Abstract
BACKGROUND:
Immunotherapy treatment for coronavirus disease 2019 combined with antiviral therapy and supportive care remains under intense investigation. However, the capacity to distinguish patients who would benefit from immunosuppressive or immune stimulatory therapies remains insufficient. Here, we present a patient with severe coronavirus disease 2019 with a defective immune response, treated successfully with interleukin-7 on compassionate basis with resultant improved adaptive immune function.
CASE SUMMARY:
A previously healthy 43-year-old male developed severe acute respiratory distress syndrome due to the severe acute respiratory syndrome coronavirus 2 virus with acute hypoxemic respiratory failure and persistent, profound lymphopenia. Functional analysis demonstrated depressed lymphocyte function and few antigen-specific T cells. Interleukin-7 administration resulted in reversal of lymphopenia and improved T-cell function. Respiratory function and clinical status rapidly improved, and he was discharged home. Whole exome sequencing identified a deleterious autosomal dominant mutation in TICAM1, associated with a dysfunctional type I interferon antiviral response with increased severity of coronavirus disease 2019 disease.
CONCLUSIONS:
Immunoadjuvant therapies to boost host immunity may be efficacious in life-threatening severe coronavirus disease 2019 infections, particularly by applying a precision medicine approach in selecting patients expressing an immunosuppressive phenotype.
Keywords: case report, coronavirus disease 2019, immunotherapy, interferons, interleukin-7, toll-like receptor 3
Severe acute respiratory distress syndrome secondary to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) carries a ~50% mortality (1–4). Although preferentially affecting individuals of advanced age, younger patients with respiratory failure who require ICU admission and invasive mechanical ventilation maintain a significant risk of mortality. Prospective and retrospective cohorts of patients with coronavirus disease 2019 (COVID-19) have demonstrated mortality rates between 20% and 40% for patients 40–50 years who are admitted to an ICU and approaches 45% if they require intubation (5–7). Severity of disease in COVID-19 is linked to a dysregulated host immune response to the SARS-CoV-2 virus (8–10), which has led to the initiation of several clinical trials investigating the use of immunomodulatory agents as adjuvant therapy alongside antiviral medications (11, 12). There have been several different immunopathologic mechanisms described in the literature to date, with each potentially benefitting from a distinct targeted therapy. Among these theories, cytokine storm and cellular adaptive immune suppression are the most frequently described in the literature (11, 13). One important indicator of cellular adaptive immune dysfunction is lymphopenia. Lymphopenia (< 1 × 103 cells/µL) is predictive of severity and poor outcome, whereas severe lymphopenia (< 0.5 × 103 cells/µL) is associated with a 12-fold increased risk of mortality (14–16). Furthermore, the functional capacity of circulating lymphocytes was assessed using an ex vivo stimulation assay (17) and is severely impaired in patients with severe COVID-19, as evidenced by defective T-cell production of interferon (IFN)–γ after T-cell receptor agonist stimulation (18). Additionally, impairments to immune function and antiviral host defense have been linked to subtle inborn errors of immunity. There are reports that 3.5% of patients with life-threatening COVID-19 pneumonia have deleterious variants in genes involved in type 1 IFN signaling, further supporting therapies that restore immune function (19–21).
Herein, we describe the use of interleukin (IL)–7 in a critically ill patient with severe COVID-19 disease, evidence of adaptive immune dysfunction, and the discovery of a genetic defect in Toll-like receptor (TLR)-3.
CASE DESCRIPTION
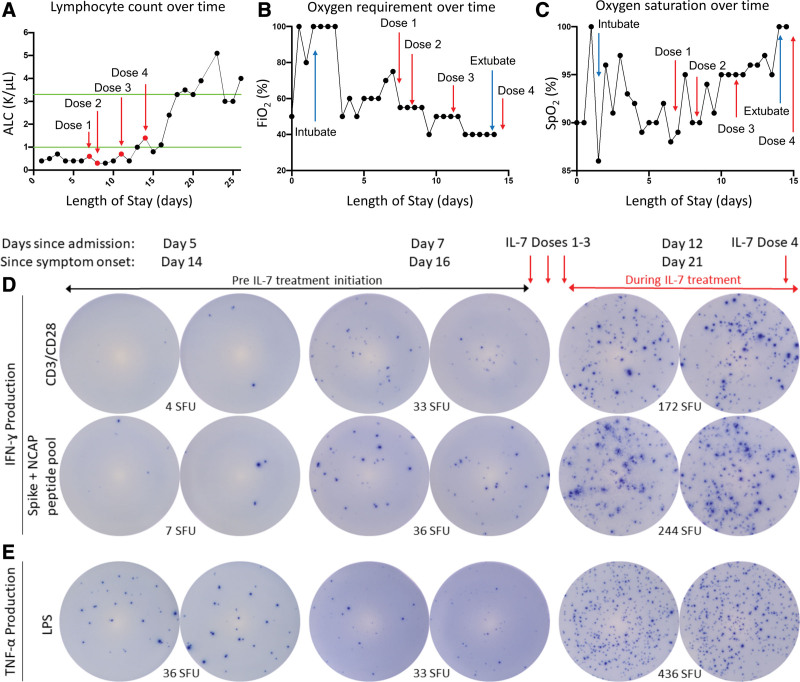
A 43-year-old male with no significant comorbidities presented with severe hypoxemic respiratory failure due to SARS-CoV-2. The patient began experiencing cough, fever, and myalgias 9 days prior to hospitalization and tested positive 2 days after symptom onset. He was prescribed 7 days of dexamethasone, 6 mg daily, at that time by his primary care provider. Despite treatment with high-flow oxygen, convalescent plasma, remdesivir, and continued dexamethasone, the patient developed rapidly worsening hypoxemia necessitating endotracheal intubation and mechanical ventilation within 24 hours of hospitalization. The patient developed refractory hypoxemia requiring 100% Fio2, high positive end-expiratory pressure (PEEP), and inhaled epoprostenol to maintain adequate oxygen saturations. His absolute lymphocyte count (ALC) was 0.4 × 103 cells/µL, and his lymphopenia persisted, ranging from 0.4 × 103 to 0.7 × 103 cells/µL (Fig. 1A).
Figure 1.
Effect of interleukin (IL)–7 on clinical and immunologic variables in critically ill coronavirus disease 2019 patient. A, A test dose of IL-7 (3 μg/kg) was administered followed by three additional doses of 10 μg/kg as indicated by red arrows. IL-7 increased the absolute lymphocyte count (ALC) from ~400 lymphocytes/μL to over 5,000 lymphocytes/μL. Green lines indicate upper and lower limit of normal for ALC; IL-7 was well tolerated and did not show any evidence of worsening lung inflammation as indicated by observing a decreasing Fio2 (B) and improving oxygen saturation (C). Blue arrows in (B) and (C) indicate day of intubation and extubation. Immunologic function of patient immune effector cells was tested by enzyme-linked immunospot (ELISpot). T-cell interferon (IFN)–γ production was tested in peripheral blood mononuclear cells (PBMCs) stimulated with anti-CD3/CD28 before and after IL-7 therapy administration to the patient. 2.5 × 104 PBMCs are plated for T-cell functional assay. Note the dramatic increase in the number of lymphocytes that produce IFN-γ (as represented by number of spot forming units [SFUs]) in upper row after IL-7 therapy (D, top row); severe acute respiratory syndrome coronavirus 2 spike, and nucleocapsid peptide antigens were incubated overnight in patient PBMCs before and after IL-7 therapy. 1 × 105 PBMCs are plated to assess antigen-specific T cells. Note the dramatic increase in the number of IFN-γ–producing lymphocytes (SFU) reacting specifically to the viral antigens after IL-7 (D, second row); innate immune function was tested by ELISpot assay for tumor necrosis factor (TNF)–α in PBMCs stimulated with lipopolysaccharide (LPS). 2.5 × 103 PBMCs are plated to assess innate immune function. Note the marked increase in the number of TNF-α–producing cells (SFU) after IL-7 (E). CD = cluster of differentiation, Spo2 = oxygen saturation, NCAP = nucleocapsid protein.
Lymphocyte function was assessed by the enzyme-linked immunospot (ELISpot) (Cellular Technology Limited, Shaker Heights, OH) assay using ex vivo stimulated peripheral blood mononuclear cells (PBMCs) as previously described (Missouri Baptist Medical Center Institutional Review Board Approval number 1132) (18, 22, 23). Cellular function is determined by the number of cells secreting cytokines. Cluster of differentiation (CD)-3/CD28 stimulated IFN-γ indicates adaptive immune function, and lipopolysaccharide-stimulated tumor necrosis factor (TNF)–α is used to indicate innate immune function (18). IFN-γ production in response to SARS-CoV-2 spike glycoprotein and nucleocapsid peptide pool (JPT, Berlin, Germany) is used to determine the patient’s antigen-specific T-cell response.
On days 5 and 7 post admission, ELISpot showed marked suppression of lymphocyte function (Fig. 1D), and TNF-α production was not elevated, suggesting that the patient did not have an overactive innate response or cytokine storm (Fig. 1E). Additionally, very few IFN-γ–producing SARS-CoV-2–specific T cells were detected (Fig. 1D). One week into hospitalization, the patient remained persistently febrile with continued need for mechanical ventilation requiring 75% Fio2 with a PEEP of 14 cm H2O (Fig. 1, A–C). He completed 5 days of remdesivir and continued dexamethasone treatment. In light of the patient’s failure to improve, the predicted prognosis, and lymphopenia with suppressed lymphocyte function, administration of IL-7 was considered on compassionate basis. Preliminary studies of COVID-19 disease patients treated with IL-7 demonstrated that IL-7 increased ALCs two- to three-fold and was well tolerated (24). Other studies showed that ex vivo addition of IL-7 to PBMCs from critically ill COVID 19 disease patients doubled lymphocyte IFN-γ production (18).
Given the patient’s ex vivo ELISpot response, approval was sought from the Federal Drug Administration (FDA) and obtained via emergency compassionate use authorization (Approved IND number 155018). Consent was obtained, and a test dose of 3 μg/kg of recombinant human IL-7 (RevImmune Inc, Paris, France) was administered via intramuscular injection and was well tolerated (Fig. 1). Twenty-four hours later, 10 μg/kg of IL-7 was administered followed by two additional doses of IL-7 separated by 72 hours.
After initiation of IL-7 therapy, the patient’s clinical status steadily improved, and mechanical ventilation was discontinued on day 15 after ICU admission. The patient was discharged home on day 24 of hospitalization (Fig. 1, B and C). Paralleling the patient’s clinical improvement, his ALC increased ~10-fold to a maximum of 5.1 × 103 lymphocytes/μL (Fig. 1A). Circulating lymphocyte and monocyte function also improved concomitant with initiation of IL-7 treatment as evidenced by multifold increases in the number of IFN-γ–producing T cells and TNF-α–producing cells (Fig. 1, D and E). Importantly, given their critical role in viral elimination, the number of SARS-CoV-2 spike protein and nucleocapsid specific T cells improved over 30-fold. Additionally, serial plasma cytokines were obtained and analyzed (Invitrogen ProcartaPlex 9-plex Luminex panel; Thermo Fisher Scientific, Waltham, MA). IL-6 levels were elevated with levels of 152 and 98 pg/mL prior to initiation of IL-7 therapy and subsequently decreased to 30 and 6 pg/mL. IL-1β, IL-2, IL-10, IL-17, and TNF-α were not significantly elevated at any timepoint. Interestingly, the patient maintained high levels of COVID-19–specific antibodies throughout his hospitalization (Leinco Technologies, St. Louis, MO) (Table 1).
TABLE 1.
Plasma Cytokine and SARS-CoV-2 Antibody Levels
| Analytes | Concentration | |||
|---|---|---|---|---|
| Pre IL-7, pg/mL | Post IL-7, pg/mL | |||
| Day 5 | Day 7 | Day 12 | Day 21 | |
| IL-1β | 0.16 | Undetectable | Undetectable | Undetectable |
| IL-2 | 0.25 | 0.17 | Undetectable | Undetectable |
| IL-6 | 152 | 98 | 30 | 6 |
| IL-10 | 3.55 | 5.53 | 2.71 | 0.83 |
| IL-12 | Undetectable | 0.03 | Undetectable | Undetectable |
| IL-17 | 0.11 | 0.16 | 0.14 | 0.12 |
| IFN-γ | 0.37 | 1.23 | Undetectable | Undetectable |
| Tumor necrosis factor-α | 0.05 | 0.04 | Undetectable | Undetectable |
| IFN-α (all subtypes) | Undetectable | Undetectable | Undetectable | Undetectable |
| IFN-β | 360 | 338 | 130 | 4.1 |
| Coronavirus disease immunoglobulin G/M/A | High positive | High positive | High positive | High positive |
IFN = interferon, IL = interleukin.
Given his severity of illness at hospital presentation without significant comorbidities and reports of associated inborn errors of immunity, whole exome sequencing was performed. A heterozygote genetic variant in TICAM1 (p.S60C) was found. TICAM1 encodes for Toll/interleukin-1 receptor homology domain–containing adapter-inducing IFN-β, an adapter protein involved in TLR3 responses. The p.S60C loss of function variant was recently reported to associate with COVID-19 severity (19, 20). Enzyme-linked immunosorbent assays were performed for plasma type I IFN levels and demonstrated undetectable levels of all subtypes of IFN-α (R&D Systems, Minneapolis, MN) throughout his hospitalization, as well as IFN-β levels of 360 and 338 pg/mL prior to IL-7 treatment, and 130 and 4.1pg/mL following treatment (Table 1). Both IFN-α and IFN-β levels in a cohort of seven healthy control subjects approached a mean of 0 pg/mL. IFN-α in 10 COVID-19 patients with disease severity comparable with that of the patient demonstrated levels ranging from 0 to 93 pg/mL; IFN-β levels from 50 to 811pg/mL. Informed consent for publication was obtained from the patient.
DISCUSSION
We demonstrate the use of IL-7 as an immunoadjuvant therapy in the treatment of COVID-19 disease. IL-7 not only restores lymphocyte counts but reverses T-cell exhaustion as evidenced by increased lymphocyte ex vivo IFN-γ production, essential for effective host immune response to pathogens. A previous report of compassionate use of IL-7 in critically ill COVID-19 disease patients with severe lymphopenia showed that IL-7 was safe, reversed the profound lymphopenia, and was well tolerated (24–26). Importantly, previous studies from our group reported that using the ex vivo stimulatory ELISpot assay in critically ill COVID-19 disease patients, we demonstrated that patients whose lymphocytes failed to produce IFN-γ upon stimulation trended toward mortality. Additionally, ex vivo stimulation of these patients’ cells with IL-7 restored lymphocyte IFN-γ production (18).
Severity of disease in COVID-19 is often associated with a dysfunctional type I IFN (IFN-α and IFN-β) antiviral response. Several inborn errors of type I IFN signaling as well as autoantibodies have been identified in relation to severe cases, in addition to numerous additional cases without a known underlying cause (19, 20). Type I IFN signaling occurs locally in the lungs as well as systemically, to activate the innate and adaptive immune response to a viral pathogen. Defective type I IFN signaling enables unrelenting nuclear factor kappa-light-chain-enhancer of activated B cells-driven systemic inflammation with elevated circulating levels of IL-6 and TNF-α, promoting increased local tissue damage and multisystem organ dysfunction (27). IL-6/signal transducer and activator of transcription-3 signaling additionally can cause an immunosuppressive phenotype with decreased antigen presentation by mononuclear cells and suppressed lymphocyte function (28). Therefore, we hypothesize that the dysfunctional viral clearance observed in severe COVID-19 could be linked to decreased type I IFN signaling and unbalanced inflammation, leading to innate and adaptive immunosuppression. Our patient was found to be lacking in circulating plasma IFN-α, while also demonstrating elevated IL-6 levels and a dysfunctional ex vivo T-cell response to stimulation. Immunotherapy with IL-7 improves cellular adaptive immune function, promoting proliferation and activation of effector T cells, which will improve viral clearance and restore immune homeostasis. The TICAM1 mutation is a defect in TLR3 signaling. IFN-γ (type II IFN) is the integral effector molecule downstream of TLR3. In a patient with defective TLR3 signaling, it is conceivable that essentially bypassing this receptor pathway using an immune stimulant such as recombinant human IL-7 could restore IFN-γ expression and therefore improved immune function and T-cell activation during an acute infection (29).
The presence of the loss of function mutation in TICAM1 shows the importance of the host response to COVID-19 infection. This patient was previously healthy, like those reported to have heterozygous mutations in genes involved in type I IFN responses. Having genetic information available may guide future therapies in critically ill patients as recently reported (30).
The patient described in this report maintained a robust anti–SARS-CoV-2 antibody response from day 5 post admission to day 21. Although some reports describe weak early antibody production to correlate with severity of disease (31), others describe no correlation between antibody levels and severity of disease, with a trend toward higher levels in more severe patients (32). Antibody levels are unlikely to be positively predictive of outcomes, and therefore, immunotherapy to stimulate the T-cell adaptive response should be considered.
Another noteworthy observation in this case is the precision medicine approach using a functional immune assay (ELISpot) to evaluate a patient’s response and candidacy for an immune enhancing therapy (i.e., IL-7). For our patient, the ELISpot assay showed severe depression of adaptive immunity, indicating that a restorative therapy might be useful in restoring lymphocyte function. Previously, our group used the ELISpot assay to show that IL-7 restored lymphocyte function in critically ill COVID-19 disease patients. The present study advances the utility of such an approach, especially for precisely curated therapies applied to a patient’s functional immune state. In this case, IL-7 administration improved both T-cell IFN-γ production as well as the number of SARS-CoV-2-specific T cells.
In conclusion, administration of IL-7 to a critically ill COVID-19 patient markedly improved patient immunity and increased SARS-CoV-2–specific lymphocytes, thereby potentially enhancing viral elimination. Presently, IL-7 is available from RevImmune with an FDA expanded access protocol for compassionate use (EAP IND number 151107). Administration of IL-7 alone or in combination with other therapies warrants serious consideration for COVID-19 patients with evidence of immunosuppression.
ACKNOWLEDGMENTS
We would like to acknowledge the regulatory assistance by Ms. Tiffany Hamilton and Ms. Sara Butler at Missouri Baptist Medical Center, delivery and administration of interleukin (IL)–7 by pharmacists Drs. Natalie Freiberger and Kathryn Vehe, and compassionate use drug monitoring by Ramona Nelson, RN. Additionally, we are most grateful for the oversight of the entire acquisition of approval, assistance in regulatory management, and delivery of IL-7 to the patient by nurse manager, Jane Blood, RN. Finally, we want to directly acknowledge the excellent bedside clinical care by the nurses and therapists at Missouri Baptist Medical Center ICU, and most of all, the patient and his family, for which his clinical outcome is most important to this report. Enzyme-linked immunospot, enzyme-linked immunosorbent assay, and flow cytometry studies were performed in the Hotchkiss laboratory by Monty B. Mazer, MD, Andrew Walton, and Dale Osborne. Luminex panel was performed at Washington University Bursky Center for Human Immunology and Immunotherapy Program’s Immunomonitoring Core laboratory by Diane Bender, PhD, and Hailey Freres. Molecular genetics was performed by Brooke Sadler, PhD, in the Institution of Clinical and Translational Sciences at Washington University in St. Louis.
Footnotes
Drs. Mazer, Hotchkiss, and Remy contributed to literature search, data analysis, writing, editing, development of concept, coordination and administration of medication. Drs. Caldwell, Turnbull, Brakenridge, Moldawer, and Di Paola contributed to writing, editing, and development of concept. Miles, Blood, and Dr. Hess contributed to data analysis, editing, and figure generation. Dr. Sadler performed genetic testing and analysis for this study. Drs. Martin, Botney, Bosanquet, Striker, and Anand contributed to coordination and administration of medication, editing, and study execution.
Dr. Turnbull has received funding from the National Institute of Health (NIH), National Institute of General Medical Sciences (NIGMS) GM133756. Dr. Morre is chief scientist for RevImmune, the company that generously provided interleukin-7 for this patient. Dr. Moldawer has received funding from the NIH, NIGMS GM139046. Dr. Di Paola has received funding from the NIH, National Heart, Lung, Blood Institute HL139825. Dr. Hotchkiss has received funding from the NIH, NIGMS GM126928. Dr. Remy has received funding from the NIH, NIGMS GM129763, and the National Center for Advancing Translational Sciences UL1TR0002345. The remaining authors have disclosed that they do not have any conflicts of interest.
This work was performed at Missouri Baptist Medical Center, St. Louis, MO, and Washington University School of Medicine, St. Louis, MO.
REFERENCES
- 1.Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. : Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med. 2021; 203:54–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quah P, Li A, Phua J: Mortality rates of patients with COVID-19 in the intensive care unit: A systematic review of the emerging literature. Crit Care. 2020; 24:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auld SC, Caridi-Scheible M, Blum JM, et al. : ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020; 48:e799–e804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pepe M, Maroun-Eid C, Romero R, et al. : Clinical presentation, therapeutic approach, and outcome of young patients admitted for COVID-19, with respect to the elderly counterpart. Clin Exp Med. 2021; 21:249–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021; 47:60–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alharthy A, Aletreby W, Faqihi F, et al. : Clinical characteristics and predictors of 28-day mortality in 352 critically ill patients with COVID-19: A retrospective study. J Epidemiol Glob Health. 2021; 11:98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu K, He J, Wu Y, et al. : Dysregulated adaptive immune response contributes to severe COVID-19. Cell Res. 2020; 30:814–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blot M, Bour JB, Quenot JP, et al. ; LYMPHONIE study group. The dysregulated innate immune response in severe COVID-19 pneumonia that could drive poorer outcome. J Transl Med. 2020; 18:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin C, Zhou L, Hu Z, et al. : Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020; 71:762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esmaeilzadeh A, Elahi R: Immunobiology and immunotherapy of COVID-19: A clinically updated overview. J Cell Physiol. 2021; 236:2519–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, et al. : Emerging treatment strategies for COVID-19 infection. Clin Exp Med. 2021; 21:167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamilloux Y, Henry T, Belot A, et al. : Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020; 19:102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry B, Cheruiyot I, Vikse J, et al. : Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID-19: A meta-analysis. Acta Biomed. 2020; 91:e2020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan L, Wang Q, Zhang D, et al. : Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct Target Ther. 2020; 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Hu B, Hu C, et al. : Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazer MB, C Caldwell C, Hanson J, et al. : A whole blood enzyme-linked immunospot assay for functional immune endotyping of septic patients. J Immunol. 2021; 206:23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remy KE, Mazer M, Striker DA, et al. : Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. 2020; 5:140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Bastard P, Liu Z, et al. ; COVID-STORM Clinicians; COVID Clinicians; Imagine COVID Group; French COVID Cohort Study Group; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort; NIAID-USUHS/TAGC COVID Immunity Group. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020; 370:eabd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastard P, Rosen LB, Zhang Q, et al. ; HGID Lab; NIAID-USUHS Immune Response to COVID Group; COVID Clinicians; COVID-STORM Clinicians; Imagine COVID Group; French COVID Cohort Study Group; Milieu Intérieur Consortium; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020; 370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu W, Metcalf JP: The Role of Type I IFNs in influenza: Antiviral superheroes or immunopathogenic villains? J Innate Immun. 2020; 12:437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verhoef PA, Kannan S, Sturgill JL, et al. : Severe acute respiratory syndrome-associated coronavirus 2 infection and organ dysfunction in the ICU: Opportunities for translational research. Crit Care Explor. 2021; 3:e0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazer MB, Caldwell CC, Hanson J, et al. : A whole blood enzyme-linked immunospot assay for functional immune endotyping of septic patents. J Immunol. 2021; 206:23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laterre PF, Francois B, Collienne C, et al. : Association of interleukin 7 immunotherapy with lymphocyte counts among patients with severe coronavirus disease 2019 (COVID-19). JAMA Netw Open. 2020; 3:e2016485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackall CL, Fry TJ, Gress RE: Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011; 11:330–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monneret G, de Marignan D, Coudereau R, et al. : Immune monitoring of interleukin-7 compassionate use in a critically ill COVID-19 patient. Cell Mol Immunol. 2020; 17:1001–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadjadj J, Yatim N, Barnabei L, et al. : Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020; 369:718–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohno Y, Kitamura H, Takahashi N, et al. : IL-6 down-regulates HLA class II expression and IL-12 production of human dendritic cells to impair activation of antigen-specific CD4(+) T cells. Cancer Immunol Immunother. 2016; 65:193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negishi H, Osawa T, Ogami K, et al. : A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proc Natl Acad Sci U S A. 2008; 105:20446–20451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lévy R, Bastard P, Lanternier F, et al. : IFN-α2a therapy in two patients with inborn errors of TLR3 and IRF3 Iinfected with SARS-CoV-2. J Clin Immunol. 2021; 41:26–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenti MV, Aronico N, Pellegrino I, et al. : Depletion of circulating IgM memory B cells predicts unfavourable outcome in COVID-19. Sci Rep. 2020; 10:20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan AT, Linster M, Tan CW, et al. : Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021; 34:108728. [DOI] [PMC free article] [PubMed] [Google Scholar]