Abstract
Purpose:
The aim of this study was to evaluate the efficiency and safety of methimazole (MMI) and propylthiouracil (PTU) in the treatment of hyperthyroidism.
Methods:
Articles were searched through the PubMed, EMBASE, Cochrane Library, Web of Science, CNKI, Wanfang, and QVIP. The primary outcomes were clinical efficacy and thyroid hormone levels in MMI and PTU groups. The secondary outcomes were liver function indexes and adverse reactions in MMI and PTU groups. Results were expressed as weighted mean difference (WMD) or odds ratio (OR) with 95% confidence intervals (CIs). The Begg test was applied to assess the publication bias.
Results:
Totally, 16 randomized controlled trials were retained in this meta-analysis with 973 patients receiving MMI and 933 receiving PTU. The levels of triiodothyronine (T3) (WMD = −1.321, 95% CI: −2.271 to −0.372, P = .006), thyroxine (T4) (WMD = −37.311, 95% CI: −61.012 to −13.610, P = .002), Free T3 (FT3) (WMD = −1.388, 95% CI: −2.543 to −0.233, P = .019), Free T4 (FT4) (WMD = −3.613, 95% CI: −5.972 to −1.255, P = .003), and the risk of liver function damage (OR = 0.208, 95% CI: 0.146–0.296, P < .001) in the MMI group were lower than those in the PTU group. The thyroid-stimulating hormone level (WMD = 0.787, 95% CI: 0.380–1.194, P < .001) and the risk of hypothyroidism (OR = 2.738, 95% CI: 1.444–5.193, P = .002) were higher in the MMI group than those in the PTU group.
Conclusions:
Although MMI might have higher risk of hypothyroidism than PTU, the efficacy of MMI may be better than PTU in patients with hyperthyroidism regarding reducing T3, T4, FT3, and FT4 levels, decreasing the risk of liver function damage and increasing the level of thyroid-stimulating hormone.
Register number:
osf.io/ds637 (https://osf.io/search/).
Keywords: hyperthyroidism, meta-analysis, methimazole, propylthiouracil
1. Introduction
Hyperthyroidism is one of the most common endocrine diseases that caused by excessive production of thyroid hormones.[1] Excessive thyroid hormones inhibits the production of serum thyroid-stimulating hormone (TSH).[2] The prevalence of hyperthyroidism is reported to be up to 1.3% in iodine sufficient areas.[3] Higher incidence of it was obtained in females than that in males with the female-to-male ratio of about 5 to 10:1.[4] Hyperthyroidism is clinically manifested by goiter, protruding eyeballs and increased basal metabolic rate.[5] Hyperthyroidism progresses rapidly and once diagnosed, treatment must be taken as soon as possible.
Evidences indicated that hyperthyroidism can elevate the risk of multiple comorbidities, such as cardiovascular, pulmonary diseases, and psychiatric diseases.[6–8] The association between hyperthyroidism and excess mortality has been confirmed by several studies.[9,10] Nowadays, anti-thyroid drugs (ATDs) are one of the main methods for the treatment of patients with hyperthyroidism, which can preserve the function of thyroid hormone production and have low possibility of hypothyroidism.[5] Methimazole (MMI) and propylthiouracil (PTU) are 2 most extensively used ATDs for patients with hyperthyroidism.[11] MMI and PTU are effective inhibitors of thyroid iodide peroxidase, which can catalyze the biosynthesis of thyroid hormone from the initial step.[12] MMI exerts its function by inhibiting the peroxidase activity in the thyroid, and then suppressing the synthesis of triiodothyronine (T3) and thyroxine (T4)[13] PTU has an inhibitory effect on peroxidase and the iodization of tyrosine in thyroid, thereby restrains the synthesis of T4. Meanwhile, PTU can interfere with the transformation from T4 to T3, which decreases the level of serum Free T3 (FT3).[14,15]
Although MMI and PTU were validated to have effects on treating hyperthyroidism, they might have adverse reactions. Previously, a study has demonstrated that PTU has a high risk of adverse reactions compared with MMI in the treatment of hyperthyroidism.[16] Meanwhile, another study has suggested that PTU and MMI has a similar risk of adverse events during the treatment of hyperthyroidism.[17] These controversial results require additional studies to make it clear about the clinical outcomes of hyperthyroidism patients after the treatment of PTU and MMI. This meta-analysis was performed to better understand the efficacy and safety of PTU and MMI in the treatment of hyperthyroidism.
2. Methods
2.1. Search strategy
The study was conducted on July 1st, 2020. Articles were searched through the PubMed, EMBASE, Cochrane Library, Web of Science, CNKI, Wanfang, and QVIP. English database was searched on July 9th, 2020, whereas Chinese database was searched on July 14th, 2020. We included the terms “Hyperthyroidism” OR “Hyperthyroid” OR “Hyperthyroids” OR “Primary Hyperthyroidism” OR “Hyperthyroidism, Primary” AND “Antithyroid Agents” OR “Agents, Antithyroid” OR “Thyroid Antagonists” OR “Antagonists, Thyroid” OR “Antithyroid Drugs” OR “Drugs, Antithyroid” OR “Goitrogens” OR “Antithyroid Effect” OR “Effect, Antithyroid” OR “Antithyroid Effects” OR “Effects, Antithyroid” OR “methylthiouracil” OR “Alkiron” OR “4-Methyl-2-thiouracil” OR “Thimecil” OR “Metacil” OR “propylthiouracil” OR “6-Propyl-2-Thiouracil” OR “6 Propyl 2 Thiouracil” OR “methimazole” OR “1-Methyl-2-mercaptoimidazole” OR “1 Methyl 2 mercaptoimidazole” OR “Merkazolil” OR “Tiamazol” OR “Thiamazole” OR “Thimazol” OR “Mercasolyl” OR “Mercazolyl” OR “Methymazol” OR “Methylmercaptoimidazole” OR “Mercazol” OR “Mercazole” OR “Metisol” OR “Metizol” OR “Tapazole” OR “Tirodril” OR “Strumazol” OR “Thiamazol Henning” OR “Henning, Thiamazol” OR “Thiamazol Hexal” OR “Hexal, Thiamazol” OR “Thyrozol” OR “Favistan” OR “Methizol” OR “carbimazole” OR “Carbimazole Henning” OR “Neo-Thyreostat” OR “Neomercazole” OR “Neo-Mercazole” OR “Neo Tomizol”. The retrieval style in PubMed was Search: ((((((((((carbimazole [Title/Abstract]) OR (Carbimazole Henning [Title/Abstract])) OR (Neo-Thyreostat [Title/Abstract])) OR (Neomercazole [Title/Abstract])) OR (Neo-Mercazole [Title/Abstract])) OR (Neo Tomizol [Title/Abstract])) OR (((((((((((((((((((((((((methimazole [Title/Abstract]) OR (1-Methyl-2-mercaptoimidazole [Title/Abstract])) OR (1 Methyl 2 mercaptoimidazole [Title/Abstract])) OR (Merkazolil [Title/Abstract])) OR (Tiamazol [Title/Abstract])) OR (Thiamazole [Title/Abstract])) OR (Thimazol [Title/Abstract])) OR (Mercasolyl [Title/Abstract])) OR (Mercazolyl [Title/Abstract])) OR (Methymazol [Title/Abstract])) OR (Methylmercaptoimidazole [Title/Abstract])) OR (Mercazol [Title/Abstract])) OR (Mercazole [Title/Abstract])) OR (Metisol [Title/Abstract])) OR (Metizol [Title/Abstract])) OR (Tapazole [Title/Abstract])) OR (Tirodril [Title/Abstract])) OR (Strumazol [Title/Abstract])) OR (Thiamazol Henning [Title/Abstract])) OR (Henning, Thiamazol [Title/Abstract])) OR (Thiamazol Hexal [Title/Abstract])) OR (Hexal, Thiamazol [Title/Abstract])) OR (Thyrozol [Title/Abstract])) OR (Favistan [Title/Abstract])) OR (Methizol [Title/Abstract]))) OR (((propylthiouracil [Title/Abstract]) OR (6-Propyl-2-Thiouracil [Title/Abstract])) OR (6 Propyl 2 Thiouracil [Title/Abstract]))) OR (((((methylthiouracil [Title/Abstract]) OR (Alkiron [Title/Abstract])) OR (4-Methyl-2-thiouracil [Title/Abstract])) OR (Thimecil [Title/Abstract])) OR (Metacil [Title/Abstract]))) OR (((((((((((Antithyroid Agents [Title/Abstract]) OR (Agents, Antithyroid [Title/Abstract])) OR (Thyroid Antagonists [Title/Abstract])) OR (Antagonists, Thyroid [Title/Abstract])) OR (Antithyroid Drugs [Title/Abstract])) OR (Drugs, Antithyroid [Title/Abstract])) OR (Goitrogens [Title/Abstract])) OR (Antithyroid Effect [Title/Abstract])) OR (Effect, Antithyroid [Title/Abstract])) OR (Antithyroid Effects [Title/Abstract])) OR (Effects, Antithyroid [Title/Abstract]))) AND (((((Hyperthyroidism [Title/Abstract]) OR (Hyperthyroid [Title/Abstract])) OR (Hyperthyroids [Title/Abstract])) OR (Primary Hyperthyroidism [Title/Abstract])) OR (Hyperthyroidism, Primary [Title/Abstract])). The retrieved literatures were imported into EndNoteX9, and the literatures after preliminary screening were conducted by reading the title and abstract. Then, the literatures that did not meet the requirements were excluded after reading the full text, and the remaining literature was finally included in this study. Our study was performed according to Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines, for the Institutional Review Board's approval or the informed consent are not necessarily required for meta-analysis.
2.2. Eligibility criteria
Inclusion criteria were first hyperthyroidism patients. The diagnostic criteria of hyperthyroidism are based on clinical symptoms: metabolic syndromes including heat unbearable, sweat, flustered, hand shake, easy to hunger, hyperphagia, emaciation characterized by goiter, ophthalmic sign, among others; and laboratory examinations: the serum levels of T3 and T4, FT3, free T4 (FT4) are increased, and the serum level of TSH is decreased; second, experimental group: treated with MMI, control group: treated with PTU; third, randomized controlled trials (RCTs); fourth, English and Chinese literatures.
Exclusion criteria were: animal experiments; articles with different study topics with our study; articles impossible to extract data; conference articles, dissertations, case reports, meta-analyses, and reviews.
2.3. Methodological quality appraisal
For the RCTs included in this study, the modified Jadad scale was used to evaluate their qualities,[18] which has a total score of 7 with 1 to 3 as low quality and 4 to 7 as high quality (Supplementary Table 1–2). Additionally, the Cochrane Collaboration's tool for assessing risk of bias in RCTs was applied to evaluate the quality of included studies.[19] The tool involved in Random Sequence Generation, Allocation Concealment, Blinding of Participants and Personnel, Blinding of Outcome Assessment, Incomplete Outcome Data Addressed, Free of Selective Reporting, and Free of Other Bias. Each was classified as “Yes,” “No,” or “?.” The results of the quality evaluation of included studies were shown in Supplementary Table 3 and Supplementary Figure 6. Moreover, the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was applied to measure the overall quality of evidence included in our study.[20] Evidence was evaluated through two aspects including Decrease quality of evidence (Study limitation, Indirectness, Inconsistency, Imprecision, and Publication bias) and increased quality of evidence (Large magnitude of effect, Residual confounding, and dose–response gradient). The detailed results were depicted in Supplementary Table 4.
2.4. Data collection process
All data were assessed by 2 reviewers (ST and LC) who extracted data including author, year, country, length of study, interventions (MMI or PTU), sex, age, number of study subjects, and outcomes indicators: clinical efficacy (effective rate and drug withdrawal rate); thyroid hormone levels (TSH, T3, T4, FT3, FT4, thyrotropin receptor antibody [TRAb] and thyroid peroxidase antibody [TPOAb]); liver function indexes (alanine aminotransferase [ALT], aspartate aminotransferase [AST], and alkaline phosphatase [ALP] levels), and adverse reactions (hypothyroidism, liver function damage, rash, pruritus, and leukopenia) (Table 1). When disagreements existed between the 2 reviewers, a consensus was achieved by consulting a third person (LJ).
Table 1.
Characteristics of articles involved in this meta-analysis.
| Author | Year | Country | Length of study | Groups | Intervention | N | Male/Female | Jadad score | Outcomes |
| Homsanit et al[22] | 2001 | Thailand | 3 mo | MMI | 15 mg once/day | 35 | 4/31 | 5 | 3, 4, 5, 13 |
| PTU | 150 mg once/day | 36 | 5/31 | ||||||
| He[21] | 2004 | China | 3 mo | MMI | 15 mg once/day | 15 | 5/10 | 4 | 3, 4, 7, 8, 13 |
| PTU | 150 mg once/day | 15 | 4/11 | ||||||
| Nakamura[23] | 2007 | Japan | 3 mo | MMI | 30 mg once/day | 98 | 25/73 | 6 | 14, 15, 17 |
| PTU | 300 mg once/day | 81 | 11/70 | ||||||
| Otsuka[24] | 2012 | Japan | 3 mo | MMI | 30 mg once/day | 144 | 21/123 | 4 | 2, 14, 15 |
| PTU | 300 mg once/day | 120 | 11/109 | ||||||
| Ma[35] | 2014 | China | 3 mo | MMI | 10 mg, 3 times /day for 30 days; then 10 mg, twice/d for 15 days; then 15 mg, once/day for 45 days | 50 | 24/76 | 4 | 5, 6, 7, 8, 9 |
| PTU | 100 mg, 3 times/day for 30 day; then 100 mg, twice/day for 15 days; then 50 mg, 3 times/days for 45 days | 50 | |||||||
| Xiang[29] | 2014 | China | 2 y | MMI | 20 mg, once/day for a month; then 2.5 mg, once/day for 1–2 year | 23 | 8/15 | 4 | 10, 14, 15, 17 |
| PTU | 100 mg, 3 times/day for a month; then 50 mg, once/day for 1–2 y | 23 | 6/17 | ||||||
| Wang[33] | 2015 | China | 2 y | MMI | 10 mg, 3 times/day | 60 | 31/29 | 3 | 1, 8, 9, 11, 12, 13, 17 |
| PTU | 100 mg, 3 times/day | 60 | 27/33 | ||||||
| He[32] | 2016 | China | 1.5 y | MMI | 30 mg/day, 3 times/day and then 5–10 mg/day, 3 times/day | 50 | 23/27 | 2 | 3, 4, 5 |
| PTU | 100 mg, 3 times/day; then 5–100 mg, 3 times/day | 50 | 22/28 | ||||||
| Liang[31] | 2016 | China | 3 mo | MMI | 30 mg/day; then 5–10 mg/day | 40 | 0/40 | 5 | 10, 11, 12 |
| PTU | 300mg/d; then 50–100 mg/day | 40 | 0/40 | ||||||
| Wang[25] | 2016 | China | 6 mo | MMI | 10 mg, 3 times/day | 50 | 19/31 | 4 | 1, 5, 6, 7, 11, 12, 13, 14, 15, 17 |
| PTU | 100 mg, 3 times/day | 50 | 17/33 | ||||||
| Bai[36] | 2017 | China | 3 mo | MMI | 10 mg, 3 times/day for 3 wk; then 10 mg, twice/day for 2 wk; then 10 mg, once/day for 3 mo | 45 | 23/22 | 2 | 5, 6, 7, 13, 14, 15, 16, 17 |
| PTU | 100, 3 times/day for 3 wk; then 100 mg, 1–2 times/day for 2 wk; then 50 mg, once/day for 3 mo | 45 | 24/21 | ||||||
| Ma[26] | 2017 | China | 3 mo | MMI | 30 mg/day; then 5–10 mg/day | 128 | 50/78 | 3 | 14 |
| PTU | 300 mg/day; then 50–100 mg/day | 128 | 60/68 | ||||||
| Xu[28] | 2017 | China | 3 mo | MMI | 10 mg, twice/day for 3 mo | 45 | 15/30 | 5 | 5, 6, 7, 10, 11,12, 14 |
| PTU | 100 mg, 3 times/day for 3 mo | 45 | 16/29 | ||||||
| Chen[30] | 2018 | China | 1 y | MMI | 30 mg, once/day; then 5–10 mg/day for 1 y | 60 | 26/34 | 4 | 3, 4, 5, 6, 10, 11,12, 14 |
| PTU | 250 mg/day; 40–90 mg/day for 1 y | 60 | 25/35 | ||||||
| Wu[34] | 2018 | China | 1 y | MMI | 20–40 mg, once or twice | 34 | 15/19 | 3 | 1, 5, 6, 7, 14, 15, 16, 17 |
| PTU | 300 mg; then 150–400 mg | 34 | 14/20 | ||||||
| Yang[27] | 2019 | China | 6 mo | MMI | 30 mg/day; then 5–10 mg/day | 96 | 34/62 | 4 | |
| PTU | 300 mg/day; then 50–100 mg/day | 96 | 30/66 | 5, 6, 7, 10 |
2.5. Objectives
The primary objective was to compare the outcomes of patients receiving MMI or PTU including clinical efficacy (effective rate and drug withdrawal rate) and thyroid hormone levels (T3 level, T4 level, TSH level, FT3 level, FT4 level, TRAb level, and TPOAb level). The secondary outcomes were liver function indexes ALP level, ALT level, and AST level) and adverse reactions (hypothyroidism, liver function damage, rash, pruritus, and leukocytopenia). Subgroup analysis was conducted according to length of study, literature quality, and the results of Cochrane bias of risk evaluation.
2.6. Statistical analysis
Stata15.1 software (Stata Corporation, College Station, TX) was employed for statistical analysis in this meta-analysis. The weighted mean difference (WMD) was used as the effect index for measurement data while odds ratio (OR) were utilized as the effect index for the enumeration data with respective 95% confidence intervals (CIs). Heterogeneity test was performed for each outcome, and random-effects model analysis was performed when the heterogeneity was high (I2 ≥50%), otherwise, fixed-effects model analysis was adopted. When the difference was statistically significant and the heterogeneity was high (I2 ≥50%), the research time and literature quality were subjected to subgroup analysis. Meta-regression analysis was used to explore the source of heterogeneity. Sensitivity analysis was performed for all outcomes through reducing the literature by one and see whether the final conclusion has changed. The Begg test was applied to assess the publication bias. A difference of P < .05 was statistically significant.
3. Results
3.1. Included studies
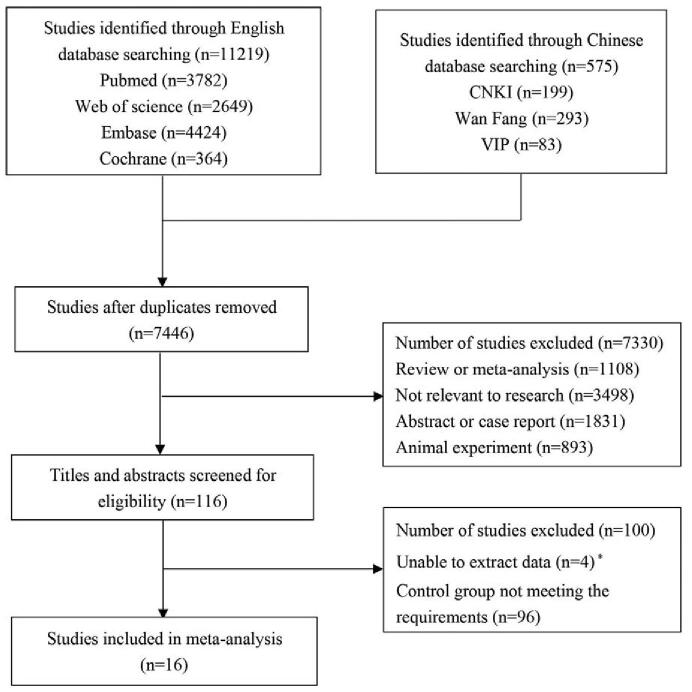
According to the search strategy, 11,219 articles were identified through searching English database and 575 articles were identified through retrieving Chinese database. After removing the duplicates, 7446 articles were included. Then 1108 reviews or meta-analysis, 3498 irrelevant researches, 1831 abstracts or case reports, and 893 animal experiments were eliminated. After screening the titles and abstracts, 4 articles unable to extract data and 96 articles with control group not meeting the requirements were excluded. Finally, 16 RCTs were retained.[21–36] In total, 1906 subjects were involved in this study with 973 receiving MMI and 933 receiving PTU. Figure 1 displayed the screen process of the articles.
Figure 1.
The screen process of the included articles.
3.2. Overall meta-analysis
The results in this meta-analysis showed that the levels of T3 (WMD = −1.321, 95% CI: −2.271 to −0.372, P = .006), T4 (WMD = −37.311, 95% CI: −61.012 to −13.610, P = .002), FT3 (WMD = −1.388, 95% CI: −2.543 to −0.233, P = .019), FT4 (WMD = −3.613, 95% CI: −5.972 to −1.255, P = .003), and the risk of liver function damage (OR = 0.208, 95% CI: 0.146–0.296, P < .001) in the MMI treatment group were lower than those in the PTU treatment group. The TSH level (WMD = 0.787, 95% CI: 0.380–1.194, P < .001) and the risk of hypothyroidism (OR = 2.738, 95% CI: 1.444–5.193, P = .002) were higher in the MMI treatment group than those in the PTU treatment group. No significant differences were obtained regarding the effective rate (OR = 0.427, 95% CI: 0.021–8.638), the drug withdrawal rate (OR = 1.135, 95% CI: 0.516–2.498), the levels of TRAb (95% CI: −28.085 to −3.288), TPOAb (WMD = 11.540, 95% CI: −5.873 to −28.952), ALP (WMD = −4.708, 95% CI: −19.606 to −10.189), ALT (WMD = −1.786, 95% CI: −8.078 to −4.506), AST (WMD = −2.149, 95% CI: −10.750 to −6.453), and the risk of rash (OR = 1.419, 95% CI: 0.980–2.056), pruritus (OR = 0.247, 95% CI: 0.099–1.220), leukocytopenia (OR = 0.887, 95% CI: 0.487–1.615) between the MMI treatment group and the PTU treatment group, all P > .05 (Table 2).
Table 2.
Overall data of the meta-analysis.
| Outcomes | Indicators | WMD/OR (95% CI) | P | I2 |
| Thyroid hormone levels | T3, nmol/L (4) | |||
| Overall | −1.321 (−2.271 to −0.372) | .006 | 96.4 | |
| Sensitivity | −1.321 (−2.271 to −0.372) | |||
| Study time | ||||
| 3 mo | −2.017 (−2.359 to −1.674) | <.001 | 0.0 | |
| ≥1 y | −0.583 (−1.021 to −0.145) | .009 | 68.5 | |
| Literature quality | ||||
| High quality | −1.474 (−2.762 to −0.185) | .025 | 97.5 | |
| Low quality | −0.890 (−1.403 to −0.377) | .001 | NA | |
| Blinding of outcome assessment | ||||
| Yes | −1.474 (−2.762 to −0.185) | .025 | 97.5 | |
| No | −0.890 (−1.403 to −0.377) | .001 | NA | |
| T4, nmol/L (4) | ||||
| Overall | −37.311 (−61.012 to −13.610) | .002 | 98.2 | |
| Sensitivity | −37.311 (−61.012 to −13.610) | |||
| Study time | ||||
| 3 mo | −60.064 (−79.052 to −41.076) | <.001 | 58.4 | |
| ≥1 y | −15.340 (−36.123 to 5.442) | .148 | 97.8 | |
| Literature quality | ||||
| High quality | −42.640 (−84.080 to −1.199) | .044 | 98.4 | |
| Low quality | −26.130 (−31.940 to 20.320) | <.001 | NA | |
| Blinding of outcome assessment | ||||
| Yes | −42.640 (−84.080 to −1.199) | .044 | 98.4 | |
| No | −26.130 (−31.940 to 20.320) | <.001 | NA | |
| TSH, μIU/mL (9) | ||||
| Overall | 0.787 (0.380–1.194) | <.001 | 98.0 | |
| Sensitivity | 0.787 (0.380–1.194) | |||
| Study time | ||||
| 3 mo | 1.385 (−0.374 to 3.145) | .123 | 98.8 | |
| 6 mo | 0.105 (−0.107 to 0.316) | .332 | 65.5 | |
| ≥1 y | 0.516 (0.284 to 0.747) | <.001 | 55.4 | |
| Literature quality | ||||
| High quality | 0.641 (0.045 to 1.237) | .035 | 98.1 | |
| Low quality | 1.116 (0.233 to 1.999) | .013 | 96.6 | |
| Blinding of outcome assessment | ||||
| Yes | 1.191 (−0.172 to 2.554) | .087 | 98.8 | |
| No | 0.439 (0.132 to 0.746) | .005 | 94.2 | |
| FT3, pmol/L (8) | ||||
| Overall | −1.388 (−2.543 to −0.233) | .019 | 97.7 | |
| Sensitivity | −1.388 (−2.543 to −0.233) | |||
| Study time | ||||
| 3 mo | −1.133 (−3.094 to 0.828) | .258 | 97.8 | |
| 6 mo | −1.532 (−4.609 to 1.545) | .329 | 99.2 | |
| 1 y | −1.767 (−2.992 to −0.542) | .005 | 92.1 | |
| Literature quality | ||||
| High quality | −1.077 (−2.537 to 0.384) | .149 | 98.1 | |
| Low quality | −2.311 (−2.667 to −1.955) | <.001 | 0.0 | |
| Blinding of outcome assessment | ||||
| Yes | −2.791 (−3.351 to −2.230) | <.001 | 56.3 | |
| No | −0.618 (−1.851 to 0.614) | .326 | 97.2 | |
| FT4, pmol/L (9) | ||||
| Overall | −3.613 (−5.972 to −1.255) | .003 | 98.6 | |
| Sensitivity | −3.613 (−5.972 to −1.255) | |||
| Study time | ||||
| 3 months | −3.254 (−6.664 to 0.156) | .061 | 98.5 | |
| 6 mo | −3.590 (−10.116 to 2.937) | .281 | 98.3 | |
| 1 y | −4.573 (−7.442 to −1.704) | .002 | 91.2 | |
| Literature quality | ||||
| High quality | −3.979 (−8.071 to 0.114) | .057 | 98.8 | |
| Low quality | −3.388 (−8.600 to 1.823) | .203 | 98.0 | |
| Blinding of outcome assessment | ||||
| Yes | −1.807 (−4.280 to 0.0.665) | .152 | 98.2 | |
| No | −6.759 (−7.448 to −6.071) | <.001 | 0.0 | |
| TRAb, U/L (3) | ||||
| Overall | −12.398 (−28.085 to 3.288) | .121 | 97.2 | |
| Sensitivity | −12.398 (−28.085 to 3.288) | |||
| TPOAb, IU/mL (2) | ||||
| Overall | 11.540 (−5.873 to 28.952) | .194 | 0.0 | |
| Sensitivity | 11.540 (−5.873 to 28.952) | |||
| Liver function indexes | ALP, U/L (4) | |||
| Overall | −4.708 (−19.606 to 10.189) | .536 | 96.8 | |
| Sensitivity | −4.708 (−19.606 to 10.189) | |||
| ALT, U/L (4) | ||||
| Overall | −1.786 (−8.078 to 4.506) | .578 | 98.2 | |
| Sensitivity | −1.786 (−8.078 to 4.506) | |||
| AST, U/L (4) | ||||
| Overall | −2.149 (−10.750 to 6.453) | .624 | 98.4 | |
| Sensitivity | −2.149 (−10.750 to 6.453) | |||
| Clinical efficacy | Effective rate (2) | |||
| Overall | 0.427 (0.021 to 8.638) | .579 | 67.6 | |
| Sensitivity | 0.427 (0.021 to 8.638) | |||
| Drug withdrawal rate (2) | ||||
| Overall | 1.135 (0.516 to 2.498) | .753 | 66.8 | |
| Sensitivity | 1.135 (0.516 to 2.498) | |||
| Adverse reactions | Hypothyroidism (6) | |||
| Overall | 2.738 (1.444 to 5.193) | .002 | 26.5 | |
| Sensitivity | 2.738 (1.444 to 5.193) | |||
| Liver function damage (9) | ||||
| Overall | 0.208 (0.146 to 0.296) | <.001 | 19.3 | |
| Sensitivity | 0.208 (0.146 to 0.296) | |||
| Rash (8) | ||||
| Overall | 1.419 (0.980 to 2.056) | .064 | 0.0 | |
| Sensitivity | 1.419 (0.980 to 2.056) | |||
| Pruritus (3) | ||||
| Overall | 0.247 (0.099 to 1.220) | .099 | 0.0 | |
| Sensitivity | 0.247 (0.099 to 1.220) | |||
| Leukocytopenia (5) | ||||
| Overall | 0.887 (0.487 to 1.615) | .696 | 13.7 | |
| Sensitivity | 0.887 (0.487 to 1.615) | |||
| Recurrence | (2) | |||
| Overall | 0.420 (0.061 to 2.904) | .379 | 0.0 | |
| Sensitivity | 0.420 (0.061 to 2.904) |
3.3. Clinical efficacy
3.3.1. Effective rate
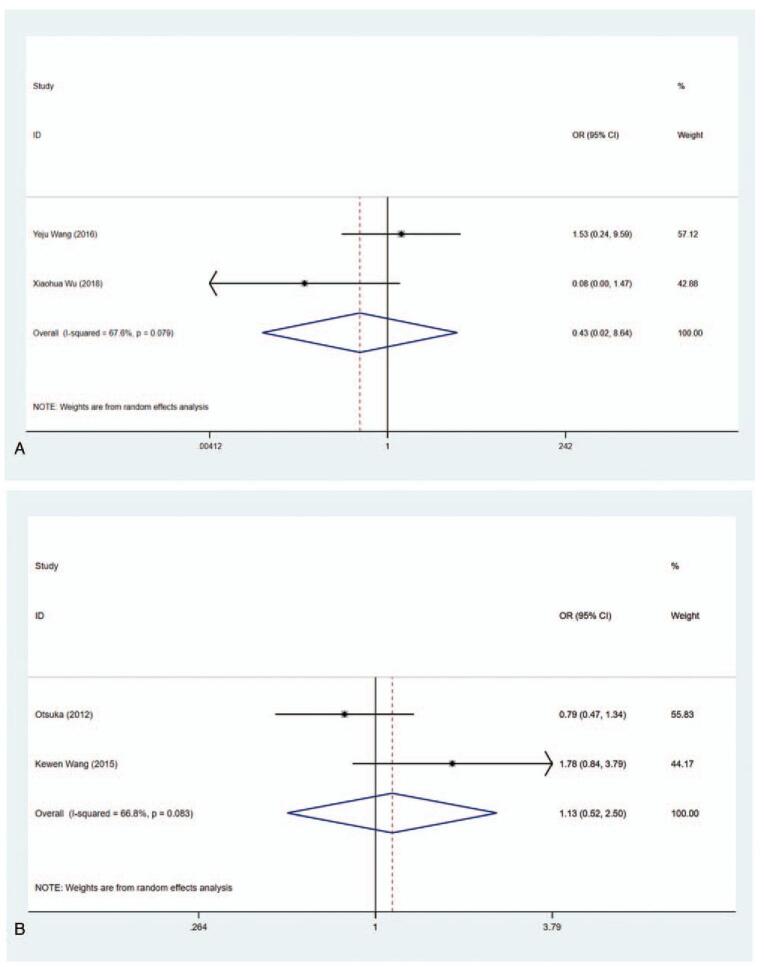
Effective rate = (cured + improved)/total number of cases. Cured means that the symptoms and signs of hyperthyroidism disappear completely and the thyroid hormone level returns to normal. Improved means that the symptoms and signs of hyperthyroidism disappeared, and the serum thyroid hormone level decreased, but still did not return to the normal level. Invalid means that the symptoms and signs of hyperthyroidism repeatedly existed or worsened, and the serum thyroid hormone level never decreased. In total, 2 articles included the data about the effective rate of MMI and PTU. Heterogeneity in the studies showed statistically significant difference (I2 = 67.6%), so the random-effect model was used for pooled analysis. The results depicted that there was no difference in clinical efficacy between the MMI group and the PTU group (OR = 0.427, 95% CI: 0.021–8.638, P = .579) (Fig. 2A, Table 2).
Figure 2.
Forest plot for effective rate (A) and drug withdrawal rate (B).
3.3.2. Drug withdrawal rate
The data on drug withdrawal rate were described in 2 articles (I2 = 66.8%). Similar drug withdrawal rate was obtained in the MMI group and PTU group with no statistical significance (OR = 1.135, 95% CI: 0.516–2.498, P = .753) (Fig. 2B, Table 2).
3.4. Thyroid hormone levels
3.4.1. T3 level, (nmol/L)
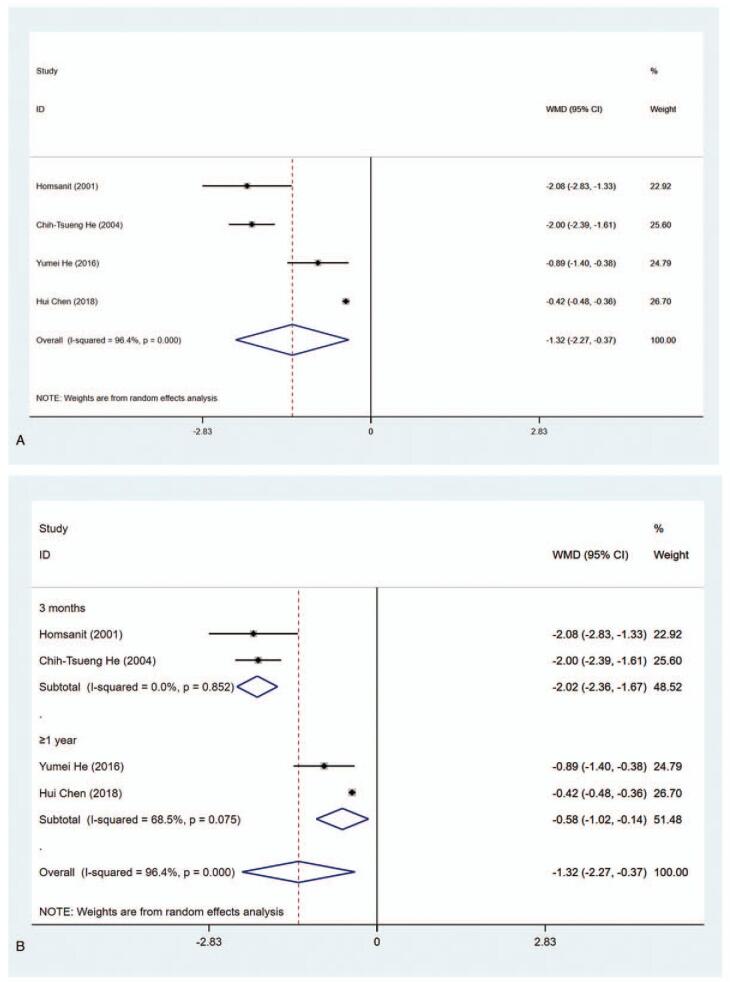
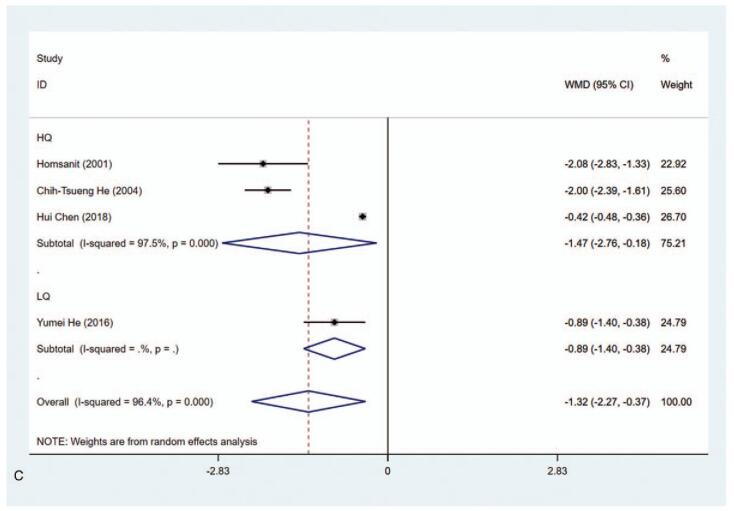
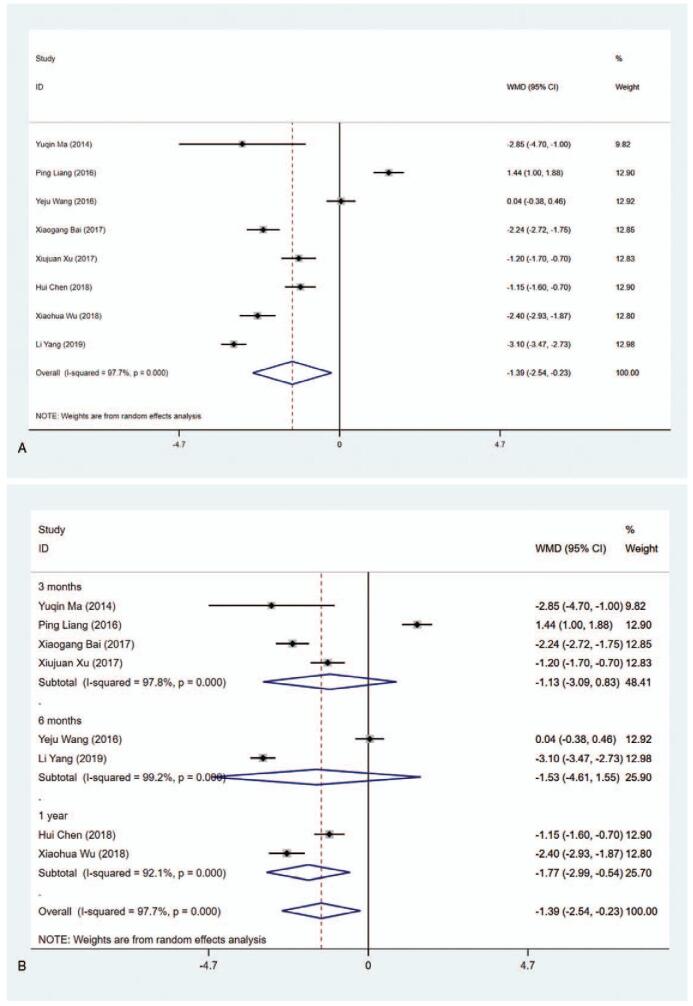
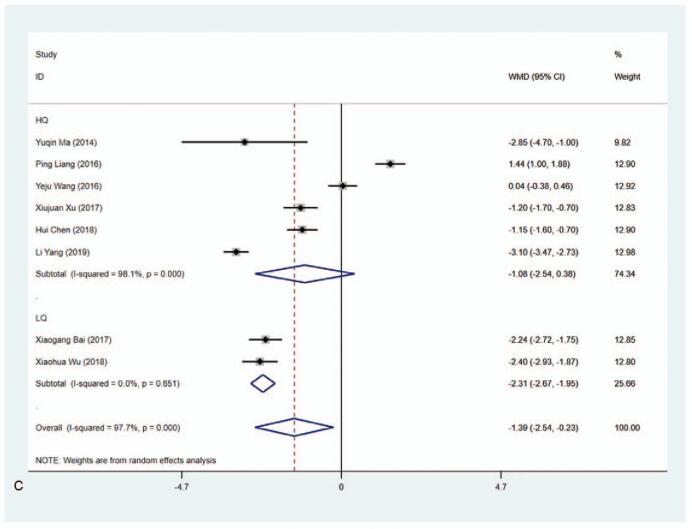
Four studies had sufficient data for assessing T3 level (nmol/L) in the MMI group and PTU group. The results elucidated that T3 level in the MMI treatment group was lower than that of PTU treatment group (WMD = −1.321, 95% CI: −2.271 to −0.372, P = .006) (Fig. 3 A, Table 2). The sensitivity analysis showed that WMD = −1.321 (95% CI: −2.271 to −0.372). As substantial heterogeneity was observed in the pooled data (I2 = 96.4%), subgroup analysis was conducted. According to length of study and literature quality, there were significant differences in 3 months (WMD = −2.017, 95% CI: −2.359 to −1.674, P < .001), ≥1 year (WMD = −0.583, 95% CI: −1.021 to −0.145, P < .001), high quality (WMD = −1.474, 95% CI: −2.762 to −0.185, P = .025), and low quality (WMD = −0.890, 95% CI: −1.403 to −0.377, P = .001). (Fig. 3 B and C, Table 2). To explore the sources of heterogeneity, meta-regression was performed concerning length of study (3 months vs ≥1 year) and literature quality (high quality vs low quality). The results demonstrated that length of study and literature quality were not associated with the heterogeneity (P > .05).
Figure 3.
Forest plot for T3 level (A), length of study (B) and literature quality (C).
According to the results of the Cochrane Collaboration's tool for assessing risk of bias in RCTs, 6 studies presented high risk of bias in Blinding of Outcome Assessment. Subgroup analysis was also conducted based on the results of Blinding of Outcome Assessment. The data depicted that there were significant differences in Blinding of Outcome Assessment (Yes) (WMD = −1.474, 95% CI: −2.762 to −0.185, P = .025) and Blinding of Outcome Assessment (No) (WMD = −0.890, 95% CI: −1.403 to −0.377, P = .001) groups (Supplementary Figure 1, Table 2). The results suggested that T3 level in the MMI treatment group was lower than that of PTU treatment group.
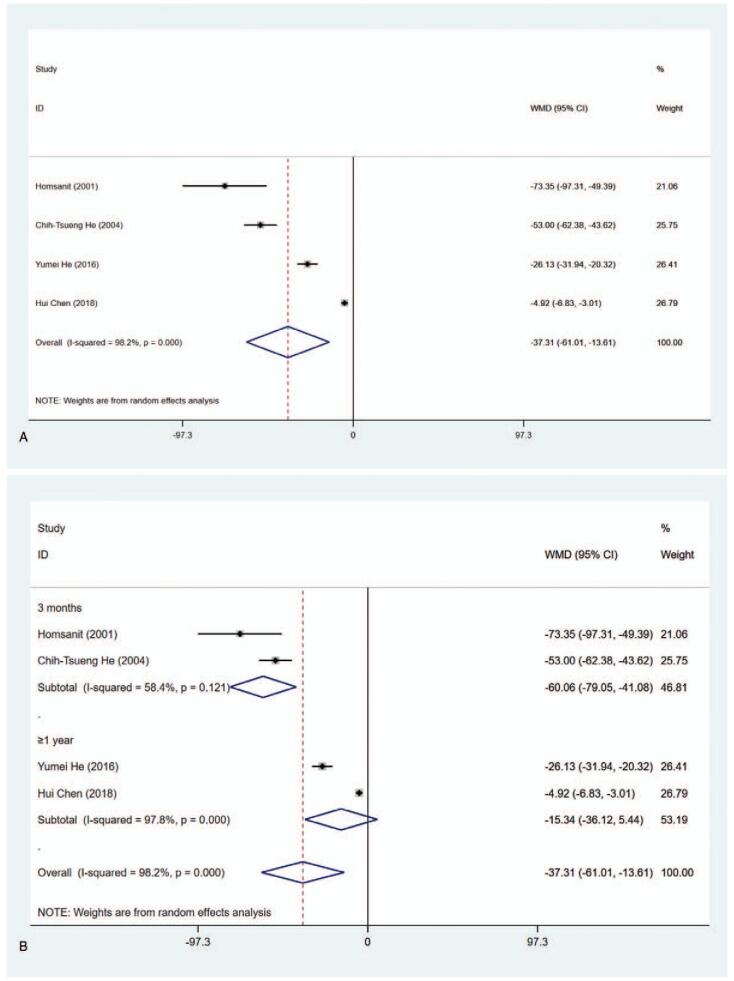
3.4.2. T4 level (nmol/L)
The data about the level of T4 (nmol/L) have been reported in 4 articles. The pooled analysis of data revealed that the level of T4 in the MMI treatment group was lower than that in the PTU treatment group (WMD = −37.311, 95% CI: −61.012 to −13.610, P = .002) (Fig. 4 A, Table 2). The sensitivity analysis showed that WMD = −37.311 (95% CI: −61.012 to −13.610). Subgroup analysis was conducted in regarding with length of study and literature quality due to the substantial heterogeneity (I2 = 98.2%). As shown in Figure 4 B and C and Table 2, significant differences were observed in 3 months (WMD = −60.064, 95% CI: −79.052 to −41.076, P < .001), high quality (WMD = −42.640, 95% CI: −84.080 to −1.199, P = .044) and low quality (WMD = −26.130, 95% CI: −31.940 to −20.320, P < .001). Meta-regression was conducted on length of study (3 months vs ≥1 year) and literature quality (high quality vs low quality), showing that length of study and literature quality had no relevant to the heterogeneity (P > .05). In addition, significant differences were also seen in Blinding of Outcome Assessment (Yes) (WMD = −42.640, 95% CI: −84.080 to −1.199, P = .044) and Blinding of Outcome Assessment (No) (WMD = −26.130, 95% CI: −31.940 to −20.320, P < .001) groups (Supplementary Figure 2, Table 2), indicating that T4 level in the MMI treatment group was lower than that of PTU treatment group.
Figure 3 (Continued).
Forest plot for T3 level (A), length of study (B) and literature quality (C).
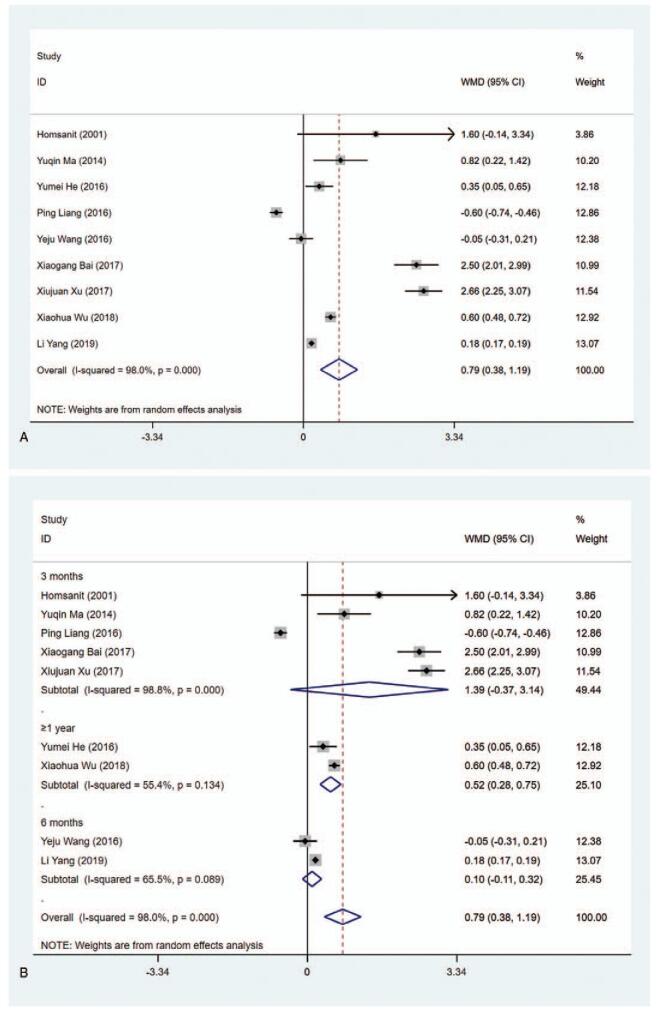
3.4.3. TSH level (μIU/mL)
The data on the level of TSH (μIU/mL) were available in 9 studies. According to the results of the pooled data analysis, the TSH level was higher in the MMI treatment group than that in the PTU treatment group (WMD = 0.787, 95% CI: 0.380–1.194, P < .001) (Fig. 5 A, Table 2). The sensitivity analysis showed that WMD = 0.787 (95% CI: 0.380–1.194). The heterogeneity test results showed statistically significant difference (I2 = 98.0%). Subgroup analysis indicated the differences were statistically significant in ≥1 year (WMD = 0.516, 95% CI: 0.284–0.747, P < .001), high quality (WMD = 0.641, 95% CI: 0.045–1.237, P = .035), and low quality (WMD = 1.116, 95% CI: 0.233–1.999, P = .013) (Fig. 5 B and C, Table 2). The results of meta-regression analysis on length of study (3 vs 6 months or 3 months vs ≥1 year) and literature quality (high quality vs low quality) disclosed that length of study and literature quality were not the influencing factors of the heterogeneity (P > .05). Besides, subgroup analysis in risk of bias concerning Blinding of Outcome Assessment showed evident difference in Blinding of Outcome Assessment (No) group (WMD = 0.439, 95% CI: 0.132–0.746, P = .005) (Supplementary Figure 3, Table 2), implying that T3 level in the MMI treatment group was lower than that of PTU treatment group in studies with risk of bias in Blinding of Outcome Assessment.
Figure 4.
Forest plot for T4 level (A), length of study (B) and literature quality (C).
3.4.4. FT3 level (pmol/L)
Eight studies included the data about FT3 level (pmol/L). The pooled data indicated that the FT3 level in the MMI treatment group was lower than that in the PTU treatment group (WMD = −1.388, 95% CI:−2.543 to −0.233, P = .019) (Fig. 6 A, Table 2). The sensitivity analysis showed that WMD−1.388 (95% CI: −2.543 to −0.233). As the heterogeneity between studies was considerable (I2 = 97.7%), subgroup analysis was conducted based on length of study and literature quality. The results showed that 1 year (WMD = −1.767, 95% CI: −2.992 to −0.542, P = .005) and low quality (WMD = −2.311, 95% CI:−2.667 to -1.955, P < .001) presented statistical differences (Fig. 6 B and C, Table 2). The results of meta-regression revealed that length of study (3 vs 6 months or 3 months vs 1 year) and literature quality (high quality vs low quality) had no effect on the heterogeneity (P > .05). Additionally, we found significant difference of MMI and PTU in subgroup analysis in terms of Blinding of Outcome Assessment (Yes) (WMD = −2.791, 95% CI: −3.351 to −2.230, P < .001), illustrating that FT3 level in the MMI treatment group was lower than that in the PTU treatment group in literatures with no risk of bias in Blinding of Outcome Assessment according to the Cochrane Collaboration's tool for assessing risk of bias in RCTs (Supplementary Figure 4, Table 2).
Figure 4 (Continued).
Forest plot for T4 level (A), length of study (B) and literature quality (C).
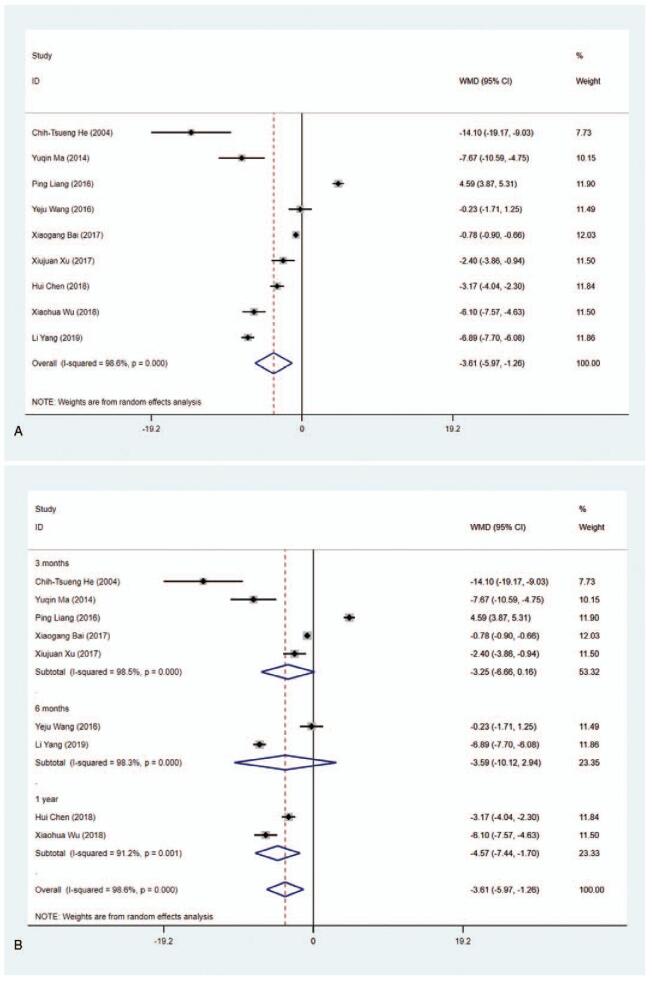
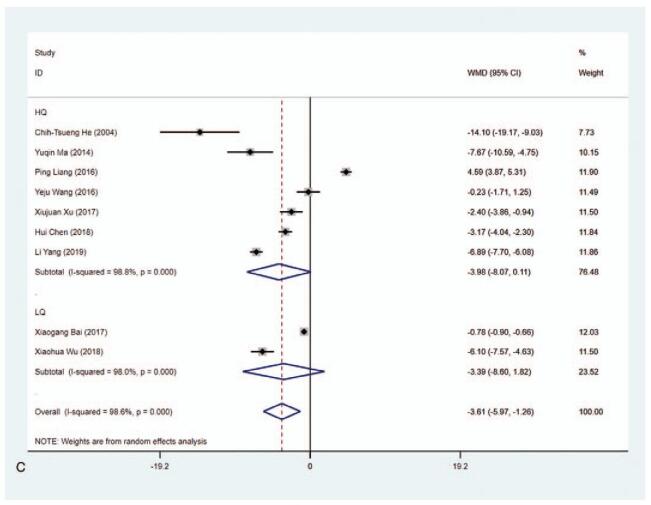
3.4.5. FT4 level (pmol/L)
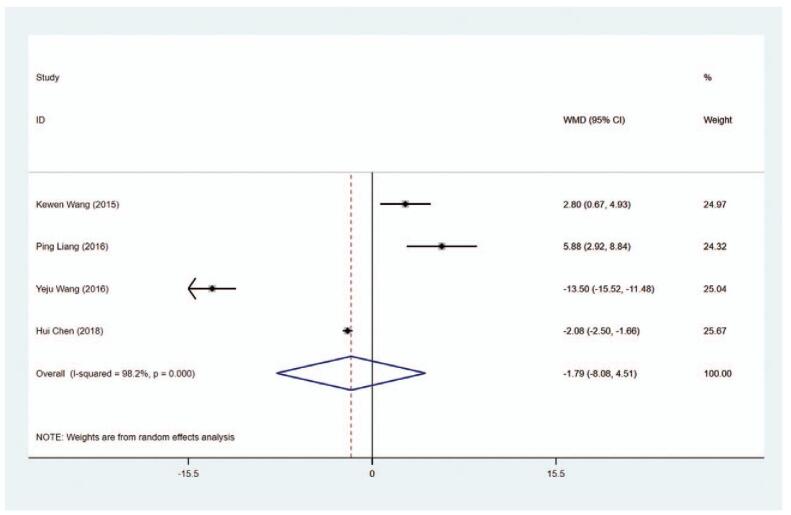
A total of 9 articles reported the level of FT4 (pmol/L) and the pooled data exhibited that the level of FT4 was lower in the MMI treatment group than that in the PTU treatment group (WMD−3.613, 95% CI: −5.972 to −1.255, P = .003) (Fig. 7 A, Table 2). The sensitivity analysis showed that WMD−3.613 (95% CI: −5.972 to −1.255). The heterogeneity test results showed statistically significant difference (I2 = 98.6%). Subgroup analysis was carried out due to the substantial heterogeneity, demonstrating that there was significant difference in 1 year (WMD−4.573, 95% CI: −7.442 to −1.704, P = .002) (Fig. 7 B and C, Table 2). The length of study (3 vs 6 months or 3 months vs 1 year) and literature quality (high quality vs low quality) were not the sources of the heterogeneity according to the results from meta-regression. Subgroup analysis concerning the risk of bias in Blinding of Outcome Assessment according to the Cochrane Collaboration's tool for assessing risk of bias in RCTs was also performed to identify the level of FT4 in MMI and PTU treatment groups. The data delineated that in studies in Blinding of Outcome Assessment (No) group, the level of FT4 was lower in the MMI treatment group than that in the PTU treatment group (WMD = −6.759, 95% CI: −7.448 to −6.071, P < .001) (Supplementary Figure 5, Table 2).
Figure 5.
Forest plot for TSH level (A), length of study (B) and literature quality (C).
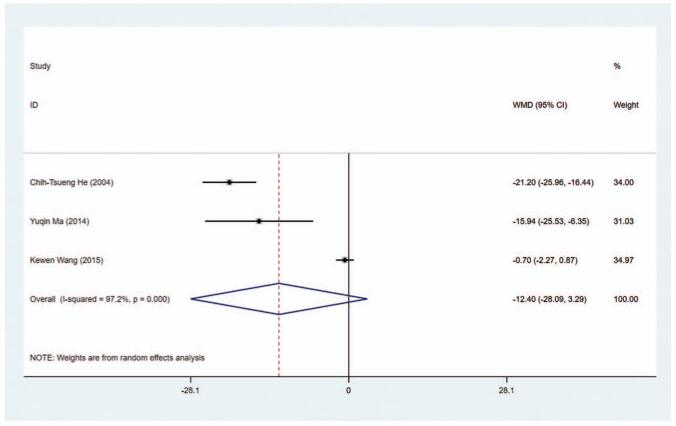
3.4.6. TRAb level
TRAb level (U/L) as an outcome index was detected in 3 studies (I2 = 97.2%). The WMD of the pooled data in all studies was -12.398 (95% CI: −28.085 to −3.288, P = .121), indicating there was no statistical significance on TRAb level between the MMI treatment group and the PTU treatment group (Fig. 8, Table 2). The sensitivity analysis showed that WMD = −12.398 (95% CI: −28.085 to −3.288).
Figure 5 (Continued).
Forest plot for TSH level (A), length of study (B) and literature quality (C).
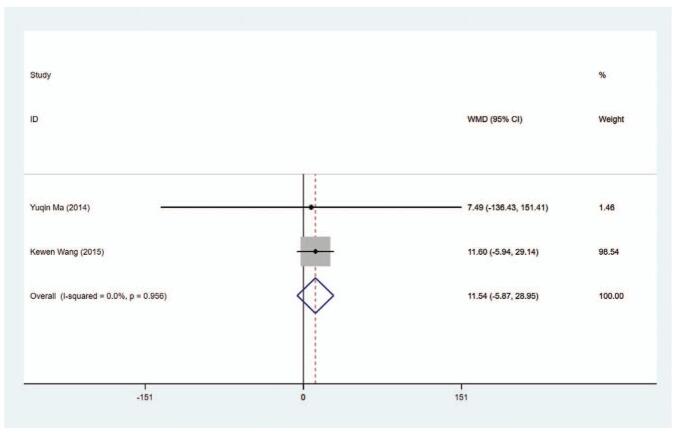
3.4.7. TPOAb level
Totally, 2 experiments provided information about TRAb level (IU/mL) in patients. The results of heterogeneity test showed no statistically significant difference (I2 = 0.0%), so fixed-effect model was used for pooled data analysis. The results of pooled data showed that the TPOAb level had no significant difference in between the MMI treatment group and the PTU treatment group (WMD = 11.540, 95% CI: −5.873 to −28.952, P = .194) (Fig. 9, Table 2). The sensitivity analysis showed that WMD = 11.540 (95% CI: −5.873 to −28.952).
Figure 6.
Forest plot for FT3 level (A), length of study (B) and literature quality (C).
3.5. Liver function indexes
3.5.1. ALP level
ALP level (U/L) was noticed in 4 trials. The results of the pooled data delineated that the ALP level was similar in the MMI treatment group and PTU treatment group (WMD = −4.708, 95% CI: −19.606 to −10.189, P = .536) (Fig. 10, Table 2). The sensitivity analysis showed that (WMD = −4.708, 95% CI: −19.606 to −10.189). To investigate the source of heterogeneity (I2 = 96.8%), meta-regression was performed on length of study, and the results indicated that length of study had no association with the heterogeneity (P > .05).
Figure 6 (Continued).
Forest plot for FT3 level (A), length of study (B) and literature quality (C).
3.5.2. ALT level
Four articles collected the data on ALT level (U/L) in patients. The WMD of the pooled data was −1.786 (95% CI: −8.078 to −4.506, P = .578), demonstrating the ALT level exhibited no significant difference in the MMI group and the PTU group (Figure 11, Table 2). The sensitivity analysis showed that WMD = −1.786 (95% CI: −8.078 to −4.506).
Figure 7.
Forest plot for FT4 level (A), length of study (B) and literature quality (C).
3.5.3. AST level
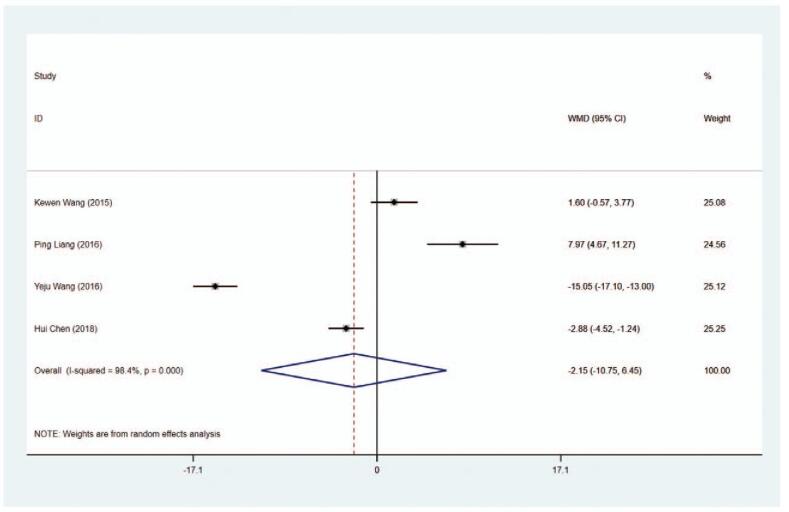
Data concerning AST level (U/L) were obtained from 4 studies. As shown in Figure 12 and Table 2, no difference was obtained in AST levels between the MMI treatment group and the PTU treatment group (WMD = −2.149, 95% CI: −10.750 to −6.453, P = .624). The sensitivity analysis showed that WMD–2.149, (95% CI: −10.750 to −6.453).
Figure 7 (Continued).
Forest plot for FT4 level (A), length of study (B) and literature quality (C).
3.6. Adverse reactions
3.6.1. Hypothyroidism
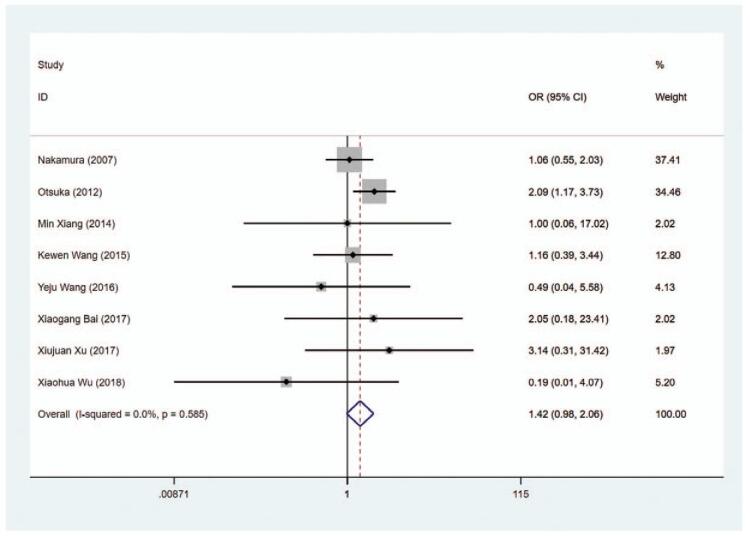
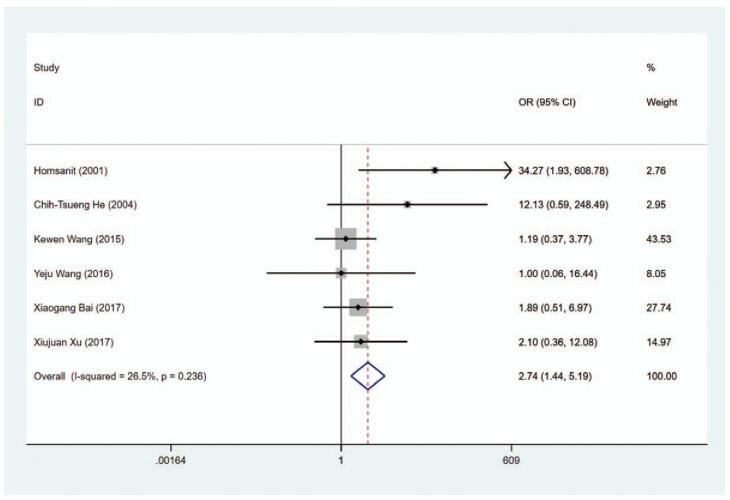
The risk of hypothyroidism was analyzed in 6 trials and the results indicated that the risk of hypothyroidism was higher in the MMI treatment group than in the PTU treatment group (OR = 2.738, 95% CI 1.444–5.193, P = .002) (Fig. 13, Table 2). The sensitivity analysis showed that OR = 2.738 (95% CI: 1.444–5.193).
Figure 8.
Forest plot for TRAb level.
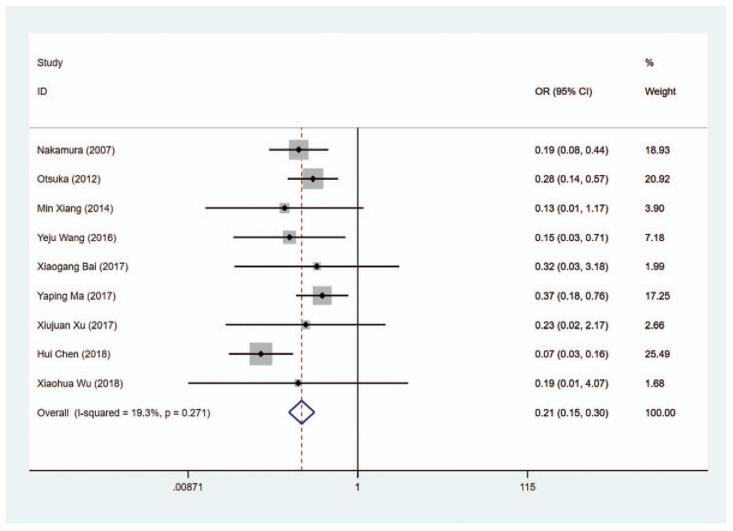
3.6.2. Liver function damage
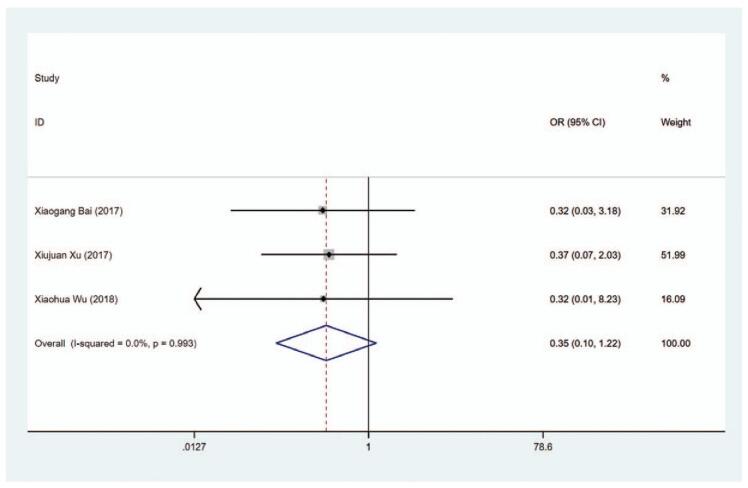
The definition of liver function damage refers to when AST and ALT more than double the upper limit of the reference range.[37] The data on liver function damage were extracted from 9 studies. We observed that the risk of liver function damage in the MMI treatment group was lower than that in the PTU treatment group (OR = 0.208, 95% CI: 0.146–0.296, P < .001) (Fig. 14, Table 2). The sensitivity analysis showed that OR = 0.208 (95% CI: 0.146–0.296).
Figure 9.
Forest plot for TPOAb level.
Figure 14.
Forest plot for the risk of liver function damage.
3.6.3. Rash
A total of 8 articles included the data about rash in the patients. The pooled data revealed that there was no significant difference regarding the risk of rash in the MMI treatment group and the PTU treatment group (OR = 1.419, 95% CI: 0.980–2.056, P = .064) (Fig. 15, Table 2). The sensitivity analysis showed that OR = 1.419 (95% CI: 0.980–2.056).
Figure 10.
Forest plot for ALP level.
Figure 15.
Forest plot for the risk of rash.
3.6.4. Pruritus
The data on the risk of pruritus in patients were available in 3 trials. As displayed in Figure 16 and Table 2, no significant difference was shown in the risk of pruritus between the MMI treatment group and the PTU treatment group (OR = 0.247, 95% CI: 0.099–1.220, P = .099). The sensitivity analysis showed that OR = 0.247 (95% CI: 0.099–1.220).
Figure 11.
Forest plot for ALT level.
Figure 16.
Forest plot for the risk of pruritus.
3.6.5. Leukocytopenia
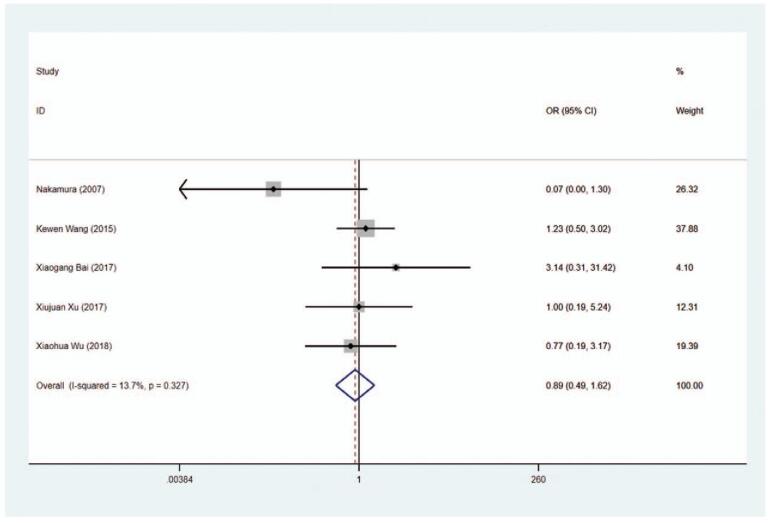
A total of 5 studies analyzing the risk of leukocytopenia were included. The pooled data indicated that the risk of leukocytopenia was similar in the MMI treatment group and the PTU treatment group (OR = 0.887, 95% CI: 0.487–1.615, P = .696) (Fig. 17, Table 2). The sensitivity analysis showed that OR = 0.887 (95% CI: 0.487–1.615).
Figure 12.
Forest plot for AST level.
Figure 17.
Forest plot for the risk of leukocytopenia.
3.7. Recurrence of hyperthyroidism
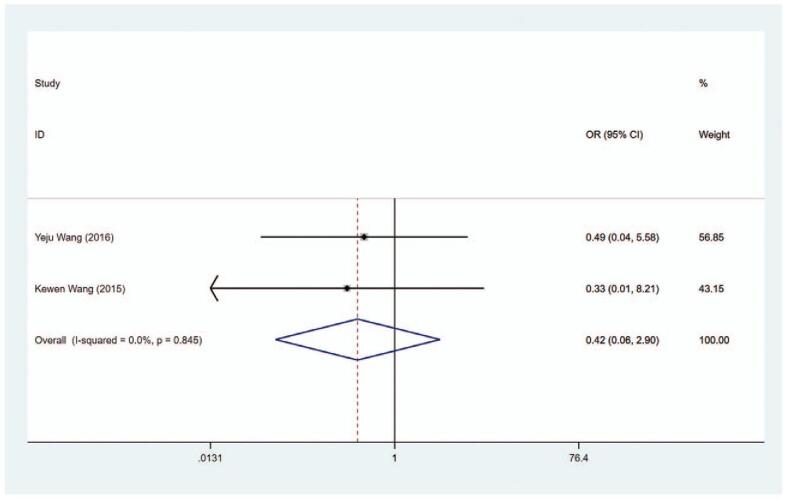
In total, 2 articles explored the recurrence of hyperthyroidism. The pooled data depicted that the risk of recurrence of hyperthyroidism was comparable in the MMI treatment group and the PTU treatment group (OR = 0.420, 95% CI: 0.061–2.904, P = .379) (Fig. 18, Table 2). The sensitivity analysis showed that OR = 0.420 (95% CI: 0.061–2.904).
Figure 13.
Forest plot for the risk of hypothyroidism.
Figure 18.
Forest plot for the recurrence of hyperthyroidism.
4. Discussion
This meta-analysis compared the efficacy and safety of MMI and PTU in the treatment of hyperthyroidism. The results showed that the levels of T3, T4, FT3, FT4 and the risk of liver function damage in the MMI treatment group were lower than those in the PTU treatment group. The TSH level and the risk of hypothyroidism were higher in the MMI treatment group than those in the PTU treatment group. The findings of our study might offer a reference for the treatment of hyperthyroidism regarding ATDs.
T3 and T4 are members of iodine-containing tyrosine, 90% of them can bind to plasma proteins composed of thyroxin-binding globulin when released to blood, and only a few of them are in free state, becoming FT3 and FT4.[38] The increase of T3 and T4 will inhibit the secretion of TSH. TSH serves as the first line indicator for evaluating thyroid function and the best index for screening overt and subclinical hyperthyroidism.[39] MMI suppresses the peroxidase system in thyroid cells to inhibit the iodization of tyrosine which can decrease the expression of T3, T4 and increase the expression of TSH; PTU inhibits the process of transformation of T4 into T3 and further elevates the level of TSH.[40] In our study, the levels of T3, T4, FT3 and FT4 in the MMI treatment group were lower than those in the PTU treatment group, whereas the level of TSH level was higher in the MMI treatment group than those in the PTU treatment group. This indicates that MMI is superior to PTU in the treatment of hyperthyroidism and can more effectively reduce the synthesis of T3 and T4. This conclusion was supported by a study from He et al indicating that MMI treatment induced a more rapid decrease of serum T3 levels than PTU treated patients.[21] Okamura et al emphasized that MMI treatment had better effect on reducing the level T3 in serum than PTU treatment.[37] That maybe because MMI had better effect on the substrate for T3 manufacture from T4. Heterogeneities existed in the results of T3, T4, TSH, FT3, and FT4 levels and subgroup analysis and sensitive analysis were conducted. The data depicted that significant differences were observed in 3 months, ≥1 year, high quality and low quality in T3 level, 3 months, high quality and low quality in T4 levels, ≥1 year, high quality and low quality in TSH level, 1 year and low quality in FT3 level and 1 year in FT4 level. However, meta-regression indicated the sources of the heterogeneity were not because of the length of study (3 vs 6 months or 3 months vs 1 year) and literature quality (high quality vs low quality). Additionally, based on the results of the Cochrane Collaboration's tool for assessing risk of bias in RCTs [19], subgroup analysis was also conducted based on the results of Blinding of Outcome Assessment. The data indicated that the evident differences were shown in T3 and T4 levels in Blinding of Outcome Assessment (Yes) and Blinding of Outcome Assessment (No). Statistical differences were also found in FT3 level in Blinding of Outcome Assessment (Yes) group. Besides, in Blinding of Outcome Assessment (No) group, the levels of TSH and FT4 were also significantly different between MMI and PTU groups. The reason of this may be due to Blinding of Outcome Assessment is only one of the items of the Cochrane Collaboration's tool for assessing risk of bias in RCTs.
In our study, we found the risk of liver function damage in the MMI treatment group were lower than those in the PTU treatment group. Liver function damage is a pivotal adverse event of PTU and MMI treatment in hyperthyroidism patients.[41] PTU may have higher risk of liver function damage than MMI. A study from Liaw et al reported that subclinical and asymptomatic liver injury can be commonly induced by PTU.[42] Tamagno revealed that PTU treatment has a higher risk of hepatotoxicity than MMI.[43] According to the results from the report of Russo et al, PTU ranked the third leading cause of drug-induced liver failure requiring transplants with 23 cases receiving liver transplants between 1990 and 2007 in the United States.[44] This may be because PTU can lead to active metabolites, resulting in the injury of the hepatocellular and the increase of ALT in serum. Accordingly, regular measurement of the liver function for hyperthyroidism patients undergoing PTU treatment is of great value and effective measures should be taken in time when transaminase or bilirubin rise obviously. The risk of hypothyroidism was higher in the MMI treatment group than those in the PTU treatment group in our meta-analysis. In previous study, 10 mg daily administration of MMI was found to cause spontaneous hypothyroidism in 2 patients with diffuse goiter among 36 participates.[45] These findings implied that the clinicians might be careful with the dose of MMI in patients to avoid hypothyroidism.
The implication of the present study was that we identified MMI might be superior to PTU in terms of reducing T3, T4, FT3, and FT4 levels, decreasing the risk of liver function damage and increasing the level of TSH. However, some limitations existed in this study. First, this study lacked the detailed analysis on sex differences in all patients as hyperthyroidism was reported to have higher incidence in females. Secondly, the functions of MMI and PTU vary dose-dependently. The doses of MMI and PTU in all the studies were not completely unification. Thirdly, publish bias was presented in the present study because the positive results were published more easily than negative results. Besides, in the clinic, more drugs will emerge for treating hyperthyroidism and the efficacy and safety of these drugs might be analyzed by network meta-analysis to identify the best drugs for treating patients with hyperthyroidism. These limitations implied that the results of our study should be interpreted with caution.
5. Conclusions
This meta-analysis compared the efficacy and safety of MMI and PTU in treating hyperthyroidism. The results of it indicated that the efficacy and safety of MMI was better than PTU in patients with hyperthyroidism regarding reducing T3, T4, FT3, and FT4 levels, decreasing the risk of liver function damage and increasing the level of TSH. The findings of the present study might serve as a guide for clinicians in the treatment of hyperthyroidism.
Acknowledgments
The authors thank the participants included in our study for their contributions.
Author contributions
All authors participated in conceiving this study. ST and LC wrote of the manuscript. Data assessment and extraction were completed by ST, LC and LJ. LC, LJ, and XF were contributed to data analysis. ST and XF critically reviewed and edited the manuscript. All authors read and approved the final manuscript.
Conceptualization: Shuang Tan.
Data curation: Long Chen, Likun Jin, Xiaomin Fu.
Formal analysis: Long Chen, Likun Jin, Xiaomin Fu.
Writing – original draft: Shuang Tan.
Writing – review & editing: Shuang Tan, Long Chen, Likun Jin, Xiaomin Fu.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, ATDs = anti-thyroid drugs, CIs = confidence intervals, FT3 = Free T3, FT4 = free T4, GRADE = Grading of Recommendations, Assessment, Development and Evaluation, MMI = methimazole, OR = odds ratio, PTU = propylthiouracil, RCTs = randomized controlled trials, T3 = triiodothyronine, T4 = thyroxine, TSH = thyroid stimulating hormone, WMD = weighted mean difference.
How to cite this article: Tan S, Chen L, Jin L, Fu X. The efficiency and safety of methimazole and propylthiouracil in hyperthyroidism: A meta-analysis of randomized controlled trials. Medicine. 2021;100:30(e26707).
Register number: osf.io/ds637 (https://osf.io/search/).
Funding: Project supported by Beijing Xicheng District Health Commission of China for Youth Science and Technology Personnel Training (Grant No. xwkx2020–02).
The authors report no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
1 = effective rate, 2 = drug withdrawal rate, 3 = T3 level, 4 = T4 level, 5 = TSH level, 6 = FT3 level, 7 = FT4 level, 8 = TRAb level, 9 = TPOAb level, 10 = ALP level, 11 = ALT level, 12 = AST level, 13 = hypothyroidism, 14 = liver function damage, 15 = rash, 16 = pruritus, 17 = leukocytopenia, HQ = high quality, LQ = low quality, MMI = methimazole, N = number of cases, PTU = propylthiouracil.
ALP = alkaline phosphatase, ALT = alalanine aminotransferase, AST = aspartate aminotransferase, CIs = confidence intervals, FT3 = Free T3, FT4 = Free T4, OR = odds ratio , T3 = triiodothyronine, T4 = thyroxine, TPOAb = thyroid peroxidase antibody, TRAb = thyrotropin receptor antibody, TSH = thyroid-stimulating hormone, WMD = weighted mean difference.
References
- [1].Bahn Chair RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid 2011;21:593–646. [DOI] [PubMed] [Google Scholar]
- [2].Costilla M, Macri Delbono R, Klecha A, Cremaschi GA, Barreiro Arcos ML. Oxidative stress produced by hyperthyroidism status induces the antioxidant enzyme transcription through the activation of the Nrf-2 factor in lymphoid tissues of Balb/c Mice. Oxid Med Cell Longev 2019;2019:7471890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol 2018;14:301–16. [DOI] [PubMed] [Google Scholar]
- [4].Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull 2011;99:39–51. [DOI] [PubMed] [Google Scholar]
- [5].De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet (London, England) 2016;388:906–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dekkers OM, Horváth-Puhó E, Cannegieter SC, Vandenbroucke JP, Sørensen HT, Jørgensen JO. Acute cardiovascular events and all-cause mortality in patients with hyperthyroidism: a population-based cohort study. Eur J Endocrinol 2017;176:01–9. [DOI] [PubMed] [Google Scholar]
- [7].Brandt F, Thvilum M, Almind D, et al. Morbidity before and after the diagnosis of hyperthyroidism: a nationwide register-based study. PLoS One 2013;8:e66711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brandt F, Thvilum M, Almind D, et al. Hyperthyroidism and psychiatric morbidity: evidence from a Danish nationwide register study. Eur J Endocrinol 2014;170:341–8. [DOI] [PubMed] [Google Scholar]
- [9].Brandt F, Almind D, Christensen K, Green A, Brix TH, Hegedüs L. Excess mortality in hyperthyroidism: the influence of preexisting comorbidity and genetic confounding: a danish nationwide register-based cohort study of twins and singletons. J Clin Endocrinol Metab 2012;97:4123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Collet TH, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med 2012;172:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sue M, Akama T, Kawashima A, et al. Propylthiouracil increases sodium/iodide symporter gene expression and iodide uptake in rat thyroid cells in the absence of TSH. Thyroid 2012;22:844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hashizume Y, Yamaki T, Hidaka H. Effect of anti-thyroid agents, methimazole and propylthiouracil, on brain noradrenaline content. Br J Pharmacol 1977;59:157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Emiliano AB, Governale L, Parks M, Cooper DS. Shifts in propylthiouracil and methimazole prescribing practices: antithyroid drug use in the United States from 1991 to 2008. J Clin Endocrinol Metab 2010;95:2227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abuid J, Larsen PR. Triiodothyronine and thyroxine in hyperthyroidism. Comparison of the acute changes during therapy with antithyroid agents. J Clin Invest 1974;54:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cooper DS, Saxe VC, Meskell M, Maloof F, Ridgway EC. Acute effects of propylthiouracil (PTU) on thyroidal iodide organification and peripheral iodothyronine deiodination: correlation with serum PTU levels measured by radioimmunoassay. J Clin Endocrinol Metab 1982;54:101–7. [DOI] [PubMed] [Google Scholar]
- [16].Azizi F. The safety and efficacy of antithyroid drugs. Expert Opin Drug Saf 2006;5:107–16. [DOI] [PubMed] [Google Scholar]
- [17].Wang MT, Lee WJ, Huang TY, Chu CL, Hsieh CH. Antithyroid drug-related hepatotoxicity in hyperthyroidism patients: a population-based cohort study. Br J Clin Pharmacol 2014;78:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin Trials 1996;17:01–12. [DOI] [PubMed] [Google Scholar]
- [19].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 2011;64:407–15. [DOI] [PubMed] [Google Scholar]
- [21].He CT, Hsieh AT, Pei D, et al. Comparison of single daily dose of methimazole and propylthiouracil in the treatment of Graves’ hyperthyroidism. Clin Endocrinol 2004;60:676–81. [DOI] [PubMed] [Google Scholar]
- [22].Homsanit M, Sriussadaporn S, Vannasaeng S, Peerapatdit T, Nitiyanant W, Vichayanrat A. Efficacy of single daily dosage of methimazole vs. propylthiouracil in the induction of euthyroidism. Clin Endocrinol 2001;54:385–90. [DOI] [PubMed] [Google Scholar]
- [23].Nakamura H, Noh JY, Itoh K, Fukata S, Miyauchi A, Hamada N. Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by Graves’ disease. J Clin Endocrinol Metab 2007;92:2157–62. [DOI] [PubMed] [Google Scholar]
- [24].Otsuka F, Noh JY, Chino T, et al. Hepatotoxicity and cutaneous reactions after antithyroid drug administration. Clin Endocrinol 2012;77:310–5. [DOI] [PubMed] [Google Scholar]
- [25].Wang Y. Comparison of efficacy between methiazole and propylthiouracil in the treatment of elderly hyperthyroidism. Prac Geriatr 2016;30:749–51. [Google Scholar]
- [26].Ma Y. Comparison of the effects of methimazole and prothiouracil on liver function in patients with hyperthyroidism. Lab Med Clin 2017;14:221–3. [Google Scholar]
- [27].Yang L. Curative effect of anti-thyroid drug therapy on bone biochemical indexes and bone mineral density in the treatment of Graves’ hyperthyroidism. Labeled Immunoassays and Clinical Medicine 2019;29:498–501. [Google Scholar]
- [28].Xu X. Effect of methimazole and propylthiouracil on beta-2 globulin and thyroid hormone levels in the treatment of hyperthyroidism. Chin J Biochem Pharm 2017;37:317–9. [Google Scholar]
- [29].Xiang M. Effects of methimazole and propylthiouracil on bone mineral density in patients with secondary osteoporosis due to hyperthyroidism. Practical Pharmacy And Clinical Remedies 2014;17:1431–4. [Google Scholar]
- [30].Chen H. Effects of methimazole on thyroid function and liver function in patients with hyperthyroidism. Guizhou Med J 2018;42:1194–5. [Google Scholar]
- [31].Liang P. Effects of prothiouracil on thyroid function and liver function in patients with hyperthyroidism complicated with pregnancy. Chin J Biochem Pharm 2016;36:102–4. [Google Scholar]
- [32].He Y. Effects of PTU and MMI on arterial blood flow in patients with hyperthyroidism. Mod Med J 2016;7:987–90. [Google Scholar]
- [33].Wang K. Analysis of curative effect of different anti-thyroid drugs on hyperthyroidism. Practical Pharmacy And Clinical Remedies 2015;18:157–60. [Google Scholar]
- [34].Wu X. Clinical observation of two different antithyroid drugs for hyperthyroidism. Int J Lab Med 2018;39:505–7. [Google Scholar]
- [35].Ma Y. Comparison between methimazole and prothiouracil in the treatment of Graves’ hyperthyroidism. Shandong Med J 2014;54:90–2. [Google Scholar]
- [36].Bai X. Comparison of clinical efficacy and safety of methimazole versus prothiouracil in the treatment of hyperthyroidism. Guizhou Med J 2017;41:945–6. [Google Scholar]
- [37].Okamura K, Ikenoue H, Shiroozu A, Sato K, Yoshinari M, Fujishima M. Reevaluation of the effects of methylmercaptoimidazole and propylthiouracil in patients with Graves’ hyperthyroidism. J Clin Endocrinol Metab 1987;65:719–23. [DOI] [PubMed] [Google Scholar]
- [38].Bakhshandeh M, Hashemi B, Mahdavi SR, Nikoofar A, Edraki HR, Kazemnejad A. Evaluation of thyroid disorders during head-and-neck radiotherapy by using functional analysis and ultrasonography. Int J Radiat Oncol Biol Phys 2012;83:198–203. [DOI] [PubMed] [Google Scholar]
- [39].Ross DS, Ardisson LJ, Meskell MJ. Measurement of thyrotropin in clinical and subclinical hyperthyroidism using a new chemiluminescent assay. J Clin Endocrinol Metab 1989;69:684–8. [DOI] [PubMed] [Google Scholar]
- [40].Kobaly K, Mandel SJ. Hyperthyroidism and pregnancy. Endocrinol Metab Clin North Am 2019;48:533–45. [DOI] [PubMed] [Google Scholar]
- [41].El-Kareem MA, Derwish WA, Moustafa HM. Response rate and factors affecting the outcome of a fixed dose of RAI-131 therapy in Graves’ disease: a 10-year Egyptian experience. Nuclear Med Commun 2014;35:900–7. [DOI] [PubMed] [Google Scholar]
- [42].Liaw YF, Huang MJ, Fan KD, Li KL, Wu SS, Chen TJ. Hepatic injury during propylthiouracil therapy in patients with hyperthyroidism. A cohort study. Ann Intern Med 1993;118:424–8. [DOI] [PubMed] [Google Scholar]
- [43].Tamagno G. Hyperthyroidism and antithyroid medications: a friend and an enemy of psychosis. Expert Opinion Drug Safety 2014;13:01–3. [DOI] [PubMed] [Google Scholar]
- [44].Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transplant 2004;10:1018–23. [DOI] [PubMed] [Google Scholar]
- [45].Azizi F, Abdi H, Cheraghi L, Amouzegar A. Treatment of subclinical hyperthyroidism in the elderly: comparison of radioiodine and long-term methimazole treatment. Thyroid 2021;31:545–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.