Abstract
Baricitinib is a Janus kinase (JAK) inhibitor that selectively blocks against JAK1 and JAK2 signaling. This study aimed to determine the effect of baricitinib on disease activity based on musculoskeletal ultrasound in patients with rheumatoid arthritis (RA).
A total of 20 patients with RA receiving baricitinib for 24 weeks were assessed. Ultrasound scores of gray scale and power Doppler synovitis, joint effusion, and bone erosion in each patient were assessed between baseline and 24 weeks for 27 affected joints. Disease activity in RA was evaluated using the disease activity score for 28-joint count with erythrocyte sediment rate (DAS28-ESR), simplified disease activity index (SDAI), and clinical disease activity index (CDAI).
Treatment with baricitinib for 12 weeks and 24 weeks significantly decreased disease activity composites such as DAS28-ESR, SDAI, and CDAI (P < .001 for all). Treatment with baricitinib for 24 weeks improved ultrasound-detected gray-scale and power Doppler synovitis and joint effusion compared to baseline (P = .002, P = .030, and P = .002, respectively). Bone erosion scores were not different between baseline and 24 weeks (P = .317). There were no differences in ultrasound abnormalities for improvement based on DAS28-ESR. Changes in power Doppler score were significantly associated with changes in DAS28-ESR (β = 0.590, P = .044), but not SDAI and CDAI.
This study demonstrates that baricitinib treatment has a favorable effect on ultrasound-detected abnormalities including synovitis and bone erosion in patients with RA.
Keywords: baricitinib, disease activity, rheumatoid arthritis, synovitis, ultrasound
1. Introduction
Rheumatoid arthritis (RA) is a complex autoimmune disease characterized by involvement of articular and extra-articular structures that ultimately leads to functional disability and poor quality of life.[1] The main target of RA treatment is to improve clinical symptoms and signs, suppress inflammatory changes within affected joints, and prevent structural joint damage. Reliable clinical measures have been developed to assess arthritis in clinical trials and daily practice, such as the disease activity score in 28 joints erythrocyte sedimentation rate (DAS28-ESR), simplified disease activity index (SDAI), and clinical disease activity index (CDAI).[2] Furthermore, musculoskeletal ultrasound is an important imaging tool used to assess disease activity and monitor treatment response.[3] Ultrasound has been recognized as an imaging biomarker to evaluate treatment monitoring in patients with RA treated with biological disease-modifying antirheumatic drugs (bDMARDs) or targeted synthetic DMARDs.[4,5,6]
Baricitinib is a small-molecule Janus kinase (JAK) inhibitor that selectively suppresses intracellular cell signaling against JAK1 and JAK2, but has weak activity against JAK3.[7] It was developed to treat patients with refractory RA who do not respond to conventional DMARDs. Extensive evidence has demonstrated that baricitinib has safety and clinical efficacy in managing patients with RA.[8,9,10,11] Several studies demonstrated that baricitinib blocked structural joint damage through significant inhibition of radiographic progression in patients with RA and clinical improvement.[9,12,13] Ultrasound findings after short-term baricitinib treatment for 3 months were associated with clinical disease activity markers.[14] However, there is still a lack of data using ultrasound to evaluate treatment response to baricitinib. The aim of this study is to assess the clinical usefulness of ultrasound in monitoring disease activity in patients with RA treated with baricitinib for 24 weeks.
2. Materials and methods
2.1. Study populations
This study included 20 patients who met the 1987 American College of Rheumatology revised classification criteria for RA and were enrolled from September 2019 to July 2020.[15] Subjects who participated in the study were patients who started baricitinib treatment due to insufficient response to treatment with conventional synthetic disease modifying antirheumatic drugs (csDMARDs) and/or bDMARDs. All patients received baricitinib 4 mg for 24 weeks and completed ultrasound examinations both at baseline and at 24 weeks were included in the study. This study was approved by the Institutional Review Board at Daegu Catholic University Medical Center (CR-20-154-L).
2.2. Collection of clinical information
Clinical data at baseline included age, gender, and disease duration at study enrollment. At the first administration of baricitinib, other medications used simultaneously were identified, such as methotrexate, corticosteroids, and nonsteroidal anti-inflammatory drugs.
The disease activity of patients with RA was assessed at both baseline and at 24 weeks after enrollment. Markers for disease activity included tender joint count (TJC), swollen joint count (SJC), patients global visual analog scale (VAS, mm), physician VAS, C-reactive protein (mg/L), and erythrocyte sediment rate (ESR, mm/h). After using these individual parameters, disease activity indexes including the DAS28-ESR, SDAI, and CDAI were calculated using the following formulas: DAS28 = 0.56 × √(TJC28) + 0.28 × √(SJC28) + 0.70 × ln (ESR) + 0.014 × patient global VAS (0–100 mm), SDAI = TJC28 + SJC28 + CRP (mg/L)/10 + patient global VAS (0– 100 mm)/10 + physician global VAS (0–100 mm)/10, and CDAI = TJC28 + SJC28 + patient global VAS (0–100 mm)/10 + physician global VAS (0–100 mm)/10.
2.3. Ultrasound assessment
Ultrasound-based joint assessment was conducted by evaluating 1 or 2 of the most painful joints at baseline and then reassessing the same affected joint after 24 weeks of baricitinib treatment (Fig. 1). One rheumatologist (June UH) who completed a training program provided by the Korean College of Rheumatology performed ultrasound examinations. Ultrasound examinations used the ACUSON S2000 Ultrasound System (Siemens Healthineers, Seoul, Korea) with a 5 to 14 MHz linear transducer (14L5).
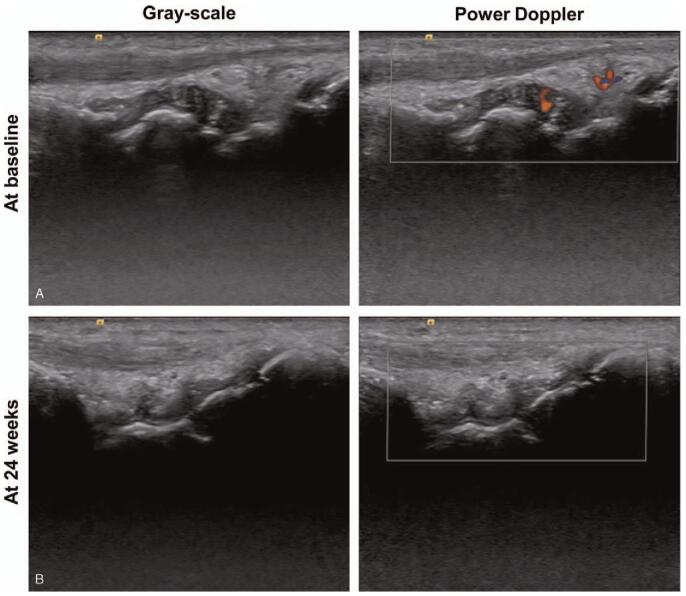
Figure 1.
Ultrasound images at baseline and 24 wk after baricitinib treatment in rheumatoid arthritis. Representative figure for gray-scale and power Doppler ultrasound images of wrist joint in a patient with rheumatoid arthritis. (A) Gray-scale synovial hypertrophy and joint effusion with widening of joint space and Doppler synovitis was also noted at baseline. (B) Gray-scale synovial hypertrophy and joint effusion within joint space was decreased and power Doppler signal was disappeared after treatment with baricitinib 4 mg for 24 wk.
The 27 joints included in the joint ultrasound assessment were knee (n = 4), elbow (n = 4), foot (n = 1), shoulder (n = 10), wrist (n = 5), and hand joints (n = 3). Ultrasound was performed at standardized joint positions and a probe application was used for each joint. Ultrasound scoring of affected joints for joint effusion, gray-scale synovial hypertrophy, power Doppler synovitis, and bone erosion was performed according to the definition provided by Szkudlarek et al (Supplemental Digital Content Table 1).[16]
Intraclass correlation coefficient with 95% confidential interval (CI) for intraobserver reliability was calculated; 0.945 (95% CI 0.846–0.981) for joint effusion, 0.832 (95% CI 0.571–0.940) for gray-scale synovial hypertrophy, 0.822 (95% CI 0.549–0.936) for power Doppler synovitis, 0.896 (95% CI 0.719–0.964) for bone erosion.
2.4. Statistical analysis
Data were described as number with percentage (%) for nominal variables and median with interquartile range (IQR) for continuous variables. The difference in ultrasound findings such as joint effusion, gray-scale synovitis, power Doppler synovitis, and bone erosion between baseline and 24 weeks was assessed by Wilcoxon signed rank test. The differences in changes of ultrasound findings among 3 treatment response groups (good, moderate, and no improvement based on DAS28-ESR) were assessed by Kruskal–Wallis test. Correlation between changes of DAS28-ESR and ultrasound findings at 24 weeks was calculated using multivariate regression analysis after adjusting confounding factors. Statistical significance was considered to be P < .05. Statistical analyses were performed using IBM SPSS Statistics 19.0 (IBM Corp., Armonk, NY).
3. Results
3.1. Baseline characteristics of enrolled patients
A total of 20 patients who were treated with baricitinib for 24 weeks was enrolled in this study (Table 1). The median age of patients was 53.5 years (IQR 47.5 – 61.3). Most patients were female (n = 15, 75.0%). The median disease duration was 85.0 months (IQR 31.5 – 158.8). Disease activity markers including SJC, TJC, patient VAS, physician VAS, ESR, and CRP are illustrated in Table 1. Five patients who failed to achieve clinical response by bDMARDs and 15 patients who were not exposed to any bDMARDs were included in this study. Twenty-seven joints in 20 patients were assessed in this study, including knee (n = 4), elbow (n = 4), foot (n = 1), shoulder (n = 10), wrist (n = 5), and hand joints (n = 3).
Table 1.
Baseline characteristics in enrolled patients (n = 20).
| Variables | Results |
| Age (yr) | 53.5 (47.5–61.3) |
| Sex, female (n, %) | 15 (75.0) |
| Disease duration (mo) | 85.0 (31.5–158.8) |
| Hypertension (n, %) | 3 (15.0) |
| Diabetes mellitus (n, %) | 1 (5.0) |
| RF positivity (n, %) | 15 (75.0) |
| Anti-CCP antibody positivity (n, %) | 17 (85.0) |
| Disease activity markers | |
| Swollen joint count | 4.0 (2.0–6.5) |
| Tender joint count | 7.0 (2.3–10.8) |
| Patient VAS | 70.0 (60.0–80.0) |
| Physician VAS | 60.0 (50.5–66.3) |
| ESR (mm/h) | 33.5 (24.3–64.8) |
| CRP (mg/L) | 16.3 (4.3–31.9) |
| csDMARDs (n, %) | |
| Methotrexate | 20 (100.0) |
| Hydrochloroquine | 2 (10.0) |
| Sulfasalazine | 12 (60.0) |
| Leflunomide | 8 (40.0) |
| Tacrolimus | 2 (10.0) |
| bDMARDs (n, %)∗ | 5 (25.0) |
| Corticosteroid (n, %) | 19 (95.0) |
| Corticosteroid (mg/d) | 5.0 (2.5–5.0) |
| NSAIDs (n, %) | 20 (100.0) |
| Joint assessment (n = 27) | |
| Knee | 4 (14.8) |
| Elbow | 4 (14.8) |
| Foot | 1 (3.7) |
| Shoulder | 10 (37.0) |
| Wrist | 5 (18.5) |
| Hand | 3 (11.1) |
3.2. Clinical response to baricitinib treatment for 24 weeks
Treatment with baricitinib markedly improved disease activity assessed by DAS28-ESR, SDAI, and CDAI (Table 2). DAS28-ESR, SDAI, and CDAI at 12 weeks significantly improved compared to baseline (P < .001 for all). At 24 weeks, DAS28-ESR, SDAI, and CDAI were even more improved compared to baseline (P < .001 for all). Treatment response based on DAS28-ESR was good for 13 patients, moderate for 4 patients, and no improvement for 3 patients.
Table 2.
Changes of clinical response after baricitinib treatment for 12 and 24 wk.
| Baseline | 12 weeks | P-value∗ | 24 weeks | P-value∗ | |
| DAS28-ESR | 5.62 (4.50–5.96) | 3.89 (2.75–4.21) | P < .001 | 2.95 (2.30–3.90) | P < .001 |
| SDAI | 24.2 (19.3– 30.8) | 8.75 (6.85–12.22) | P < .001 | 4.93 (3.19–8.10) | P < .001 |
| CDAI | 22.3 (17.9–30.4) | 8.60 (6.70–12.00) | P < .001 | 4.80 (2.95–7.83) | P < .001 |
3.3. Ultrasound findings after treatment with baricitinib for 24 weeks
Differences in ultrasound findings between baseline and 24 weeks after baricitinib are illustrated in Figure 2. Baricitinib for 24 weeks induced significant improvement of gray-scale synovitis, power Doppler synovitis, and joint effusion compared to baseline (2.00 [IQR 1.00–3.00] vs 1.00 [IQR 0.00–2.00], P = .002; 1.00 [IQR 0.00–2.00] vs 0.00 [IQR 0.00–1.00], P = .030; 3.00 [IQR 2.00–3.00] vs 2.00 [IQR 1.0–3.0], P = .002, respectively). In addition, bone erosion score was not different with baricitinib treatment for 24 weeks (P = .317).
Figure 2.
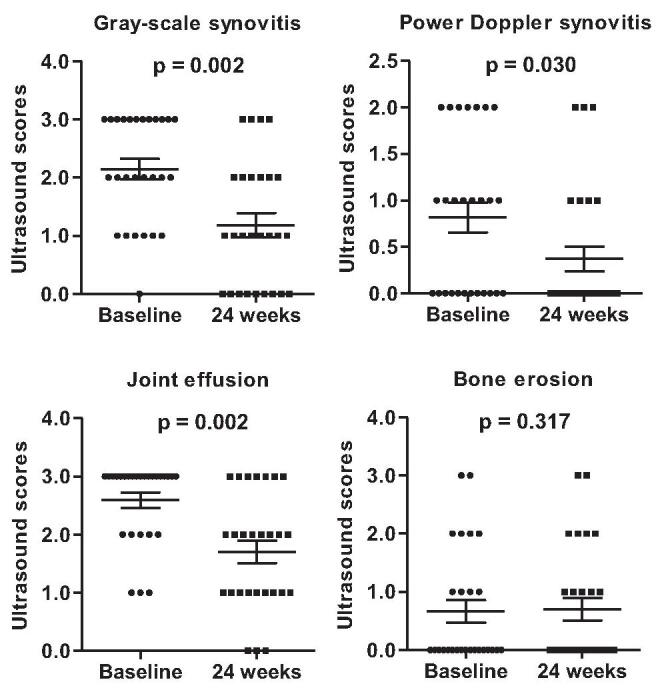
Comparison of ultrasound findings between baseline and 24 wk after baricitinib treatment.
In analysis of the correlation between disease activity composites and ultrasound findings, multivariate regression analysis showed that power Doppler score was related to DAS28-ESR (β = 0.590, P = .044), but not SDAI and CDAI (Table 3). The relationship between disease activity composites and gray-scale synovitis was insufficient. Changes in ultrasound findings were compared based on DAS28-ESR improvement (Fig. 3). There were no significant changes in gray-scale synovitis, power Doppler synovitis, joint effusion, and bone erosion scores among patients with good, moderate, and no improvement (P > .05 for all).
Table 3.
Correlation between changes of DAS28-ESR and synovitis scores for 24 wk.
| Changes of synovitis scores | ||
| ΔGray-scale score | ΔPower Doppler score | |
| ΔDAS28-ESR | ||
| Univariate analysis | β = 0.014, P = .954 | β = 0.523, P = .018 |
| Multivariate analysis∗ | β = 0.043, P = .850 | β = 0.590, P = .044 |
| ΔSDAI | ||
| Univariate analysis | β = 0.227, P = .255 | β = 0.412, P = .033 |
| Multivariate analysis∗ | β = 0.324, P = .124 | β = 0.558, P = .078 |
| ΔCDAI | ||
| Univariate analysis | β = 0.220, P = .271 | β = 0.406, P = .036 |
| Multivariate analysis∗ | β = 0.308, P = .152 | β = 0.539, P = .095 |
Figure 3.
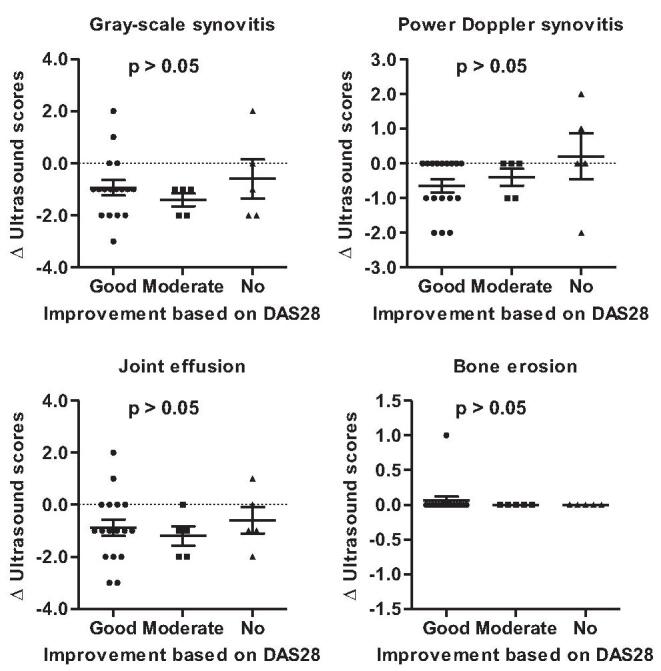
Comparison of changes in ultrasound findings based on DAS28-ESR improvement. DAS28-ESR = disease activity score for 28-joint count with erythrocyte sediment rate.
4. Discussion
The synovial immunologic and inflammatory response of RA is tightly controlled by a variety of cellular and humoral process.[1] The JAK-signal transducer and activator of transcription (STAT) signaling pathway plays a role in mediating the function of inflammatory cytokines and growth factors associated with synovial inflammation and bone destruction in RA.[1,17] Tight regulation of the JAK signaling pathway has emerged as a crucial therapeutic strategy in RA. Four phase 3, randomized, double-blind, multicenter studies demonstrated that baricitinib markedly improved clinical symptoms and signs in RA.[8,9,10,11] However, there is insufficient efficacy data for ultrasound-based synovial inflammation using gray-scale and power Doppler synovitis for bone damage after treatment with baricitinib. This study showed that baricitinib treatment for 24 weeks significantly improved ultrasound-detected joint effusion, gray-scale, and power Doppler synovitis and inhibits progression of bone erosion in patients with RA.
Synovial tissue and fibroblasts play a crucial role in the inflammatory response in RA and further induce destructive cartilage and bone damages. Therefore, synovial tissue and fibroblasts are potential therapeutic targets in RA. Experimental evidence showed that JAK-STAT proteins are highly expressed in synovial fibroblasts and tissues.[18,19] Tofacitinib, an oral JAK inhibitor that selectively inhibits JAK1 and JAK3 with less selectivity against JAK2, was approved for management of RA.[20] JAK inhibition with tofacitinib suppressed the inflammatory response of rats with adjuvant-induced arthritis, illustrating decreased infiltration of synovial sub-lining and lining layers.[21] Another study showed that increased synovial lining hyperplasia in a rabbit model of chronic antigen induced arthritis was significantly inhibited by tofacitinib.[22] Changes in musculoskeletal images after treatment with tofacitinib were investigated using ultrasound. In a phase 4 study using ultrasound to assess treatment response in patients with RA who took tofacitinib 5 mg bid, gray-scale synovial hypertrophy and synovitis with power Doppler signal significantly improved at 12 weeks of tofacitinib treatment (https://clinicaltrials.gov/ct2/show/NCT02321930). Another study revealed that ultrasound-based score after 12 weeks of tofacitinib treatment was closely related to clinical and laboratory measures of disease activity.[23]
There is some experimental evidence regarding the therapeutic effect of baricitinib on synovial inflammation. Interferon-γ-induced gliostatin production was significantly suppressed by treatment with baricitinib in human fibroblast-like synoviocytes.[24] In an in vitro experiment about the effect of different JAK inhibitors on proliferation of RA synoviocytes, tofacitinib, baricitinib, and peficitinib markedly blocked proliferation of synoviocytes stimulated by interleukin-1β, but not filgotinib.[25] Torikai et al showed that 3 months of baricitinib improved clinical disease activity based on CDAI and also significantly decreased gray-scale and power Doppler scores at 1 and 3 months compared to baseline.[14] Consistently, our study also confirmed that baricitinib is an effective therapeutic strategy for regulation of synovial inflammation in RA. This was supported by improved DAS28-ESR at 3 and 6 months compared to baseline and markedly decreased ultrasound-based gray-scale and power Doppler scores. Interestingly, we found no changes in bone erosion score for 6 months after treatment with baricitinib. This result implicates that baricitinib suppresses deterioration of bone erosion in RA.
Power Doppler in ultrasound is an imaging tool to assess active synovial inflammation by detecting increased microvascular blood in affected joints. Furthermore, it is being used more frequently to evaluate and monitor disease activity in RA.[26] There are some debates regarding the association between power Doppler intensity and clinical disease activity composites. On ultrasound evaluation of 50 patients with RA in clinical remission, there was an association between power Doppler and CDAI (P = .005), but not DAS28-ESR (P = .11).[27] In contrast, power Doppler was not associated with disease activity indexes such as DAS28-ESR, CDAI, and SDAI.[28] In this study, we observed that changes of power Doppler signal between baseline and 24 weeks were related to changes of DAS28-ESR, but not SDAI and CDAI. This implicates that power Doppler signal based on ultrasound might reflect disease activity relatively well in RA.
Cumulative evidence suggests that the JAK-STAT pathway is involved in bone metabolism.[17] JAK family proteins consisting of JAK1, JAK2, JAK3, and tyrosine kinase 2 induce production of pro-inflammatory cytokines through activation of STAT proteins.[29] Some researchers investigated whether the JAK signal pathway plays a crucial role in osteoclastogenesis in RA-like inflammatory arthritis. Tofacitinib did not show an inhibitory effect on RANKL-induced osteoclast formation or differentiation in inflammatory arthritis animal models.[30,31] In contrast, tofacitinib treatment markedly prevented bone erosion in Wistar rats with adjuvant-induced arthritis compared to rats with arthritis not receiving tofacitinib.[21] Conversely, the role of baricitinib in bone remodeling is not clearly identified in RA. Murakami et al demonstrated that baricitinib inhibited osteoclast differentiation from bone marrow cells under stimulation with 1,25-dihydroxyvitamin D3 and prostaglandin E2 and suppressed RANKL expression in osteoblastic cells.[32] They proposed that inhibition of JAK1 and JAK2 is responsible for induction of RANKL, which blocks osteoclast formation in inflammatory arthritis. In the RA-BUILD study, tofacitinib treatment for 24 weeks reduced radiographic progression of structural joint damage compared to a placebo group.[9] Consistently, our study showed that there was no further increase in bone erosion in affected joints after baricitinib treatment for 24 weeks. This result suggests that baricitinib can prevent and reduce bony structural damage in RA.
This study involves some limitations. First, ultrasound-detected findings were evaluated for only 1 or 2 joints showing the most severe abnormalities in each patient. Evaluation of these joints assessed using ultrasound does not fully reflect the overall inflammatory changes of other affected joints. Second, the study population may be too small to prove relevant changes in ultrasound and clinical findings. However, prior power calculation revealed that 27 painful joints in 20 patients would be appropriate to confirm the changes of ultrasound-detected abnormalities. The statistical power (1-β) was set to 0.8. Third, the absence of a control group treated with only csDMARDs without baricitinib may be a limitation to determine the superiority of baricitinib to ultrasound findings.
In conclusion, our data provided evidence that treatment with baricitinib 4 mg for 24 weeks showed a beneficial effect on synovial inflammation and bone erosion in patients with RA with inadequate response to csDMARDs or bDMARDs. Long-term follow-up study is needed to confirm if the therapeutic effect of baricitinib on relieving ultrasound findings remains.
Author contributions
Conceptualization: Seong-Kyu Kim.
Data curation: Seong-Kyu Kim, Ui Hong Jung, Ji-Won Kim, Jung-Yoon Choe.
Investigation: Seong-Kyu Kim, Ui Hong Jung, Ji-Won Kim, Jung-Yoon Choe.
Methodology: Seong-Kyu Kim, Ui Hong Jung, Ji-Won Kim, Jung-Yoon Choe.
Supervision: Seong-Kyu Kim.
Writing – original draft: Seong-Kyu Kim.
Writing – review & editing: Seong-Kyu Kim.
Supplementary Material
Footnotes
Abbreviations: CDAI = clinical disease activity index, CRP = C-reactive protein, DAS = disease activity, DMARDs = disease-modifying antirheumatic drugs, ESR = erythrocyte sediment rate, JAK = Janus kinase, RA = rheumatoid arthritis, SDAI = simplified disease activity index, SJC = swollen joint count, STAT = signal transducer and activator of transcription, TJC = tender joint count, VAS = visual analog scale.
How to cite this article: Kim SK, Jung UH, Kim JW, Choe JY. The beneficial effect of baricitinib on ultrasound-detected synovial inflammation and bone damage in rheumatoid arthritis: preliminarily data from single center-based observational study for 24 weeks. Medicine. 2021;100:30(e26739).
The authors have no funding and conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Supplemental digital content is available for this article.
Data was described as median (interquartile range) or number (percentages, %).
bDMARDs = biologic disease modifying anti-rheumatic drugs, CCP = cyclic citrullinated peptide, CDAI = clinical disease activity index, CRP = C-reactive protein, csDMARDs = conventional synthetic disease modifying antirheumatic drugs, DAS = disease activity score, ESR = erythrocyte sedimentation rate, NSAIDs = nonsteroidal anti-inflammatory drugs, RF = rheumatoid factor, SDAI = simplified disease activity index, VAS = visual analog scale.
bDMARDs included tocilizumab (n = 2) and adalimumab (n = 3).
P-values vs baseline.
Wilcoxon signed rank test.
CDAI = clinical disease activity index, DAS28-ESR = disease activity score in 28 joints erythrocyte sedimentation rate, SDAI = simplified disease activity index.
Adjusted with age, gender, disease duration.
Univariate and multivariate regression analysis.
CDAI = clinical disease activity index, DAS28-ESR = disease activity score in 28 joints erythrocyte sedimentation rate, SDAI = simplified disease activity index.
References
- [1].McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- [2].England BR, Tiong BK, Bergman MJ, et al. 2019 Update of the American College of Rheumatology Recommended Rheumatoid Arthritis Disease Activity Measures. Arthritis Care Res (Hoboken) 2019;71:1540–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Filippucci E, Cipolletta E, Mashadi Mirza R, et al. Ultrasound imaging in rheumatoid arthritis. Radiol Med 2019;124:1087–100. [DOI] [PubMed] [Google Scholar]
- [4].Nishino A, Kawashiri SY, Koga T, et al. Ultrasonographic efficacy of biologic and targeted synthetic disease-modifying antirheumatic drug therapy in rheumatoid arthritis from a multicenter rheumatoid arthritis ultrasound prospective cohort in Japan. Arthritis Care Res (Hoboken) 2018;70:1719–26. [DOI] [PubMed] [Google Scholar]
- [5].Umeda M, Fukui S, Nakashima Y, et al. Ultrasound disease activity of bilateral wrist and finger joints at three months reflects the clinical response at six months of patients with rheumatoid arthritis treated with biologic disease-modifying anti-rheumatic drugs. Mod Rheumatol 2017;27:252–6. [DOI] [PubMed] [Google Scholar]
- [6].Kawashiri SY, Fujikawa K, Nishino A, et al. Ultrasound-detected bone erosion is a relapse risk factor after discontinuation of biologic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis whose ultrasound power Doppler synovitis activity and clinical disease activity are well controlled. Arthritis Res Ther 2017;19:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shi JG, Chen X, Lee F, et al. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J Clin Pharmacol 2014;54:1354–61. [DOI] [PubMed] [Google Scholar]
- [8].Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243–52. [DOI] [PubMed] [Google Scholar]
- [9].Dougados M, van der Heijde D, Chen YC, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis 2017;76:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017;376:652–62. [DOI] [PubMed] [Google Scholar]
- [11].Fleischmann R, Schiff M, van der Heijde D, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol 2017;69:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].van der Heijde D, Durez P, Schett G, et al. Structural damage progression in patients with early rheumatoid arthritis treated with methotrexate, baricitinib, or baricitinib plus methotrexate based on clinical response in the phase 3 RA-BEGIN study. Clin Rheumatol 2018;37:2381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].van der Heijde D, Dougados M, Chen YC, et al. Effects of baricitinib on radiographic progression of structural joint damage at 1 year in patients with rheumatoid arthritis and an inadequate response to conventional synthetic disease-modifying antirheumatic drugs. RMD Open 2018;4:e000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Torikai E, Suzuki D. Ultrasound evaluation for monitoring response to baricitinib in rheumatoid arthritis patients at early stage after treatment. Ann Rheum Dis 2019;78: Suppl 2: 375. [Google Scholar]
- [15].Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- [16].Szkudlarek M, Court-Payen M, Jacobsen S, et al. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum 2003;48:955–62. [DOI] [PubMed] [Google Scholar]
- [17].Li J. JAK-STAT and bone metabolism. JAKSTAT 2013;2:e23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Migita K, Izumi Y, Torigoshi T, et al. Inhibition of Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathway in rheumatoid synovial fibroblasts using small molecule compounds. Clin Exp Immunol 2013;174:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Walker JG, Ahern MJ, Coleman M, et al. Changes in synovial tissue Jak-STAT expression in rheumatoid arthritis in response to successful DMARD treatment. Ann Rheum Dis 2006;65:1558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Traynor K. FDA approves tofacitinib for rheumatoid arthritis. Am J Health Syst Pharm 2012;69:2120. [DOI] [PubMed] [Google Scholar]
- [21].Vidal B, Cascão R, Finnilä MAJ, et al. Effects of tofacitinib in early arthritis-induced bone loss in an adjuvant-induced arthritis rat model. Rheumatology (Oxford) 2018;57:1461–71. [DOI] [PubMed] [Google Scholar]
- [22].Pérez-Baos S, Gratal P, Barrasa JI, et al. Inhibition of pSTAT1 by tofacitinib accounts for the early improvement of experimental chronic synovitis. J Inflamm (Lond) 2019;16:02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yu C. Clinical and musculoskeletal ultrasound assessment of therapeutic response to tofacitinib in patients with rheumatoid arthritis: real-world clinical experience from a single centre in Hong Kong. Ann Rheum Dis 2018;77: Suppl 2: 1289. [Google Scholar]
- [24].Joyo Y, Waguri-Nagaya Y, Kawaguchi Y, et al. The JAK inhibitor (baricitinib) inhibits IFNg-induced gliostatin expression in human fibroblast-like synoviocytes. Ann Rheum Dis 2019;78: Suppl 2: 1497.31413004 [Google Scholar]
- [25].Diller M, Hasseli R, Hülser ML, et al. Targeting activated synovial fibroblasts in rheumatoid arthritis by peficitinib. Front Immunol 2019;10:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bhasin S, Cheung PP. The role of power Doppler ultrasonography as disease activity marker in rheumatoid arthritis. Dis Markers 2015;2015:325909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].El-Serougy EM, Eesa NN, El-Azizi HM, et al. Power Doppler ultrasound in the evaluation of hand joints in rheumatoidarthritis patients in clinical remission: association with composite indexscores and functional status. Egyptian Rheumatol 2019;41:07–10. [Google Scholar]
- [28].Geng Y, Han J, Deng X, Zhang Z. Presence of power Doppler synovitis in rheumatoid arthritis patients with synthetic and/or biological disease-modifying anti-rheumatic drug-induced clinical remission: experience from a Chinese cohort. Clin Rheumatol 2014;33:1061–6. [DOI] [PubMed] [Google Scholar]
- [29].Darnell JE, Jr. STATs and gene regulation. Science 1997;277:1630–5. [DOI] [PubMed] [Google Scholar]
- [30].Mori T, Miyamoto T, Yoshida H, et al. IL-1β and TNFα-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int Immunol 2011;23:701–12. [DOI] [PubMed] [Google Scholar]
- [31].LaBranche TP, Jesson MI, Radi ZA, et al. JAK inhibition with tofacitinib suppresses arthritic joint structural damage through decreased RANKL production. Arthritis Rheum 2012;64:3531–42. [DOI] [PubMed] [Google Scholar]
- [32].Murakami K, Kobayashi Y, Uehara S, et al. A Jak1/2 inhibitor, baricitinib, inhibits osteoclastogenesis by suppressing RANKL expression in osteoblasts in vitro. PLoS One 2017;12:e0181126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.