Abstract
Background:
Sodium glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have been demonstrated to be able to improve the cardiovascular and renal prognosis in patients with type 2 diabetes (T2D). However, the relative efficacy of various SGLT2 inhibitors and GLP-1 RAs on cardiorenal outcomes is unestablished.
Methods:
We searched PubMed and Embase for relevant cardiovascular or renal outcome trials (CVOTs). Endpoints of interest were major adverse cardiovascular events (MACE), stroke, myocardial infarction (MI), cardiovascular death (CVD), all-cause death (ACD), kidney function progression (KFP), and hospitalization for heart failure (HHF). Bayesian network meta-analysis was conducted to produce pooled hazard ratio (HR) and 95% confidence interval (CI). We calculated the probability values of surface under the cumulative ranking curve to rank active and placebo interventions.
Results:
Fourteen COVTs were included in analysis. Sotagliflozin (HR 0.76, 95% CI 0.61–0.94), subcutaneous semaglutide, and albiglutide lowered MACE versus lixisenatide among others. Sotagliflozin (HR 0.59, 95% CI 0.40–0.89), canagliflozin, and empagliflozin lowered HHF versus subcutaneous semaglutide among others. Dapagliflozin and empagliflozin lowered KFP versus exenatide among others. Empagliflozin and oral semaglutide lowered CVD versus dapagliflozin among others. Sotagliflozin (HR 0.65, 95% CI 0.47–0.91) and albiglutide lowered MI versus ertugliflozin among others. Sotagliflozin (HR 0.56, 95% CI 0.37–0.85) and subcutaneous semaglutide lowered stroke versus empagliflozin among others. Oral semaglutide and empagliflozin lowered ACD versus subcutaneous semaglutide among others. The maximum surface under the cumulative ranking curve values followed sotagliflozin, subcutaneous semaglutide, and albiglutide in lowering MACE; sotagliflozin, canagliflozin, and empagliflozin in lowering HHF; dapagliflozin and empagliflozin in lowering KFP; empagliflozin and oral semaglutide in lowering CVD; sotagliflozin and albiglutide in lowering MI; sotagliflozin and subcutaneous semaglutide in lowering stroke; and oral semaglutide and empagliflozin in lowering ACD.
Conclusions:
This updated network meta-analysis reproduced the findings in the first network meta-analysis, and moreover revealed that sotagliflozin was one of the most effective drugs as for lowering MI, stroke, MACE, and HHF, whereas ertugliflozin was not. These findings will provide the according evidence regarding the usage of specific SGLT2 inhibitors and GLP-1 RAs in T2D patients for prevention of specific cardiorenal endpoints.
Keywords: cardiorenal outcomes, GLP-1 RAs, network meta-analysis, SGLT2 inhibitors, type 2 diabetes
1. Introduction
More and more sodium glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have been demonstrated to be able to improve the cardiovascular and renal prognosis in patients with type 2 diabetes (T2D). However, the relative efficacy of various SGLT2 inhibitors and GLP-1 RAs on cardiorenal outcomes is unestablished. Not long ago, our group evaluated the relative efficacy of three SGLT2 inhibitors (ie, canagliflozin, dapagliflozin, and empagliflozin) and seven GLP-1 RAs (ie, lixisenatide, dulaglutide, liraglutide, albiglutide, exenatide, subcutaneous semaglutide, and oral semaglutide) on several cardiorenal outcomes among T2D patients, by conducting a network meta-analysis[1] which included 4 cardiovascular or renal outcome trials (CVOTs) of SGLT2 inhibitors[2–5] and 7 CVOTs of GLP-1 RAs.[6–12] Nowadays, 3 new CVOTs [13–15] respectively assessing sotagliflozin[14,15] and ertugliflozin[13] in T2D patients are available. However, the 2 SGLT2 inhibitors of sotagliflozin and ertugliflozin failed to be assessed in our first network meta-analysis.[1] Hence, we intended to implement an updated network meta-analysis by incorporating all the CVOTs of SGLT2 inhibitors and GLP-1 RAs including the 3 new ones,[13–15] to assess the relative cardiorenal effects of five SGLT2 inhibitor and 7 GLP-1 RA interventions in T2D patients.
2. Methods
This network meta-analysis is reported according to the PRISMA extension statement for network meta-analyses.[16] The PRISMA checklist for this article is provided in Table S1 (Supplemental Digital Content, which shows the PRISMA checklist).
2.1. Inclusion and exclusion criteria
The databases of PubMed and Embase were searched for relevant randomized trials from the start date of databases to March 15th, 2021. The according search strategies for the 2 databases are provided in Table S2 (Supplemental Digital Content, which presents the whole search strategies). Studies eligible to be included in analysis were CVOTs comparing any SGLT2 inhibitor or GLP-1 RA with placebo in adult individuals with T2D. Seven critical endpoints of interest for this study were major adverse cardiovascular events (MACE), fatal or nonfatal stroke, fatal or nonfatal myocardial infarction (MI), cardiovascular death (CVD), all-cause death (ACD), kidney function progression (KFP), and hospitalization for heart failure (HHF).
2.2. Study selection and risk of bias assessment
Two authors separately performed study selection and data extraction. The pre-specified data to be extracted from included studies included study name and publication year, type of interventions and comparators, and study outcomes. According to the Cochrane risk of bias tool,[17] included trials were assessed for risk of bias in terms of the following 7 types of risks: risk of selection bias (concerning random sequence generation), risk of detection bias (concerning blinding of outcome assessment), risk of performance bias (concerning blinding of participants and personnel), risk of selection bias (concerning allocation concealment), risk of attrition bias (concerning incomplete outcome data), risk of reporting bias (concerning selective reporting), and risk of other bias. Any disagreements between the 2 authors occurring in study selection, data extraction, and risk of bias assessment were addressed by discussion or the involvement of a third author.
2.3. Statistical analysis
Using the trial-level data of hazard ratios (HRs) and 95% confidence intervals (CIs) extracted from included studies, we performed Bayesian network meta-analysis with a fixed-effects model. Statistical heterogeneity across studies was measured by I2 statistic. In this network meta-analysis, we included only placebo-controlled trials, and therefore there was only indirect evidence between various active interventions. Thus, it was not necessary to perform test for the inconsistency between direct and indirect evidence. The probability values of surface under the cumulative ranking curve (SUCRA) were calculated to rank active and placebo interventions in terms of various cardiorenal endpoints, and a radar plot was drawn to present these SUCRA values. We implemented network meta-analysis in the R (version 3.6.0) and JAGS (version 4.3.0) software, whereas we made the radar plot in the Stata/SE (version 15.1) software.
2.4. Ethical statement
The data analyzed in this study were extracted from previously published studies, and therefore ethical approval was not necessary.
3. Results
After study selection (Figure S1, Supplemental Digital Content which shows the process of study selection), we included 14 placebo-controlled CVOTs[2–15] in quantitative synthesis. Included trials consisted of 7 ones of SGLT2 inhibitors[2–5,13–15] and 7 ones of GLP-1 RAs,[6–12] all of which were with low risk of bias (Figure S2, Supplemental Digital Content which presents the result of risk of bias assessment). A total of five SGLT2 inhibitor interventions (ie, sotagliflozin, ertugliflozin, canagliflozin, dapagliflozin, and empagliflozin) and 7 GLP-1 RA interventions (ie, lixisenatide, dulaglutide, liraglutide, albiglutide, exenatide, subcutaneous semaglutide, and oral semaglutide) were assessed in this network meta-analysis.
3.1. Network meta-analyses
Sotagliflozin (HR 0.76, 95% CI 0.61–0.94), subcutaneous semaglutide (HR 0.73, 95% CI 0.55–0.96), and albiglutide (HR 0.77, 95% CI 0.63–0.93) lowered the risk of MACE versus lixisenatide among others. The detailed results regarding MACE are shown in Table 1. Sotagliflozin (HR 0.59, 95% CI 0.40–0.89), canagliflozin (HR 0.58, 95% CI 0.38–0.87), and empagliflozin (HR 0.59, 95% CI 0.37–0.92) lowered the risk of HHF versus subcutaneous semaglutide among others. The detailed results regarding HHF are shown in Table 2. Dapagliflozin (HR 0.60, 95% CI 0.47–0.78) and empagliflozin (HR 0.61, 95% CI 0.43–0.87) lowered the risk of KFP versus exenatide among others. The detailed results regarding KFP are shown in Table 3. Empagliflozin (HR 0.63, 95% CI 0.47–0.84) and oral semaglutide (HR 0.50, 95% CI 0.26–0.94) lowered the risk of CVD versus dapagliflozin among others. The detailed results regarding CVD are shown in Table 4. Sotagliflozin (HR 0.65, 95% CI 0.47–0.91) and albiglutide (HR 0.72, 95% CI 0.55–0.95) lowered the risk of MI versus ertugliflozin among others. The detailed results regarding MI are shown in Table 5. Sotagliflozin (HR 0.56, 95% CI 0.37–0.85) and subcutaneous semaglutide (HR 0.55, 95% CI 0.32–0.94) lowered the risk of stroke versus empagliflozin among others. The detailed results regarding stroke are shown in Table 6. Oral semaglutide (HR 0.49, 95% CI 0.27–0.89) and empagliflozin (HR 0.65, 95% CI 0.43–0.96) lowered the risk of ACD versus subcutaneous semaglutide among others. The detailed results regarding ACD are shown in Table 7.
Table 1.
Pairwise comparison results from network meta-analysis on major adverse cardiovascular events.
| Albiglutide | 1.07 (0.9–1.28) | 1.19 (1–1.42) | 1.13 (0.94–1.35) | 1.1 (0.9–1.35) | 1.24 (1.03–1.51) | 1.17 (0.99–1.38) | 1.12 (0.93–1.33) | 1.31 (1.07–1.59) | 1.01 (0.71–1.46) | 1.28 (1.11–1.48) | 0.99 (0.79–1.23) | 0.95 (0.72–1.26) |
| 0.93 (0.78–1.11) | Canagliflozin | 1.11 (0.96–1.28) | 1.05 (0.9–1.22) | 1.03 (0.86–1.23) | 1.16 (0.98–1.37) | 1.09 (0.94–1.25) | 1.04 (0.89–1.21) | 1.21 (1.03–1.44) | 0.94 (0.66–1.34) | 1.19 (1.08–1.32) | 0.92 (0.75–1.12) | 0.88 (0.68–1.15) |
| 0.84 (0.71–1) | 0.9 (0.78–1.04) | Dapagliflozin | 0.95 (0.81–1.1) | 0.92 (0.77–1.1) | 1.04 (0.88–1.23) | 0.98 (0.85–1.12) | 0.94 (0.8–1.09) | 1.1 (0.92–1.3) | 0.85 (0.6–1.21) | 1.08 (0.97–1.19) | 0.83 (0.68–1.01) | 0.8 (0.61–1.04) |
| 0.89 (0.74–1.06) | 0.95 (0.82–1.11) | 1.06 (0.91–1.23) | Dulaglutide | 0.98 (0.81–1.18) | 1.1 (0.93–1.31) | 1.03 (0.89–1.2) | 0.99 (0.84–1.16) | 1.16 (0.97–1.38) | 0.9 (0.63–1.28) | 1.14 (1.01–1.27) | 0.88 (0.71–1.07) | 0.84 (0.64–1.1) |
| 0.91 (0.74–1.11) | 0.97 (0.82–1.17) | 1.08 (0.91–1.29) | 1.02 (0.85–1.23) | Empagliflozin | 1.13 (0.93–1.37) | 1.06 (0.89–1.26) | 1.01 (0.84–1.21) | 1.19 (0.97–1.45) | 0.92 (0.64–1.32) | 1.16 (1.01–1.34) | 0.9 (0.72–1.12) | 0.86 (0.65–1.15) |
| 0.8 (0.66–0.98) | 0.86 (0.73–1.02) | 0.96 (0.81–1.13) | 0.91 (0.76–1.08) | 0.89 (0.73–1.08) | Ertugliflozin | 0.94 (0.8–1.1) | 0.9 (0.75–1.07) | 1.05 (0.87–1.27) | 0.81 (0.57–1.17) | 1.03 (0.9–1.18) | 0.79 (0.64–0.98) | 0.76 (0.58–1.01) |
| 0.86 (0.72–1.01) | 0.92 (0.8–1.06) | 1.02 (0.89–1.17) | 0.97 (0.83–1.12) | 0.95 (0.79–1.12) | 1.07 (0.91–1.25) | Exenatide | 0.96 (0.83–1.1) | 1.12 (0.95–1.32) | 0.87 (0.61–1.23) | 1.1 (1–1.21) | 0.85 (0.7–1.03) | 0.81 (0.63–1.06) |
| 0.9 (0.75–1.07) | 0.96 (0.83–1.12) | 1.07 (0.92–1.24) | 1.01 (0.86–1.18) | 0.99 (0.82–1.19) | 1.11 (0.94–1.32) | 1.05 (0.91–1.21) | Liraglutide | 1.17 (0.98–1.4) | 0.91 (0.64–1.29) | 1.15 (1.03–1.28) | 0.89 (0.72–1.08) | 0.85 (0.65–1.11) |
| 0.77 (0.63–0.93) | 0.82 (0.69–0.98) | 0.91 (0.77–1.08) | 0.86 (0.72–1.03) | 0.84 (0.69–1.03) | 0.95 (0.79–1.15) | 0.89 (0.76–1.05) | 0.85 (0.72–1.02) | Lixisenatide | 0.78 (0.54–1.11) | 0.98 (0.86–1.12) | 0.76 (0.61–0.94) | 0.73 (0.55–0.96) |
| 0.99 (0.69–1.42) | 1.06 (0.75–1.51) | 1.18 (0.83–1.67) | 1.11 (0.78–1.58) | 1.09 (0.76–1.56) | 1.23 (0.86–1.76) | 1.15 (0.81–1.63) | 1.1 (0.77–1.56) | 1.29 (0.9–1.85) | Osemaglutide | 1.27 (0.91–1.77) | 0.97 (0.67–1.41) | 0.94 (0.62–1.42) |
| 0.78 (0.68–0.9) | 0.84 (0.76–0.93) | 0.93 (0.84–1.03) | 0.88 (0.79–0.99) | 0.86 (0.74–0.99) | 0.97 (0.85–1.11) | 0.91 (0.83–1) | 0.87 (0.78–0.97) | 1.02 (0.89–1.17) | 0.79 (0.57–1.1) | Placebo | 0.77 (0.65–0.91) | 0.74 (0.58–0.95) |
| 1.01 (0.81–1.26) | 1.09 (0.9–1.33) | 1.21 (0.99–1.47) | 1.14 (0.93–1.4) | 1.12 (0.89–1.39) | 1.26 (1.02–1.56) | 1.18 (0.97–1.43) | 1.13 (0.92–1.38) | 1.32 (1.07–1.64) | 1.03 (0.71–1.49) | 1.3 (1.1–1.54) | Sotagliflozin | 0.96 (0.71–1.29) |
| 1.05 (0.8–1.4) | 1.13 (0.87–1.48) | 1.26 (0.96–1.64) | 1.19 (0.91–1.56) | 1.16 (0.87–1.54) | 1.31 (0.99–1.73) | 1.23 (0.95–1.6) | 1.17 (0.9–1.54) | 1.38 (1.04–1.82) | 1.07 (0.71–1.61) | 1.35 (1.06–1.73) | 1.04 (0.77–1.4) | Ssemaglutide |
Table 2.
Pairwise comparison results from network meta-analysis on hospitalization for heart failure.
| Albiglutide | 0.9 (0.64–1.27) | 1.03 (0.73–1.45) | 1.31 (0.93–1.85) | 0.92 (0.62–1.35) | 0.99 (0.67–1.45) | 1.32 (0.94–1.87) | 1.23 (0.87–1.72) | 1.35 (0.93–1.98) | 1.21 (0.65–2.26) | 1.41 (1.06–1.88) | 0.93 (0.67–1.29) | 1.56 (0.98–2.49) |
| 1.11 (0.79–1.56) | Canagliflozin | 1.14 (0.88–1.48) | 1.45 (1.11–1.89) | 1.01 (0.73–1.4) | 1.09 (0.8–1.49) | 1.47 (1.13–1.91) | 1.36 (1.05–1.76) | 1.5 (1.1–2.04) | 1.34 (0.75–2.39) | 1.56 (1.3–1.88) | 1.03 (0.81–1.31) | 1.73 (1.15–2.62) |
| 0.97 (0.69–1.37) | 0.88 (0.68–1.14) | Dapagliflozin | 1.27 (0.98–1.66) | 0.89 (0.64–1.23) | 0.96 (0.7–1.31) | 1.29 (0.99–1.67) | 1.19 (0.92–1.54) | 1.31 (0.96–1.79) | 1.18 (0.66–2.1) | 1.37 (1.14–1.65) | 0.9 (0.71–1.15) | 1.52 (1–2.29) |
| 0.76 (0.54–1.08) | 0.69 (0.53–0.9) | 0.79 (0.6–1.02) | Dulaglutide | 0.7 (0.5–0.97) | 0.75 (0.55–1.03) | 1.01 (0.78–1.32) | 0.94 (0.72–1.21) | 1.03 (0.76–1.41) | 0.92 (0.52–1.65) | 1.08 (0.89–1.3) | 0.71 (0.55–0.9) | 1.19 (0.79–1.8) |
| 1.09 (0.74–1.62) | 0.99 (0.71–1.36) | 1.12 (0.81–1.55) | 1.43 (1.03–1.98) | Empagliflozin | 1.08 (0.74–1.55) | 1.45 (1.05–2) | 1.34 (0.97–1.84) | 1.48 (1.03–2.12) | 1.32 (0.72–2.45) | 1.54 (1.18–2.01) | 1.01 (0.74–1.38) | 1.71 (1.09–2.68) |
| 1.01 (0.69–1.49) | 0.92 (0.67–1.25) | 1.04 (0.76–1.43) | 1.33 (0.97–1.82) | 0.93 (0.64–1.34) | Ertugliflozin | 1.34 (0.98–1.84) | 1.24 (0.91–1.7) | 1.37 (0.96–1.96) | 1.23 (0.67–2.25) | 1.43 (1.11–1.84) | 0.94 (0.7–1.27) | 1.58 (1.01–2.48) |
| 0.76 (0.53–1.06) | 0.68 (0.52–0.89) | 0.78 (0.6–1.01) | 0.99 (0.76–1.29) | 0.69 (0.5–0.96) | 0.74 (0.54–1.02) | Exenatide | 0.93 (0.71–1.2) | 1.02 (0.75–1.39) | 0.92 (0.51–1.63) | 1.06 (0.88–1.28) | 0.7 (0.55–0.89) | 1.18 (0.78–1.78) |
| 0.82 (0.58–1.15) | 0.74 (0.57–0.96) | 0.84 (0.65–1.09) | 1.07 (0.82–1.39) | 0.75 (0.54–1.03) | 0.8 (0.59–1.1) | 1.08 (0.83–1.4) | Liraglutide | 1.1 (0.81–1.5) | 0.99 (0.55–1.77) | 1.15 (0.96–1.38) | 0.76 (0.6–0.97) | 1.27 (0.84–1.92) |
| 0.74 (0.5–1.08) | 0.67 (0.49–0.91) | 0.76 (0.56–1.04) | 0.97 (0.71–1.32) | 0.68 (0.47–0.97) | 0.73 (0.51–1.04) | 0.98 (0.72–1.34) | 0.91 (0.67–1.23) | Lixisenatide | 0.9 (0.49–1.63) | 1.04 (0.81–1.33) | 0.69 (0.51–0.92) | 1.16 (0.74–1.8) |
| 0.83 (0.44–1.54) | 0.75 (0.42–1.33) | 0.85 (0.48–1.52) | 1.08 (0.61–1.93) | 0.76 (0.41–1.39) | 0.81 (0.44–1.5) | 1.09 (0.61–1.95) | 1.01 (0.57–1.81) | 1.12 (0.61–2.04) | Osemaglutide | 1.16 (0.67–2.02) | 0.77 (0.43–1.36) | 1.29 (0.67–2.5) |
| 0.71 (0.53–0.95) | 0.64 (0.53–0.77) | 0.73 (0.61–0.88) | 0.93 (0.77–1.12) | 0.65 (0.5–0.85) | 0.7 (0.54–0.9) | 0.94 (0.78–1.13) | 0.87 (0.72–1.04) | 0.96 (0.75–1.23) | 0.86 (0.5–1.49) | Placebo | 0.66 (0.56–0.77) | 1.11 (0.77–1.6) |
| 1.08 (0.78–1.49) | 0.97 (0.76–1.24) | 1.11 (0.87–1.41) | 1.41 (1.11–1.8) | 0.99 (0.72–1.34) | 1.06 (0.79–1.43) | 1.43 (1.12–1.82) | 1.32 (1.03–1.68) | 1.46 (1.08–1.95) | 1.31 (0.74–2.31) | 1.52 (1.29–1.78) | Sotagliflozin | 1.68 (1.13–2.51) |
| 0.64 (0.4–1.02) | 0.58 (0.38–0.87) | 0.66 (0.44–1) | 0.84 (0.56–1.27) | 0.59 (0.37–0.92) | 0.63 (0.4–0.99) | 0.85 (0.56–1.28) | 0.78 (0.52–1.18) | 0.87 (0.56–1.35) | 0.77 (0.4–1.5) | 0.9 (0.63–1.3) | 0.59 (0.4–0.89) | Ssemaglutide |
Table 3.
Pairwise comparison results from network meta-analysis on kidney function progression.
| Canagliflozin | 0.83 (0.63–1.11) | 1.34 (1.08–1.66) | 0.85 (0.59–1.23) | 1.27 (0.92–1.75) | 1.38 (1.09–1.76) | 1.23 (0.96–1.57) | 1.32 (1–1.75) | 1.57 (1.3–1.91) | 1.12 (0.7–1.78) | 1.01 (0.69–1.47) |
| 1.2 (0.9–1.6) | Dapagliflozin | 1.6 (1.27–2.03) | 1.02 (0.7–1.49) | 1.53 (1.1–2.12) | 1.66 (1.28–2.15) | 1.47 (1.13–1.92) | 1.58 (1.18–2.13) | 1.89 (1.52–2.34) | 1.34 (0.83–2.16) | 1.21 (0.82–1.78) |
| 0.75 (0.6–0.93) | 0.62 (0.49–0.79) | Dulaglutide | 0.64 (0.46–0.88) | 0.95 (0.73–1.25) | 1.04 (0.87–1.23) | 0.92 (0.76–1.1) | 0.99 (0.79–1.24) | 1.18 (1.07–1.29) | 0.84 (0.54–1.29) | 0.75 (0.54–1.06) |
| 1.18 (0.81–1.7) | 0.98 (0.67–1.44) | 1.57 (1.13–2.18) | Empagliflozin | 1.5 (1–2.24) | 1.63 (1.15–2.3) | 1.44 (1.02–2.05) | 1.55 (1.07–2.26) | 1.85 (1.35–2.53) | 1.31 (0.78–2.24) | 1.19 (0.75–1.86) |
| 0.79 (0.57–1.08) | 0.65 (0.47–0.91) | 1.05 (0.8–1.37) | 0.67 (0.45–1) | Ertugliflozin | 1.09 (0.81–1.45) | 0.96 (0.72–1.3) | 1.04 (0.75–1.43) | 1.24 (0.96–1.59) | 0.88 (0.53–1.44) | 0.79 (0.52–1.19) |
| 0.72 (0.57–0.92) | 0.6 (0.47–0.78) | 0.97 (0.81–1.15) | 0.61 (0.43–0.87) | 0.92 (0.69–1.23) | Exenatide | 0.89 (0.72–1.09) | 0.95 (0.74–1.22) | 1.14 (0.99–1.31) | 0.81 (0.51–1.27) | 0.73 (0.51–1.04) |
| 0.82 (0.64–1.05) | 0.68 (0.52–0.89) | 1.09 (0.91–1.31) | 0.69 (0.49–0.98) | 1.04 (0.77–1.4) | 1.13 (0.91–1.4) | Liraglutide | 1.08 (0.83–1.39) | 1.28 (1.09–1.5) | 0.91 (0.58–1.43) | 0.82 (0.57–1.18) |
| 0.76 (0.57–1) | 0.63 (0.47–0.85) | 1.01 (0.81–1.26) | 0.64 (0.44–0.93) | 0.96 (0.7–1.33) | 1.05 (0.82–1.34) | 0.93 (0.72–1.2) | Lixisenatide | 1.19 (0.97–1.46) | 0.85 (0.53–1.36) | 0.76 (0.52–1.12) |
| 0.64 (0.52–0.77) | 0.53 (0.43–0.66) | 0.85 (0.77–0.93) | 0.54 (0.4–0.74) | 0.81 (0.63–1.04) | 0.88 (0.76–1.01) | 0.78 (0.67–0.91) | 0.84 (0.69–1.03) | Placebo | 0.71 (0.46–1.09) | 0.64 (0.46–0.89) |
| 0.89 (0.56–1.43) | 0.75 (0.46–1.21) | 1.2 (0.77–1.86) | 0.76 (0.45–1.29) | 1.14 (0.69–1.87) | 1.24 (0.79–1.95) | 1.1 (0.7–1.74) | 1.18 (0.74–1.9) | 1.41 (0.92–2.17) | Sotagliflozin | 0.9 (0.53–1.54) |
| 0.99 (0.68–1.45) | 0.83 (0.56–1.23) | 1.33 (0.94–1.86) | 0.84 (0.54–1.33) | 1.26 (0.84–1.91) | 1.37 (0.96–1.96) | 1.22 (0.85–1.75) | 1.31 (0.89–1.92) | 1.56 (1.13–2.16) | 1.11 (0.65–1.9) | Ssemaglutide |
Table 4.
Pairwise comparison results from network meta-analysis on cardiovascular death.
| Albiglutide | 0.9 (0.67–1.2) | 1.05 (0.78–1.42) | 0.98 (0.73–1.3) | 0.67 (0.48–0.93) | 0.99 (0.73–1.34) | 0.95 (0.71–1.26) | 0.84 (0.62–1.13) | 1.05 (0.76–1.47) | 0.53 (0.27–1.02) | 1.08 (0.84–1.37) | 0.95 (0.7–1.29) | 1.05 (0.65–1.71) |
| 1.11 (0.84–1.48) | Canagliflozin | 1.17 (0.93–1.48) | 1.09 (0.88–1.35) | 0.74 (0.57–0.98) | 1.1 (0.87–1.4) | 1.05 (0.85–1.3) | 0.93 (0.74–1.17) | 1.17 (0.89–1.54) | 0.59 (0.31–1.1) | 1.2 (1.03–1.4) | 1.06 (0.83–1.35) | 1.17 (0.75–1.82) |
| 0.95 (0.7–1.28) | 0.85 (0.67–1.08) | Dapagliflozin | 0.93 (0.74–1.17) | 0.63 (0.47–0.84) | 0.94 (0.73–1.21) | 0.9 (0.71–1.13) | 0.8 (0.62–1.02) | 1 (0.75–1.33) | 0.5 (0.26–0.94) | 1.02 (0.85–1.22) | 0.9 (0.7–1.17) | 1 (0.64–1.57) |
| 1.02 (0.77–1.36) | 0.92 (0.74–1.14) | 1.08 (0.85–1.36) | Dulaglutide | 0.68 (0.52–0.9) | 1.01 (0.8–1.28) | 0.97 (0.78–1.2) | 0.86 (0.68–1.08) | 1.08 (0.82–1.41) | 0.54 (0.29–1.01) | 1.1 (0.94–1.28) | 0.97 (0.77–1.24) | 1.08 (0.69–1.67) |
| 1.5 (1.07–2.09) | 1.35 (1.02–1.76) | 1.58 (1.18–2.11) | 1.47 (1.12–1.93) | Empagliflozin | 1.48 (1.11–1.99) | 1.42 (1.08–1.86) | 1.26 (0.95–1.67) | 1.58 (1.15–2.17) | 0.79 (0.41–1.51) | 1.61 (1.28–2.02) | 1.43 (1.07–1.91) | 1.58 (0.99–2.53) |
| 1.01 (0.75–1.37) | 0.91 (0.72–1.15) | 1.06 (0.83–1.37) | 0.99 (0.78–1.26) | 0.67 (0.5–0.9) | Ertugliflozin | 0.96 (0.76–1.21) | 0.85 (0.66–1.09) | 1.06 (0.8–1.42) | 0.53 (0.28–1) | 1.09 (0.91–1.31) | 0.96 (0.74–1.25) | 1.07 (0.68–1.67) |
| 1.06 (0.79–1.41) | 0.95 (0.77–1.17) | 1.11 (0.88–1.4) | 1.03 (0.84–1.28) | 0.71 (0.54–0.92) | 1.05 (0.83–1.32) | Exenatide | 0.89 (0.71–1.11) | 1.11 (0.85–1.46) | 0.56 (0.3–1.04) | 1.14 (0.98–1.32) | 1.01 (0.79–1.27) | 1.11 (0.72–1.72) |
| 1.19 (0.88–1.61) | 1.07 (0.85–1.35) | 1.26 (0.98–1.61) | 1.17 (0.93–1.47) | 0.8 (0.6–1.06) | 1.18 (0.92–1.52) | 1.13 (0.9–1.41) | Liraglutide | 1.26 (0.95–1.66) | 0.63 (0.33–1.18) | 1.28 (1.08–1.52) | 1.13 (0.88–1.46) | 1.26 (0.8, 1.97) |
| 0.95 (0.68–1.32) | 0.85 (0.65–1.12) | 1 (0.75–1.33) | 0.93 (0.71–1.22) | 0.63 (0.46–0.87) | 0.94 (0.7–1.25) | 0.9 (0.69–1.17) | 0.8 (0.6–1.06) | Lixisenatide | 0.5 (0.26–0.96) | 1.02 (0.82–1.28) | 0.9 (0.68–1.21) | 1 (0.63–1.59) |
| 1.9 (0.98–3.67) | 1.7 (0.91–3.21) | 2 (1.06–3.8) | 1.86 (0.99–3.51) | 1.27 (0.66–2.44) | 1.88 (1–3.56) | 1.79 (0.96–3.38) | 1.59 (0.85–3.01) | 2 (1.04–3.84) | Osemaglutide | 2.04 (1.11–3.77) | 1.8 (0.96–3.42) | 2 (0.96–4.22) |
| 0.93 (0.73–1.19) | 0.83 (0.72–0.97) | 0.98 (0.82–1.17) | 0.91 (0.78–1.06) | 0.62 (0.5–0.78) | 0.92 (0.77–1.1) | 0.88 (0.76–1.02) | 0.78 (0.66–0.92) | 0.98 (0.78–1.22) | 0.49 (0.26–0.9) | Placebo | 0.89 (0.73–1.07) | 0.98 (0.65–1.48) |
| 1.05 (0.78–1.43) | 0.94 (0.74–1.2) | 1.11 (0.86–1.43) | 1.03 (0.81–1.31) | 0.7 (0.52–0.94) | 1.04 (0.8–1.35) | 0.99 (0.78–1.26) | 0.88 (0.68–1.13) | 1.11 (0.83–1.48) | 0.55 (0.29–1.05) | 1.13 (0.94–1.36) | Sotagliflozin | 1.11 (0.7–1.74) |
| 0.95 (0.59–1.53) | 0.85 (0.55–1.33) | 1 (0.64–1.56) | 0.93 (0.6–1.44) | 0.63 (0.4–1.01) | 0.94 (0.6–1.47) | 0.9 (0.58–1.39) | 0.79 (0.51–1.24) | 1 (0.63–1.59) | 0.5 (0.24–1.04) | 1.02 (0.68–1.54) | 0.9 (0.58–1.42) | Ssemaglutide |
Table 5.
Pairwise comparison results from network meta-analysis on myocardial infarction.
| Albiglutide | 1.17 (0.91–1.52) | 1.19 (0.94–1.5) | 1.28 (0.98–1.68) | 1.16 (0.86–1.56) | 1.39 (1.06–1.82) | 1.29 (1.02–1.63) | 1.15 (0.89–1.47) | 1.37 (1.06–1.77) | 1.57 (0.94–2.63) | 1.33 (1.1–1.62) | 0.91 (0.65–1.26) | 1.08 (0.72–1.62) |
| 0.85 (0.66–1.1) | Canagliflozin | 1.01 (0.82–1.25) | 1.09 (0.85–1.4) | 0.99 (0.75–1.3) | 1.18 (0.92–1.52) | 1.1 (0.89–1.36) | 0.98 (0.78–1.23) | 1.17 (0.92–1.48) | 1.34 (0.81–2.22) | 1.14 (0.96–1.34) | 0.77 (0.56–1.06) | 0.92 (0.62–1.36) |
| 0.84 (0.66–1.07) | 0.99 (0.8–1.23) | Dapagliflozin | 1.08 (0.86–1.36) | 0.98 (0.75–1.27) | 1.17 (0.92–1.48) | 1.09 (0.9–1.31) | 0.97 (0.79–1.19) | 1.16 (0.93–1.44) | 1.32 (0.81–2.18) | 1.12 (0.98–1.28) | 0.76 (0.56–1.03) | 0.91 (0.62–1.33) |
| 0.78 (0.6–1.02) | 0.92 (0.71–1.18) | 0.93 (0.74–1.17) | Dulaglutide | 0.91 (0.68–1.21) | 1.08 (0.83–1.41) | 1.01 (0.81–1.27) | 0.9 (0.7–1.14) | 1.07 (0.83–1.38) | 1.23 (0.73–2.05) | 1.04 (0.86–1.26) | 0.71 (0.51–0.98) | 0.84 (0.56–1.26) |
| 0.86 (0.64–1.16) | 1.01 (0.77–1.34) | 1.02 (0.79–1.33) | 1.1 (0.83–1.48) | Empagliflozin | 1.19 (0.89–1.6) | 1.12 (0.86–1.44) | 0.99 (0.75–1.3) | 1.18 (0.89–1.57) | 1.36 (0.81–2.3) | 1.15 (0.92–1.44) | 0.78 (0.55–1.11) | 0.93 (0.61–1.41) |
| 0.72 (0.55–0.95) | 0.85 (0.66–1.09) | 0.86 (0.68–1.08) | 0.92 (0.71–1.21) | 0.84 (0.62–1.12) | Ertugliflozin | 0.93 (0.74–1.18) | 0.83 (0.64–1.06) | 0.99 (0.77–1.28) | 1.14 (0.68–1.9) | 0.96 (0.8–1.16) | 0.65 (0.47–0.91) | 0.78 (0.52–1.17) |
| 0.77 (0.61–0.98) | 0.91 (0.74–1.12) | 0.92 (0.76–1.11) | 0.99 (0.79–1.24) | 0.9 (0.69–1.16) | 1.07 (0.85–1.35) | Exenatide | 0.89 (0.72–1.08) | 1.06 (0.86–1.31) | 1.22 (0.74–1.99) | 1.03 (0.91–1.17) | 0.7 (0.52–0.95) | 0.84 (0.57–1.22) |
| 0.87 (0.68–1.12) | 1.03 (0.81–1.29) | 1.04 (0.84–1.27) | 1.12 (0.88–1.43) | 1.01 (0.77–1.33) | 1.21 (0.94–1.55) | 1.13 (0.92–1.38) | Liraglutide | 1.2 (0.95–1.51) | 1.37 (0.83–2.26) | 1.16 (0.99–1.36) | 0.79 (0.58–1.08) | 0.94 (0.64–1.39) |
| 0.73 (0.56–0.94) | 0.86 (0.68–1.08) | 0.86 (0.7–1.07) | 0.93 (0.72–1.2) | 0.84 (0.64–1.12) | 1.01 (0.78–1.3) | 0.94 (0.76–1.16) | 0.84 (0.66–1.05) | Lixisenatide | 1.15 (0.69–1.9) | 0.97 (0.82–1.15) | 0.66 (0.48–0.91) | 0.79 (0.53–1.16) |
| 0.64 (0.38–1.07) | 0.75 (0.45–1.24) | 0.76 (0.46–1.24) | 0.81 (0.49–1.36) | 0.74 (0.44–1.24) | 0.88 (0.53–1.47) | 0.82 (0.5–1.34) | 0.73 (0.44–1.2) | 0.87 (0.53–1.45) | Osemaglutide | 0.85 (0.53–1.37) | 0.58 (0.33–1) | 0.69 (0.38–1.24) |
| 0.75 (0.62–0.91) | 0.88 (0.75–1.04) | 0.89 (0.78–1.02) | 0.96 (0.8–1.16) | 0.87 (0.7–1.09) | 1.04 (0.86–1.26) | 0.97 (0.85–1.1) | 0.86 (0.73–1.01) | 1.03 (0.87–1.22) | 1.18 (0.73–1.9) | Placebo | 0.68 (0.52–0.89) | 0.81 (0.57–1.16) |
| 1.1 (0.79–1.54) | 1.3 (0.94–1.78) | 1.31 (0.97–1.77) | 1.41 (1.02–1.96) | 1.28 (0.9–1.82) | 1.53 (1.1–2.12) | 1.43 (1.06–1.92) | 1.27 (0.92–1.73) | 1.52 (1.1–2.08) | 1.74 (1–3) | 1.47 (1.12–1.93) | Sotagliflozin | 1.19 (0.76–1.86) |
| 0.92 (0.62–1.39) | 1.09 (0.74–1.61) | 1.1 (0.75–1.61) | 1.18 (0.79–1.77) | 1.07 (0.71–1.64) | 1.28 (0.86–1.92) | 1.2 (0.82–1.75) | 1.06 (0.72–1.57) | 1.27 (0.86–1.88) | 1.46 (0.81–2.64) | 1.23 (0.86–1.76) | 0.84 (0.54–1.31) | Ssemaglutide |
Table 6.
Pairwise comparison results from network meta-analysis on stroke.
| Albiglutide | 0.97 (0.7–1.35) | 1.17 (0.85–1.63) | 0.88 (0.63–1.24) | 1.37 (0.93–2.03) | 1.23 (0.85–1.79) | 0.99 (0.71–1.38) | 1 (0.71–1.4) | 1.3 (0.84–2.02) | 0.86 (0.39–1.9) | 1.16 (0.88–1.52) | 0.77 (0.51–1.17) | 0.76 (0.44–1.29) |
| 1.03 (0.74–1.43) | Canagliflozin | 1.21 (0.93–1.57) | 0.91 (0.69–1.2) | 1.41 (1–1.98) | 1.27 (0.92–1.75) | 1.02 (0.78–1.33) | 1.03 (0.78–1.35) | 1.34 (0.9–1.99) | 0.89 (0.41–1.91) | 1.2 (0.99–1.44) | 0.79 (0.54–1.14) | 0.78 (0.47–1.27) |
| 0.85 (0.62–1.18) | 0.83 (0.64–1.08) | Dapagliflozin | 0.75 (0.57–0.99) | 1.17 (0.84–1.64) | 1.05 (0.77–1.44) | 0.84 (0.65–1.1) | 0.85 (0.65–1.12) | 1.11 (0.75–1.64) | 0.73 (0.34–1.58) | 0.99 (0.83–1.19) | 0.65 (0.45–0.94) | 0.64 (0.39–1.06) |
| 1.13 (0.8–1.59) | 1.1 (0.83–1.46) | 1.33 (1.01–1.75) | Dulaglutide | 1.55 (1.09–2.2) | 1.39 (1–1.94) | 1.12 (0.84–1.49) | 1.13 (0.85–1.52) | 1.47 (0.98–2.21) | 0.97 (0.45–2.12) | 1.32 (1.07–1.62) | 0.87 (0.59–1.27) | 0.85 (0.51–1.42) |
| 0.73 (0.49–1.08) | 0.71 (0.51–1) | 0.86 (0.61–1.2) | 0.64 (0.45–0.92) | Empagliflozin | 0.9 (0.61–1.32) | 0.72 (0.51–1.01) | 0.73 (0.52–1.03) | 0.95 (0.61–1.48) | 0.62 (0.28–1.4) | 0.85 (0.64–1.13) | 0.56 (0.37–0.85) | 0.55 (0.32–0.94) |
| 0.81 (0.56–1.18) | 0.79 (0.57–1.09) | 0.95 (0.69–1.3) | 0.72 (0.51–1) | 1.11 (0.76–1.63) | Ertugliflozin | 0.8 (0.58–1.11) | 0.81 (0.59–1.13) | 1.06 (0.69–1.62) | 0.7 (0.32–1.54) | 0.94 (0.73–1.22) | 0.62 (0.41–0.94) | 0.61 (0.36–1.04) |
| 1.01 (0.73–1.41) | 0.98 (0.75–1.29) | 1.19 (0.91–1.55) | 0.89 (0.67–1.19) | 1.39 (0.99–1.95) | 1.25 (0.9–1.72) | Exenatide | 1.01 (0.77–1.34) | 1.32 (0.89–1.96) | 0.87 (0.4–1.88) | 1.18 (0.97–1.43) | 0.78 (0.54–1.13) | 0.76 (0.47–1.25) |
| 1 (0.71–1.4) | 0.97 (0.74–1.28) | 1.17 (0.89–1.54) | 0.88 (0.66–1.18) | 1.37 (0.97–1.94) | 1.23 (0.89–1.7) | 0.99 (0.75–1.31) | Liraglutide | 1.3 (0.87–1.94) | 0.86 (0.4–1.86) | 1.16 (0.95–1.42) | 0.77 (0.53–1.12) | 0.76 (0.46–1.24) |
| 0.77 (0.49–1.19) | 0.75 (0.5–1.11) | 0.9 (0.61–1.33) | 0.68 (0.45–1.02) | 1.05 (0.67–1.64) | 0.95 (0.62–1.46) | 0.76 (0.51–1.13) | 0.77 (0.52–1.15) | Lixisenatide | 0.66 (0.29–1.51) | 0.89 (0.63–1.27) | 0.59 (0.37–0.94) | 0.58 (0.33–1.03) |
| 1.17 (0.53–2.57) | 1.13 (0.52–2.43) | 1.37 (0.63–2.94) | 1.03 (0.47–2.22) | 1.6 (0.71–3.54) | 1.43 (0.65–3.16) | 1.15 (0.53–2.47) | 1.17 (0.54–2.51) | 1.52 (0.66–3.43) | Osemaglutide | 1.35 (0.64–2.84) | 0.89 (0.39–2.01) | 0.88 (0.37–2.12) |
| 0.86 (0.66–1.13) | 0.84 (0.69–1.01) | 1.01 (0.84–1.21) | 0.76 (0.62–0.93) | 1.18 (0.89–1.56) | 1.06 (0.82–1.37) | 0.85 (0.7–1.03) | 0.86 (0.7–1.05) | 1.12 (0.79–1.59) | 0.74 (0.35–1.56) | Placebo | 0.66 (0.48–0.91) | 0.65 (0.41–1.03) |
| 1.3 (0.86–1.98) | 1.27 (0.87–1.84) | 1.53 (1.06–2.21) | 1.15 (0.79–1.69) | 1.79 (1.17–2.74) | 1.61 (1.07–2.41) | 1.29 (0.89–1.87) | 1.3 (0.89–1.9) | 1.69 (1.06–2.71) | 1.12 (0.5–2.53) | 1.51 (1.1–2.08) | Sotagliflozin | 0.99 (0.56–1.72) |
| 1.32 (0.78–2.27) | 1.29 (0.79–2.11) | 1.55 (0.95–2.55) | 1.17 (0.71–1.94) | 1.81 (1.06–3.14) | 1.63 (0.96–2.75) | 1.31 (0.8–2.15) | 1.32 (0.8–2.19) | 1.72 (0.97–3.06) | 1.14 (0.47–2.74) | 1.54 (0.97–2.44) | 1.02 (0.58–1.78) | Ssemaglutide |
Table 7.
Pairwise comparison results from network meta-analysis on all-cause death.
| Albiglutide | 0.9 (0.72–1.13) | 0.98 (0.78–1.23) | 0.95 (0.76–1.19) | 0.72 (0.55–0.93) | 0.98 (0.77–1.25) | 0.91 (0.72–1.13) | 0.89 (0.71–1.13) | 0.99 (0.76–1.29) | 0.54 (0.32–0.91) | 1.05 (0.87–1.28) | 1 (0.78–1.28) | 1.11 (0.74–1.65) |
| 1.11 (0.89–1.4) | Canagliflozin | 1.09 (0.92–1.29) | 1.05 (0.89–1.25) | 0.79 (0.64–0.99) | 1.09 (0.9–1.32) | 1.01 (0.85–1.19) | 0.99 (0.83–1.19) | 1.1 (0.88–1.37) | 0.6 (0.36–0.99) | 1.17 (1.03–1.32) | 1.11 (0.91–1.36) | 1.23 (0.85–1.79) |
| 1.02 (0.81–1.28) | 0.92 (0.77–1.09) | Dapagliflozin | 0.97 (0.82–1.14) | 0.73 (0.59–0.91) | 1 (0.83–1.21) | 0.92 (0.78–1.09) | 0.91 (0.76–1.09) | 1.01 (0.81–1.26) | 0.55 (0.33–0.91) | 1.08 (0.95–1.21) | 1.02 (0.84–1.24) | 1.13 (0.78–1.64) |
| 1.05 (0.84–1.32) | 0.95 (0.8–1.12) | 1.03 (0.87–1.22) | Dulaglutide | 0.75 (0.61–0.94) | 1.03 (0.86–1.25) | 0.96 (0.81–1.13) | 0.94 (0.79–1.13) | 1.04 (0.84–1.3) | 0.57 (0.34–0.94) | 1.11 (0.99–1.25) | 1.05 (0.87–1.28) | 1.17 (0.81–1.7) |
| 1.4 (1.07–1.82) | 1.26 (1.01–1.57) | 1.37 (1.1–1.7) | 1.32 (1.07–1.64) | Empagliflozin | 1.37 (1.08–1.73) | 1.27 (1.02–1.57) | 1.25 (0.99–1.57) | 1.38 (1.07–1.79) | 0.75 (0.44–1.27) | 1.47 (1.23–1.76) | 1.4 (1.1–1.78) | 1.55 (1.04–2.3) |
| 1.02 (0.8–1.3) | 0.92 (0.76–1.12) | 1 (0.83–1.21) | 0.97 (0.8–1.17) | 0.73 (0.58–0.92) | Ertugliflozin | 0.92 (0.76–1.12) | 0.91 (0.75–1.12) | 1.01 (0.8–1.28) | 0.55 (0.33–0.92) | 1.07 (0.93–1.25) | 1.02 (0.82–1.27) | 1.13 (0.77–1.65) |
| 1.1 (0.88–1.38) | 0.99 (0.84–1.18) | 1.08 (0.92–1.28) | 1.05 (0.89–1.23) | 0.79 (0.64–0.98) | 1.08 (0.9–1.31) | Exenatide | 0.99 (0.83–1.18) | 1.09 (0.88–1.36) | 0.59 (0.36–0.98) | 1.16 (1.04–1.3) | 1.1 (0.91–1.34) | 1.22 (0.84–1.77) |
| 1.12 (0.88–1.41) | 1.01 (0.84–1.21) | 1.09 (0.92–1.31) | 1.06 (0.89–1.27) | 0.8 (0.64–1.01) | 1.09 (0.89–1.34) | 1.01 (0.85–1.21) | Liraglutide | 1.11 (0.88–1.39) | 0.6 (0.36–1) | 1.18 (1.03–1.35) | 1.12 (0.91–1.37) | 1.24 (0.85–1.81) |
| 1.01 (0.78–1.32) | 0.91 (0.73–1.14) | 0.99 (0.79–1.23) | 0.96 (0.77–1.19) | 0.72 (0.56–0.94) | 0.99 (0.78–1.25) | 0.91 (0.74–1.14) | 0.9 (0.72–1.14) | Lixisenatide | 0.54 (0.32–0.92) | 1.06 (0.88–1.28) | 1.01 (0.79–1.29) | 1.12 (0.75–1.66) |
| 1.86 (1.1–3.17) | 1.68 (1.01–2.79) | 1.82 (1.1–3.03) | 1.76 (1.06–2.94) | 1.33 (0.79–2.26) | 1.82 (1.09–3.06) | 1.68 (1.02–2.8) | 1.66 (1–2.79) | 1.84 (1.09–3.13) | Osemaglutide | 1.96 (1.19–3.21) | 1.86 (1.11–3.12) | 2.06 (1.13–3.76) |
| 0.95 (0.78–1.15) | 0.85 (0.76–0.97) | 0.93 (0.83–1.05) | 0.9 (0.8–1.01) | 0.68 (0.57–0.82) | 0.93 (0.8–1.08) | 0.86 (0.77–0.96) | 0.85 (0.74–0.97) | 0.94 (0.78–1.13) | 0.51 (0.31–0.84) | Placebo | 0.95 (0.81–1.11) | 1.05 (0.74–1.5) |
| 1 (0.78–1.28) | 0.9 (0.74–1.1) | 0.98 (0.8–1.19) | 0.95 (0.78–1.15) | 0.72 (0.56–0.91) | 0.98 (0.79–1.21) | 0.91 (0.75–1.1) | 0.89 (0.73–1.1) | 0.99 (0.78–1.26) | 0.54 (0.32–0.9) | 1.05 (0.9–1.23) | Sotagliflozin | 1.11 (0.75–1.63) |
| 0.9 (0.6–1.35) | 0.81 (0.56–1.18) | 0.88 (0.61–1.28) | 0.86 (0.59–1.24) | 0.65 (0.43–0.96) | 0.89 (0.6–1.3) | 0.82 (0.56–1.18) | 0.81 (0.55–1.18) | 0.89 (0.6–1.33) | 0.49 (0.27–0.89) | 0.95 (0.67–1.35) | 0.9 (0.61–1.33) | Ssemaglutide |
3.2. Rankings according to SUCRA values
As is shown in Figure 1, the maximum SUCRA values followed sotagliflozin, subcutaneous semaglutide, and albiglutide in lowering MACE; followed sotagliflozin, canagliflozin, and empagliflozin in lowering HHF; followed dapagliflozin and empagliflozin in lowering KFP; followed empagliflozin and oral semaglutide in lowering CVD; followed sotagliflozin and albiglutide in lowering MI; followed sotagliflozin and subcutaneous semaglutide in lowering stroke; and followed oral semaglutide and empagliflozin in lowering ACD.
Figure 1.
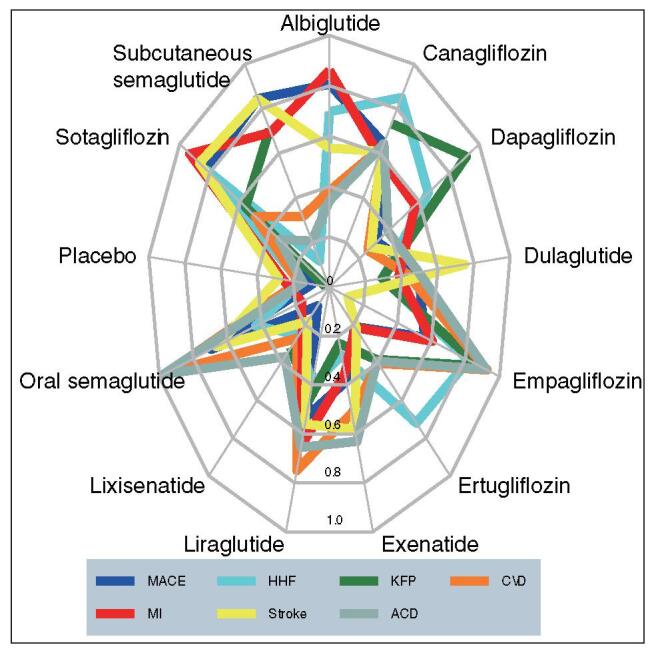
Radar plot of SUCRA values of 12 drug interventions for preventing 7 cardiorenal outcomes in T2D patients. ACD = all-cause death, CVD = cardiovascular death, HHF = hospitalization for heart failure, MI = myocardial infarction, KFP = kidney function progression, MACE = major adverse cardiovascular events, SUCRA = surface under the cumulative ranking curve, T2D = type 2 diabetes.
4. Discussion
This updated network meta-analysis revealed that different members of the two new classes of hypoglycemic agents (ie, SGLT2 inhibitors and GLP-1 RAs) have different benefits on specific cardiorenal outcomes in T2D patients. To be more specific, sotagliflozin, subcutaneous semaglutide and albiglutide had the greatest effect in lowering MACE; sotagliflozin, canagliflozin, and empagliflozin had the greatest effect in lowering HHF; dapagliflozin and empagliflozin had the greatest effect in lowering KFP; empagliflozin and oral semaglutide had the greatest effect in lowering CVD; sotagliflozin and albiglutide had the greatest effect in lowering MI; sotagliflozin and subcutaneous semaglutide had the greatest effect in lowering stroke; and oral semaglutide and empagliflozin had the greatest effect in lowering ACD.
Compared to our first network meta-analysis[1] that failed to assess sotagliflozin and ertugliflozin, this updated network meta-analysis additionally revealed the fact that sotagliflozin was one of the most effective drugs as for lowering MI, stroke, MACE, and HHF, whereas ertugliflozin was not one of the most effective drugs as for lowering any cardiorenal endpoint. A previous network meta-analysis[18] assessed the relative efficacy of different GLP-1 RAs on cardiorenal endpoints but failed to assess different SGLT2 inhibitors, whereas one other network meta-analysis[19] assessed the relative efficacy of different SGLT2 inhibitors on cardiorenal endpoints but failed to assess different GLP-1 RAs. Moreover, another network meta-analysis[20] assessed the relative efficacy of several different GLP-1 RAs and SGLT2 inhibitors on the only endpoint of MACE, but failed to consider the heart failure, renal failure, and individual death endpoints. Therefore, the present network meta-analysis provided the most comprehensive analysis regarding the relative efficacy of different SGLT2 inhibitors and GLP-1 RAs on various cardiorenal endpoints in T2D patients.
The greatest strength of this network meta-analysis is that all the included studies had low risk of bias. Oppositely, this network meta-analysis has the 2 main weaknesses as follows. First, all the included studies were placebo-controlled trials and therefore all network meta-analyses conducted in this study were based on indirect comparisons. Thus, active-controlled trials comparing different SGLT2 inhibitors and GLP-1 RAs are urgently needed to validate the estimators of the comparative efficacy produced in this network meta-analysis. Second, we failed to find out the possible mechanisms for different SGLT2is with the different efficacy on various cardiorenal outcomes. This needs to be further researched.
In conclusion, this updated network meta-analysis reproduced the findings in the first network meta-analysis, and moreover revealed that sotagliflozin was one of the most effective drugs as for lowering MI, stroke, MACE, and HHF, whereas ertugliflozin was not. These findings will provide the according evidence regarding the usage of specific SGLT2 inhibitors and GLP-1 RAs in T2D patients for prevention of specific cardiorenal endpoints.
Author contributions
Conceptualization: Xue-Yan Duan.
Data curation: Xue-Yan Duan, Shu-Yan Liu, Dao-Gen Yin.
Formal analysis: Shu-Yan Liu.
Validation: Dao-Gen Yin.
Writing – original draft: Xue-Yan Duan.
Writing – review & editing: Shu-Yan Liu, Dao-Gen Yin.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ACD = all-cause death, CI = confidence interval, CVD = cardiovascular death, CVOTs = cardiovascular or renal outcome trials, GLP-1 RAs = glucagon-like peptide 1 receptor agonists, HHF = hospitalization for heart failure, HR = hazard ratio, KFP = kidney function progression, MACE = major adverse cardiovascular events, MI = myocardial infarction, SGLT2 = sodium glucose cotransporter 2, SUCRA = surface under the cumulative ranking curve, T2D = type 2 diabetes.
How to cite this article: Duan XY, Liu SY, Yin DG. Comparative efficacy of 5 sodium glucose cotransporter 2 inhibitor and 7 glucagon-like peptide 1 receptor agonists interventions on cardiorenal outcomes in type 2 diabetes patients: A network meta-analysis based on cardiovascular or renal outcome trials. Medicine. 2021;100:30(e26431).
Funding: None.
The authors report no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
Results are presented as hazard ratios (95% confidence intervals) of the column-defining treatment versus the row-defining treatment. Red bold font indicates statistical significance. Osemaglutide = oral semaglutide, Ssemaglutide = subcutaneous semaglutide.
Results are presented as hazard ratios (95% confidence intervals) of the column-defining treatment versus the row-defining treatment. Red bold font indicates statistical significance. Osemaglutide = oral semaglutide, Ssemaglutide = subcutaneous semaglutide.
Results are presented as hazard ratios (95% confidence intervals) of the column-defining treatment versus the row-defining treatment. Red bold font indicates statistical significance. Ssemaglutide = subcutaneous semaglutide.
Results are presented as hazard ratios (95% confidence intervals) of the column-defining treatment versus the row-defining treatment. Red bold font indicates statistical significance. Osemaglutide = oral semaglutide, Ssemaglutide = subcutaneous semaglutide.
Results are presented as hazard ratios (95% confidence intervals) of the column-defining treatment versus the row-defining treatment. Red bold font indicates statistical significance. Osemaglutide = oral semaglutide, Ssemaglutide = subcutaneous semaglutide.
Results are presented as hazard ratios (95% confidence intervals) of the column-defining treatment versus the row-defining treatment. Red bold font indicates statistical significance. Osemaglutide = oral semaglutide, Ssemaglutide = subcutaneous semaglutide.
Results are presented as hazard ratios (95% confidence intervals) of the column-defining treatment versus the row-defining treatment. Red bold font indicates statistical significance. Osemaglutide = oral semaglutide, = Ssemaglutide = subcutaneous semaglutide.
References
- [1].Wei XB, Wei W, Ding LL, et al. Comparison of the effects of 10 GLP-1 RA and SGLT2 inhibitor interventions on cardiovascular, mortality, and kidney outcomes in type 2 diabetes: a network meta-analysis of large randomized trials. Prim Care Diabetes 2021;15:208–11. [DOI] [PubMed] [Google Scholar]
- [2].Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- [3].Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57. [DOI] [PubMed] [Google Scholar]
- [4].Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- [5].Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. [DOI] [PubMed] [Google Scholar]
- [6].Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–30. [DOI] [PubMed] [Google Scholar]
- [9].Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- [10].Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–29. [DOI] [PubMed] [Google Scholar]
- [11].Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–51. [DOI] [PubMed] [Google Scholar]
- [12].Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–57. [DOI] [PubMed] [Google Scholar]
- [13].Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020;383:1425–35. [DOI] [PubMed] [Google Scholar]
- [14].Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 2021;384:129–39. [DOI] [PubMed] [Google Scholar]
- [15].Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–28. [DOI] [PubMed] [Google Scholar]
- [16].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [17].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alfayez OM, Almohammed OA, Alkhezi OS, et al. Indirect comparison of glucagon like peptide-1 receptor agonists regarding cardiovascular safety and mortality in patients with type 2 diabetes mellitus: network meta-analysis. Cardiovasc Diabetol 2020;19:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Täger T, Atar D, Agewall S, et al. Comparative efficacy of sodium-glucose cotransporter-2 inhibitors (SGLT2i) for cardiovascular outcomes in type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials. Heart Fail Rev 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qiu M, Ding LL, Wei XB, et al. Comparative efficacy of glucagon-like peptide 1 receptor agonists and sodium glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular events in type 2 diabetes: a network meta-analysis. J Cardiovasc Pharmacol 2021;77:34–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


