Abstract
Background
Global variation in susceptibility to diabetes, insulin sensitivity, and regimen intensity poses a challenge for clinicians regarding the optimal choice of insulin therapy. The current study was carried out to see the relative safety and efficacy of currently available long-acting insulins among the type 2 diabetic Asian population.
Methods
A systematic literature search was done using various search engines (PubMed, Cochrane, Google Scholar, Scopus, and Embase) and included published randomized controlled trials (RCTs) in English before December 2019. Further, a manual search was performed by screening the reference list of the identified articles.
Results
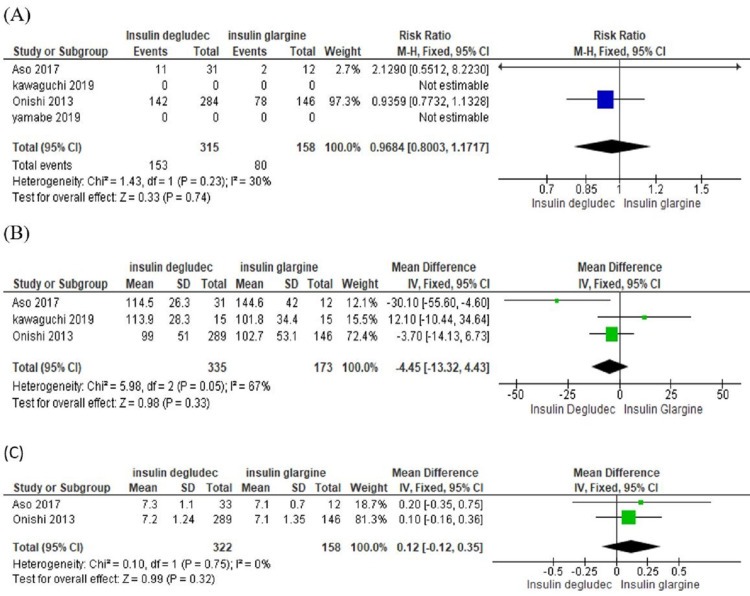
We included four RCTs with 534 participants (349 in the insulin degludec group and 185 in the insulin glargine group) with type 2 diabetes mellitus (T2DM). Results show that both insulin glargine and degludec are equally efficacious in reducing fasting blood glucose (mean difference is -4.45, confidence interval -13.32- 4.43, I2=67%) and HbA1c (glycosylated hemoglobin) (mean difference is 0.12, confidence interval -0.12-0.35, I2=0%). However, insulin glargine was associated with lower risks of hypoglycemia (risk ratio = 0.9684, confidence interval- 0.8003- 1.1717, I2=30%).
Conclusion
Insulin glargine and degludec are comparable in achieving glycemic control with fewer hypoglycemic episodes in the insulin glargine-treated group.
Keywords: glycemic control, hypoglycemia, insulin degludec, insulin glargine, long-acting insulin, type 2 diabetes
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic non-communicable disease characterized by progressive B- cell dysfunction [1]. It occurs due to hyperglycemia and insulin resistance leading to increased insulin demands of tissue that culminates into over-functioning of B-cells and ultimately leading to B-cell failure [2].
According to International Diabetes Federation's IDF Diabetes Atlas 2019, globally, 463 million people live with diabetes mellitus, and out of that, 88 million are from the South East Asia region (SEAR) only. By 2045, it is expected to increase to 51% globally and 74% in SEAR [3]. Race and ethnic differences variate the susceptibility to diabetes, insulin sensitivity, and regimen intensity which poses a challenge regarding the optimal choice of second-line therapy for clinicians [4-6]. The Asian population is at higher risk of developing type 2 diabetes mellitus (DM) than the European population and develops diabetes at a lower BMI [7-9]. In the South-East Asian diabetic population, 90% of the population has T2DM, which is preventable [10].
Glycemic control is the only way to reduce microvascular and macrovascular complications of DM, which can be attained by effective pharmacotherapeutic agents [11]. Currently, available treatment options include oral antidiabetic drugs and insulin. Oral antidiabetic drugs are often limited in their efficacy to reduce HbA1c beyond 1-2% [12]. Insulin is the only drug that can reduce HbA1c to exceptionally lower levels and maintain it near normal. [13]. Newer long-acting insulins are more stable and efficacious than conventional insulin [14].
There are conflicting results in earlier studies like a meta-analysis by Su W et al., which found that glargine was superior to degludec [15]. Contrary to this, a meta-analysis by Zhou W et al. found a degludec much better than glargine [16]. In view of the conflicting data regarding safety and efficacy, it is required to compare and prioritize the treatment so far as long-acting insulin use is concerned. It was altogether more important to undertake studies in the Asian population as earlier meta-analyses hardly included this population. This study aimed to evaluate the safety and efficacy of currently available long-acting insulin (insulin degludec and glargine) in T2DM Asian patients.
Materials and methods
Data sources with search strategy
A systematic literature search was done as per the PRISMA protocol (preferred reporting items for systematic reviews and meta-analysis) [17] (Figure 1) and PICO (participant, intervention, comparison, and outcome) format.
Figure 1. PRISMA flow chart.
PRISMA: preferred reporting items for systematic reviews and meta-analysis
Our search strategy included all relevant published randomized controlled trials (RCTs) before December 2019 from PubMed, Cochrane, Google Scholar, and Embase. Articles in the English language and having an Asian population were included. Keywords that were used for the search were (“insulin Degludec” OR “degludec” OR “IDeg”) AND (“insulin glargine” OR “insulin” “glargine” OR “IGlar”) AND (“randomized” OR “randomly”) AND (“diabetes” OR “diabetes mellitus” OR "type 2 diabetes mellitus, OR “type 2 diabetes”) AND (“Asians”). Further, a manual search was performed by screening the reference list of the identified articles.
Inclusion criteria
1) Patient population: All studies enrolling Asian type 2 diabetes patients
2) Intervention: insulin degludec
3) Comparison: insulin glargine
4) Study design: RCTs (parallel-group and cross-over study designs)
5) Outcomes: Safety of insulins was assessed with a risk of hypoglycemia (episodes >1) of documented hypoglycemia (<70 mg/dl) and efficacy was assessed by improvement or change in the HbA1c or fasting plasma glucose
Exclusion criteria
1) Articles on animal studies and studies conducted outside Asia
2) Review articles, observational, quasi-experimental studies, and case series
3) Studies with less than 12 weeks duration of intervention
Data extraction
Two reviewers (PY and MG) screened the studies for eligibility and data extraction independently. Any disagreements were resolved by discussion with a third reviewer (RK). The data were extracted from eligible studies without modification of original data onto pre-existing data collection forms: study location, year of publication, sample size, types of sample selection and allocation to two groups (intervention and comparator or control group), methods, types of administration of intervention and control, and primary outcomes. Authors of selected articles were contacted for the required data for analysis. The search file was imported into Zotero library (Center for History and New Media, George Mason University, Fairfax County, USA). After removing duplicate items, to exclude studies, we used Rayyan (Qatar Computer Research Institute, Doha, Qatar) [18].
Evaluation of the risk of bias (quality) assessment
The risk of bias has been evaluated with the Cochrane Risk of Bias Assessment Tool. Two reviewers (PY and MG) assessed the risk of bias independently, and discrepancy at any point was also discussed with the third reviewer (YB) also. The risk of bias assessment has been calculated undertaking blinding (masking) of participants, researcher and outcomes, allocation concealment, randomization details, data outcome, and data reporting [19]. Quality assessments have been done with GRADEpro GDT software (McMaster University and Evidence Prime, Inc., Hamilton, Canada). Relevant file has been imported from RevMan software (Cochrane Training, London, UK) to the "Summary of Findings" table GRADE Profiler to create a "Summary of Findings" table [20]. The summary of the intervention effect and a measure of the quality of evidence was noted in the table.
Data analysis
Four RCTs that suit the eligibility criteria were enrolled [21-24]. Onishi et al. considered the confirmed hypoglycemia as plasma glucose level <3.1 mmol/L or 55.86 mg/dl and found lower hypoglycemia rates in degludec treated patients as opposed to the glargine group [23]. These findings are reinforced by Aso Y et al., with a similar improvement in glycemic control in both groups but a low risk of hypoglycemia with degludec, considering plasma glucose level as <70 mg/dl for confirmed hypoglycemia [21]. In contrast, Kawaguchi Y et al. [22] and Yamabe M et al. [24] considered the confirmed hypoglycemia as plasma glucose level <70 mg/dl and observed that insulin glargine and insulin degludec are equivalent long-acting insulin analogs and insulin glargine had lesser episodes of hypoglycemia. They noted the mean percentage of time in the target glucose range (70-179 mg/dL) and considered hypoglycemia as <70 mg/dL, with flash glucose monitoring weekly. Authors of two studies, Kawaguchi Y et al, 2019 [22] and Yamabe M et al. 2019 [24], have been contacted for the number of events with hypoglycemia episodes and post-intervention data for change in HbA1c (mean and standard deviation) for the relevant data required in the meta-analysis, but the unavailability of data reinforces the decision to exclude these studies from the final analysis.
Relevant findings were tabulated and displayed based on the forest plots. The I2 statistic was used to test statistical heterogeneity, which may arise due to inter-trial variability, with >75% values representing important heterogeneity with a fixed-effects model (95% confidence interval, p-value < 0.05). For calculating efficacy, the mean difference between the insulin degludec and glargine group was calculated to show differential mean changes in HbA1c and fasting plasma glucose (FPG) levels. For calculating safety between insulin degludec and glargine group, the risk ratio (RR) was calculated. We have not observed significant heterogeneity for hypoglycemia and glycemic control incidence. Sensitivity analysis was performed to control fasting plasma glucose, although we observed similar heterogeneity with a random-effect model. Subgroup analysis could not be possible due to the limited number of studies.
Results
The Meta-analysis was performed using the RevMan version 5.4 software. Finally, four RCTs with a total of 534 participants (349 in the insulin degludec group and 185 in the insulin glargine group) with T2DM were eligible for inclusion in this meta-analysis. Characteristics of participants in each trial are described in Table 1.
Table 1. Characteristics of participants in each trial.
| Author and year of the study | Setting | Population | Number of Participants | Duration of Trial | Mean Age of Participants | Duration of Diabetes | Gender Distribution |
| Aso Y et al., 2017 [21] | Single-Center Study | Japan | 45 participants [33 in insulin degludec group and 12 in glargine group] | 24 weeks | 64.0±13.6 in insulin degludec and 64.7±15.7 in insulin glargine | 10 years in degludec and 13 years in insulin glargine | Female participants 55% |
| Kawaguchi Yet al., 2019 [22] | Single-Center Study | Japan | 30 patients [15 patients in each group] | 6 months | 69.5 ± 11.3 years | 18.3 ± 11.3 years | Male participants (60%) |
| Onishi Y et al., 2013 [23] | A Multi-center Study | Hong Kong, Japan, Malaysia, South Korea, Taiwan, and Thailand | 435 participants [289 in insulin degludec group and 146 in glargine group] | 26 weeks | 58.6 years | 11.6 Years | Male participants (53.56%) |
| Yamabe M et al., 2019 [24] | Single-Center Study | Japan | 24 participants [12 in each group] | 5 months | 70.7 ± 7.6 (years) | 14.0 ± 9.3 (years) | Male (50%) |
The summary of findings with the grade of evidence are shown in Table 2. This table has displayed the certainty of evidence and grade as high, moderate, and low for each outcome variable.
Table 2. GRADEpro summary of findings.
aheterogeneity; blimited studies; cwide confidence interval; *significant effect; ⨁◯ symbols indicating certainty of evidence
CI: confidence interval; MD: mean difference; HbA1C: glycosylated hemoglobin; RR: risk ratio
| Patient or population: Type 2 diabetic patients | |||||
| Setting: Asian population | |||||
| Intervention: Insulin degludec | |||||
| Comparison: insulin glargine | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | The certainty of the evidence (GRADE) | |
| The risk with insulin glargine | The risk with Insulin degludec | ||||
| Fasting blood sugar | The mean fasting blood sugar was 0 | MD 4.45 lower (13.32 lower to 4.43 higher) | - | 508 (3 studies) | ⨁⨁⨁◯ MODERATE a, b |
| Decrease in HbA1c | The mean decrease in HbA1c was 0 | MD 0.12 higher (0.12 lower to 0.35 higher) | - | 480 (2 studies | ⨁⨁⨁◯ MODERATE b |
| Risk of hypoglycemia | 506 per 1,000 | 491 per 1,000 (405 to 592) | RR 0.97 (0.80 to 1.17) | 473 (2 studies) | ⨁⨁◯◯ LOW a, b, c |
Figure 2A shows the forest plot for hypoglycemia risk with RR = 0.9684, suggesting that hypoglycemia is less with glargine, CI of RR (0.8003- 1.1717) with I2=30%, which shows mild heterogeneity among studies.
Figure 2. Forest plot shows the comparison of insulin degludec and glargine .
A) Incidence of hypoglycemia; B) Control of fasting plasma glucose; C) Decrease in HbA1c
CI: Confidence Interval; SD: Standard Deviation [19]
Figure 2B shows the forest plot which states the no statistically significant difference in fasting plasma glucose between insulin degludec and glargine [mean difference is -4.45, confidence interval -13.32- 4.43] with I2=67%, p-value=0.02, which shows heterogeneity among studies.
Figure 2C shows the forest plot which states no statistically significant difference in HbA1c between insulin degludec and insulin glargine [mean difference is 0.12, confidence interval -0.12-0.35], I2=0%.
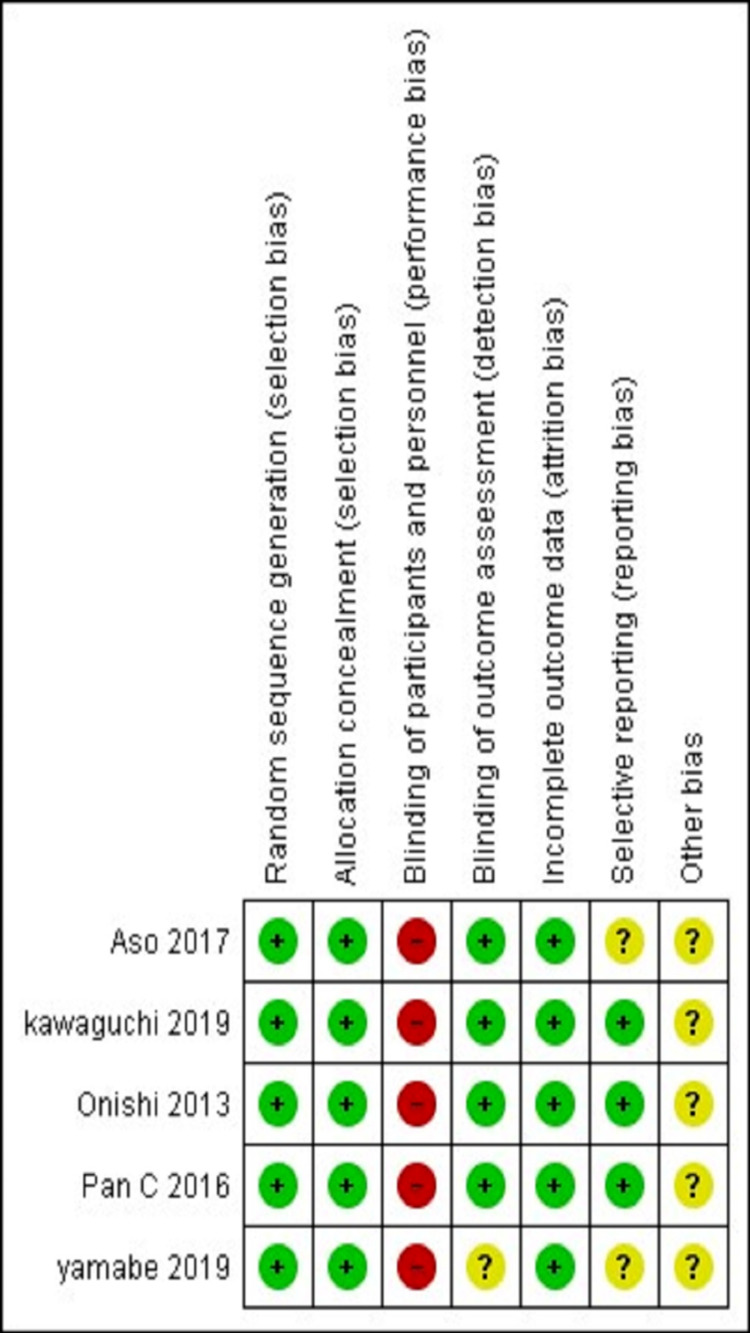
The risk of bias graph and summary have been displayed in Figure 3 and Figure 4, respectively, which included random sequence generation, allocation concealment, detection bias, attrition bias, reporting bias, and others in each study.
Figure 3. Risk of bias graph.
Review authors' judgements about each risk of bias item presented as percentages across all included studies [19]
Figure 4. Risk of bias summary.
Review authors' judgments about each risk of bias item for each included study [19]
Funnel plots have not been created due to the limited number of studies in the analysis.
Discussion
Optimum glycemic control in T2DM patients represents a challenge even with advanced pharmacologic treatment [11]. Evidence suggested that insulin therapy has been convincingly superior in attaining target glycemic control over oral anti-diabetic drugs [12]. However, its use is accompanied by hypoglycemia episodes; long-acting insulin therapy is a safe and effective way of treating diabetes mellitus [13].
In the present meta-analysis, the efficacy and safety of currently available long-acting insulin (insulin degludec and glargine) were compared in type 2 diabetes mellitus Asian patients. A total of four high-quality RCTs were included to evaluate outcome measures. In the present study, both insulins effectively reduced fasting plasma glucose and HbA1c.
We have noted no statistically significant mean difference in fasting plasma glucose (mean difference is -4.45, confidence interval -13.32- 4.43) and HbA1c reduction (mean difference is 0.12, confidence interval -0.12-0.35) between both groups. For comparing safety between the groups, RR = 0.9684, suggestive that risk of hypoglycemia is less with glargine, CI of RR (0.8003- 1.1717) with I2=30%. A meta-analysis by Heller S et al. showed a lower rate of hypoglycemia in the degludec group [25]. Findings of this study are supported by Onishi et al. and Pan C et al. both these investigators considered the confirmed hypoglycemia as plasma glucose level <3.1 mmol/L or 55.86 mg/dl and did find lower events of hypoglycemia in degludec treated patients as opposed to glargine group [23, 26]. These findings are reinforced by Aso Y et al. also with a similar improvement in glycemic control with both insulins but a lower risk of hypoglycemia with degludec, considering higher plasma glucose level (<70 mg/dl) for confirmed hypoglycemia [21]. Kawaguchi Y et al. [22] and Yamabe M et al. [24] also considered the confirmed hypoglycemia as plasma glucose level <70 mg/dl and observed that insulin glargine and insulin degludec are equivalent long-acting insulin analogs and insulin glargine has lesser episodes of hypoglycemia. A meta-analysis by Ratner RE et al. also concluded that similar improvements in glycemic control could be achieved along with fewer hypoglycemic episodes with insulin glargine [27]. The findings of Ratner RE et al. and Rodbard HW et al. were conflicting, as later concluded that insulin degludec had fewer hypoglycemic events than insulin glargine [28], although clinical efficacy was similar. Even Russell-Jones D et al. reported a higher clinical safety and efficacy of insulin degludec than insulin glargine among patients with T2DM [29]. A randomized controlled trial among T2DM patients also concluded that insulin degludec improved glycemic control similarly to insulin glargine with a lower risk of hypoglycemia [30].
It is apparent from the studies that most researchers found a lower hypoglycaemic profile in patients treated with insulin degludec. These results could be partially explained by the cut-off value of plasma glucose to define hypoglycemia as a value of 55.86 mg/dl or less than the plasma sugar of 70 mg/dl is expected to exclude a fair number of patients with hypoglycemia. Our findings are contrary to most available literature, as, in the current meta-analysis, we found that patients with insulin glargine were associated with lower rates of hypoglycemia. However, both insulins had equal propensity to achieve short-term and long-term glycemic goals.
This meta-analysis is novel work as there were only a few studies conducted in the Asian population to compare the safety and efficacy of currently available long-acting insulin (insulin degludec and glargine) in type 2 diabetes mellitus patients.
Limitations
Studies included in this meta-analysis were open-label design, and results represent heterogeneity.
Conclusions
This meta-analysis concluded similar efficacy of currently available long-acting insulin (insulin degludec and glargine) in T2DM patients in the Asian region, with lower hypoglycemia episodes with insulin glargine. It also found a limited number of trials in the Asian population, suggesting the need for future research for comparing the relative safety and efficacy of currently available insulins.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 2.Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Unger RH, Grundy S. https://pubmed.ncbi.nlm.nih.gov/3888754/ Diabetologia. 1985;28:119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- 3.IDF ATLAS 2019 International Diabetes Federation. [Jun;2020 ];IDF ATLAS 2019 International Diabetes Federation. https://www.diabetesatlas.org/en/ 2020
- 4.Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Diabetes Care. 2013;36:1789–1796. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Impact of race/ethnicity on the efficacy and safety of commonly used insulin regimens: a post hoc analysis of clinical trials in type 2 diabetes mellitus. Davidson JA, Lacaya LB, Jiang H, Heilmann CR, Scism-Bacon JL, Gates JR, Jackson JA. Endocr Pract. 2010;16:818–828. doi: 10.4158/EP09285.OR. [DOI] [PubMed] [Google Scholar]
- 6.Treatment of type 2 diabetes mellitus worldwide: baseline patient characteristics in the global DISCOVER study. Gomes MB, Rathmann W, Charbonnel B, et al. Diabetes Res Clin Pract. 2019;151:20–32. doi: 10.1016/j.diabres.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Diabetes in Asia: epidemiology, risk factors, and pathophysiology. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 8.Global obesity: trends, risk factors and policy implications. Malik VS, Willett WC, Hu FB. Nat Rev Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 9.Epidemiology and determinants of type 2 diabetes in South Asia. Hills AP, Arena R, Khunti K, et al. Lancet Diabetes Endocrinol. 2018;6:966–978. doi: 10.1016/S2213-8587(18)30204-3. [DOI] [PubMed] [Google Scholar]
- 10.Addressing Asia's fast growing diabetes epidemic. [Jun;2020 ];https://www.who.int/bulletin/volumes/95/8/17-020817/en/ Bull World Health Organ. 2017 95:550–551. doi: 10.2471/BLT.17.020817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glycemic control in insulin-treated patients with type 2 diabetes: empowerment perceptions and diabetes distress as important determinants. Chen SY, Hsu HC, Wang RH, Lee YJ, Hsieh CH. Biol Res Nurs. 2019;21:182–189. doi: 10.1177/1099800418820170. [DOI] [PubMed] [Google Scholar]
- 12.Beneficial effects of insulin on glycemic control and beta-cell function in newly diagnosed type 2 diabetes with severe hyperglycemia after short-term intensive insulin therapy. Chen HS, Wu TE, Jap TS, Hsiao LC, Lee SH, Lin HD. Diabetes Care. 2008;31:1927–1932. doi: 10.2337/dc08-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hypoglycemia in type 1 diabetes mellitus. Cryer PE. Endocrinol Metab Clin North Am. 2010;39:641–654. doi: 10.1016/j.ecl.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Insulin, medicines, & other diabetes treatments | NIDDK. [Jun;2020 ];https://www.niddk.nih.gov/health-information/diabetes/overview/insulin-medicines-treatments 2020
- 15.Meta-analysis and cost-effectiveness analysis of insulin glargine 100 u/m l versus insulin degludec for the treatment of type 2 diabetes in China. Su W, Li C, Zhang L, Lin Z, Tan J, Xuan J. https://pubmed.ncbi.nlm.nih.gov/31482483. Diabetes Ther. 2019;10:1969–1984. doi: 10.1007/s13300-019-00683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Insulin degludec, a novel ultra-long-acting basal insulin versus insulin glargine for the management of type 2 diabetes: a systematic review and meta-analysis. Zhou W, Tao J, Zhou X, Chen H. Diabetes Ther. 2019;10:835–852. doi: 10.1007/s13300-019-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reprint--preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Moher D, Liberati A, Tetzlaff J, Altman DG. Phys Ther. 2009;89:873–880. [PubMed] [Google Scholar]
- 18.Rayyan-a web and mobile app for systematic reviews. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RevMan | Cochrane Training. [Jun;2020 ];RevMan. Review Manager. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman 2020
- 20.GRADEpro GDT: GRADEpro Guideline Development Tool. [Jun;2020 ];https://gradepro.org/ 2020
- 21.Effect of insulin degludec versus insulin glargine on glycemic control and daily fasting blood glucose variability in insulin-naïve Japanese patients with type 2 diabetes: I'D GOT trial. Aso Y, Suzuki K, Chiba Y, et al. Diabetes Res Clin Pract. 2017;130:237–243. doi: 10.1016/j.diabres.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Efficacy and safety of insulin glargine 300 U/mL vs insulin degludec in patients with type 2 diabetes: a randomized, open-label, cross-over study using continuous glucose monitoring profiles. Kawaguchi Y, Sawa J, Sakuma N, Kumeda Y. J Diabetes Investig. 2019;10:343–351. doi: 10.1111/jdi.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Insulin degludec compared with insulin glargine in insulin-naïve patients with type 2 diabetes: a 26-week, randomized, controlled, pan-Asian, treat-to-target trial. Onishi Y, Iwamoto Y, Yoo SJ, Clauson P, Tamer SC, Park S. J Diabetes Investig. 2013;4:605–612. doi: 10.1111/jdi.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comparison of insulin glargine 300 U/mL and insulin degludec using flash glucose monitoring: a randomized cross-over study. Yamabe M, Kuroda M, Hirosawa Y, Kamino H, Ohno H, Yoneda M. J Diabetes Investig. 2019;10:352–357. doi: 10.1111/jdi.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A meta-analysis of rate ratios for nocturnal confirmed hypoglycaemia with insulin degludec vs. insulin glargine using different definitions for hypoglycaemia. Heller S, Mathieu C, Kapur R, Wolden ML, Zinman B. Diabet Med. 2016;33:478–487. doi: 10.1111/dme.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A multinational, randomized, open-label, treat-to-target trial comparing insulin degludec and insulin glargine in insulin-naïve patients with type 2 diabetes mellitus. Pan C, Gross JL, Yang W, et al. Drugs R D. 2016;16:239–249. doi: 10.1007/s40268-016-0134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Ratner RE, Gough SC, Mathieu C, et al. Diabetes Obes Metab. 2013;15:175–184. doi: 10.1111/dom.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reduced risk of hypoglycemia with insulin degludec versus insulin glargine in patients with type 2 diabetes requiring high doses of basal insulin: a meta-analysis of 5 randomized begin trials. Rodbard HW, Gough S, Lane W, Korsholm L, Bretler DM, Handelsman Y. Endocr Pract. 2014;20:285–292. doi: 10.4158/EP13287.OR. [DOI] [PubMed] [Google Scholar]
- 29.Insulin degludec results in lower rates of nocturnal hypoglycaemia and fasting plasma glucose vs. insulin glargine: a meta-analysis of seven clinical trials. Russell-Jones D, Gall MA, Niemeyer M, Diamant M, Del Prato S. Nutr Metab Cardiovasc Dis. 2015;25:898–905. doi: 10.1016/j.numecd.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Low-volume insulin degludec 200 units/ml once daily improves glycemic control similarly to insulin glargine with a low risk of hypoglycemia in insulin-naive patients with type 2 diabetes: a 26-week, randomized, controlled, multinational, treat-to-target trial: the BEGIN LOW VOLUME trial. Gough SC, Bhargava A, Jain R, Mersebach H, Rasmussen S, Bergenstal RM. Diabetes Care. 2013;36:2536–2542. doi: 10.2337/dc12-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]