Abstract
Background:
Human endogenous retrovirus-H long terminal repeat-associating protein 2 (HHLA2), a newly discovered member of the B7 family, is overexpressed in numerous tumors. However, the prognostic impact of HHLA2 in human cancers remains controversial. Thus, we performed this meta-analysis to explore the prognostic value of HHLA2 in Chinese patients with solid tumors.
Methods:
PubMed, Embase, Web of Science, Chinese National Knowledge Infrastructure, and WanFang databases were systematically searched for eligible studies that evaluated the impact of HHLA2 on overall survival (OS) in patients with cancer. Hazard ratios (HRs) and 95% confidence intervals (CIs) were combined to evaluate the association between HHLA2 expression and OS in solid tumors. Odds ratios (ORs) and 95% CIs were pooled to assess the correlation between HHLA2 expression and clinicopathological characteristics in solid tumors.
Results:
A total of 12 studies, including 15 cohorts and 1747 patients, were included in this meta-analysis. We found that high HHLA2 expression was significantly associated with shorter OS (HR = 1.65, 95% CI: 1.12–2.43). Subgroup analysis by cancer type demonstrated that high HHLA2 expression was associated with poor OS in patients with clear cell renal cell carcinoma (HR = 3.42, 95% CI: 2.39–4.91), gastric cancer (HR = 2.03, 95% CI: 1.31–3.16), intrahepatic cholangiocarcinoma (HR = 1.77, 95% CI: 1.24–2.53), lung cancer (HR = 2.14, 95% CI: 1.33–3.44) and other cancer types (HR = 2.08, 95% CI: 1.34–3.24), but not in patients with epithelial ovarian cancer (HR = 0.52, 95% CI: 0.08–3.56). Nevertheless, high HHLA2 expression was associated with better OS in patients with pancreatic ductal adenocarcinoma (HR = 0.45, 95% CI: 0.32–0.64). Furthermore, high HHLA2 expression was associated with old age (OR = 1.30, 95% CI: 1.03–1.63), lymph node metastasis (OR = 1.99, 95% CI: 1.41–2.81), and vascular invasion (OR = 1.69, 95% CI: 1.18–2.42).
Conclusions:
HHLA2 may serve as a potential prognostic biomarker for solid tumors in Chinese population, by predict the prognosis of cancer patients based on their tumor types.
Keywords: HHLA2, meta-analysis, overall survival, solid tumor
1. Introduction
Malignant tumors have a high incidence and mortality rate, and are the major public health problem worldwide, leading to heavy medical and socioeconomic burdens.[1] Despite substantial advances in surgical, radio- and chemo- therapies in recent decades, poor tumor prognosis remains a tremendous challenge for researchers and clinicians.[2] As a Consequence, the development of novel therapeutics for solid tumors is needed.
Cancer immunotherapy is a rapidly developing cancer treatment designed to stimulate the immune system to fight and eliminate tumors.[3,4] Among various immunotherapy strategies, immune checkpoint blockade seems to be an effective approach.[5] Immune checkpoint proteins are surface molecules on certain immune cell populations that activate or inhibit immune function when engaged to their ligands.[5] Several checkpoint inhibitors have been extensively studied for cancer treatment, and some have been used in clinical trials in cancer patients, such as antibodies against cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), or its ligand PD-L1.[6–8] However, the response rate of anti-PD-1 and anti-CTLA4 antibody therapy is rather low in some types of cancer.[9–12] Hence, the development of other immune checkpoint molecules for immunotherapy to improve the survival rate of cancer patients is urgently required.
Human endogenous retrovirus-H long terminal repeat-associating protein 2 (HHLA2), also known as B7H7 or B7-H5, is a newly discovered member of the B7 family.[13] HHLA2 effectively suppresses the activity of CD4+ and CD8+ T cells and reduces the expression of T cell cytokines, such as interferon-γ, tumor necrosis factor-α, interleukin (IL)-5, IL-10, IL-13, IL-17α and IL-22.[14] In addition, HHLA2 is an inducer of the inflammatory response of antigen-presenting cells.[15] HHLA2 is widely expressed in a large proportion of tumor specimens, such as lung cancer, breast cancer, thyroid cancer, ovarian cancer, liver cancer, esophagus cancer, pancreatic cancer, bladder cancer, renal carcinoma, prostate cancer, and colon cancer.[16] Furthermore, recent studies have revealed that high HHLA2 protein expression in primary tumor tissues is associated with poor prognosis of cancer patients.[13,17–22] However, the prognostic significance of HHLA2 is inconclusive.[9,23–26] Thus, we performed this meta-analysis to investigate the association between HHLA2 protein expression and prognosis in patients with cancer.
2. Materials and methods
This meta-analysis was performed in accordance with the preferred reporting items for systematic reviews and meta-analyses guidelines.[27] Ethical approval was not required, as the study was conducted based on existing literature and did not involve direct human or animal participation.
2.1. Search strategy
A comprehensive electronic search of PubMed, Embase, Web of Science, Chinese National Knowledge Infrastructure, and WanFang databases was performed to identify relevant studies that focused on the prognostic value of HHLA2 in cancer patients prior to November 2020. The following search terms were included “human endogenous retrovirus-H long terminal repeat-associating protein 2” or “HHLA2” or “B7H7” or “B7H5” and “tumor” or “cancer” or “neoplasm” “carcinoma” and “prognosis” or “survival” or “outcome”. The citation lists of the identified studies were also screened for other pertinent studies.
2.2. Study selection criteria
Publications were included in this meta-analysis based on the following selection criteria:
-
1.
studies originated from China and focused on Chinese population;
-
2.
tumors were diagnosed by histological or pathological examinations;
-
3.
the expression level of HHLA2 in tumor tissues was detected by immunohistochemistry (IHC) and divided into “positive” and “negative” or “high” and “low” groups;
-
4.
the correlation between HHLA2 expression and overall survival (OS) was assessed;
-
5.
hazard ratios (HRs) with 95% confidence intervals (CIs) were reported or could be calculated based on sufficient data.
Studies were excluded according to the following criteria:
-
1.
reviews, case reports, conference abstracts, letters or editorials;
-
2.
mRNA expression was measured in cancer tissue;
-
3.
patients were not divided into two groups based on HHLA2 expression; and
-
4.
studies without sufficient data to estimate the HR and corresponding 95% CI.
2.3. Data extraction and quality assessment
Data were extracted and examined from the included manuscripts by two independent reviews (ZCM and XJ). Any disagreement in the literature assessment was resolved through consensus with a third author (YJ). The following data were extracted: the clinicopathological characteristics, first author's name, publication year, country, cancer type, clinical stage, follow-up time, sample size, cut-off value, number and proportion of patients with high HHLA2 expression, analysis method, language, HR and 95% CI. If univariate and multivariate HRs existed, the latter was selected to minimize bias.
The methodological quality of each recruited study was evaluated independently by two investigators (ZCM and YJ), according to the Newcastle–Ottawa Scale (NOS). The scale included three dimensions as follows: selection (0–4), comparability (0–2), and outcome assessment (0–3), with a total score of 0–9.[28] Articles with a NOS score of ≥ 6 were considered to be of high quality.
2.4. Statistical analysis
The Stata version 12.0 software (StataCorp LLC, College Station, Texas, USA) was used to analyze the correlation between HHLA2 expression and OS, and to evaluate the clinicopathological significance of HHLA2 expression in human cancers. Heterogeneity among the included studies was measured by the I2 and Chi-square Q tests. When significant heterogeneity (I2 > 50% or P < .05) existed, the random effects model was used for the meta-analysis. Otherwise, a fixed effects model was adopted. Subgroup analysis was performed to examine the potential sources of statistical heterogeneity. Sensitivity analysis was conducted by sequentially omitting each individual cohort to evaluate the stability of the results. The potential publication bias was investigated using the funnel plot and Begg/Egger test. A P-value of < .05 was considered statistically significant.
3. Results
3.1. Study selection and characteristics
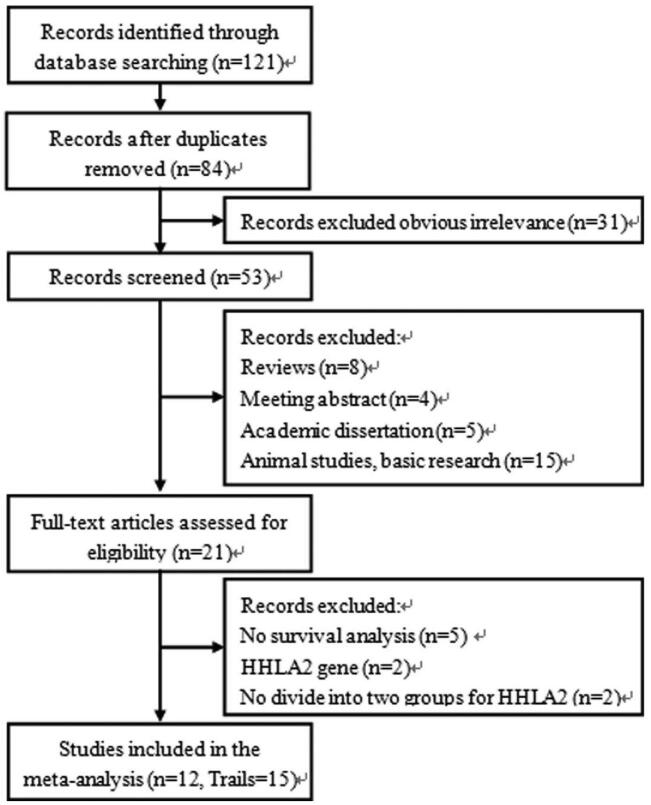
A flow diagram showing our literature search and screening strategy is presented in Figure 1. A total of 121 articles were initially obtained according to the search strategies. After removing 37 duplicate studies and 31 apparently irrelevant articles, the remaining 53 records were screened by reading the titles and abstracts. Further, 32 studies were excluded (8 for reviews, 4 for meeting abstract, 5 for academic dissertations, 15 for animal studies or basic research). Twenty-one full-text studies were evaluated for eligibility, and finally 12 articles with 15 cohorts were included in the meta-analysis.
Figure 1.
Flow diagram of the study selection process and specific reasons for exclusion of the studies in the meta-analysis.
All of these studies were published between 2018 and 2020, and all the patients included in these studies were from China. They consisted of the following cancer types: clear cell renal cell carcinoma (ccRCC),[13,19] gastric cancer (GC),[17,18] epithelial ovarian cancer (EOC),[9,26] pancreatic ductal adenocarcinoma (PDAC),[23,24] oral squamous cell carcinoma (OSCC),[25] intrahepatic cholangiocarcinoma (ICC),[20] lung adenocarcinoma,[21] lung squamous cell carcinoma,[21] and colorectal cancer (CRC).[22] The sample sizes ranged from 63 to 403, with a total of 1747 patients. HHLA2 expression was measured by IHC in all cohorts. HRs and the corresponding 95% CIs of OS were obtained by the multivariate analysis in 13 cohorts and univariate analysis or Kaplan–Meier curves in 2 cohorts. The NOS scores of all these studies were between 6 and 8 points, indicating that each article was of high quality. Further detailed descriptions of these eligible articles are listed in Table 1.
Table 1.
Main characteristics of the eligible studies.
| Study | Region | Duration | Cancer type | Clinical stage | Follow up | Number | Detection method | Cut-off value | HHLA2-high (%) | Analysis method | Language | Quality |
| Zhou QH 2020 (T) | China | 2006–2013 | ccRCC | I–IV | Until Dec 2019 | 206 | IHC | 20% | 91 (44.2) | Multivariate | English | 8 |
| Zhou QH 2020 (V) | China | 2006–2013 | ccRCC | I–IV | Until Dec 2019 | 197 | IHC | 20% | 81 (41.1) | Multivariate | English | 8 |
| Wei L 2020 | China | NR | GC | I–IV | NR | 124 | IHC | ≥8 | 66 (53.2) | Multivariate | English | 7 |
| Fu Y 2020 | China | 2009–2013 | EOC | I–IV | Until Mar 2018 | 119 | IHC | >31.51% | 60 (50.4) | Univariate | English | 6 |
| Hu C 2020 | China | NR | GC | I–IV | NR | 71 | IHC | ≥8 | 52 (73.2) | Multivariate | English | 7 |
| Chen Q 2019 | China | 2012–2017 | PDAC | I–IV | NR | 136 | IHC | NR | 93 (68.3) | Multivariate | English | 7 |
| Yan H 2019 | China | 2013–2014 | PDAC | I–III | Until Nov 2017 | 92 | IHC | >5% | 71 (77.2) | Univariate | English | 6 |
| Chen L 2019 | China | 2006–2008 | ccRCC | I–IV | NR | 87 | IHC | >90 | 26 (29.9) | Multivariate | English | 7 |
| Xiao Y 2019 | China | 2008–2017 | OSCC | I–III | NR | 201 | IHC | 85.4 | 138 (68.7) | Multivariate | English | 7 |
| Shi YY 2019 | China | 2007–2011 | EOC | I–IV | NR | 64 | IHC | >0% | 11 (17.2) | Multivariate | Chinese | 7 |
| Jing CY 2019 (T) | China | 2005–2014 | ICC | I–III | Until May 2017 | 153 | IHC | ≥5 | 75 (49.0) | Multivariate | English | 8 |
| Jing CY 2019 (V) | China | 2005–2014 | ICC | I–III | Until May 2017 | 65 | IHC | ≥5 | 44 (67.7) | Multivariate | English | 8 |
| Zhang YQ 2018 (LA) | China | 2004–2009 | LA | I–IV | Until Aug 2017 | 94 | IHC | ≥100 | 31 (33.0) | Multivariate | Chinese | 8 |
| Zhang YQ 2018 (LSCC) | China | 2004–2009 | LSCC | I–IV | Until Aug 2017 | 75 | IHC | ≥135 | 15 (20.0) | Multivariate | Chinese | 8 |
| Zhu Z 2018 | China | 2005–2013 | CRC | I–III | Until Aug 2016 | 63 | IHC | >9 | 30 (47.6) | Multivariate | English | 8 |
3.2. Association of HHLA2 expression with OS
The random effect model was used to calculate the pooled HR and 95% CI because of apparent statistical heterogeneity (I2 = 83.0%, P < .001). The results indicated that high expression of HHLA2 in human tumor tissue was associated with poor OS compared to low expression of HHLA2 (HR = 1.65, 95% CI: 1.12–2.43, P = .011) (Fig. 2, Table 2).
Figure 2.
Forest plot of studies evaluating hazard ratios of high HHLA2 expression and the overall survival of cancer patients.
Table 2.
Summary of the meta-analysis results.
| Categories | Trials (patients) | HR (95%CI) | I2 (%) | Ph | Z | Pz |
| OS (All) | 15 (1747) | 1.65 (1.12–2.43) | 83.0 | <.001 | 2.56 | .011 |
| Cancer type | ||||||
| ccRCC | 3 (490) | 3.42 (2.39–4.91)F | 0.0 | .840 | 6.70 | <.001 |
| GC | 2 (195) | 2.03 (1.31–3.16)F | 0.0 | .461 | 3.16 | .002 |
| EOC | 2 (183) | 0.52 (0.08–3.56) | 76.4 | .040 | 0.67 | .506 |
| PDAC | 2 (228) | 0.45 (0.32–0.64)F | 0.0 | .960 | 4.46 | <.001 |
| ICC | 2 (218) | 1.77 (1.24–2.53)F | 5.4 | .304 | 3.15 | .002 |
| LC | 2 (169) | 2.14 (1.33–3.44)F | 28.5 | .237 | 3.13 | .002 |
| Others | 2 (264) | 2.08 (1.34–3.24)F | 0.0 | .821 | 3.26 | .001 |
| Clinical stage | ||||||
| Stage I–IV | 10 (1173) | 1.76 (1.06–2.92) | 83.3 | <.001 | 2.19 | .029 |
| Stage I–III | 5 (574) | 1.46 (0.77–2.77) | 84.8 | <.001 | 1.16 | .246 |
| Sample size | ||||||
| ≥100 | 7 (1136) | 1.65 (0.97–2.80) | 85.2 | <.001 | 1.85 | .064 |
| <100 | 8 (611) | 1.64 (0.89–3.02) | 83.2 | <.001 | 1.58 | .114 |
| HHLA2-high (%) | ||||||
| ≥50% | 8 (808) | 1.26 (0.77–2.06) | 83.7 | <.001 | 0.93 | .353 |
| <50% | 7 (939) | 2.48 (1.65–3.73) | 57.3 | .029 | 4.36 | <.001 |
| Analysis method | ||||||
| Multivariate | 13 (1536) | 1.92 (1.32–2.79) | 77.3 | <.001 | 3.42 | .001 |
| Univariate | 2 (211) | 0.72 (0.29–1.81) | 85.4 | .009 | 0.69 | .489 |
Considering the significant heterogeneity, we performed subgroup analysis to explore the potential factors that may cause heterogeneity. We classified the included cohorts and conducted subgroup analysis based on cancer type, clinical stage, sample size, proportion of patients with high HHLA2expression, and analysis method (Table 2). Subgroup analysis by cancer type revealed that HHLA2 overexpression was correlated with poor OS in patients with ccRCC (HR = 3.42, 95% CI: 2.39–4.91), GC (HR = 2.03, 95% CI: 1.31–3.16), ICC (HR = 1.77, 95% CI: 1.24–2.53), lung cancer (LC) (HR = 2.14, 95% CI: 1.33–3.44) and other cancer types (HR = 2.08, 95% CI: 1.34–3.24), but not in patients with EOC (HR = 0.52, 95% CI: 0.08–3.56). Nevertheless, high HHLA2 expression was related to better OS in patients with PDAC (HR = 0.45, 95% CI: 0.32–0.64). In addition, subgroup analysis according to cancer type showed that there was no significant heterogeneity within each subgroup, except for the EOC subgroup. When subgroup analysis was performed according to clinical stage, sample size, proportion of patients with high HHLA2 expression and analysis method, the heterogeneity within each subgroup did not change significantly. Therefore, cancer type may be the source of heterogeneity.
3.3. Sensitivity analysis and publication bias
We performed sensitivity analysis by successively ignoring individual cohort studies, and the results showed that no individual cohort affected the relationship between HHLA2 expression and OS in patients with solid tumors (Fig. 3). Moreover, no publication bias was detected, which was verified by the Begg test (P = .075), Egger's test (P = .507), and funnel plot (Fig. 4). Therefore, our results were robust and reliable.
Figure 3.
Sensitivity analysis for evaluating the effects of individual studies on the relationship between HHLA2 expression and overall survival in patients with solid cancer.
Figure 4.
Begg's funnel plots for assessing potential publication bias on the relationship between HHLA2 expression and overall survival in patients with solid tumors.
3.4. Association of expression with clinicopathological parameters
Meta-analysis of the association between HHLA2 expression and clinicopathological parameters showed a significant relationship between high HHLA2 expression and old age (OR = 1.30, 95% CI: 1.03–1.63), lymph node metastasis (OR = 1.99, 95% CI: 1.41–2.81), and vascular invasion (OR = 1.69, 95% CI: 1.18–2.42). However, a similar relationship was not observed between HHLA2 expression and gender (OR = 1.21, 95% CI: 0.91–1.61), tumor size (OR = 1.28, 95% CI: 0.86–1.90), clinical stage (OR = 1.68, 95% CI: 0.94–3.00), tumor depth (OR = 1.67, 95% CI: 0.78–3.59) and distant metastasis (OR = 1.52, 95% CI: 0.83–2.79). (Table 3)
Table 3.
Meta-analysis of HHLA2 and clinicopathological features in cancer patients.
| Categories | Trials (Patients) | OR (95%CI) | I2(%) | Ph | Z | Pz |
| Age (young vs old) | 12 (1308) | 1.30 (1.03–1.63) | 0.0 | .525 | 2.24 | .025 |
| Gender (male vs female) | 9 (1004) | 1.21 (0.91–1.61) | 0.0 | .780 | 1.34 | .181 |
| Tumor size (small vs large) | 5 (465) | 1.28 (0.86–1.90) | 38.4 | .165 | 1.20 | .231 |
| Clinical stage (I–II vs. III–IV) | 13 (1391) | 1.68 (0.94–3.00)R | 77.7 | <.001 | 1.77 | .077 |
| Tumor depth (T0–T2 vs T3–T4) | 6 (510) | 1.67 (0.78–3.59)R | 58.6 | .034 | 1.32 | .188 |
| Lymph node metastasis (negative vs positive) | 9 (787) | 1.99 (1.41–2.81) | 26.5 | .208 | 3.93 | <.001 |
| Distant metastasis (negative vs positive) | 7 (611) | 1.52 (0.83–2.79) | 24.9 | .239 | 1.36 | .174 |
| vascular invasion (negative vs positive) | 7 (901) | 1.69 (1.18–2.42) | 0.0 | .548 | 2.86 | .004 |
4. Discussion
In the past several decades, many studies have focused on identifying novel diagnostic and prognostic markers of tumors in order to advance treatment and outcomes by providing information for clinical decision-making.[29–33] The prognostic significance of HHLA2 expression has been evaluated in a variety of solid tumors. Here, we aimed to summarize and evaluate the results of published studies and extract valuable information that can be used in clinical decision-making for human solid tumors.
A total of 12 studies, including 15 cohorts and 1747 patients, were included. Our results demonstrated that high HHLA2 expression predicted poor OS in Chinese patients with cancers. Furthermore, sensitivity analysis and publication bias proved that the results were robust and reliable. However, a significant heterogeneity existed among these studies. Considering the apparent heterogeneity, subgroup analysis was performed. Subgroup analysis showed that clinical stage, sample size, proportion of patients with high HHLA2 expression and analysis method did not change heterogeneity significantly. Subgroup analysis by cancer type showed that HHLA2 overexpression was correlated with poor OS in patients with ccRCC, GC, ICC, LC, and other cancer types, but not in patients with EOC. Nevertheless, high HHLA2 expression was related to better OS in patients with PDAC. In addition, the cancer type subgroup significantly reduced the heterogeneity within each subgroup. Thus, cancer type was the main source of heterogeneity and HHLA2 expression may play a different role in different types of cancer, especially in patients with PDAC. The mechanism should be further studied to reveal the influence of HHLA2 expression on the types of cancer.
HHLA2 is involved in tumor immunosuppressive mechanisms and is a checkpoint for inhibition.[9] HHLA2 inhibits CD4 + and CD8 + T cell proliferation and cytokine production by binding to putative receptors in various immune cells.[34] In addition, HHLA2 binds to receptors on T cells and other immune cells to evade immune attacks.[35] Furthermore, HHLA2 binds to CD28H, which is expressed in naive T cells, natural killer cells, endothelial cells and epithelial cells, but not in activated T cells, and plays an important a role in angiogenesis, cell-cell interaction, and cell migration.[36,37] However, the HHLA2/CD28H interaction can also co-stimulate T cell proliferation and cytokine production via the AKT pathway.[18,38] Therefore, HHLA2 may have dual immune functions depending on the immune environment, receptor engagement or blockade, or interaction with different receptors, which is similar to the other members of the B7 family, such as B7-H3 and B7x.[24,39] In view of the conflicting results shown in HHLA2 so far, continued research and further understanding of these pathways is urgently needed to understand the causes of these differences among different tumors, to provide new strategies for tumor immunotherapy.
Several limitations should be noted in the present study. First, all the patients included in this study were from China, which affected the applicability of the results to a certain extent. Second, the cut-off values of high HHLA2 expression were inconsistent, which may affect the effectiveness of HHLA2 as a predictor of cancer prognosis. Third, HR and 95% CI in some studies were calculated by extracting data from Kaplan–Meier curves rather than directly from the original literature, which inevitably led to small statistical deviations. Fourth, HHLA2 may act differently in patients with PDAC than in other cancer types, and PDAC patients were also included in the study. Given the limitations of this study, further well-designed studies that include evaluation of more tumor types with a larger sample size are needed.
In summary, high HHLA2 expression was associated with poor OS in Chinese patients with solid tumors, and might be used as a potential prognostic marker and tumor treatment target. However, high HHLA2 expression was related to better OS in patients with PDAC. Therefore, more mechanistic studies are needed for further analysis.
Author contributions
Conceptualization: Xiaohong Zhang.
Data curation: Chuanmeng Zhang, Jie Xu.
Investigation: Chuanmeng Zhang, Jie Xu, Jun Ye.
Software: Chuanmeng Zhang, Jie Xu.
Supervision: Xiaohong Zhang.
Writing – original draft: Chuanmeng Zhang, Jun Ye.
Writing – review & editing: Chuanmeng Zhang, Xiaohong Zhang.
Footnotes
Abbreviations: ccRCC = clear cell renal cell carcinoma, CIs = confidence intervals, CRC = colorectal cancer, CTLA-4 = cytotoxic T lymphocyte-associated antigen-4, EOC = epithelial ovarian cancer, GC = gastric cancer, HHLA2 = Human endogenous retrovirus-H long terminal repeat-associating protein 2, HR = Hazard ratio, ICC = intrahepatic cholangiocarcinoma, IHC = immunohistochemistey, IL = interleukin, LC = lung cancer, NOS = Newcastle-Ottawa Scale, ORs = odds ratios, OS = overall survival, OSCC = oral squamous cell carcinoma, PD-1 = programmed cell death protein 1, PDAC = pancreatic ductal adenocarcinoma,.
How to cite this article: Zhang C, Xu J, Ye J, Zhang X. Prognostic value of HHLA2 expression in solid tumors: A meta-analysis based on the Chinese population. Medicine. 2021;100:30(e26789).
This study was funded by Taizhou People's Hospital Medical Innovation Team Foundation (CXTDA201901, CXTDA201904), and Taizhou People's Hospital Mandatory Project (ZL202020).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
ccRCC = clear cell renal cell carcinoma, CRC = colorectal cancer, EOC = epithelial ovarian cancer, GC = gastric cancer, HHLA2 = human endogenous retrovirus-H long terminal repeat-associating protein 2, ICC = intrahepatic cholangiocarcinoma, IHC = immunohistochemistry, LA = lung adenocarcinoma, LSCC = lung squamous cell carcinoma, NR = none reported, OSCC = oral squamous cell carcinoma, PDAC = pancreatic ductal adenocarcinoma, T = Training cohort, V = validation cohort.
ccRCC = clear cell renal cell carcinoma, CI = confidence interval, EOC = epithelial ovarian cancer, GC = gastric cancer, HHLA2 = human endogenous retrovirus-H long terminal repeat-associating protein 2, HR = hazard ratio, ICC = intrahepatic cholangiocarcinoma, LC = lung cancer, OS overall survival, PDAC = pancreatic ductal adenocarcinoma.
All pooled HRs were calculated from random-effect model except for cells marked with (fixedF); Ph = P value for heterogeneity based on Q test; Pz = P value for statistical significance based on Z test.
All pooled ORs were calculated from fixed-effect model except for cells marked with (randomR). Ph denotes P value for heterogeneity based on Q test.
Pz denotes P value for statistical significance based on Z test. OR odds ratio; CI = confidence interval.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:07–30. [DOI] [PubMed] [Google Scholar]
- [2].Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016;25:16–27. [DOI] [PubMed] [Google Scholar]
- [3].Boor P, Sideras K, Biermann K, et al. HHLA2 is expressed in pancreatic and ampullary cancers and increased expression is associated with better post-surgical prognosis. Br J Cancer 2020;122:1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abdel Ghafar MT, Morad MA, El-Zamarany EA, et al. Autologous dendritic cells pulsed with lysate from an allogeneic hepatic cancer cell line as a treatment for patients with advanced hepatocellular carcinoma: a pilot study. Int Immunopharmacol 2020;82:106375. [DOI] [PubMed] [Google Scholar]
- [5].Qi Y, Deng G, Xu P, et al. HHLA2 is a novel prognostic predictor and potential therapeutic target in malignant glioma. Oncol Rep 2019;42:2309–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pujade-Lauraine E, Fujiwara K, Dychter SS, et al. Avelumab (anti-PD-L1) in platinum-resistant/refractory ovarian cancer: JAVELIN Ovarian 200 Phase III study design. Fut Oncol 2018;14:2103–13. [DOI] [PubMed] [Google Scholar]
- [7].Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pietzner K, Nasser S, Alavi S, et al. Checkpoint-inhibition in ovarian cancer: rising star or just a dream? J Gynecol Oncol 2018;29:e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fu Y, Ding Y, Liu J, et al. B7-H7 is a prognostic biomarker in epithelial ovarian cancer. Trans Cancer Res 2020;9:5360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ring KL, Pakish J, Jazaeri AA. Immune checkpoint inhibitors in the treatment of gynecologic malignancies. Cancer J 2016;22:101–7. [DOI] [PubMed] [Google Scholar]
- [11].Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev 2014;23:2965–70. [DOI] [PubMed] [Google Scholar]
- [12].Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent ipilimumab (Anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2013;33:828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhou Q, Li K, Chen X, et al. HHLA2 and PD-L1 co-expression predicts poor prognosis in patients with clear cell renal cell carcinoma. J Immunother Cancer 2019;8:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhao R, Chinai JM, Buhl S, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. PNAS 2013;110:9879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhong C, Lang Q, Yu J, et al. Phenotypical and potential functional characteristics of different immune cells expressing CD28H/B7-H5 and their relationship with cancer prognosis. Clin Exp Immunol 2020;200:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Janakiram M, Chinai JM, Fineberg S, et al. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res 2015;21:2359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wei L, Tang L, Chang H, et al. HHLA2 overexpression is a novel biomarker of malignant status and poor prognosis in gastric cancer. Human Cell 2020;33:116–22. [DOI] [PubMed] [Google Scholar]
- [18].Hu C, Xu Z, Chen S, et al. Overexpression of B7H5/CD28H is associated with worse survival in human gastric cancer. J Cell Mol Med 2020;24:1360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen L, Zhu D, Feng J, et al. Overexpression of HHLA2 in human clear cell renal cell carcinoma is significantly associated with poor survival of the patients. Cancer Cell Int 2019;19: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jing C, Fu Y, Yi Y, et al. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. J Immunother Cancer 2019;7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yuqing Z, Lujun C, Xiao Z, et al. The expression of human endogenous retrovirus subfamily H long terminal repeat associating protein 2 in lung cancer tissue and it's clinical significance. Chin J Exp Surg 2018;35:2142–6. [Google Scholar]
- [22].Zhu Z, Dong W. Overexpression of HHLA2, a member of the B7 family, is associated with worse survival in human colorectal carcinoma. Onco Targets Ther 2018;11:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen Q, Wang J, Chen W, et al. B7-H5/CD 28H is a co-stimulatory pathway and correlates with improved prognosis in pancreatic ductal adenocarcinoma. Cancer Sci 2019;110:530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yan H, Qiu W, Koehne De Gonzalez AK, et al. HHLA2 is a novel immune checkpoint protein in pancreatic ductal adenocarcinoma and predicts post-surgical survival. Cancer Lett 2019;442:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xiao Y, Li H, Yang L, et al. The Expression patterns and associated clinical parameters of human endogenous retrovirus-h long terminal repeat-associating protein 2 and transmembrane and immunoglobulin domain containing 2 in oral squamous cell carcinoma. Dis Markers 2019;2019:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shi YY, Xu GC, Bz Z. Expression and clinical significance of HHLA2 in epithelial ovarian cancer. Prog Obstet Gynecol 2019;28:438–41. [Google Scholar]
- [27].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [29].Abdel Ghafar MT, Elkhouly RA, Elnaggar MH, et al. Utility of serum neuropilin-1 and angiopoietin-2 as markers of hepatocellular carcinoma. J Investig Med 2021;1744–2020. [DOI] [PubMed] [Google Scholar]
- [30].Habib EM, Nosiar NA, Eid MA, et al. Circulating miR-146a expression predicts early treatment response to imatinib in adult chronic myeloid leukemia. J Investig Med 2021;69:333–7. [DOI] [PubMed] [Google Scholar]
- [31].Abdel Ghafar MT, Gharib F, Al-Ashmawy GM, et al. Serum high-temperature-required protein A2: a potential biomarker for the diagnosis of breast cancer. Gene Rep 2020;20:100706. [Google Scholar]
- [32].Abdel Ghafar MT, Gharib F, Abdel-Salam S, et al. Role of serum metadherin mRNA expression in the diagnosis and prediction of survival in patients with colorectal cancer. Mol Biol Rep 2020;47:2509–19. [DOI] [PubMed] [Google Scholar]
- [33].El-Guindy DM, Wasfy RE, Abdel Ghafar MT, et al. Oct4 expression in gastric carcinoma: association with tumor proliferation, angiogenesis and survival. J Egyptian Natl Cancer Ins 2019;31: [DOI] [PubMed] [Google Scholar]
- [34].Cheng H, Borczuk A, Janakiram M, et al. Wide expression and significance of alternative immune checkpoint molecules, B7x and HHLA2, in PD-L1-negative human lung cancers. Clin Cancer Res 2018;24:1954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fu Y, Wu C, Jiang J. Research progress on the expression and mechanism of costimulatory molecule B7-H7 in tumors. Chinese J Clin Lab Sci 2020;38:363–6. [Google Scholar]
- [36].Janakiram M, Chinai JM, Zhao A, et al. HHLA2 and TMIGD2: new immunotherapeutic targets of the B7 and CD28 families. Oncoimmunology 2015;4:e1026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xiao Y, Freeman GJ. A new B7:CD28 family checkpoint target for cancer immunotherapy: HHLA2. Clin Cancer Res 2015;21:2201–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhu Y, Yao S, Iliopoulou BP, et al. B7-H5 costimulates human T cells via CD28H. Nat Comm 2043;4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ni L, Dong C. New B7 family checkpoints in human cancers. Mol Cancer Ther 2017;16:1203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]