, it is well-known that heat stress during incubation, particularly during the last week, may result in poor chick quality due to poorly absorbed yolk sacs, leading to unhealed navels (du Preez, 2007; Hamidu et al., 2018; van der Wagt et al., 2020).
Previous studies of OF hatching have focused on fast-growing broilers, but OF hatching also seems to agree with the organic farming principles, where animal welfare is given priority. Therefore, the aim of the present study was to examine how hatching OF of slower growing organic broilers affects hatchability, chick quality, mortality, litter quality, gait, footpad dermatitis, and a range of slaughter parameters. Hatchery-hatched chicks subjected to hatchery procedures and transport was used for comparison. We expected to find positive effects of OF hatching on mortality, gait, footpad dermatitis, and slaughter parameters, but negative effects on hatchability and chick quality due to the delicate control of the microclimate surrounding the eggs being more challenging in the barn than at the hatchery. Furthermore, the effect of transport was examined on chick quality of the hatchery-hatched chicks, with the expectation that chick reflex would be poorer in transported chicks. The effect of hatching location on behavior, first week growth, fear level, and use of an outdoor range was also examined (Jessen et al., 2021).
MATERIALS AND METHODS
The experiment was performed on an organic broiler farm in Denmark from August 2019 to January 2020. The study was performed according to legislation and guidelines concerning organic farming in Denmark (Landbrugsstyrelsen, 2020).
Ethics Statement
The experiment was carried out according to the guidelines of the Danish Animal Experiments Inspectorate with respect to animal experimentation and care of animals under study.
Animals and Housing
Six replicates of 2 mixed-sex treatment flocks of organic broiler chickens (Hubbard JA57 ColorYield) took part in the study, each treatment flock consisting of approximately 3,600 chickens. Males were slaughtered on d 52 to 56 (D52-56, termed D53) and females on D60-63 (termed D60). Catching before slaughter was performed manually by experienced catchers trained in sex-sorting. All chickens were slaughtered at the same commercial slaughter plant located 21.5 km/13.4 miles from the farm.
The farm consisted of 3 buildings, placed adjacent to each other with a 290-m long range in-between, each building housing 2 barns. A plywood wall (H: 63 cm) was placed in the middle of each barn, sectioning the 2 treatment flocks, and in extension of the wall, a vertical net was hung to prevent the chickens from mixing.
There were 3 pop-holes (L × H: 3.5 m × 0.5 m, each) in each section, which were opened on D35, and each treatment flock had immediately thereafter access to a covered veranda (L × W: 22 m × 4 m) and a range (W × L: approximately 125 m × 325 m), each range being separated with a wire mesh fence (H: 1 m). For full details on animals and housing, see Jessen et al. (2021).
Treatments
The study involved 2 treatments. One treatment consisted of chicks undergoing conventional hatchery processing and transport (HC), that is, they hatched at the hatchery, were sorted, vaccinated, transported on conveyer belts, crated, and transported as day-old for approximately one hour before unloading at the farm, while the other treatment consisted of chicks hatched OF using the One2Born system (One2Born, Uden, the Netherlands). The eggs of both treatments originated from the same parental flock, were stored at the same time in the same storage room and incubator at the hatchery (DanHatch A/S, Vrå, Denmark). After candling on E18, the fertilized OF eggs were transported to the barn and the HC eggs to the hatcher in the hatchery. Upon arrival to the barn, the OF eggs were placed in one of 2 sections, leaving room for the HC chicks to be placed in the remaining section on D0. The sections in which each treatment flock was placed alternated for each replication to minimize any location bias. The period from hatching to arrival at the barn was 5 to 25 h for HC chicks, depending on whether they hatched early or late (personal communication, production manager Kim Risgaard Larsen, DanHatch, Vrå, Denmark).
Immediately before arrival of HC chicks, the hatched OF chicks were picked up by hand and sorted into first and second grade, and chicks with either beak deformities, missing eyes or exposed brains, navel infections, lameness, ectopic viscera, or any other abnormality, which decreased the health and welfare of the chick, were culled. The unhatched eggs were placed in a CO2-gas chamber to kill embryos potentially still alive. To ensure similar group sizes of the 2 treatment flocks, any surplus chicks were culled. After sorting, a veterinarian and 2 assistants vaccinated first grade chicks for Marek's disease and Gumboro, coccidiosis and infectious bronchitis according to the vaccination program for slower growing broilers.
Two of the barns were used for 2 replicates each, and the other 2 for one replicate each. The replicates were placed consecutively with 14 d in between each replicate, with the exception that replicates 3 and 4 were separated by 28 d. For additional information on the treatments in the present study, see Jessen et al. (2021).
Data Collection
Data on several parameters were collected, mainly during early life and slaughter, as described in the following paragraphs. The hours and days indicated for different types of data collection are in relation to placement of HC chicks. Up until slaughter of males, data collection was done on mixed-sex flocks (sex was only determined during the chick quality assessment), whereas data collected after D56 were from female chickens.
Hatchability, Grading, and Chick Quality
For both treatments, the number of fertilized eggs placed OF or in the hatcher, the number of unhatched eggs, and the number of second grade chicks were registered. The sorting of chicks into first and second grade was done for the HC chicks by the hatchery personnel and by the farmer and assistants under his supervision for OF chicks. For the OF flocks, the reason for being sorted as second grade was noted for each chick. The quality of the remaining chicks was assessed on D0 using the Pasgar score (Boerjan, 2002) for a random subsample of 100 per treatment. This was done twice for the HC chicks (different subsamples): first at the hatchery and then after arrival to the barn where the assessment was done for both treatments. As such, 100 OF chicks and 200 HC chicks were assessed in total per replication. The same observer did all chick quality assessments, regardless of location. Five criteria were examined (Table 1), and for each abnormality in these criteria, one point was subtracted from the maximum score of 10, ultimately scoring the chick on its overall quality. The lowest possible score was 5 after subtracting a point for each criterion. In addition, each chick was weighed manually on a scale (VIBRA CJ-6200CE, SHINKO DENSHI CO., LTD, Tokyo, Japan; precision ± 0.1 g), and the sex was determined based on the length of the primary remiges and the coverlets (Supplementary Figure S1).
Table 1.
Criteria for assessing chick quality according to the Pasgar scoring system (Boerjan, 2002).
| Criteria | Suboptimal if: |
|---|---|
| Reflex | Chick needed >2 s to bring itself to an upright position after being placed on its back. |
| Navel | Navel was closed with a small white knob, large black knob, otherwise open or smeared with albumen or if there were remnants of yolk. |
| Hocks | Hocks were red, swollen, or malformed. |
| Beak | There was a red dot, if the nostrils were contaminated with albumen, or if the beak was malformed |
| Yolk sac | There was no yolk left, or if yolk sac was too large. |
Mortality
The farmer registered the daily mortality for each treatment flock up until the day of slaughter. For the first seven days, it was also noted whether the chick was found dead (and the presumed cause of death) or had been culled (and reason for culling, e.g., non-starter, deformities, etc.). To determine whether the chick had accessed feed and/or water, the crop content was assessed by external palpation using a 4-point scale (Henriksen et al., 2016).
Gait
The gait of 120 OF chickens and 120 HC chickens was assessed on the day before slaughter of males and females (termed D52 and D59), respectively. Approximately 80 chickens were assessed in the barn, 20 in the veranda and 20 in the outdoor area. During the gait scoring of the females, the pop-holes to the veranda were closed, that is, all 120 chickens were assessed inside the barn. The gait was assessed using the Bristol scale, which spans from 0 to 5 according to increasing difficulty of walking (Kestin et al., 1992; Table 2).
Table 2.
Criteria for assessing litter quality (Welfare Quality, 2009), gait score (Kestin et al., 1992), and footpad dermatitis (Ekstrand et al., 1998).
| Scores | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Litter quality | Completely dry; easily moved and separated |
Dry, but not easily shifted | Leaves boot imprint; forms loosely connected ball | Sticks to bottom of boot; forms readily into a ball | Stick to boot once crust is broken |
- |
| Gait score |
No abnormality; fluid motion | Slight defect, difficult to define | Identifiable defect not hindering movement | Obvious gait defect hindering acceleration ability and maneuverability | Severe gait defect; only moves when forced to and only for few steps | Complete lameness; unable to support weight |
| Footpad dermatitis |
No lesions, scar, or a mild discoloration of <4 mm2 | Minor lesion, discoloration of >4 mm2, superficial lesion or ulcer with epidermal necrosis through to dermis of < 4 mm2 | Severe lesion with epidermal necrosis through to dermis of <4 mm2 + other lesions and discoloration or ulcer with epidermal necrosis >4 mm2 | - | - | - |
When assessing gait in the barn, the observer would start in the left proximate corner of the section and slowly walk up the first corridor. During assessment, whichever chickens in front of the observer were scored. When the end of the corridor was reached, the adjacent corridor was skipped to avoid scoring chickens twice. Additionally, the observer would walk in one side of the corridor, such that chickens would be encouraged to flee in the direction from which the observer had come from. When in the veranda, the observer would go to the barn wall and wait 5 min for the chickens to get accustomed to the presence of a human. Then the observer would walk along the wall, such that chickens would be encouraged to flee in the direction of the outdoor area, which allowed more time for gait scoring than if they fled inside the barn. The location of gait scoring in the outdoor area depended on the distribution of the chickens, but chickens close to the veranda were avoided to reduce the risk of assessing the same chickens twice. All gait assessments were done by the same observer who was trained and had gait scored more than 7,000 broilers prior to the start of the present study. The observer displayed high intraobserver reliability (Cohen's kappa coefficient: κ = 0.90; Landis and Koch, 1977).
Litter Quality
The litter quality was assessed the day before slaughter of males and females, respectively, in a random spot in six locations throughout the section of each treatment by visual inspection using the Welfare Quality scoring protocol (Welfare Quality®, 2009; Table 2). All assessments were done by the same observer. The litter quality was categorized on a scale from 0 to 4: the higher the score, the denser and more humid the litter was.
Footpad Dermatitis
From each treatment flock, 100 feet from each of the sexes were collected from the slaughter plant (2,400 in total). They were assessed on a scale from 0 to 2, according to increasing severity of the lesion (Ekstrand et al., 1998; Table 2). All assessments were done by the same observer having a high intraobserver reliability (κ = 0.80).
Parameters Collected at Slaughter
Data on average live weight, summed footpad score, proportion of chickens with breast blisters, proportion of chickens with skin injuries, and number of rejections for males and females from both treatments were provided by the slaughter plant. In the official veterinarian control at Danish slaughter plants, footpad dermatitis is assessed by a veterinarian or other trained staff using the same protocol as described above (Ekstrand et al., 1998), with the exception that score 1 is replaced by score 0.5. From each flock, a random sample of 50 feet from the first third of a flock and 50 feet from the final third was assessed, and the sum of scores of these 100 feet was the measure provided by the slaughter plant.
Statistical Analyses
Data were processed and analyzed using R 4.0.0 software (R Core Team, 2020). The statistical significance level used was 0.05, whereas P-values between 0.05 and 0.10 (0.05 ≤ P < 0.10) were considered trends toward significance. P-values from post-hoc pairwise comparisons for significant factors with three or more categories were adjusted for multiple testing according to Benjamini and Hochberg (1995), controlling the false discovery rate. To acquire least-squares means and pairwise comparisons, the function ‘emmeans’ from the package ‘emmeans’ v. 1.4.7 was applied.
The number of hatched eggs was determined using 2 different approaches. One method of calculation was according to commercial practice where hatchability is determined as number of hatched first grade chicks divided by number of placed eggs, thus regarding second grade chicks as unhatched eggs. When using the second method of calculation, hatchability was determined as number of hatched first grade and second grade chicks divided by number of placed eggs. Hatchability was examined with a mixed effects logistic regression, that is, generalized linear mixed effects model (GLMM) with binomial distribution and logit link function, using the ‘glmer’ function from the ‘lme4’ package v. 1.1-23 (Bates et al., 2015). The rate of unhatched eggs (mean number per replication among placed eggs) and rate of second grade chicks (mean number per replication among hatched eggs) were analyzed with a Poisson GLMM using ‘glmer’ and with log(placed eggs) and log(hatched eggs) as offsets, respectively. These rates are presented per 1,000 eggs to make them easier to compare and interpret. All 3 models included treatment as fixed effect and replication as random effect.
Chick quality was analyzed with a mixed effects logistic regression after combining the scores to a dichotomous outcome (yes/no) for abnormalities, that is, having a score below 10 or equal to 10. In addition to treatment, sex was assessed as fixed effect and replication was included as random effect. Percentages and standard error for each observed level of score (7/8, 9, and 10) was determined by corresponding dichotomizations and a model only including treatment as fixed effect. For the assessment of HC chicks before and after transport to the farm, a before/after variable was included as fixed effect instead of treatment, and both the Pasgar score and the chick reflex on its own were analyzed. For chick reflex, however, a standard logistic regression was applied with replication included as a factor.
Mortality within the first week and throughout the study was examined with mixed effects logistic regression. The causes of chicks dying the first week were evaluated by normal linear mixed effects model (body weight) and mixed effects logistic regression (nonstarter, empty crop, found dead by barn staff). All models included treatment as fixed effect and replication as random effect.
Gait scores were analyzed by an ordinal mixed effects logistic regression using the function ‘clmm’ from the ‘ordinal’ package v. 2019.12-10. Replication was included as random effect and the fixed effects of treatment, age, and their interaction were examined. Results are shown as odds ratios (ORs) and 95% confidence intervals (CIs) and are calculated from odds of belonging to gait score k or lower (compared to higher than k). The proportional odds assumption means that the effect of treatment and age are the same across all odds, that is, no matter which groups of gait scores are compared, the OR between treatments (or among ages) remains the same.
Litter quality scores were analyzed like gait score (see above). Footpad dermatitis scorings were analyzed for effect of treatment by an ordinal mixed effect logistic regression. In the analyses of footpad dermatitis, 5 out of the 24 samples of feet (6 replicates × 2 treatments × 2 sexes) were by mistake not collected. Furthermore, insufficient markings of the samples obstructed discrimination of the 2 sexes from each flock.
Data on parameters collected at slaughter were analyzed with mixed effects models including replication as random effect and investigating the fixed effects of treatment, sex, and their interaction. The interaction term was removed when not statistically significant. Live weight, the log-transformed summed footpad dermatitis score, and the percentage of chickens with skin injuries were analyzed with normal distribution using ‘lmer’ from ‘lme4’. Number of rejections was analyzed with negative binomial distribution using ‘glmmTMB’ from ‘glmmTMB’ package v. 1.0.1 (Brooks et al., 2017). For the percentage of chickens with breast blisters score, the random effect of replication could not be estimated, and this outcome was therefore analyzed with a normal linear model using the ‘lm’ function from the ‘stats’ base package.
RESULTS
Hatchability and Chick Quality
Results on hatchability, rate of unhatched eggs, and rate of second grade chicks are shown in Table 3. When using the standard method applied at hatcheries for calculating the hatchability, that is, considering the second grade chicks as unhatched, the hatchability was found to be higher for OF than HC. If both first grade and second grade chicks were regarded as hatched, then hatchability did not differ between treatments. Compared to HC, the number of second grade chicks was lower in OF, whereas the number of unhatched eggs did not differ between treatments.
Table 3.
Hatchability (%), number of unhatched eggs (rate per replication), and number of second grade chicks (rate among hatched eggs per replication) for chickens hatched on-farm (OF) and in a hatchery (HC).
| Parameter1 | OF | HC | Ratio | Statistics |
|---|---|---|---|---|
| Hatchability (%) | 95.3 ± 0.44 | 94.8 ± 0.49 | 0.90 (0.82–0.97) | = 6.69, P = 0.0097 |
| Unhatched eggs (‰) | 35.7 ± 3.93 | 36.6 ± 4.03 | 1.03 (0.93–1.13) | = 0.30, P = 0.59 |
| Second grade chicks (‰) | 11.6 ± 0.93 | 16.1 ± 1.17 | 1.38 (1.18–1.63) | = 15.9, P < 0.0001 |
Response estimates are back transformed model means, and the effects are shown as odds ratio (OR [95%CI]) for hatchability and rate ratio (RR) for unhatched eggs and second grade chicks,comparing HC with OF.
The quality of OF chicks was worse compared to the quality of HC chicks after arrival ( = 11.1, P = 0.0009; OR: 1.79, 95% CI: 1.27–2.52), with the odds of having at least one abnormality from the Pasgar scoring system being higher for OF chicks. There was no effect of sex ( = 0.05, P = 0.82; OR (male vs. female): 1.04, 95% CI: 0.74–1.46). For both treatments, most of the chicks were of the highest chick quality, that is, score 10 (OF: 83.8 ± 2.1%, HC: 90.2 ± 1.5%). Score 9 was given to 14.3 ± 1.9% of the OF chicks and 9.3 ± 1.4% of the HC chicks. The percentages of score 8 were 1.8 ± 0.8% for OF chicks and 0.5 ± 0.3% for HC chicks. Score 7 was found only in 0.2% of OF and none of HC chicks. No chicks were scored 6 or 5. The issues causing a decrease in chick quality were suboptimal navel (OF: 8.2%; HC: 5.3%), yolk sac (OF: 5.3%; HC: 2.7%), reflex (OF: 3.5%; HC: 0.3%), hocks (OF: 1.5%; HC: 0%), and beak (OF: 0.2%; HC: 1.2%). Thus, only beak abnormalities were more common in HC than OF, but this was the least frequently observed abnormality.
In HC chicks, the odds of having at least one abnormality after transport did not differ from the odds before transport (Pasgar score: = 0.47, P = 0.49; after vs. before OR: 1.15, 95% CI: 0.78–1.69). Neither did transport affect the odds of presenting a suboptimal reflex (Reflex: χ2=0.07; P = 0.80; after vs. before OR: 1.14, 95% CI: 0.41–3.18). Furthermore, there was no effect of sex (Pasgar score: = 0.05, P = 0.82; OR (male vs. female): 0.96, 95% CI: 0.65–1.41). The effect of body weight, however, was significant (Pasgar score: = 5.17, P = 0.023; OR per extra gram: 1.07, 95% CI: 1.01–1.13), with the odds of having at least one abnormality being higher for heavier chicks.
Mortality
The first week mortality was higher in HC than OF (HC vs. OF OR: 2.41, 95% CI: 1.90–3.06, = 57.2, P < 0.0001), being 0.99 ± 0.18% for HC chicks and 0.41 ± 0.08% for OF chicks. The mean body weights of the OF and HC chicks that died during the first week were 39.2 ± 1.25 g and 37.7 ± 0.99 g, respectively. Most dead chicks were assessed to be nonstarters (OF: 83.4 ± 5.9%, HC: 91.3 ± 3.2%) due to low body weight, and of the dead chicks assessed, 59.2 ± 15.2% of OF chicks and 60.7 ± 14.3% of HC chicks were found to have empty crops. In addition, most chicks were found dead by the barn staff compared to being culled (OF: 75.9 ± 4.9%, HC: 82.2 ± 3.0%).
The total mortality during the rearing period was also higher in HC than OF (OR (HC vs. OF): 1.47, 95% CI: 1.27–1.69, = 28.7, P < 0.0001), being 2.20 ± 0.20% for HC chickens and 1.51 ± 0.14% for OF chickens.
Gait Score
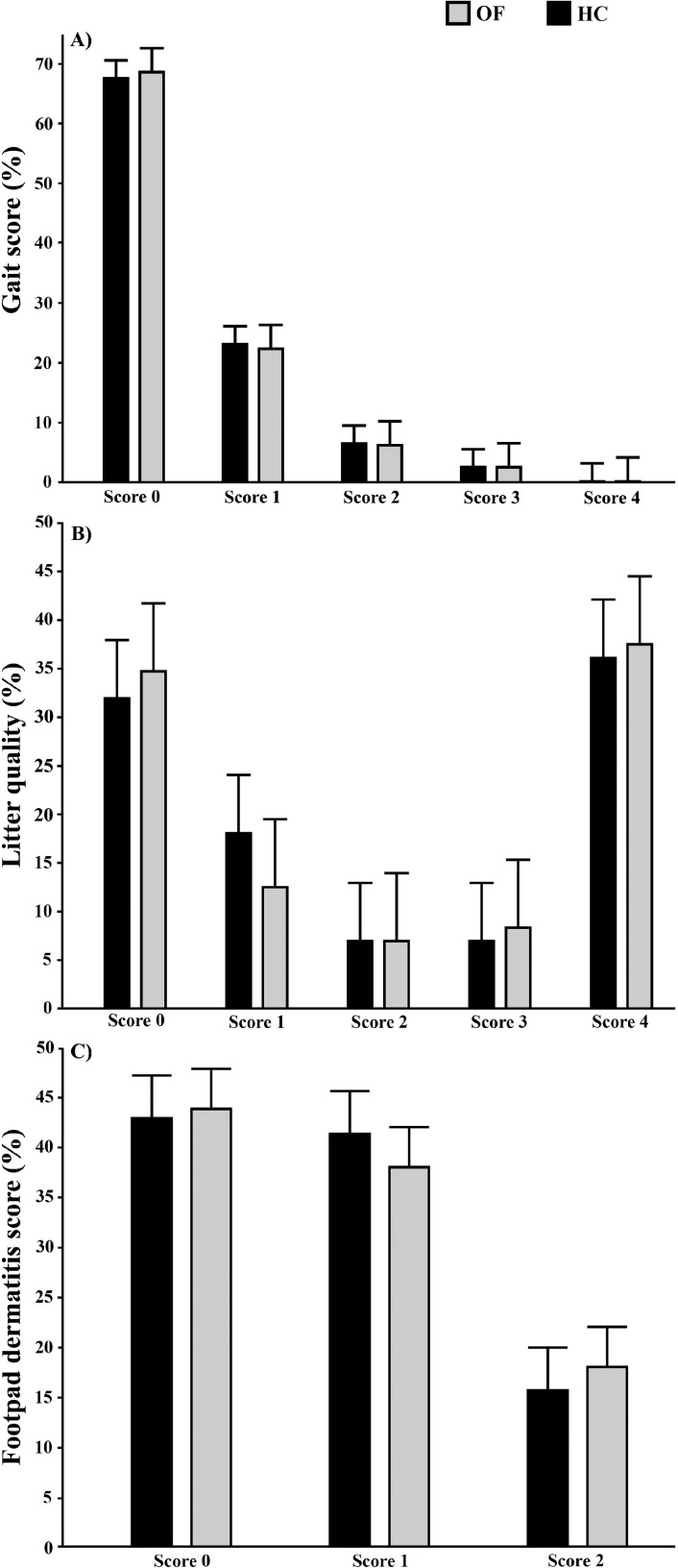
No significant interaction between treatment and age was found for gait score ( = 1.76, P = 0.18). The odds of having a lower gait score, that is, better gait, did not differ between treatments ( = 0.33, P = 0.57; OR [OF vs. HC]: 1.05, 95% CI: 0.90–1.22; Figure 1A). In contrast, a large difference was seen between ages ( = 66.8, P < 0.0001) where the odds of having a lower gait score, that is, better gait, increased with age (OR [D59 vs. D52]: 1.92, 95% CI: 1.64–2.25), that is, females on D59 had better gait scores than the mixed-sex flocks on D52. No chickens were assessed as a score 5 and only 3 chickens in each treatment were assessed as a score 4 (0.2%).
Figure 1.
Relative frequencies (%) of gait (A), litter quality (B), and footpad dermatitis (C) scores for chickens hatched on-farm (OF) and in a hatchery (HC).
Litter Quality
No significant interaction between treatment and age was found for litter quality ( = 0.10, P = 0.75). The odds of a lower score, that is, drier litter, did not differ between treatments ( = 0.01, P = 0.91; OR [OF vs. HC]: 0.97, 95% CI: 0.53–1.76; Figure 1B) or between ages ( = 0.18, P = 0.67; OR [D52 vs. D59]: 1.14, 95% CI: 0.62–2.08).
Footpad Dermatitis
The odds of having a lower footpad dermatitis score, that is, less footpad dermatitis, did not differ between treatments ( = 0.22, P = 0.64; OR (OF vs. HC): 1.04, 95% CI: 0.88–1.24; Figure 1C).
Parameters Collected at Slaughter
The estimates of the different parameters obtained at the slaughter plant are shown in Table 4. The interaction between treatment and sex tended to be significant for breast blisters (F1,20 = 3.17, P = 0.090) with fewer incidences of breast blisters for OF males compared to HC males (t20 = -2.56; P = 0.038), whereas no difference was found between females from the 2 treatments (t20 = −0.04; P = 0.97). For all the remaining parameters, the interaction between treatment and sex was not significant (P ≥ 0.19). No effect of treatment was found for live body weight at slaughter age (F1,16 = 0.13, P = 0.73), summed footpad dermatitis score (F1,16 = 0.94, P = 0.35), skin injuries (F1,16 = 0.01, P = 0.91), and number of rejections ( = 0.12, P = 0.73). Differences between sexes were found for the summed footpad dermatitis score (F1,16 = 5.26, P = 0.036) and skin injuries (F1,16 = 10.2, P = 0.0056) with more females than males suffering from footpad dermatitis, whereas the opposite was true for skin injuries. In contrast, sex had no influence on live body weight at slaughter age (F1,16 = 1.14, P = 0.30) and the number of rejections ( = 0.41, P = 0.52).
Table 4.
Estimates of breast blisters (%), live weight (g), summed footpad dermatitis scores, skin injuries (%) and rejections (N) for chickens hatched on-farm (OF) and in a hatchery (HC). Data were obtained from the slaughter plant.
| Parameter | Sex | OF1 | HC1 |
|---|---|---|---|
| Breast blisters (%) | M | 1.83 ± 0.51a | 3.67 ± 0.51b |
| F | 1.58 ± 0.51 | 1.61 ± 0.51 | |
| Live weight (g) | M | 2033 ± 39.3 | 2039 ± 39.3 |
| F | 1990 ± 39.3 | 2008 ± 39.3 | |
| Summed footpad dermatitis scores | M | 8.8 ± 3.1 | 12.3 ± 4.3 |
| F | 17.8 ± 6.2 | 21.7 ± 7.6 | |
| Skin injuries (%) | M | 2.50 ± 0.46 | 2.33 ± 0.46 |
| F | 1.14 ± 0.46 | 1.22 ± 0.46 | |
| Rejections (No.) | M | 4.68 ± 2.11 | 2.98 ± 1.40 |
| F | 4.03 ± 1.82 | 5.02 ± 2.24 |
a-bOnly the prevalence of breast blisters in males differed between the treatments (P < 0.05).
The model estimates presented are mean±SE.
DISCUSSION
In the present study, positive effects of hatching slow-growing broilers OF were found on mortality and hatchability, as lower mortality was found throughout the rearing period, and, when using the commercial practice of calculation, hatchability was higher. The only negative effect found of OF hatching was a lower chick quality. None of the clinical welfare measures (gait and footpad dermatitis) or production parameters collected at slaughter differed between OF and HC chickens, except for a weak tendency of HC males to have more breast blisters than OF males.
In the few studies reporting hatchability in OF hatching systems, different methods of calculation have been applied. The standard method of calculating hatchability applied at hatcheries is to exclude second grade chicks from the number of hatched chicks, that is, count them in as unhatched. van de Ven et al. (2009) found a higher hatchability in the OF system compared to the hatchery, similar to our findings when using the standard method, but van de Ven et al. (2009) included second grade chicks as hatched chicks in the calculation of hatchability OF, whereas they were excluded in the hatchability calculated for the hatchery chicks. In contrast, de Jong et al. (2019) found no difference in hatchability when using the standard method for calculation for both treatments. The present study and Souza da Silva et al. (2021) found a similar result when second grade chicks were included as hatched chicks. Thus, one may question whether hatching OF in fact does result in better hatchability, or whether it is an artifact of some second grade chicks being overlooked during the sorting at the farm. In support of this, the staff at the hatchery was more experienced in performing this selection, and the settings for sorting were better optimized at the hatchery. Nevertheless, the results on hatchability from the present and previous studies demonstrate that hatching OF is a viable concept.
In line with other studies, a reduced chick quality was found for chicks hatched OF (van de Ven et al., 2012; de Jong et al., 2019, 2020). Souza da Silva et al. (2021) reported conflicting results as they found that OF hatched chicks had worse navel and hocks, but were longer, the latter being considered a positive chick quality indicator. The difference between treatments could be linked to more second grade chicks being sorted out at the hatchery compared to at the farm, again insinuating that some second grade chicks were overlooked during the sorting at the farm. However, van de Ven et al. (2012) have shown that second grade chicks have a high early mortality (62.5% at D7). As the first week mortality in the present study was in fact lower, not higher, for the OF chicks, we find no support for the suggestion that the reduced quality of OF chicks was due to more second grade chicks being included in the OF flocks. Further supporting this is that second grade chicks have poorer growth and slaughter weight than first grade chicks (van de Ven et al., 2012), but we found no difference in slaughter weight between OF and HC chickens in the present study, which is in agreement with most previous studies (van de Ven et al., 2011; de Jong et al., 2019, 2020). Only Souza da Silva et al. (2021) report a difference in slaughter weight between chicks hatched either OF or at the hatchery, but with the OF hatched chicks weighing more.
Alternatively, the reduced quality of OF chicks may be attributed to suboptimal temperature or humidity conditions during transport on E18 and the hatching period on the farm, where it is more problematic to delicately manage the microclimate compared to in the hatchery facilities. Heat stress during incubation can lead to poorly absorbed yolk sacs, decreasing the chick quality due to unhealed navels (du Preez, 2007; Hamidu et al., 2018; van der Wagt et al., 2020). Like reporting from previous studies (van de Ven et al., 2012; de Jong et al., 2019), enlarged residual yolk sacs and open navels were the 2 abnormalities most frequently registered during the chick quality assessment in the present study. One study has found unhealed navels to result in higher chick mortality, likely due to being the entry point of pathogenic bacteria (Fasenko and O'Dea, 2008). However, other studies report no or limited evidence of a relationship between Pasgar score and performance or mortality (Willemsen et al., 2008; van de Ven et al., 2012). In the present study, the higher odds of poor chick quality in OF chicks on D0 did not decrease their first week mortality, or the total mortality. In contrast, both were lower compared to the mortality of HC chicks, which is in accordance with findings in previous studies (van de Ven et al., 2009; de Jong et al., 2020). Thus, we found no indications that reduced chick quality, based on the Pasgar score, negatively affects animal welfare, but due to conflicting results this topic merits further research, preferably including other welfare indicators in addition to mortality and examinations at the level of individuals.
Interestingly, we found that heavier chicks had higher odds of having at least one abnormality in the Pasgar scoring system. In support of this, chick quality assessed either using Tona or Pasgar score has been found in previous studies to be lower in heavier chicks (Willemsen et al., 2008; van de Ven et al., 2012). In these studies, the heavier chicks originated from older breeders. It is well known that the age of the breeder flock influences egg composition, egg weight, and chick weight at hatch with the latter 2 increasing with breeder age (Suarez et al., 1997; Tona et al., 2004; Nangsuay et al., 2011, 2013). In the present study, breeder age varied between the replicates, and we therefore speculate whether the heavier chicks with lower Pasgar scores were offspring from older breeders.
Disorientation has been reported in broiler chicks after exposure to hatchery procedures, leading to reduced righting time in the reflex test (Knowles et al., 2004). We were interested in knowing whether transport would have further negative effects on the chick reflex, and perhaps even lead to a reduction in overall chick quality measured as Pasgar score, which includes chick reflex as one of 5 parameters assessed. However, no such effect was found, that is, the 1-h of transport that the HC chicks were exposed to, did not reduce chick quality.
No difference between treatments were found on gait score, and neither have previous studies of hatching OF found any effects on walking abilities (de Jong et al., 2019, 2020; Giersberg et al., 2021). The finding that females had better gait scores than the mixed-sex flocks, although they were 1 wk older, aligns with the results found in a survey of walking ability in Danish organic broiler flocks (Tahamtani et al., 2018). It likely reflects that growth rate, which is higher in males than in females, and gait score are positively correlated (Kestin et al., 2001; Angel, 2007). We found no difference between treatments in the use of an outdoor area (reported in Jessen et al., 2021), which aligns well with the lack of treatment effect on gait score, as leg health is a major contributing factor in whether or not an outdoor area will be properly utilized by broilers (Taylor et al., 2020).
Footpad dermatitis is considered painful (Michel et al., 2012) and has been shown to cause behavioral changes in turkeys (Mayne et al., 2007; Hocking and Wu, 2013; Sinclair et al., 2015). In contrast to findings in fast-growing broilers (de Jong et al., 2019, 2020), OF hatching of the slower-growing broilers in the present study did not improve litter quality or reduce occurrence of footpad dermatitis. The cause of the improved litter condition during previous studies of OF hatching has not been established. However, it has been suggested that early feeding benefits development of the gastrointestinal system, improving the texture of excreta (de Jong et al., 2020). If this is the cause of the reduced footpad dermatitis observed in fast-growing broilers, then a possible explanation of the lack of effect of OF hatching on footpad dermatitis in the present study could be a better intestinal integrity per se in slower-growing broilers compared to fast-growing broilers. Other potentially influencing factors that differ between organic and conventional farming are stocking density and feed composition. Whichever the cause, it may also have been counteracted by the outdoor access of the broilers in the present study. During the experimental period in the present study, the average monthly rainfall was high (101 ± 21 mm, measured at the nearest official weather station (2020), increasing the risk of broilers bringing in moist after visits to the outdoor area in addition to the difficulties of managing relative humidity indoor with open pop-holes. Even in broilers housed without outdoor access, a positive correlation has been demonstrated between outdoor relative humidity and occurrence of footpad dermatitis (Ekstrand and Carpenter, 1998). Further studies are needed for clarification of the reasons for the diverging results on footpad dermatitis in fast-growing and slower-growing broilers.
The assessments of footpad dermatitis performed at the slaughter plant were in agreement with the more thorough assessments of feet evaluated in the laboratory. No previous studies have reported the effect of hatching location on rejection rates, but skin injuries was assessed by Giersberg et al. (2021), although in live fast-growing broilers, and in accordance with the present study, they found no differences between OF and hatchery-hatched chickens. Furthermore, Giersberg et al. (2021) examined for breast blisters, but found only one occurrence during the entire study. In contrast, we found that 1.6 TO 3.7% of the broilers had breast blisters, with the hatchery-hatched males having, for unknown reasons, about twice as high an occurrence than the OF hatched males and females from the 2 treatments.
The post-hatch deprivation period was relatively short in the present study (5–25 h depending on hatching time within the hatching window), which is nearly within the 24 h limit previously suggested to be the threshold where weight at slaughter is not negatively affected by fasting post-hatch (Gonzales et al., 2003). The longer the deprivation period, the larger the consequences for growth and mortality (Gonzales et al., 2003; de Jong et al., 2017). By extension, it seems reasonable to assume that the differences found between hatching locations would be amplified, had the deprivation period of HC chicks been longer.
CONCLUSIONS
Based on the positive results on reduced mortality and increased hatchability found in the present study, hatching OF of slower-growing broilers appears to be an animal welfare friendly and viable concept. The only negative effect of OF hatching was a lower chick quality, but to what extent that impacted animal welfare is unknown, other than it did not affect mortality and performance. As knowledge on OF hatching is limited, more research should be conducted, examining welfare consequences of hatching in OF systems, including possible effects on footpad dermatitis and chick quality, and on how chick quality impact other animal welfare indicators than those addressed in the present study.
Acknowledgments
ACKNOWLEDGMENTS
We are grateful to Estelle Leroux, Agrocampus Ouest, France, for assisting with data collection and data management during her internship. We thank post doc Fernanda Tahamtani for assisting with data collection and Dr. Ingrid de Jong, Wageningen Livestock Research, the Netherlands, for valuable feedback on an earlier version of this manuscript. A special thanks to all the different segments of the broiler production chain – without their willingness to participate, we could not have conducted this study. The research described in this paper has been commissioned and funded by the Ministry of Environment and Food of Denmark as part of the “Contract between Aarhus University and Ministry of Environment and Food for the provision of research-based policy advice at Aarhus University, 2017-2020”.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2021.101292.
Appendix. Supplementary materials
REFERENCES
- Angel R. Metabolic disorders: llimitations to growth of and mineral deposition into the broiler skeleton after hatch and potential implications for leg problems. J. Appl. Poult. Res. 2007;16:138–149. [Google Scholar]
- Archer G.S., Mench J.A. Natural incubation patterns and the effects of exposing eggs to light at various times during incubation on post-hatch fear and stress responses in broiler (meat) chickens. Appl. Anim. Behav. Sci. 2014;152:44–51. [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- Bergoug H., Guinebretiere M., Tong Q., Roulston N., Romanini C.E., Exadaktylos V., Berckmans D., Garain P., Demmers T.G., McGonnell I.M., Bahr C., Burel C., Eterradossi N., Michel V. Effect of transportation duration of 1-day-old chicks on postplacement production performances and pododermatitis of broilers up to slaughter age. Poult. Sci. 2013;92:3300–3309. doi: 10.3382/ps.2013-03118. [DOI] [PubMed] [Google Scholar]
- Boerjan M. Programs for single stage incubation and chick quality. Avian Poult. Biol. Rev. 2002;13:237–238. [Google Scholar]
- Brooks M.E., Kristensen K., Benthem K.J.v., Magnusson A., Berg C.W., Nielsen A., Skaug H.J., Mächler M., Bolker B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R J. 2017;9:378–400. [Google Scholar]
- Careghi C., Tona K., Onagbesan O., Buyse J., Decuypere E., Bruggeman V. The effects of the spread of hatch and interaction with delayed feed access after hatch on broiler performance until seven days of age. Poult. Sci. 2005;84:1314–1320. doi: 10.1093/ps/84.8.1314. [DOI] [PubMed] [Google Scholar]
- de Jong I.C., Gunnink H., van Harn J. Wet litter not only induces footpad dermatitis but also reduces overall welfare, technical performance, and carcass yield in broiler chickens. J. Appl. Poult. Res. 2014;23:51–58. [Google Scholar]
- de Jong I.C., Gunnink H., van Hattum T., van Riel J.W., Raaijmakers M.M.P., Zoet E.S., van den Brand H. Comparison of performance, health and welfare aspects between commercially housed hatchery-hatched and on-farm hatched broiler flocks. Animal. 2019;13:1269–1277. doi: 10.1017/S1751731118002872. [DOI] [PubMed] [Google Scholar]
- de Jong I.C., van Hattum T., van Riel J.W., De Baere K., Kempen I., Cardinaels S., Gunnink H. Effects of on-farm and traditional hatching on welfare, health, and performance of broiler chickens. Poult. Sci. 2020;99:4662–4671. doi: 10.1016/j.psj.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong I.C., van Riel J., Bracke M.B.M., van den Brand H. A 'meta-analysis' of effects of post-hatch food and water deprivation on development, performance and welfare of chickens. PLoS One. 2017;12:20. doi: 10.1371/journal.pone.0189350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2020.DMI. 2020. Vejrarkiv [In Danish: Weather archive]. Accessed June 2020. https://www.dmi.dk/vejrarkiv/
- du Preez J.H. University of Stellenbosch; Stellenbosch, South Africa: 2007. The Effect of Different Incubation Temperatures on Chick Quality. MPhil Thesis. [Google Scholar]
- Ekstrand C., Carpenter T.E. Temporal aspects of footpad dermatitis in Swedish broilers. Acta Vet. Scand. 1998;39:229–236. doi: 10.1186/BF03547795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand C., Carpenter T.E., Andersson I., Algers B. Prevalence and control of foot-pad dermatitis in broilers in Sweden. Br. Poult. Sci. 1998;39:318–324. doi: 10.1080/00071669888845. [DOI] [PubMed] [Google Scholar]
- Fasenko G.M., O'Dea E.E. Evaluating broiler growth and mortality in chicks with minor navel conditions at hatching. Poult. Sci. 2008;87:594–597. doi: 10.3382/ps.2007-00352. [DOI] [PubMed] [Google Scholar]
- Giersberg M.F., Molenaar R., Pieters R., Boyer W., Rodenburg T.B. Effects of drop height, conveyor belt speed, and acceleration on the welfare of broiler chickens in early and later life. Poult. Sci. 2020;99:6293–6299. doi: 10.1016/j.psj.2020.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giersberg M.F., Poolen I., de Baere K., Gunnink H., van Hattum T., van Riel J.W., de Jong I.C. Comparative assessment of general behaviour and fear-related responses in hatchery-hatched and on-farm hatched broiler chickens. Appl. Anim. Behav. Sci. 2020;232 [Google Scholar]
- Giersberg M.F., Molenaar R., de Jong I.C., Souza da Silva C., van den Brand H., Kemp B., Rodenburg T.B. Effects of hatching system on the welfare of broiler chickens in early and later life. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales E., Kondo N., Saldanha E.S., Loddy M.M., Careghi C., Decuypere E. Performance and physiological parameters of broiler chickens subjected to fasting on the neonatal period. Poult. Sci. 2003;82:1250–1256. doi: 10.1093/ps/82.8.1250. [DOI] [PubMed] [Google Scholar]
- Hamidu J.A., Torres C.A., Johnson-Dahl M.L., Korver D.R. Physiological response of broiler embryos to different incubator temperature profiles and maternal flock age during incubation. 1. Embryonic metabolism and day-old chick quality. Poult. Sci. 2018;97:2934–2946. doi: 10.3382/ps/pey089. [DOI] [PubMed] [Google Scholar]
- Hedlund L., Whittle R., Jensen P. Effects of commercial hatchery processing on short- and long-term stress responses in laying hens. Sci. Rep. 2019;9:10. doi: 10.1038/s41598-019-38817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen S., Bilde T., Riber A.B. Effects of post-hatch brooding temperature on broiler behavior, welfare, and growth. Poult. Sci. 2016;95:2235–2243. doi: 10.3382/ps/pew224. [DOI] [PubMed] [Google Scholar]
- Hocking P.M., Wu K. Traditional and commercial turkeys show similar susceptibility to foot pad dermatitis and behavioural evidence of pain. Br. Poult. Sci. 2013;54:281–288. doi: 10.1080/00071668.2013.781265. [DOI] [PubMed] [Google Scholar]
- Hollemans M.S., de Vries S., Lammers A., Clouard C. Effects of early nutrition and transport of 1-day-old chickens on production performance and fear response. Poult. Sci. 2018;97:2534–2542. doi: 10.3382/ps/pey106. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Delezie E., Duchateau L., Goethals K., Ampe B., Buyse J., Tuyttens F.A.M. Impact of transportation duration on stress responses in day-old chicks from young and old breeders. Res. Vet. Sci. 2017;112:172–176. doi: 10.1016/j.rvsc.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Delezie E., Duchateau L., Goethals K., Ampe B., Lambrecht E., Gellynck X., Tuyttens F.A. Effect of post-hatch transportation duration and parental age on broiler chicken quality, welfare, and productivity. Poult. Sci. 2016;95:1973–1979. doi: 10.3382/ps/pew155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen C.T., Foldager L., Riber A.B. Effects of hatching on-farm on behaviour, first week performance, fear level and range use of organic broilers. Appl. Anim. Behav. Sci. 2021;238 doi: 10.1016/j.psj.2021.101292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestin S.C., Gordon S., Su G., Sørensen P. Relationships in broiler chickens between lameness, liveweight, growth rate and age. Vet. Rec. 2001;148:195–197. doi: 10.1136/vr.148.7.195. [DOI] [PubMed] [Google Scholar]
- Kestin S.C., Knowles T.G., Tinch A.E., Gregory N.G. Prevalence of leg weakness in broiler chickens and its relationship with genotype. Vet. Rec. 1992;131:190–194. doi: 10.1136/vr.131.9.190. [DOI] [PubMed] [Google Scholar]
- Knowles T.G., Brown S.N., Warriss P.D., Butterworth A., Hewitt L. Welfare aspects of chick handling in broiler and laying hen hatcheries. Anim. Welf. 2004;13:409–418. [Google Scholar]
- Landbrugsstyrelsen. 2020. Vejledning for økologisk jordbrugsproduktion [In Danish: Guidelines for organic farming]. Accessed June 2020. https://lbst.dk/fileadmin/user_upload/NaturErhverv/Filer/Indsatsomraader/Oekologi/Jordbrugsbedrifter/Vejledning_til_oekologisk_jordbrugsproduktion/OEkologivejledning_februar2020.pdf
- Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Mancinelli A.C., Mugnai C., Castellini C., Mattioli S., Moscati L., Piottoli L., Amato M.G., Doretti M., Dal Bosco A., Cordovani E., Abbate Y., Ranucci D. Effect of transport length and genotype on tonic immobility, blood parameters and carcass contamination of free-range reared chickens. Ital. J. Anim. Sci. 2018;17:557–564. [Google Scholar]
- Mayne R.K., Else R.W., Hocking P.M. High litter moisture alone is sufficient to cause footpad dermatitis in growing turkeys. Br. Poult. Sci. 2007;48:538–545. doi: 10.1080/00071660701573045. [DOI] [PubMed] [Google Scholar]
- Michel V., Prampart E., Mirabito L., Allain V., Arnould C., Huonnic D., Le Bouquin S., Albaric O. Histologically-validated footpad dermatitis scoring system for use in chicken processing plants. Br. Poult. Sci. 2012;53:275–281. doi: 10.1080/00071668.2012.695336. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Meijerhof R., Ruangpanit Y., Kemp B., van den Brand H. Energy utilization and heat production of embryos from eggs originating from young and old broiler breeder flocks. Poult. Sci. 2013;92:474–482. doi: 10.3382/ps.2012-02643. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Ruangpanit Y., Meijerhof R., Attamangkune S. Yolk absorption and embryo development of small and large eggs originating from young and old breeder hens. Poult. Sci. 2011;90:2648–2655. doi: 10.3382/ps.2011-01415. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Shira E.B., Sklan D., Friedman A. Impaired immune responses in broiler hatchling hindgut following delayed access to feed. Vet. Immunol. Immunopathol. 2005;105:33–45. doi: 10.1016/j.vetimm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Simon K., de Vries Reilingh G., Bolhuis J.E., Kemp B., Lammers A. Early feeding and early life housing conditions influence the response towards a noninfectious lung challenge in broilers. Poult. Sci. 2015;94:2041–2048. doi: 10.3382/ps/pev189. [DOI] [PubMed] [Google Scholar]
- Sinclair A., Weber Wyneken C., Veldkamp T., Vinco L.J., Hocking P.M. Behavioural assessment of pain in commercial turkeys (Meleagris gallopavo) with foot pad dermatitis. Br. Poult. Sci. 2015;56:511–521. doi: 10.1080/00071668.2015.1077204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza da Silva C., Molenaar R., Giersberg M.F., Rodenburg T.B., van Riel J.W., De Baere K., Van Dosselaer I., Kemp B., van den Brand H., de Jong I.C. Day-old chicken quality and performance of broiler chickens from 3 different hatching systems. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez M.E., Wilson H.R., Mather F.B., Wilcox C.J., McPherson B.N. Effect of strain and age of the broiler breeder female on incubation time and chick weight. Poult. Sci. 1997;76:1029–1036. doi: 10.1093/ps/76.7.1029. [DOI] [PubMed] [Google Scholar]
- Tahamtani F.M., Hinrichsen L.K., Riber A.B. Welfare assessment of conventional and organic broilers in Denmark, with emphasis on leg health. Vet. Rec. 2018;183:192. doi: 10.1136/vr.104817. [DOI] [PubMed] [Google Scholar]
- Taylor P.S., Hemsworth P.H., Groves P.J., Gebhardt-Henrich S.G., Rault J.L. Frequent range visits further from the shed relate positively to free-range broiler chicken welfare. Animal. 2020;14:138–149. doi: 10.1017/S1751731119001514. [DOI] [PubMed] [Google Scholar]
- Tona K., Onagbesan O., De Ketelaere B., Decuypere E., Bruggeman V. Effects of age of broiler breeders and egg storage on egg quality, hatchability, chick quality, chick weight, and chick posthatch growth to forty-two days. J. Appl. Poult. Res. 2004;13:10–18. [Google Scholar]
- Uni Z., Ferket R.P. Methods for early nutrition and their potential. Worlds Poult. Sci. J. 2019;60:101–111. [Google Scholar]
- van de Ven L.J., van Wagenberg A.V., Debonne M., Decuypere E., Kemp B., van den Brand H. Hatching system and time effects on broiler physiology and posthatch growth. Poult. Sci. 2011;90:1267–1275. doi: 10.3382/ps.2010-00876. [DOI] [PubMed] [Google Scholar]
- van de Ven L.J., van Wagenberg A.V., Groot Koerkamp P.W., Kemp B., van den Brand H. Effects of a combined hatching and brooding system on hatchability, chick weight, and mortality in broilers. Poult. Sci. 2009;88:2273–2279. doi: 10.3382/ps.2009-00112. [DOI] [PubMed] [Google Scholar]
- van de Ven L.J., van Wagenberg A.V., Uitdehaag K.A., Groot Koerkamp P.W., Kemp B., van den Brand H. Significance of chick quality score in broiler production. Animal. 2012;6:1677–1683. doi: 10.1017/S1751731112000663. [DOI] [PubMed] [Google Scholar]
- van der Wagt I., de Jong I.C., Mitchell M.A., Molenaar R., van den Brand H. A review on yolk sac utilization in poultry. Poult. Sci. 2020;99:2162–2175. doi: 10.1016/j.psj.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welfare Quality® . Welfare Quality Consortium; Lelystad, Netherlands: 2009. Welfare Quality Assessment Protocol for Poultry. [Google Scholar]
- Willemsen H., Debonne M., Swennen Q., Everaert N., Careghi C., Han H., Bruggeman V., Tona K., Decuypere E. Delay in feed access and spread of hatch: importance of early nutrition. Worlds Poult. Sci. J. 2010;66:177–188. [Google Scholar]
- Willemsen H., Everaert N., Witters A., De Smit L., Debonne M., Verschuere F., Garain P., Berckmans D., Decuypere E., Bruggeman V. Critical assessment of chick quality measurements as an indicator of posthatch performance. Poult. Sci. 2008;87:2358–2366. doi: 10.3382/ps.2008-00095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.