Abstract
Massive, irreparable rotator cuff tears in younger, more active patients present a unique treatment challenge to shoulder surgeons. In the past few years, new techniques continue to emerge to address this challenging problem. The superior capsular reconstruction technique has been accepted as an option for addressing this problem. While initial results are encouraging, pitfalls remain regarding technical challenges, healing and protracted rehabilitation due to delayed motion protocols. We present an alternative approach to the massive, irreparable rotator cuff tears using a biologic interpositional humeral -based graft.
Technique Video
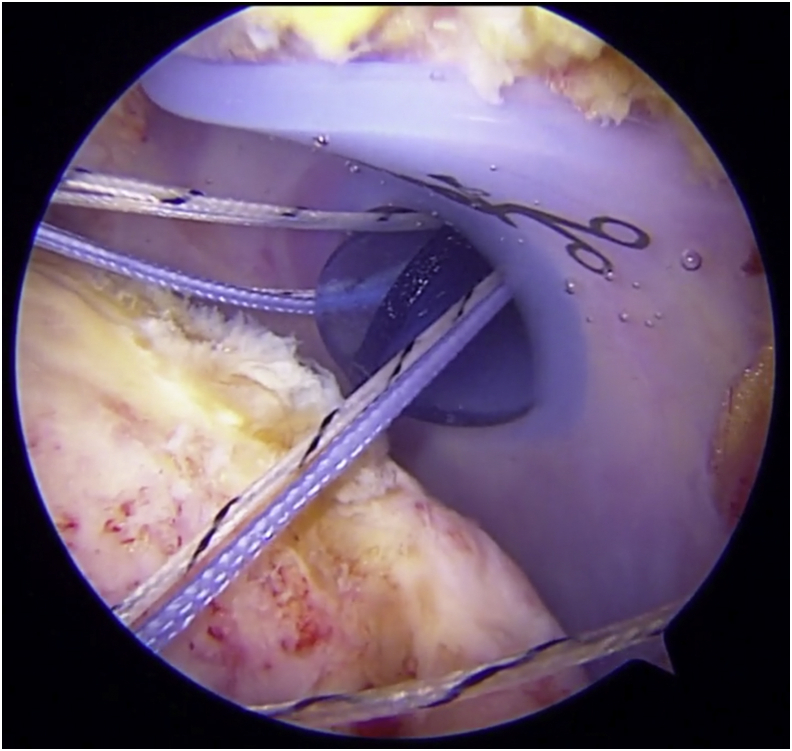
This is a patient with an irreparable rotator cuff tear with little-to-no arthritis. We begin from a posterior portal demonstrating the tear. This is followed by our technique for performing an arthroscopic biologic interpositional tuberosity graft for treatment of irreparable rotator cuff tears. Completed graft and gentle range of motion are demonstrated as well.
Introduction (With Video Illustration)
Optimal treatment for young, active patients with massive, irreparable rotator cuff tears (MIRCTs) remains controversial (Fig 1). While some can do well with nonsurgical management, others have persistent pain, weakness, and disability.1,2 Several surgical options are available to try to address this problem; however, each has inherent limitations and risks. Current options include partial repair, tendon transfers, bridging grafts, superior capsular reconstruction (SCR), bursal-sided acromial resurfacing, interpositional devices, reverse shoulder arthroplasty, and choosing to address other potential pain generators such as the biceps tendon.3, 4, 5 Clinical decision-making for which technique is employed is based on patient factors and preferences, including accounting for individual risk aversity, as well as surgeon preference.
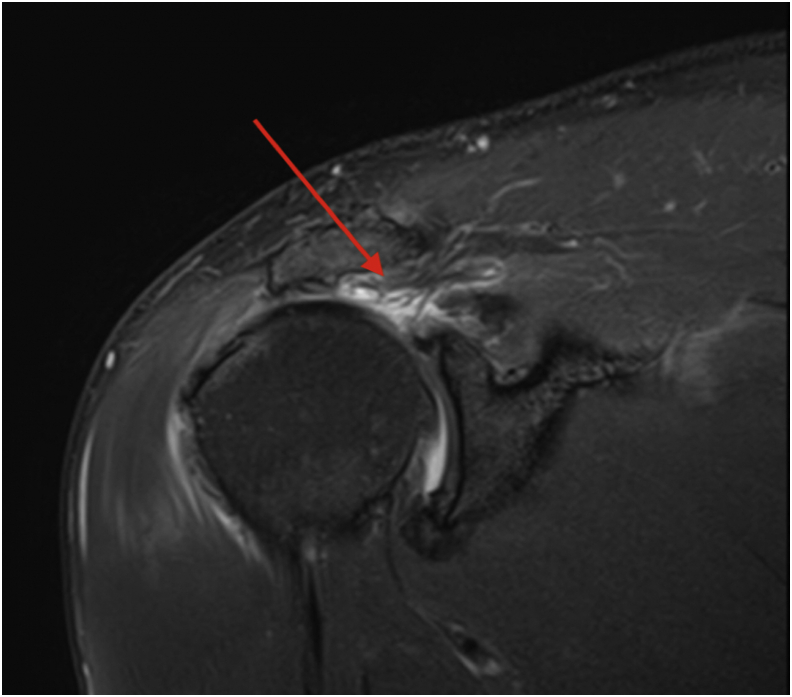
Fig 1.
Coronal magnetic resonance imaging of an irreparable rotator cuff tear retracted back to the level of the glenohumeral joint (red arrow).
Originally described by Mihata et al., SCR has evolved over the past several years into an accepted although challenging arthroscopic technique for joint preservation in this population.4,6,7 The basic principle is that a biologic graft is placed to span from the superior glenoid to the greater tuberosity. When healed at both locations under appropriate tension, this may improve glenohumeral kinematics by depressing the humeral head via a reverse trampoline effect of the graft.3,8 Mihata et al.9 has published successful results using a thick fascia lata autograft. In an effort to avoid donor-site morbidity, acellular dermal allograft has been a more popular choice in the United States. Although some authors have published successful clinical results using a 3-mm dermal allograft, the results of Mihata et al. have generally not been replicated to date.10
As surgeons have gained experience with SCR using dermal allografts, several authors have reported on both technical and biologic challenges of the procedure. Efficient facilitation of SCR has remained a challenge for many of even the most proficient arthroscopic shoulder surgeons. From a technical standpoint, glenoid anchor placement and fixation security can be a problem.8 Achieving optimal graft tensioning to accomplish the desired biomechanical effect yet avoiding overtensioning, which can adversely affect graft healing, may not be predictable.5 In addition, dermal graft tensile strength has been reported to decrease significantly following implantation.11 There are increasing reports that graft healing is not predictable, especially on the glenoid side. Less-than-optimal graft healing has now been reported by several authors who have evaluated radiographic healing.12,13 However, an interesting observation has been that many patients who have radiographic failure of the graft, either on the glenoid side or midsubstance, are found to have successful clinical outcomes if the graft has healed over the greater tuberosity.5,14,15 It is hypothesized that clinical success in this group may be related to the interpositional effect of the graft between the greater tuberosity and acromion.
In this article, we present our technique for placement of a humeral biologic interpositional graft to accomplish a similar goal of joint preservation in this unique patient cohort (Video 1). The basic premise is that we are placing a permanent interpositional graft between the acromion and greater tuberosity but also somewhat medializing the graft onto the superior humeral head. Although there are technical considerations to graft delivery and suture management, which will be reviewed, fixation principles are similar to rotator cuff repair. Relative to SCR, concerns of glenoid fixation and optimal graft tensioning are avoided. Since this technique provides essentially tensionless graft repair, postoperative rehabilitation can be accelerated relative to SCR. We also believe that most surgeons who perform arthroscopic rotator cuff repair can achieve proficiency with this technique.
Technique Description
Similar to arthroscopic rotator cuff repair, this technique can be performed with the patient in either the beach chair or lateral position. We prefer the beach chair position with sedation following an interscalene block. Following an examination under anesthesia, a diagnostic arthroscopy is performed. Any intra-articular pathology is assessed and addressed appropriately, including management of both the biceps and subscapularis if necessary.
The arthroscope is then placed in the subacromial space, and a lateral portal is created to assess reparability of the superior rotator cuff based on degree of retraction, mobility, tissue quality, and atrophy. If the rotator cuff is determined to be irreparable the following technique has been considered and used for select patients.
Standard rotator cuff portals are made with or without cannulas as indicated to access and properly visualize the superior humeral head and greater tuberosity. A spinal needle is used to confirm optimal portal placement. Typically, either a midlateral or posterior portal (usually with a 70° scope) is used as the viewing portal. A large flexible cannula (PassPort; Arthrex, Naples FL) can be placed now or following tuberosity preparation. Acromial work is left to the end of the surgery to minimize soft-tissue distension (Table 1).
Table 1.
Pearls and Pitfalls of Arthroscopic Biologic Interpositional Tuberosity Graft
| Pearls | Pitfalls |
|---|---|
| Patient selection. | Improper patient selection: contraindications include advanced arthritis, true pseudoparalysis. Expectations must be reasonable. |
| Bone preparation: decorticate 5-10 mm medial to the RC footprint. | Skiving into the joint with anchors: determine optimal angle of insertion of anchors which may include accessory anterior or posterior portals. |
| Suture management: dock suture tapes in other portals while medial row knotless sutures pass through the removable segregated insert for lateral cannula. | Suture management: utilize removable insert that creates 4 quadrants in the lateral flexible cannula. Dock sutures anterior or posterior until ready to be used for lateral row anchors. |
| Graft preparation: leave 5 mm around the sides of the graft to allow fixation to the posterior and possible anterior RC. Do not leave excess graft lateral as this may impede visualization and proper tensioning. | Improper graft sizing: increased graft is utilized (5 mm) anterior, posterior and medial, but not lateral. |
| Graft delivery: orchestrate passage of graft thru a combination of tensioning the medial row knotless sutures while pushing the graft through the large flexible cannula. | Excessive fluid extravasation may occur. Saving the acromioplasty until the end can help to avoid this. Keeping pressure within the system as low as possible also will help avoid this problem. |
| Sutures placed in the lateral corners of the graft maybe used to assist in reducing the graft prior to placing lateral row anchors. | |
| Optimize portal placement: utilize a spinal needle to determine portal location which will facilitate medial row anchor allow placement. |
Bone preparation using a bone shaver (and access channels) to enhance graft healing on the tuberosity footprint is similar to rotator cuff repair. However, our technique includes medialization of the footprint (on average 5-10 mm) to provide greater coverage of the superior humeral head. No glenoid work is required in this procedure. There is no reason to decorticate beyond where the medial row anchors can be successfully placed. To help determine ultimate medial row anchor placement and ideal portal placement to ensure proper insertion trajectory, a spinal need combined with rotation and adduction maneuvers can facilitate portal optimization. Typically, a small posterosuperior percutaneous portal is created to place the posterior medial row anchor, combined with a more standard anterosuperior portal just adjacent to the acromion. Sometimes the acromion can limit your ability to go as far medial as you would prefer without skiving into the joint. In this situation, placing the middle (if 3 anchors) and anterior medial row anchors through accessory portals (posterior or anterior) may provide a more ideal angle of insertion.
Via the appropriate portal as described previously, 2 or 3 (typically 3) medial anchors are placed with appropriate spacing. Our preference is to use anchors loaded with tape as well as a separate suture with a knotless mechanism. However, graft fixation may be performed with a number of different anchor designs and suture configurations and likely will evolve over time.
When using 3 knotless anchors with tape (Arthrex), the tape from at least one anchor may need to be exchanged to smaller diameter suture because to allow them to slide thru the lateral row anchor eyelet. If the large flexible cannula has not been previously placed into the lateral portal it should be placed at this time. Measurements are made between the medial row anchors and an estimate is made as to where lateral row anchors will be placed (Fig 2). Measurements are used to prepare the dermal graft on the back table leaving approximately 5 mm of excess graft around the periphery except for the lateral side of the graft for incorporation and rotator cuff repair (excess graft laterally can be problematic regarding optimal tensioning and visualization) (Fig 3). We strive to use a dermal graft approximately 5-6 mm in thickness if possible (Fig 4).
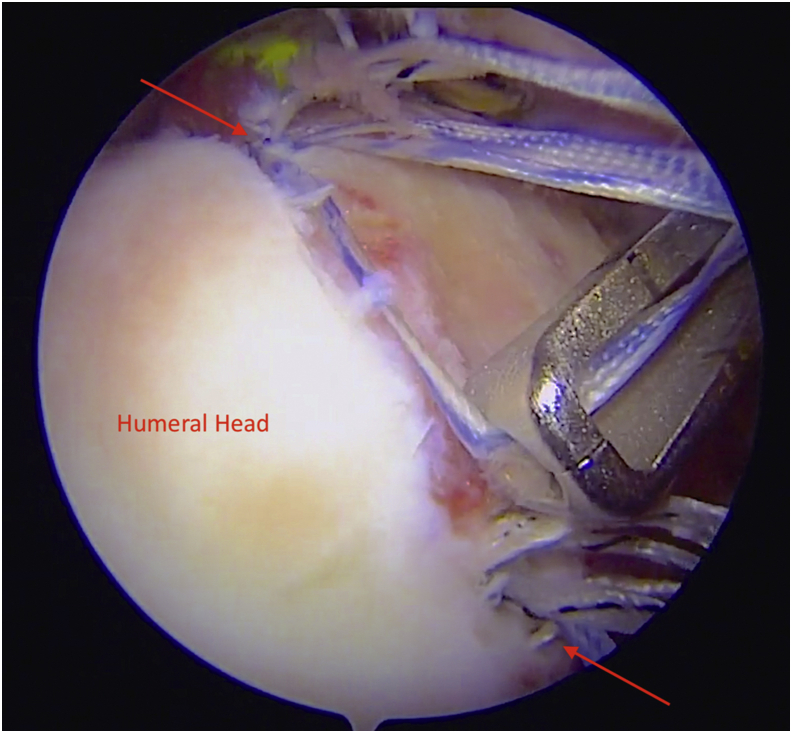
Fig 2.
Arthroscopic view of the right shoulder using 30° arthroscope from the posterior portal. With a commercially available arthroscopic measuring device (Arthrex), the distance between medial row anchors (red arrows) is measured to determine graft dimensions.
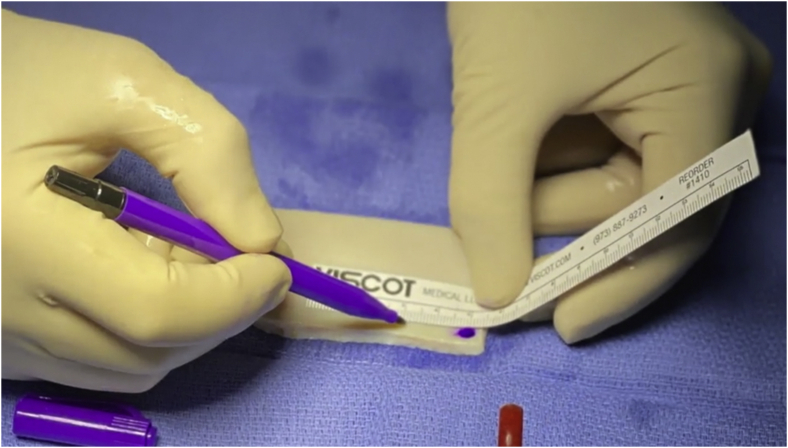
Fig 3.
Intraoperative measurements are used to prepare the dermal graft on the back table, leaving approximately 5 mm of excess graft around the periphery except for the lateral side of the graft for incorporation and rotator cuff repair (excess graft laterally can be problematic regarding optimal tensioning and visualization).
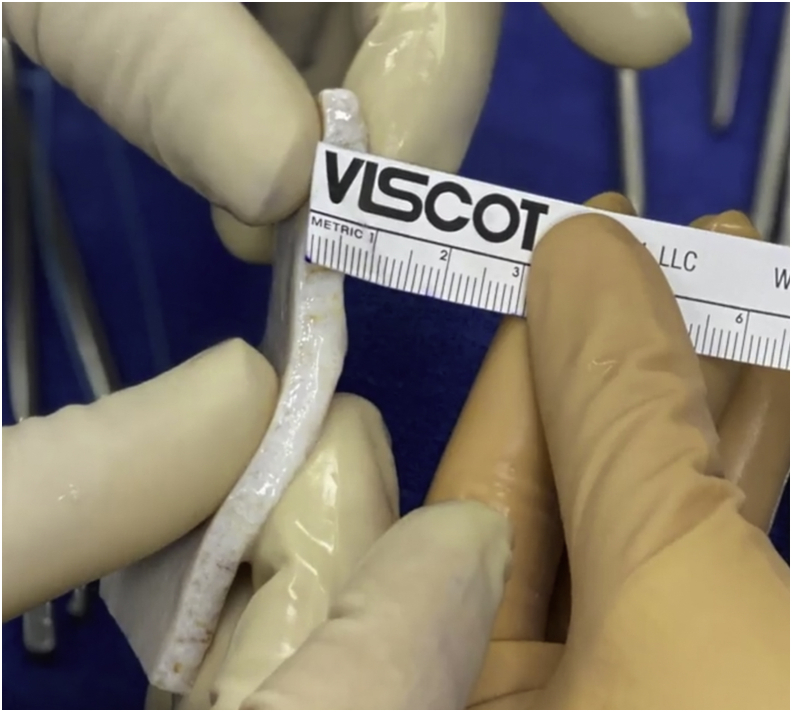
Fig 4.
The graft is held to view from the side the dermal graft thickness, which is measured with optimal thickness, 5-6 mm in this case.
A commercially available divider (Arthrex) is placed into the flexible lateral cannula to assist in suture management (Figs 5 and 6). Sutures or tapes, which will be ultimately draped over the graft for fixation, are left docked in other working portals. The 3 (or 2) knotless fixation repair sutures from medial row anchors are delivered through the individual partitions of the divided lateral cannula. Using a free needle, knotless repair sutures are placed through the medial side of the graft (simple or inverted mattress pattern) at the appropriate location so that when fully tensioned the graft will reduce to the anchors medially (Fig 7). The knotless repair sutures are shuttled through the anchors via the passing loop suture in a mattress fashion to help the graft sit appropriately. The medial row knotless repair sutures will be tensioned in tandem with graft delivery through the lateral cannula.
Fig 5.
When viewing from a posterior portal, sutures are seen exiting the shoulder through a cannula. Suture management is a key component of efficiency in this case and is facilitated by using a cannula divider.
Fig 6.
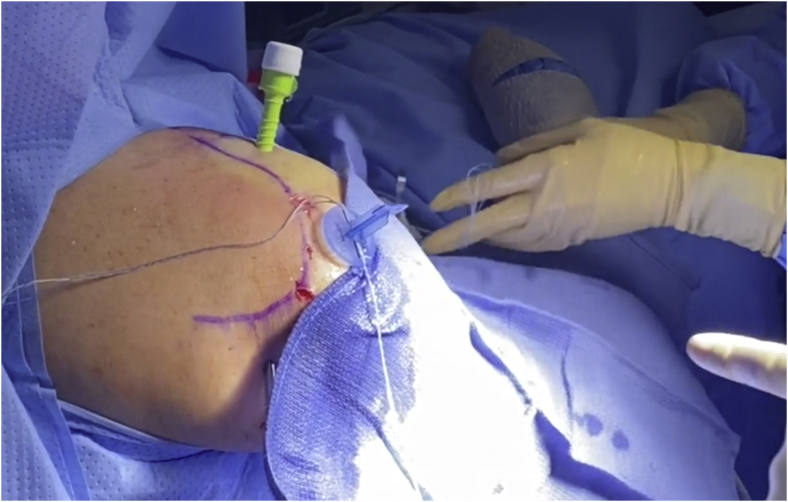
External view of a right shoulder with portal placement and suture organization using cannula divider.
Fig 7.
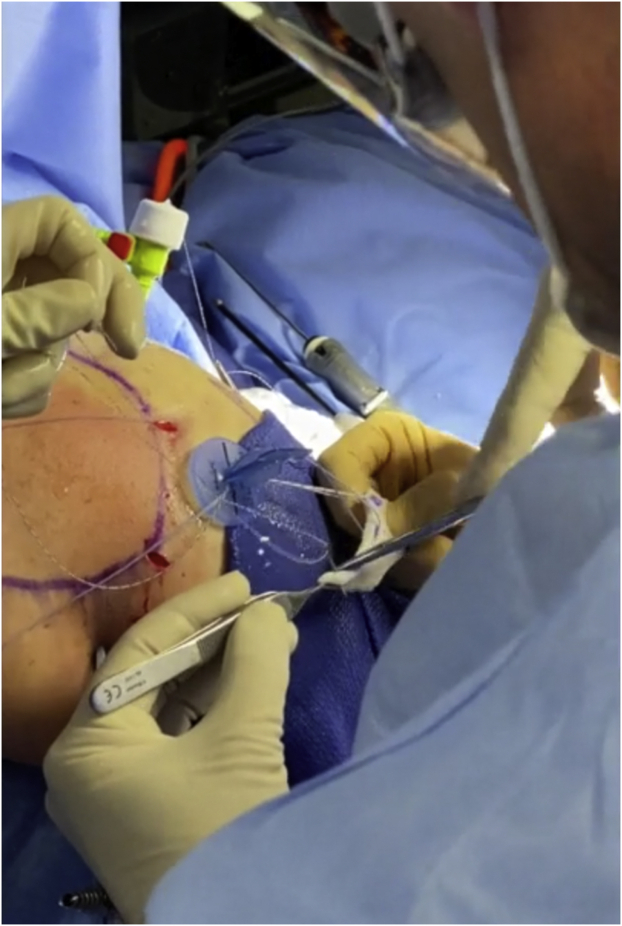
External view of right shoulder showing the surgeon passing medial row knotless sutures through graft exiting the lateral working portal.
Before graft delivery, the cannula divider is removed while aligning the graft at the cannula to avoid suture entanglement. The graft is then folded and introduced into the portal. Graft delivery is facilitated with a combination of pulling (tensioning) the knotless repair sutures and inserting the graft through the cannula with an arthroscopic instrument or hemostat. During this delivery and reduction maneuver, the medial row knotless sutures are tensioned while medial row tapes are kept docked in separate working portals to avoid tangling. Once the graft is reduced and fixed medially with the knotless locking mechanism, one limb of tape from each anchor is delivered over the top of the graft to fix laterally over the tuberosity. (Tip: In graft preparation, lateral sutures can be placed at the lateral corners of the graft and those sutures can be used to tension the graft distally as sutures and lateral row anchors are managed.) Similar to rotator cuff repair, lateral anchors are placed securing the medial row tapes or sutures, which are tensioned over the graft.
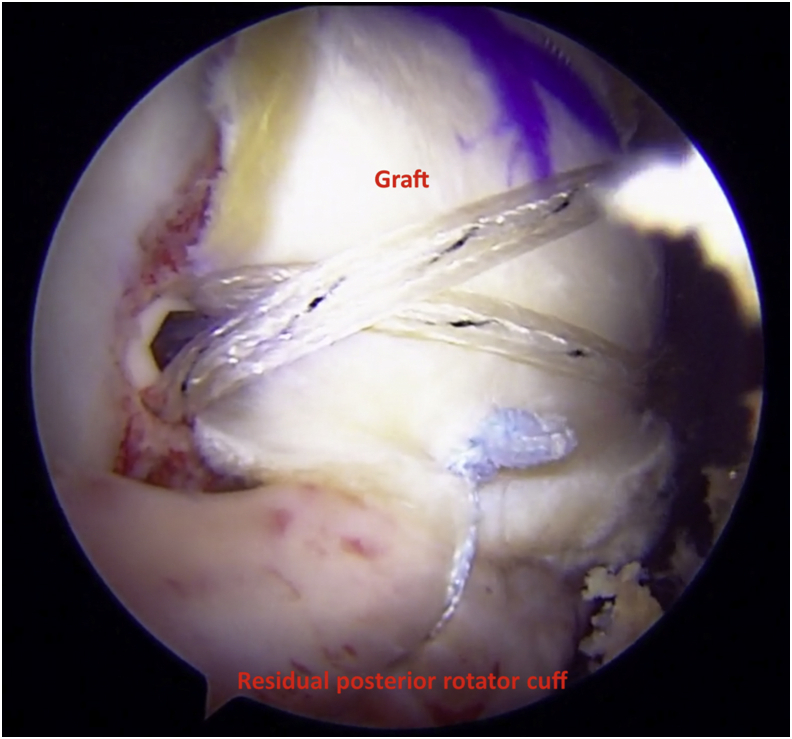
The final step of the procedure is to fix the residual rotator cuff posteriorly to the posterior tuberosity graft and, if possible, anteriorly to the residual rotator interval tissue and subscapularis to produce maximum coverage under the acromion (Figs 8 and 9). In addition, one of the tapes or sutures from the posterior and anterior medial row anchors can also be used to perform a partial cuff repair to the tuberosity.
Fig 8.
The tuberosity graft is seen here abutting the acromion and acts as a biologic interpositional spacer between acromion and greater tuberosity seen here from a posterior portal in a right shoulder.
Fig 9.
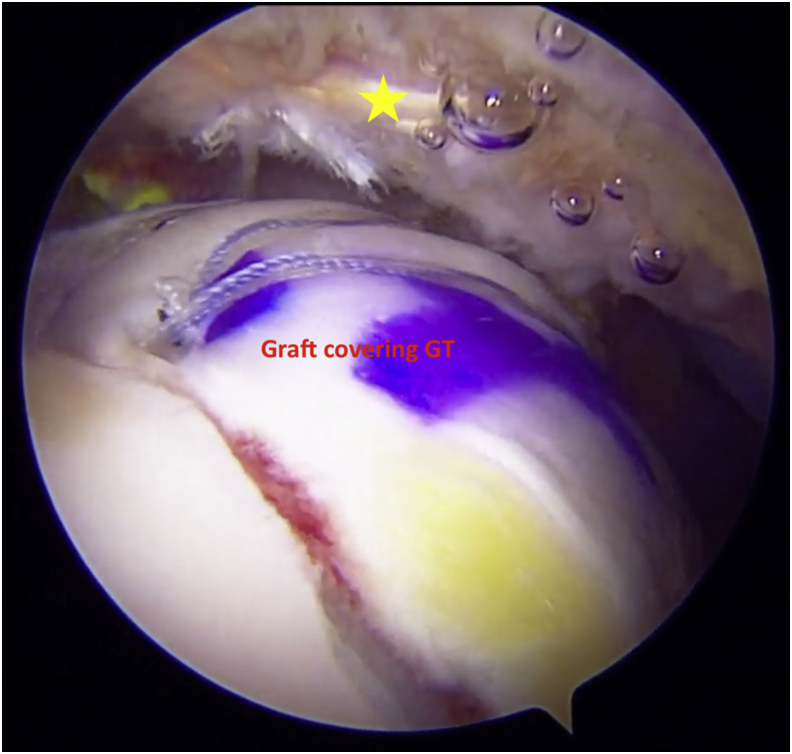
Arthroscopic view of right shoulder using 70° arthroscope from the posterior portal. The graft acts as a biologic interpositional spacer between acromion (yellow star) and greater tuberosity (GT).
At the conclusion of the procedure, the graft extends more medially than the typical medial footprint. This acts as a permanent biologic interpositional graft between the acromion and greater tuberosity.
Rehabilitation
Rehabilitation after massive rotator cuff repair and grafting procedures often involves extensive immobilization periods. Following biologic interpositional graft, patients are immobilized for 6 weeks in a sling. They are then progressed with active assisted range of motion with subsequent advancement to active range of motion as tolerated. Strengthening can begin at approximately 8 weeks postoperatively.
Discussion
Several techniques have evolved for treatment of MIRCTs in patients in whom arthroplasty is not ideal.3 Arthroscopic SCR has gained popularity, although more recently, failure modes have been described and questions remain regarding many aspects of this procedure.12,15,16 Clinical outcomes and radiographic results appear to differ in the world’s literature (Table 2).5,12,14,15,17, 18, 19 It still remains unclear whether the primary mechanism of action is through its tenodesis effect, force coupler effect, or its acting as a subacromial spacer.16 Disadvantages of SCR include generalizability of a difficult technique, glenoid fixation challenges, optimizing tensioning, and unpredictable graft healing, especially on the glenoid side.5,8,11, 12, 13 The results of Mihata et al. have generally not been replicated elsewhere, which may be the result of multiple factors, one of which may be related to graft thickness. Mihata et al. described using a much thicker graft than the common 3-mm graft currently in use for dermal allograft SCR.6,10 In our described technique, we strive to use a thicker 5- to 6-mm graft in an attempt to get closer to the original described thickness.
Table 2.
Literature-Reported Radiographic Versus Clinical Results of Superior Capsular Reconstruction
| Study | Radiographic Results | Clinical Results |
|---|---|---|
| Lacheta et al.5 (N = 21) | Graft integrity at tuberosity 100%, midsubstance 76%, and glenoid 81% | No significant difference in clinical outcome (torn vs not torn) |
| Denard et al.14 (N = 20, 59) | 45% had a completely healed Graft | 74.6% had a successfulclinical outcome |
| Burkhart and Hartzler17 (N = 10) | 70% had a completely healed Graft | 90% had a successful outcome |
| Acevedo et al.12 (N = 43) | 38% had an intact graft, 33% had a tear at the glenoid, 12% had a midsubstance tear, 14% had a tear at tuberosity, and 2% had an absent graft | No significant difference in clinical outcome between those with intact graft and those with tear at the glenoid |
| Those with a tear at the tuberosity had significantly less improvement compared with intact or glenoid tear | ||
| Mirzayan et al.15 (N = 22) | 41% of grafts intact | Significant clinical improvement for intact grafts and those that still covered the tuberosity |
| No improvement if graft torn from tuberosity | ||
| Campbell et al.18 (N = 24) | 50% intact, 33% torn from glenoid, and 17% torn elsewhere | Evidence of clinical improvement, but no significant correlation between graft integrity and clinical outcome |
| Lee et al.19 (N = 46) | 65% intact, 22% lateral tear, 7% midsubstance tear, 4% medial tear, and 2% both medial and lateral tear | Significant clinical improvement for all patients using ASES, VAS, and Constant scales |
| Significant improvement in ROM only for intact group |
ASES, American Shoulder and Elbow Surgeons; ROM, range of motion; VAS, visual analog scale.
Recently, Lacheta et al.5 reported their series of 22 patients with all grafts healed on the tuberosity site though only 81% on the glenoid side. Despite graft failure at 2.5 months, clinical outcomes were not affected. Denard et al.14 reported 1-year outcomes demonstrating 45% graft healing on postoperative magnetic resonance imaging (MRI) with approximately 70% successful outcomes in their series. Similarly, Burkhart and Hartzler17 reported only 70% intact grafts yet 90% successful outcomes. Acevedo et al.12 reported that only 38% of their patients had a completely intact SCR graft based on MRI evaluation. Of these, 33% failed from the glenoid side (despite 3 anchors), 12% had a mid-substance tear, 14% failed from the tuberosity, and 2% had complete graft absence. Mirzayan et al.15 reported 59% of their SCR grafts had torn based on MRI at a mean of 13.9 months postoperatively. In their series, as long as the tuberosity was covered with the graft on MRI, there was no difference in clinical outcomes between the grafts that had fully healed and the ones that had failed medial to the tuberosity (Table 2). Both of these groups were significantly better than if the graft failed and left the tuberosity uncovered. The authors termed this the “biologic tuberoplasty effect.” Campbell et al.18 recently reported MRI appearance of SCR and association of short-term outcomes. At a mean of 9.1 months post-op there was a 50% incidence of graft tearing (two-thirds failed at the glenoid), but there was no correlation between clinical outcome and graft integrity. Lee et al.19 reported a 35% incidence of retear at a mean follow up of 7.8 months, with 68% of these being lateral tears. The clinical outcome for those with lateral tears was significantly worse than the intact group or those with a nonlateral tear.19 These results cause one to ask why we are spending so much effort to fix the graft to the glenoid. Perhaps a humeral based graft alone acting as a biologic interpositional spacer may be sufficient with a quicker recovery.
As a result of these issues as well as additional challenges associated with SCR, alternative treatments continue to be explored. Placement of a temporary, resorbable interpositional balloon in the subacromial space has been investigated with reports of short-term success in this population.3,20, 21, 22 However, although this interpositional device holds promise, it serves as a temporary spacer, and there are reports of complications, including migration, since it is not fixated to bone.22,23 Acromial based techniques for additional buttress or spacer effect have also been described and are under investigation.8,20,24 However, many surgeons are likely to feel uncomfortable or technically challenged with fixing a graft to the undersurface of the acromion. There also remains concern regarding the potential risks of creating stress risers with acromial-based procedures.23
Our technique for placement of a humeral-sided permanent biologic interpositional graft sets out to accomplish a similar goal of clinical outcome improvement combined with joint preservation in this unique patient cohort. The primary aim is to provide pain relief. Function may be improved but is potentially related to pain relief and the ability to rehabilitate the shoulder to a greater level than achievable preoperatively. Significant arthritis, true pseudoparalysis, or an irreparable subscapularis we consider relative contraindications. Older patients who are good candidates for reverse shoulder arthroplasty are typically advised that arthroplasty may be a more predictable option.
Although several advantages to biologic interpositional graft are apparent, limitations exist. Limited data exist regarding this technique, as it is still in an early stage of data collection. It is unknown whether fixation on the tuberosity alone will provide the same biomechanical advantages to the shoulder as those techniques which fix grafts to the glenoid. Certainly, one limitation remains the management of the graft arthroscopically from technique perspective. Long-term outcomes and additional technique modifications may elucidate optimal options over time.
We are not stating that this technique is in any way better, or even equal to, alternative options including SCR. In fact, we caution the acceptance of any technique until it is shown to be safe and effective. However, if this technique can be found to be effective, then it may prove to play a role going forward in this challenging patient cohort. Potential advantages versus other options might include avoiding some of the challenges related to SCR, maintaining a fixed longer lasting subacromial spacer, expedited rehabilitation, and the ability to achieve proficiency by a larger group of surgeons.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: J.G. reports consultant, paid speaker, and royalties from Arthrex, outside the submitted work. He also reports a pending patent for a biologic interpositional graft. K.B. reports royalties from Arthrex and DePuy Mitek and consultant for Embody, outside the submitted work. He also reports a pending patent for a biologic interpositional graft. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
This is a patient with an irreparable rotator cuff tear with little-to-no arthritis. We begin from a posterior portal demonstrating the tear. This is followed by our technique for performing an arthroscopic biologic interpositional tuberosity graft for treatment of irreparable rotator cuff tears. Completed graft and gentle range of motion are demonstrated as well.
References
- 1.Rockwood C.A., Williams G.R., Burkhead W.Z. Debridement of degenerative, irreparable lesions of the rotator cuff. J Bone Joint Surg Am. 1995;77:857–866. doi: 10.2106/00004623-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Berth A., Neumann W., Awiszus F., Pap G. Massive rotator cuff tears: Functional outcome after debridement or arthroscopic partial repair. J Orthop. 2010;11:13–20. doi: 10.1007/s10195-010-0084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carver TJ, Kraeutler MJ, Smith JR, Bravman JT, McCarty EC. Nonarthroplasty surgical treatment options for massive, irreparable rotator cuff tears. Orthop J Sport Med 6:2325967118805385. [DOI] [PMC free article] [PubMed]
- 4.Curtis D.M., Lee C.S., Qin C. Superior capsule reconstruction with subacromial allograft spacer: Biomechanical cadaveric study of subacromial contact pressure and superior humeral head translation. Arthroscopy. 2020;36:680–686. doi: 10.1016/j.arthro.2019.09.047. [DOI] [PubMed] [Google Scholar]
- 5.Lacheta L., Horan M.P., Schairer W.W. Clinical and imaging outcomes after arthroscopic superior capsule reconstruction with human dermal allograft for irreparable posterosuperior rotator cuff tears: A minimum 2-year follow-up. Arthroscopy. 2020;36:1011–1019. doi: 10.1016/j.arthro.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Mihata T., Lee T.Q., Watanabe C. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29:459–470. doi: 10.1016/j.arthro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Leschinger T., Besch K., Aydin C. Irreparable rotator cuff tears: A biomechanical comparison of superior capsuloligamentous complex reconstruction techniques and an interpositional graft technique. Orthop J Sports Med. 2019;7 doi: 10.1177/2325967119864590. 2325967119864590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makovicka J.L., Patel K.A., Tokish J.M. Superior capsular reconstruction with the addition of an acromial acellular dermal allograft spacer. Arthrosc Tech. 2018;7:e1181–e1190. doi: 10.1016/j.eats.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mihata T., Bui C.N.H., Akeda M. A biomechanical cadaveric study comparing superior capsule reconstruction using fascia lata allograft with human dermal allograft for irreparable rotator cuff tear. J Shoulder Elbow Surg. 2017;26:2158–2166. doi: 10.1016/j.jse.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Savoie F.H. Editorial commentary: Shoulder superior capsular reconstruction: When a systematic review of a procedure can be misleading. Arthroscopy. 2019;35:1278–1279. doi: 10.1016/j.arthro.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Altintas B., Scibetta A.C., Storaci H.W., Lacheta L., Anderson N.L., Millett P.J. Biomechanical and histopathological analysis of a retrieved dermal allograft after superior capsule reconstruction: A case report. Arthroscopy. 2019;35:2959–2965. doi: 10.1016/j.arthro.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Acevedo D., Sidell M., Otarodifard K. Functional and MRI outcomes of superior capsule reconstruction with acellular dermal matrix. Orthop J Sports Med. 2020;8(7 suppl 6) 2325967120S00333. [Google Scholar]
- 13.LaBelle M.W., Peck M., Mengers S. Evaluating the role of graft integrity on outcomes: Clinical and imaging results following superior capsular reconstruction. J Shoulder Elbow Surg. 2020;29:e140–e141. doi: 10.1016/j.jse.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Denard P.J., Brady P.C., Adams C.R., Tokish J.M., Burkhart S.S. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy. 2018;34:93–99. doi: 10.1016/j.arthro.2017.08.265. [DOI] [PubMed] [Google Scholar]
- 15.Mirzayan R., Stone M.A., Batech M., Acevedo D.C., Singh A. Failed dermal allograft procedures for irreparable rotator cuff tears can still improve pain and function: The “biologic tuberoplasty effect.”. Orthop J Sport Med. 2019;7:1–7. doi: 10.1177/2325967119863432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tokish J.M., Makovicka J.L. The superior capsular reconstruction: Lessons learned and future directions. J Am Acad Orthop Surg. 2020;28:528–537. doi: 10.5435/JAAOS-D-19-00057. [DOI] [PubMed] [Google Scholar]
- 17.Burkhart S.S., Hartzler R.U. Superior capsular reconstruction reverses profound pseudoparalysis in patients with irreparable rotator cuff tears and minimal or no glenohumeral arthritis. Arthroscopy. 2019;35:22–28. doi: 10.1016/j.arthro.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Campbell A.L., Baron S.L., Pham H., Gyftopoulos S., Meislin R., Samim M. MRI of superior capsular reconstruction graft and associated short-term clinical outcomes in patients with massive irreparable rotator cuff tears. Clin Imaging. 2021;70:74–80. doi: 10.1016/j.clinimag.2020.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Lee S.-J., Kang S.-W., Chung I., Jang H. Which factors influence clinical outcomes after superior capsular reconstruction surgery? Orthop J Sport Med. 2020;8 doi: 10.1177/2325967120966410. 232596712096641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horneff J.G., Abboud J.A. Balloon interspace arthroplasty for irreparable rotator cuff tears. Oper Tech Orthop. 2018;28:232–237. [Google Scholar]
- 21.Lobao M.H., Canham R.B., Melvani R.T., Abboud J.A., Parks B.G., Murthi A.M. Biomechanics of biodegradable subacromial balloon spacer for irreparable superior rotator cuff tears: Study of a cadaveric model. J Bone Joint Surg Am. 2019;101:e49. doi: 10.2106/JBJS.18.00850. [DOI] [PubMed] [Google Scholar]
- 22.Verma N, Srikumaran U, Roden C, et al. Balloon Subacromial spacer vs. partial repair for massive rotator cuff tears: A randomized trial. In: AANA20. Symposium conducted at the meeting of Arthroscopy Association of North America virtually.
- 23.Stewart R.K., Kaplin L., Parada S.A., Graves B.R., Verma N.N., Waterman B.R. Outcomes of subacromial balloon spacer implantation for massive and irreparable rotator cuff tears: A systematic review. Orthop J Sport Med. 2019;7 doi: 10.1177/2325967119875717. 2325967119875717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karzel R.P. MicroAire SASI Rotator Cuff Study. Southern California Orthopedic Institute. https://info.microaire.com/rotator-cuff-study Published 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This is a patient with an irreparable rotator cuff tear with little-to-no arthritis. We begin from a posterior portal demonstrating the tear. This is followed by our technique for performing an arthroscopic biologic interpositional tuberosity graft for treatment of irreparable rotator cuff tears. Completed graft and gentle range of motion are demonstrated as well.
This is a patient with an irreparable rotator cuff tear with little-to-no arthritis. We begin from a posterior portal demonstrating the tear. This is followed by our technique for performing an arthroscopic biologic interpositional tuberosity graft for treatment of irreparable rotator cuff tears. Completed graft and gentle range of motion are demonstrated as well.