Abstract
Purpose
The purpose of this study was to investigate how axial length (AL) changes the relationship of intraocular pressure (IOP) with peripapillary vessel density (pVD) in glaucoma versus non-glaucomatous eyes.
Methods
A population-based, cross-sectional study of 2127 African Americans aged 40 years and older in Inglewood, California, were imaged with 6 × 6-mm optic disc optical coherence tomography angiography scans. There were 1028 healthy subjects (1539 eyes) and 65 subjects with glaucoma (86 eyes) who met inclusion criteria. A multivariable linear mixed effects regression model investigated the relationship of IOP on pVD after controlling for signal strength, retinal nerve fiber layer thickness, and age. These results were stratified by AL groups.
Results
Higher IOP was a significant predictor of lower pVD among subjects with glaucoma (P = 0.009), but not among healthy subjects (P = 0.26). After stratifying by the sample median AL (23.46 mm), higher IOP was associated with lower pVD among subjects with glaucoma with longer AL (≥ 23.46 mm, P = 0.005), but not among those in the shorter AL (< 23.46 mm, P = 0.45). IOP was not significantly associated with pVD among healthy subjects in either AL stratum.
Conclusions
Among subjects with glaucoma with longer AL, IOP was significantly associated with pVD. This relationship was not seen among subjects with glaucoma with shorter AL or non-glaucomatous subjects in either AL group. These findings support the hypothesis that disturbed retinal autoregulation may be present in subjects with glaucoma with longer AL. Longitudinal studies are needed to further investigate whether axial elongation increases glaucoma risk by compromising retinal autoregulation.
Keywords: autoregulation, optical coherence tomography, glaucoma posterior segment, glaucoma
The pathophysiology of glaucoma remains poorly understood, and both mechanical and vascular factors may be involved. Mechanical stress and strain caused by elevated intraocular pressure (IOP) may compress retinal ganglion cell axons at the lamina cribrosa, disrupting axonal transport, and causing apoptosis.1 However, elevated IOP is not necessary to produce glaucomatous damage, and, in fact, over half of those diagnosed with glaucoma have baseline IOP in the “normal range”2; indicating other factors contribute to glaucoma pathogenesis.2,3 Various imaging techniques have demonstrated an association between glaucoma and reduced ocular blood flow,4,5 but whether this is a causal association or a result of existing damage is not clear. Vascular dysregulation at the optic nerve head (ONH) leading to reduced ocular perfusion pressure (OPP) may be a primary cause of glaucoma in at least some cases.3 Changes in IOP itself, while contributing to mechanical stress at the ONH, may also play a role in open angle glaucoma (OAG) pathogenesis by its effect on manipulating OPP. Specifically, glaucomatous damage may result from repeated reperfusion injury and increased oxidative stress due to instability of blood flow, caused by disturbed retinal autoregulation.6 Previously, it has been shown that in normal eyes, peripapillary vessel density (pVD) as measured by optical coherence tomography angiography (OCTA) is not correlated with IOP,7 and does not change during conditions of acutely elevated IOP,8 demonstrating intact autoregulation. In contrast, glaucomatous eyes, in at least some cases, are thought to have abnormal autoregulation making them more susceptible to changes in ocular perfusion with IOP changes. However, it remains poorly understood which factors may contribute to this disturbed autoregulation in glaucomatous eyes.
Myopia is an additional well-known risk factor for glaucoma, and, long axial length (AL) is also independently associated with an increased risk of normotensive primary OAG.9 One hypothesis for this is that the retinal ganglion axons in longer eyes might be more susceptible to mechanical damage from elevated IOP due to reduced support from a thinner sclera and laminar cribrosa.10 Additionally, it is possible that in longer eyes the mechanical support for the microvasculature of the ONH and peripapillary retina is compromised. In individuals with myopia, it has been previously shown that there is reduced choroidal and retinal circulation11,12 and that the vessels here may be on stretch as demonstrated by OCTA.13 Because vascular compromise may precede the development of OAG,14 and longer AL is associated with an increased risk of both normotensive and hypertensive OAG,15 we sought to examine how the known relationship between IOP and pVD changes in longer versus shorter AL in African American subjects with and without glaucoma.
In this study, we examine the relationship of IOP with pVD, with a specific focus on how AL influences that relationship in non-glaucomatous versus glaucomatous eyes. We hypothesized that AL would modify the relationship of IOP and pVD in glaucomatous eyes and that patients with relative axial elongation would be more prone to vascular compromise with high IOP. We suspected that non-glaucomatous eyes would be less susceptible to vascular compromise with higher IOP, even in the setting of axial elongation, presumably due to better retinal autoregulation.
Methods
Study Population
This study is a population-based, cross-sectional study that included self-identified African Americans aged 40 years and older residing within 30 census tracts in an area around Inglewood, California. Written informed consent was obtained from all subjects. This study is compliant with the Health Insurance Portability and Accountability Act of 1996, and is adherent to the tenets of the Declaration of Helsinki. The institutional review board of the University of Southern California Health Sciences approved this study.
Clinical Assessment
Characteristics of this dataset are also described elsewhere as part of the African American Eye Disease Study.7,16 In brief, participants visited a local ophthalmology suite for a comprehensive evaluation of both systemic and ocular variables. Clinical evaluation was comprised of an ocular clinical examination, and clinical questionnaire to ascertain medical disease history. For each subject, an ocular clinical examination was done by a comprehensive ophthalmologist and included anterior and posterior segment evaluation as well as relevant studies for the diagnosis of glaucoma including OCT (Cirrus 5000 HD-OCT; Carl Zeiss Meditec, Dublin, CA, USA), and visual field (Humphrey Field Analyzer II Swedish Interactive Threshold Algorithm 24-2; Carl Zeiss Meditec, Dublin, CA, USA). IOP was measured three times prior to pupillary dilation with Goldmann applanation tonometry and averaged. The diagnosis of glaucoma was based on assessment of visual field data, and clinical evaluation of the optic disc demonstrating an optic nerve rim defect (such as notching or localized thinning) characteristic of glaucoma. For each subject, 6 × 6 mm OCTA (Cirrus 5000 HD-OCT with Angioplex; Carl Zeiss Meditec) images centered on the optic disc were captured, and automated segmentation software (CIRRUS 11.0; Zeiss) was used to detect boundaries of the retinal layers to create a two-dimensional en face image of the perfused radial peripapillary capillaries (RPCs). For each OCTA image, the unitless signal strength (SS) value was recorded. The RPC layer was defined as the segment between the superficial portion of the inner limiting membrane to the posterior surface of the retinal nerve fiber layer (RNFL). En face images were processed by custom software that allowed for manual input in order to quantify vessel area density by a method previously described.17 The software utilized a method that combined a global threshold, Hessian filter, and adaptive threshold to generate binary pixel vessel maps that yield quantitative indices of perfused microvasculature in MATLAB (R2017a; MathWorks, Inc., Natick, MA, USA). The ONH was excluded from quantification, as well as vessels greater than 32 µm in diameter. To obtain the value of pVD, the unitless ratio of the total amount of area of vessels (codified by white pixels) in the binary vessel map was divided by the total amount of pixels in the binary vessel map of interest.17 AL was obtained by an average of three separate A-scan ultrasound measurements (4000B A-Scan/Pachymeter; DGH Technology, Inc., Exton, PA, USA). Subjects were excluded from the study if they had vision-threatening diabetic retinopathy (including diabetic macular edema, severe nonproliferative diabetic retinopathy, or proliferative diabetic retinopathy), OCTA imaging with signal strength (SS) less than 7 out of 10, or poor OCTA image quality such as motion artifacts, media opacities (i.e. vitreous floaters), or decentration.7,18
Statistical Analysis
Linear mixed effects models were used to perform univariable and multivariable analyses to examine the association of age, SS, AL, IOP, and RNFL with pVD while controlling for inter-eye correlation.19 We focused specifically on the influence of age, AL, IOP, RNFL, and SS on pVD because these variables were previously identified to significantly impact pVD.7,18 We divided subjects into shorter and longer axial length groups based upon the median axial length (23.46 mm) of all study participants. Locally weighted scatterplot smoothing (LOESS) plots were used to examine the relationship between pVD and IOP in each AL group. Categorical variables were analyzed with χ2 tests. All hypothesis tests used an alpha of 0.05. All statistical analysis was conducted in RStudio (version 1.2.1335).
Results
We examined a total of 1093 subjects (1028 non-glaucoma subjects, and 65 subjects with glaucoma) with measurements on a total of 1539 eyes (1453 eyes without glaucoma, 86 eyes with glaucoma). Subjects were excluded for SS less than 7 out of 10 (294 eyes), or presence of other sight threatening ocular diseases (17 eyes).
Baseline characteristics of the systemic and ocular variables in the study are included in Table 1. Compared to subjects without glaucoma, subjects with glaucoma tended to be of greater age (66 ± 9.1 vs. 57.4 ± 9.7, P < 0.001), have higher IOP (17.8 ± 5.8 vs. 15.2 ± 3.0, P < 0.001), lower RNFL (73.9 ± 14.6 vs. 92.3 ± 10.9, P < 0.001), and lower pVD (0.263 ± 0.07 vs. 0.349 ± 0.04, P < 0.001). There was no difference in AL between subjects with and without glaucoma (23.6 ± 1 vs. 23.7 ± 1, P = 0.056). Signal strength was statistically different between glaucoma and non-glaucoma subjects (8.756 ± 0.88 vs. 9.323 ± 0.82, P < 0.001).
Table 1.
Baseline Systemic and Ocular Characteristics Between Non-Glaucoma and Subjects With Glaucoma
| Variables | Non-Glaucoma | Glaucoma | P Value |
|---|---|---|---|
| Total subjects (N) | 1028 | 65 | – |
| Total eyes (N) | 1453 | 86 | – |
| Female gender * | 660 (64%) | 42 (65%) | 0.79 |
| Age (years) | 57.4 ± 9.7 (40–91) | 66 ± 9.1 (45–90) | <0.001 |
| IOP (mm Hg) | 15.2 ± 3.0 (7–28) | 17.8 ± 5.8 (8–35) | <0.001 |
| AL (mm) | 23.6 ± 1 (20.6–27.5) | 23.7 ± 1 (22–27.7) | 0.056 |
| RNFL (microns) | 92.3 ± 10.9 (52–138) | 73.9 ± 14.6 (48–109) | <0.001 |
| pVD | 0.349 ± 0.04 (0.179–0.451) | 0.263 ± 0.07 (0.113–0.413) | <0.001 |
| SS | 9.323 ± 0.82 (7–10) | 8.756 ± 0.88 (7–10) | <0.001 |
All variables denoted as mean ± standard deviation except for gender* which is expressed as frequency (percent). The P-value for gender determined by χ2 test. All other P values were generated from mixed linear effects model to control for inter-eye correlation. Significance level was 0.05. IOP = intraocular pressure; AL = axial length; RNFL = retinal nerve fiber layer thickness; pVD = peripapillary vessel density; SS = signal strength (on a scale of 1–10).
As demonstrated in Table 2, significant univariable determinants of pVD among non-glaucoma subjects were age (β = −0.00165 per year, P < 0.001), AL (β = −0.0133 per mm, P < 0.001), RNFL (β = 0.00254 per µm, P < 0.001), SS (β = 0.0241, P < 0.001), and IOP (β = −0.00086 per mm Hg, P = 0.045). Among subjects with glaucoma, significant univariable determinants of pVD included IOP (β = −0.00458 per mm Hg, P < 0.001), AL (β = −0.0282 per mm, P < 0.001), RNFL (β = 0.00356 per µm, P < 0.001), and SS (β = 0.03202, P < 0.001), but not age (β = −0.00065 per year, P = 0.472).
Table 2.
Univariable Mixed Linear Effects Models of Determinants of Peripapillary Vessel Density (pVD) in Non-Glaucoma and Subjects With Glaucoma
| Non-Glaucoma | Glaucoma | |||||
|---|---|---|---|---|---|---|
| Variable | β | CI | P Value | β | CI | P Value |
| Age (years) | −0.00165 | (−0.00189 to −0.00140) | <0.001 | −0.00065 | (−0.00240 to 0.00111) | 0.472 |
| IOP (mm Hg) | −0.00086 | (−0.00170 to −0.00002) | 0.045 | −0.00458 | (−0.00694 to −0.00221) | <0.001 |
| AL (mm) | −0.01334 | (−0.01589 to −0.01079) | <0.001 | −0.02820 | (−0.04242 to −0.01398) | <0.001 |
| RNFL (micron) | 0.00254 | (0.00237 to 0.00271) | <0.001 | 0.00356 | (0.00294 to 0.00418) | <0.001 |
| SS | 0.02416 | (0.02227 to 0.02605) | <0.001 | 0.03202 | (0.01920 to 0.04483) | <0.001 |
Beta-estimate, CI, and P value generated from mixed linear effects model controlling for inter-eye correlation. Significance level was 0.05. CI = confidence interval; IOP = intraocular pressure; AL = axial length; RNFL = retinal nerve fiber layer thickness; pVD = peripapillary vessel density; SS = signal strength (on a scale of 1–10).
We were particularly interested in how mean pVD values may differ depending on IOP, AL, and the interaction between IOP and AL. Our model controlled for the influence of age, RNFL, and SS given that these covariables have previously been shown to be important determinants of pVD.7 Based on another study in our group18 that demonstrated that longer diabetes duration and male gender were associated with reduced pVD among healthy subjects, we also tested the confounding effect of diabetes, duration of diabetes, and gender; however, these variables were removed for model simplification after the presence or absence of these individual variables did not significantly change the model predictions or estimates of our variables of interest. Due to prior evidence of reduced mean central corneal thickness (CCT) in African Americans,20 we evaluated whether CCT was a confounder in our model by evaluating whether adding CCT to our models changed the beta estimates of interest, and we similarly found it was not a confounder. Also of note, measures of blood pressure, including systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure, were not associated with pVD in either the non-glaucoma18 or subjects with glaucoma. As demonstrated in Table 3, among non-glaucoma subjects, after controlling for SS, age, and RNFL, IOP (β = −0.00028 per mm Hg, P = 0.263) was not significantly associated with pVD but AL (β = −0.00443 per mm, P < 0.001) was significantly associated. Conversely, among subjects with glaucoma, after controlling for the same covariables, IOP (β = −0.00178 per mm Hg, P = 0.009) was significantly associated with pVD but AL was not (β = −0.00529 per mm, P = 0.227).
Table 3.
Multivariable Mixed Linear Regression of Determinants of pVD in All Non-Glaucoma and Subjects With Glaucoma
| Non-Glaucoma | Glaucoma | |||||
|---|---|---|---|---|---|---|
| Variable | β | CI | P Value | β | CI | P Value |
| Age (years) | −0.00083 | (−0.00099 to −0.00067) | <0.001 | −0.00088 | (−0.00174 to −0.00001) | 0.052 |
| IOP (mm Hg) | −0.00028 | (−0.00077 to 0.00021) | 0.263 | −0.00178 | (−0.00309 to −0.0004) | 0.009 |
| AL (mm) | −0.00443 | (−0.00604 to −0.00283) | <0.001 | −0.00529 | (−0.01379 to 0.00321) | 0.227 |
| RNFL (micron) | 0.00218 | (0.00205 to 0.00232) | <0.001 | 0.00300 | (0.00248 to 0.00352) | <0.001 |
| SS | 0.02053 | (0.01896 to 0.02209) | <0.001 | 0.02301 | (0.01489 to 0.03113) | <0.001 |
Beta-estimate, CI, and P-value generated from mixed linear effects model controlling for inter-eye correlation. Significance level was 0.05. CI = confidence interval; IOP = intraocular pressure; RNFL = retinal nerve fiber layer thickness; SS = signal strength.
To examine the interactive effect between IOP and AL, we divided our study population (non-glaucoma subjects and subjects with glaucoma) into longer AL and shorter AL groups using the total study population's median AL of 23.46 mm as our cut point. Baseline characteristics of these four groups are listed in Supplementary Table S1. In both the non-glaucoma and subjects with glaucoma, longer AL was associated with decreased RNFL, pVD, and SS (Supplementary Table S1). Among healthy eyes, the difference in average IOP between short (IOP = 15.2 ± 2.9 mm Hg) and long (IOP = 15.2 ± 2.9 mm Hg, P = 0.48) AL groups was not significantly different. Similarly, among glaucomatous eyes, there was no statistically significant difference in mean IOP between short (IOP = 17.2 ± 6.03 mm Hg) and long (IOP =18.4 ± 5.6 mm Hg, P = 0.65) axial length groups. Table 4 illustrates that among non-glaucomatous subjects with longer AL, IOP (β = −0.00023 per mm Hg, P = 0.53) was not a significant predictor of pVD while controlling for differences in age, RNFL, and SS. Similarly, IOP (β = −0.0004 per mm Hg, P = 0.26) was not a significant predictor among non-glaucomatous subjects with shorter AL. The lack of association between IOP and pVD was also present among the 110 non-glaucomatous subjects with IOP ≥ 20 (Range 20-28). IOP was not correlated with pVD in either long or short AL subdivisions of non-glaucoma subjects with higher IOP (Supplementary Table S2). Conversely, among subjects with glaucoma, after controlling for age, RNFL, and SS, IOP was significantly associated with pVD in the longer AL group (β = −0.00235 per mm Hg, P = 0.005), but not in the shorter AL group (IOP; β = −0.00075 per mm Hg, P = 0.455). We also performed a subanalysis restricting to IOP < 25, identifying 71 total eyes among the subjects with glaucoma, and 1376 eyes in the non-glaucoma subjects. We similarly found that the inverse association between higher IOP and lower pVD remained significant in the longer AL glaucoma group of eyes with IOP < 25 (IOP; β = −0.00356 per mm Hg, P = 0.003), but the IOP-pVD association remained non-significant for the shorter AL glaucoma group (P = 0.113) and in both AL-stratified non-glaucoma groups (short AL, P = 0.673 and long AL, P = 0.527).
Table 4.
Multivariable Mixed Linear Regression of Determinants of Peripapillary Vessel Density (pVD) of Short and Long Axial Length in Non-Glaucomatous and Glaucomatous Eyes
| Non-Glaucoma Short AL | Non-Glaucoma Long AL | |||||
|---|---|---|---|---|---|---|
| Variable | β | CI | P Value | β | CI | P Value |
| Age (years) | −0.00073 | (−0.00096 to −0.00051) | <0.001 | −0.00088 | (−0.00110 to −0.00066) | <0.001 |
| IOP (mm Hg) | −0.00040 | (−0.00109 to 0.00029) | 0.257 | −0.00023 | (−0.00092 to 0.00047) | 0.526 |
| RNFL (micron) | 0.00216 | (0.00197 to 0.00234) | <0.001 | 0.00225 | (0.00205 to 0.00245) | <0.001 |
| SS | 0.02209 | (0.01969 to 0.02448) | <0.001 | 0.02014 | (0.01804 to 0.02223) | <0.001 |
| Glaucoma Short AL | Glaucoma Long AL | |||||
| Variable | β | CI | P Value | β | CI | P Value |
| Age (years) | −0.00028 | (−0.00179 to 0.00124) | 0.723 | −0.00123 | (−0.00214 to −0.00033) | 0.011 |
| IOP (mm Hg) | −0.00075 | (−0.00269 to 0.00119) | 0.445 | −0.00235 | (−0.00391 to −0.00079) | 0.005 |
| RNFL (micron) | 0.00249 | (0.00181 to 0.00318) | <0.001 | 0.00372 | (0.00305 to 0.00439) | <0.001 |
| SS | 0.02998 | (0.01550 to 0.04446) | <0.001 | 0.02146 | (0.01273 to 0.03020) | <0.001 |
Beta-estimate, CI, and P value generated from mixed linear effects model controlling for inter-eye correlation. Significance level was 0.05. IOP = intraocular pressure; RNFL = retinal nerve fiber layer thickness; SS = signal strength. Longer axial length was defined as ≥ 23.46 mm, the median for the study population.
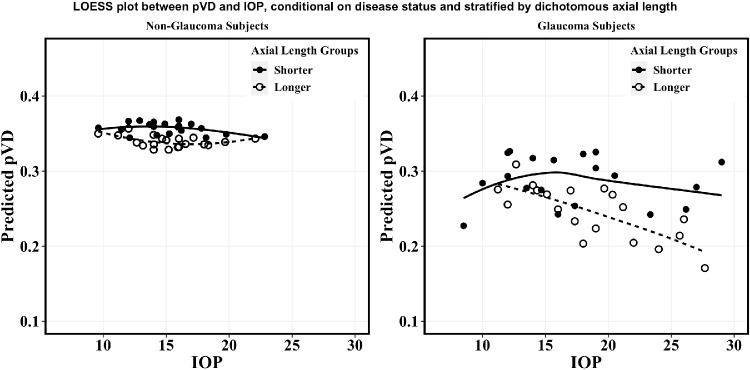
As shown in Figure A, among non-glaucoma subjects, there appears to be little change in predicted pVD per change in IOP in both shorter and longer axial length groups. Conversely, Figure B shows that among subjects with glaucoma the relationship between predicted pVD and IOP differs between AL groups. Among glaucomatous eyes with shorter ALs, overall predicted pVD exhibits little change over a range of IOPs, even among IOP extending beyond 20 mm Hg. However, among subjects with glaucoma with longer AL, pVD changed on average by greater than 0.1 units over the entire range of IOP from approximately 10 to over 30 mm Hg.
Figure.
Predicted pVD from multivariable regression after controlling for RNFL, SS, age, and inter-eye correlation as presented in Table 3 for non-glaucoma subjects (A) compared to subjects with glaucoma (B). Within each disease category, axial length (AL) was sub-divided into a shorter group (AL < 23.46, solid line, solid circle) and a longer group (AL ≥ 23.46, dashed line, hollow circle). Axial length observations were divided into 20 bins in ascending order based on IOP. For each bin, the average IOP and average pVD was determined, then plotted on top of the LOESS curve. LOESS = locally weighted scatterplot smoothing; pVD = peripapillary vessel density; RNFL = retinal nerve fiber layer; SS = signal Strength; AL = axial length; IOP = intraocular pressure (mm Hg).
Discussion
In this study, we examined the association between IOP and peripapillary vessel density with a focus on how this relationship is modulated by AL in non-glaucomatous and subjects with glaucoma. We expanded upon previous work by additionally studying the glaucoma subjects of African American Eye Disease Study (AFEDS) and examining the determinants of pVD among them.7,18 This study focused on African Americans, a particularly important population to study the contribution of vascular factors to glaucoma onset and progression for a few reasons. First, they have a disproportionately high incidence of both glaucoma21 and systemic vascular diseases, such as hypertension, coronary artery disease, and stroke.22 Second, African Americans have also been shown to have lower retrobulbar ocular blood flow and greater microvascular abnormalities at the ONH, which may contribute to glaucoma pathogenesis in this population.23 Our study found that after controlling for age, SS, and RNFL, higher IOP is significantly associated with decreased pVD in subjects with glaucoma, specifically among those with longer ALs. Interestingly, this relationship between IOP and pVD was not seen among subjects with glaucoma with shorter AL or among healthy subjects in either AL group. These findings provide preliminary support for the idea that axial elongation may increase risk for glaucoma15,24 by compromising retinal autoregulation.25
Proper vascular autoregulation at the ONH is defined by the ability to maintain appropriate perfusion to the nerve and retinal capillaries during periods of blood flow instability and IOP fluctuations.26 The precise role of vascular autoregulation in glaucoma pathogenesis remains unclear, but autoregulation occurring at the radial peripapillary region as investigated in this study is primarily mediated by the endothelium.27 There is significant data now demonstrating that with loss of retinal ganglion cells and their axons, there is associated loss of the microvasculature supplying that tissue.28 It is not completely understood whether autoregulation breakdown is a mechanism that precedes glaucoma development or occurs as a result of glaucoma. One study of non-human primates demonstrated that chronic elevations in IOP may lead to autoregulation dysfunction29; however, it is possible that understanding blood flow alterations may be dependent on the disease stage in glaucoma. Although one study showed associations with increased blood flow to the ONH in glaucoma suspects,30 others have found decreased blood flow may occur prior to structural damage in studies examining vascular blood flow at the ONH using color doppler31,32 and OCTA imaging.33 Additionally, inappropriate autoregulation of ocular blood flow at the ONH has been identified as a predictor of glaucomatous progression,26,34,35 and reduced pVD observed on OCTA is associated with reduction in RNFL thickness.28 Recent studies have demonstrated that patients with glaucoma are more likely to have phenomena of systemic vascular dysregulation, such as nailbed capillaroscopy changes,36 and these systemic changes are associated with ocular blood flow alterations.26,34 Although OCTA does not directly measure ocular blood flow, studying the density of perfused microvasculature, which we have done with pVD, and the factors that affect it, can provide insight into the autoregulatory capacity of peripapillary microvasculature between non-glaucoma and subjects with glaucoma.
We found changes in AL was not correlated with IOP among healthy individuals, consistent with findings reported previously,37–39 or among individuals with glaucoma. Among non-glaucomatous subjects, only AL, but not IOP, was significantly associated with pVD in both univariable and multivariable modeling. This finding has been similarly shown in previous studies demonstrating that longer AL is independently associated with decreased vessel density around the ONH in healthy eyes,40–42 as well as an independent risk factor for glaucoma.24 It is possible that increased AL may lead to reduced microcirculation as a result of the mechanical stretching effect on the retinal vasculature itself at the nerve head.7
In contrast, we found that among subjects with glaucoma, higher IOP was associated with decreased pVD and, in particular, higher IOP was significantly associated with decreased pVD in the longer AL glaucoma cohort, but not in the shorter AL glaucoma cohort. The specific AL cutoff to differentiate long and short AL we used was 23.46 based on the median AL of all eyes in the study. Using this cutoff, we found a significant interaction term between long and short AL among the glaucoma population (data not shown); which was not surprising given the significant association we found between IOP in the long AL cohort compared to the nonsignificant association with IOP in the short AL cohort among subjects with glaucoma in Table 4. Taken together, this supports the notion that retinal microcirculation among subjects with glaucoma is sensitive to changes in IOP among eyes with longer ALs.
Interestingly, in the Singapore Epidemiology of Eye Diseases Study (SEEDS),15 longer AL and higher IOP were associated with a synergistically increased risk of POAG compared to eyes with shorter AL and lower IOP. Specifically, individuals with either the combination of long AL and physiologic IOP or short AL and high IOP had a three times increased risk in POAG compared to individuals with short AL and normal IOP. In contrast, the joint effect of high IOP and long AL was associated with a 16 times increased risk of POAG. For comparison purposes, we also subdivided axial length by the AL definitions used in the SEEDs study (< 23.5 mm, 23.5 to 24.5 mm, 24.5–25.5 mm, > 25.5 mm). Although there were too few observations in the categories of axial length > 24.5 mm for glaucoma, we were able to compare the axial length cohort < 23.5 mm versus 23.5–24.5 mm. We found IOP was significantly associated with decreased pVD in the glaucoma population with AL between 23.5 and 24.5 mm, but not in the glaucoma group with AL < 23.5. In non-glaucoma subjects, IOP was not significantly associated with pVD in any of the SEEDs defined AL length divisions.
It is interesting that our finding of higher IOP being associated with reduced pVD was unique to the high AL glaucoma group. This relationship was not seen in low AL individuals with glaucoma, in any AL subgroup of non-glaucoma individuals, and even among those non-glaucoma eyes specifically with IOP ≥ 20. Our cross-sectional findings support (but do not prove) the idea that myopic eyes with glaucoma may be susceptible to retinal autoregulation breakdown in the setting of higher IOP. Longitudinal data is needed to verify this idea. One hypothesis as to why this may be the case is that myopic elongated eyes have thinner sclera and less structural support for its microvasculature43,44 such that there may be increased transmural pressure on the vessel walls, which may lead to vasoconstriction and reduced blood flow.45 This might be similar to that seen in cerebral autoregulation of blood flow where mechanoreceptors on the endothelium lead to vasoconstriction in the setting of increased transmural pressure.46 It is also possible that glaucomatous eyes have increased frailty of the nerve and its microvasculature and that this may further exacerbate susceptibility of blood flow reductions with increased IOP. There are two possible explanations as to why this relationship was not seen in non-glaucomatous eyes with long AL: (1) the glaucomatous eyes with longer AL have other predisposing factors that increase their susceptibility to reduced blood flow with high IOP, and (2) the glaucomatous eyes have increased frailty of the nerve due to the existing damage to the nerve and its microvasculature, which causes these eyes to be susceptible to IOP-induced blood flow reduction. Longitudinal evaluations of ocular hypertensive and glaucoma suspect subjects could help elucidate this association. Nevertheless, these findings may help to explain why increased AL is an independent risk factor for glaucoma.
There are some limitations to our study. In our glaucoma subpopulation, we were limited by power given a total of 86 eyes. With each additional significant determinant that can be included in the multivariable model, significantly more power is needed. Additionally, the IOP in 58 of the 86 glaucomatous eyes was affected by IOP lowering treatment (49 with IOP lowering drops only, 2 with SLT only, 3 with SLT and IOP lowering drops, and 4 with glaucoma surgery and IOP lowering drops), which altered the range of baseline IOP. In addition, whereas our study only looked at the radial peripapillary capillaries, which may provide insight into endothelium-related autoregulation of the retinal microvasculature, we did not study other autoregulation or autonomic regulation issues that may be occurring in retrolaminar or laminar regions of the optic nerve. Finally, our study was a cross-sectional based study where measurements were taken at one visit, thus we were unable to identify temporal relationships among these variables.
In summary, our findings suggest that AL disturbed the IOP-microvascular autoregulation relationship in subjects with glaucoma, but not in healthy subjects. Increased IOP was significantly associated with decreased retinal microcirculation among longer AL groups in subjects with glaucoma and is consistent with the possibility that longer AL eyes, with presumably reduced structural support, may have compromised vascular autoregulation. These findings corroborate other studies reporting a synergistically increased POAG risk in long AL eyes with high IOP. Longitudinal studies are needed to further investigate the mechanisms of the interactive effect of AL and IOP on peripapillary vessel density reduction and glaucomatous development. Additionally important would be studying these associations among different ethnic populations.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health Grants (Bethesda, MD; K23EY027855-01, G.M.R.; U10EY023575, R.V.), American Glaucoma Society Young Clinician Scientist grant (San Francisco, California; GMR), unrestricted grant to the USC Department of Ophthalmology from Research to Prevent Blindness (New York, New York), and Carl Zeiss Meditec (Dublin, CA; SD-OCTA device).
R.K.W. is a consultant of and receives a grant from Carl Zeiss Meditec and he has intellectual property at the Oregon Health and Science University and the University of Washington related to OCT angiography that is related to the technology and analysis methods described in parts of this manuscript. The remaining authors declare no financial disclosures.
Disclosure: J. Juliano, None; B. Burkemper, None; J. Lee, None; A. Nelson, None; V. LeTran, None; Z. Chu, None; G. Zhou, None; X. Jiang, None; R.K. Wang, Carl Zeiss Meditec (F, C, P); R. Varma, None; G.M. Richter, Carl Zeiss Meditec (F)
References
- 1. Burgoyne CF, Crawford Downs J, Bellezza AJ, Francis Suh JK, Hart RT. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005; 24(1): 39–73. [DOI] [PubMed] [Google Scholar]
- 2. Fan N, Wang P, Tang L, Liu X.. Ocular blood flow and normal tension glaucoma. Biomed Res Int. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flammer J, Orgül S.. Optic nerve blood-flow abnormalities in glaucoma. Prog Retin Eye Res. 1998; 17(2): 267–289. [DOI] [PubMed] [Google Scholar]
- 4. Gherghel D, Orgül S, Gugleta K, Gekkieva M, Flammer J.. Relationship between ocular perfusion pressure and retrobulbar blood flow in patients with glaucoma with progressive damage. Am J Ophthalmol. 2000; 130(5): 597–605. [DOI] [PubMed] [Google Scholar]
- 5. Fuchsjäger-Mayrl G, Wally B, Georgopoulos M, et al.. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Investig Ophthalmol Vis Sci. 2004; 45(3): 834–839. [DOI] [PubMed] [Google Scholar]
- 6. Flammer J, Konieczka K, Flammer AJ.. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J. 2013; 4(1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nelson AJ, Chang R, LeTran V, et al.. Ocular determinants of peripapillary vessel density in healthy African Americans: The African American Eye Disease Study. Investig Ophthalmol Vis Sci. 2019; 60(10): 3368–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Q, Jonas JB, Wang Q, et al.. Optical coherence tomography angiography vessel density changes after acute intraocular pressure elevation. Sci Rep. 2018; 8(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oku Y, Oku H, Park M, et al.. Long axial length as risk factor for normal tension glaucoma. Graefe's Arch Clin Exp Ophthalmol. 2009; 247(6): 781–787. [DOI] [PubMed] [Google Scholar]
- 10. Jonas JB, Berenshtein E, Holbach L.. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Investig Ophthalmol Vis Sci. 2004; 45(8): 2660–2665. [DOI] [PubMed] [Google Scholar]
- 11. Akyol N, Kükner AŞ, Ozdemir T, Esmerligil S.. Choroidal and retinal blood flow changes in degenerative myopia. Can J Ophthalmol. 1996; 31(3): 113–119. [PubMed] [Google Scholar]
- 12. Shimada N, Ohno-Matsui K, Harino S, et al.. Reduction of retinal blood flow in high myopia. Graefe's Arch Clin Exp Ophthalmol. 2004; 242(4): 284–288. [DOI] [PubMed] [Google Scholar]
- 13. Al-Sheikh M, Phasukkijwatana N, Dolz-Marco R, et al.. Quantitative OCT angiography of the retinal microvasculature and the choriocapillaris in myopic eyes. Investig Ophthalmol Vis Sci. 2017; 58(4): 2063–2069. [DOI] [PubMed] [Google Scholar]
- 14. Kawasaki R, Wang JJ, Rochtchina E, Lee AJ, Wong TY, Mitchell P.. Retinal vessel caliber is associated with the 10-year incidence of glaucoma: The Blue Mountains Eye Study. Ophthalmology. 2013; 120(1): 84–90. [DOI] [PubMed] [Google Scholar]
- 15. Tham Y-C, Aung T, Fan Q, et al.. Joint effects of intraocular pressure and myopia on risk of primary open-angle glaucoma: The Singapore epidemiology of eye diseases study. Sci Rep. 2016; 6(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKean-Cowdin R, Fairbrother-Crisp A, Torres M, et al.. The African American Eye Disease Study: design and methods. Ophthalmic Epidemiol. 2018; 25(4): 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chu Z, Lin J, Gao C, et al.. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J Biomed Opt. 2016; 21(6): 066008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang R, Nelson AJ, Letran V, et al.. Systemic determinants of peripapillary vessel density in healthy African Americans: The African American Eye Disease Study. Am J Ophthalmol. 2019; 207: 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ying G, Maguire MG, Glynn R, Rosner B.. Tutorial on biostatistics: statistical analysis for correlated binary eye data. Ophthalmic Epidemiol. 2018; 25(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aghaian E, Choe JE, Lin S, Stamper RL.. Central corneal thickness of Caucasians, Chinese, Hispanics, Filipinos, African Americans, and Japanese in a glaucoma clinic. Ophthalmology. 2004; 111(12): 2211–2219. [DOI] [PubMed] [Google Scholar]
- 21. Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J.. Racial variations in the prevalence of primary open-angle glaucoma: The Baltimore Eye Survey. JAMA. 1991; 266(3): 369–374. [PubMed] [Google Scholar]
- 22. Howard G, Cushman M, Howard VJ, et al.. Risk factors for intracerebral hemorrhage: The REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Stroke. 2013; 44(5): 1282–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siesky B, Harris A, Racette L, et al.. Differences in ocular blood flow in glaucoma between patients of African and European descent. J Glaucoma. 2015; 24(2): 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mitchell P, Wang JJ, Hourihan F.. The relationship between glaucoma and myopia: The Blue Mountains Eye Study. Arch Ophthalmol. 1999; 117(10): 1319–1324. [DOI] [PubMed] [Google Scholar]
- 25. Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al.. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology. 2016; 123: 2498–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grieshaber MC, Mozaffarieh M, Flammer J.. What is the link between vascular dysregulation and glaucoma? Surv Ophthalmol. 2007; 52(6): S144–S154. [DOI] [PubMed] [Google Scholar]
- 27. Singh Sohan H. Blood flow in the optic nerve head and factors that may influence it. Prog Retin Eye Res. 2001; 20(5): 595–624. [DOI] [PubMed] [Google Scholar]
- 28. Richter GM, Sylvester B, Chu Z, et al.. Peripapillary microvasculature in the retinal nerve fiber layer in glaucoma by optical coherence tomography angiography: Focal structural and functional correlations and diagnostic performance. Clin Ophthalmol. 2018; 12: 2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang L, Burgoyne CF, Cull G, Thompson S, Fortune B.. Static blood flow autoregulation in the optic nerve head in normal and experimental glaucoma. Investig Ophthalmol Vis Sci. 2014; 55(2): 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gardiner SK, Cull G, Fortune B, Wang L.. Increased optic nerve head capillary blood flow in early primary open-angle glaucoma. Investig Ophthalmol Vis Sci. 2019; 60(8): 3110–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nicolela MT, Drance SM, Rankin SJA, Buckley AR, Walman BE.. Color Doppler imaging in patients with asymmetric glaucoma and unilateral visual field loss. Am J Ophthalmol. 1996; 121(5): 502–510. [DOI] [PubMed] [Google Scholar]
- 32. Flammer J, Konieczka K.. The discovery of the Flammer syndrome: A historical and personal perspective. EPMA J. 2017; 8(2): 75–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen CL, Bojikian KD, Wen JC, et al.. Peripapillary retinal nerve fiber layer vascular microcirculation in eyes with glaucoma and single-hemifield visual field loss. JAMA Ophthalmol. 2017; 135(5): 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Emre M, Orgül S, Gugleta K, Flammer J.. Ocular blood flow alteration in glaucoma is related to systemic vascular dysregulation. Br J Ophthalmol. 2004; 88(5): 662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Galassi F, Sodi A, Ucci F, Renieri G, Pieri B, Baccini M.. Ocular hemodynamics and glaucoma prognosis. Arch Ophthalmol. 2003; 121(12): 1711–1715. [DOI] [PubMed] [Google Scholar]
- 36. Pasquale LR, Hanyuda A, Ren A, et al.. Nailfold capillary abnormalities in primary open-angle glaucoma: A multisite study. Investig Ophthalmol Vis Sci. 2015; 56(12): 7021–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nangia V, Jonas JB, Sinha A, Matin A, Kulkarni M, Panda-Jonas S. Ocular axial length and its associations in an adult population of central rural india: the Central India Eye and Medical Study. Ophthalmology. 2010; 117(7): 1360–1366. [DOI] [PubMed] [Google Scholar]
- 38. Zhou Q, Liang YB, Yin Wong T, et al.. Intraocular pressure and its relationship to ocular and systemic factors in a healthy chinese rural population: The Handan Eye Study. Ophthalmic Epidemiol. 2012; 19(5): 278–284. [DOI] [PubMed] [Google Scholar]
- 39. Memarzadeh F, Ying-Lai M, Azen SP, Varma R.. Associations with intraocular pressure in Latinos: The Los Angeles Latino Eye Study. Am J Ophthalmol. 2008; 146(1): 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qu D, Lin Y, Jiang H, et al.. Retinal nerve fiber layer (RNFL) integrity and its relations to retinal microvasculature and microcirculation in myopic eyes. Eye Vis. 2018; 5(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li M, Yang Y, Jiang H, et al.. Retinal microvascular network and microcirculation assessments in high myopia. Am J Ophthalmol. 2017; 174: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Benavente-Pérez A, Hosking SL, Logan NS, Broadway DC.. Ocular blood flow measurements in healthy human myopic eyes. Graefe's Arch Clin Exp Ophthalmol. 2010; 248(11): 1587–1594. [DOI] [PubMed] [Google Scholar]
- 43. Grudzińska E, Modrzejewska M.. Modern diagnostic techniques for the assessment of ocular blood flow in myopia: current state of knowledge. J Ophthalmol. 2018; 2018: 4694789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boote C, Sigal I, Grytz R, Hua Y, Nguyen T, Girard M.. Scleral structure and biomechanics. Prog Retin Eye Res. 2020; 74: 100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pournaras CJ, Rungger-Brändle E, Riva CE, Hardarson SH, Stefansson E.. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008; 27(3): 284–330. [DOI] [PubMed] [Google Scholar]
- 46. Peterson EC, Wang Z, Britz G.. Regulation of cerebral blood flow. Int J Vasc Med. 2011; 2011: 823525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.