Abstract
Increasing evidence suggests that the “NACHT-LRR and PYD domain-containing protein 3” (NLRP3) inflammasome plays an important role in atherosclerotic cardiovascular disease (ASCVD). Recent preclinical evidence has suggested that the NLRP3 inflammasome may play a prominent role in the pathogenesis of atrial fibrillation (AF). As such, the therapies that have shown efficacy in reducing ASCVD events may also prove beneficial in AF. In this article, we review the findings that implicate the NLRP3 inflammasome in the pathogenesis of AF, discuss existing evidence behind the use of anti-inflammatory agents for AF, and discuss the future role that colchicine and other anti-inflammatory agents may play in the prevention and treatment of AF.
Keywords: Atrial fibrillation, Catheter ablation, Colchicine, Electrophysiology, Inflammation, Prevention
Key Findings.
-
▪
The NLRP3 inflammasome may play a prominent role in the pathogenesis of atrial fibrillation (AF).
-
▪
Anti-inflammatory therapies such as colchicine have shown benefits in reducing recurrence of AF after surgery or ablation.
-
▪
Future randomized controlled trials are necessary to determine the efficacy of colchicine therapy for primary prevention and treatment of AF.
Introduction
Atrial fibrillation (AF), the most common arrhythmia worldwide, affects 2%–3% of the population and is associated with significant morbidity and mortality.1,2 AF is commonly preceded by structural remodeling of the atrial myocardium, which predisposes to impaired electrical conduction.3 The genesis of AF begins with a focal trigger of ectopic firing that initiates a reentrant wave in a vulnerable atrial substrate. The onset of AF leads to further atrial remodeling, which facilitates maintenance and progression of AF from paroxysmal AF (pAF) to persistent and longstanding persistent AF.4, 5, 6, 7
Many nonmodifiable and modifiable risk factors, such as age, sex, ion channel mutations, hypertension, diabetes, obesity, and obstructive sleep apnea, have been linked to the progression of AF.3 However, whether there is a common fundamental mechanism leading to clinical AF remains unknown.8 Enhanced inflammatory signaling has been previously proposed as one potential link in the pathogenesis of AF.9 The association between inflammation and AF was first noted over 20 years ago with the high incidence rate of postoperative AF (up to 50%) after cardiac surgery.10 In studies of postoperative AF, elevated inflammatory markers such as interleukin-1β (IL-1β), IL-6, and C-reactive protein (CRP) temporally correlated with onset of AF. In addition, randomized controlled trials (RCTs) demonstrated that prophylactic anti-inflammatory therapies after cardiac surgery also reduced the risk of postoperative AF.11 Additional prospective cohort studies also suggested that higher CRP levels predicted onset of nonsurgical AF and lone AF in the general population.9 However, Mendelian randomization studies of patients with increased CRP levels as a result of genetic polymorphisms did not increase the incidence of AF, suggesting that CRP levels were a marker of increased risk but did not play a pathophysiological role in the genesis of AF.12 As such, it remained unclear if inflammatory processes precipitated AF or were simply a marker of increased risk.
A recent study by Yao and colleagues13 suggests that the “NACHT-LRR and PYD domain-containing protein 3” (NLRP3) inflammasome may play a causal role in the pathogenesis of AF. In this article, we review the findings from this study, discuss the evidence behind the use of anti-inflammatory agents for AF, and discuss the future role that colchicine may play in the prevention and treatment of AF.
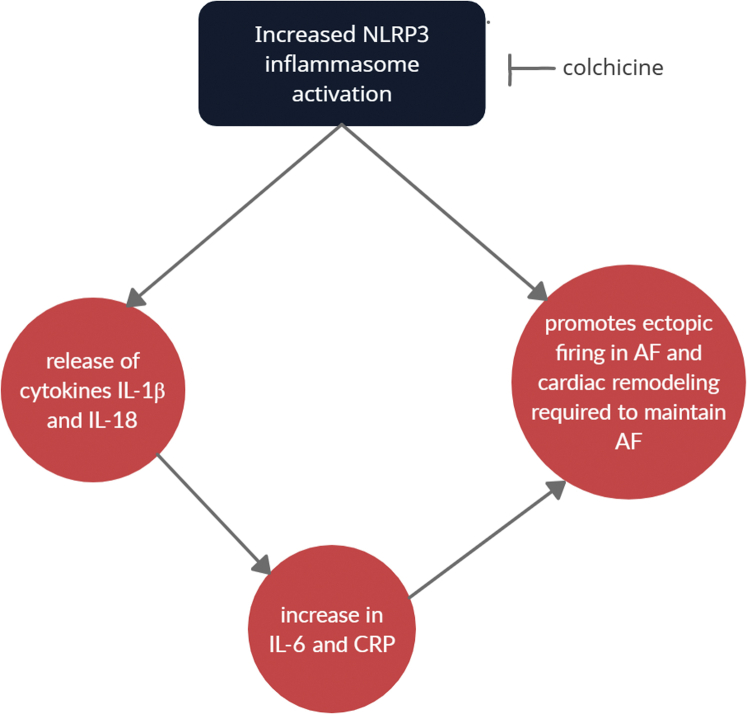
Inflammasomes are intracellular protein complexes that are responsible for activation of inflammatory signaling.14 They form in response to cellular detection of a broad range of signals including microbial motifs, danger signals, environmental irritants, etc, and serve as a first line of defense against pathogens.4 NLRP3 is a type of inflammasome that has been noted in numerous cell types including immune cells, epithelial cells, and cardiomyocytes. Activation of the NLRP3 inflammasome causes the release of cytokines such as IL-1β and IL-18, which leads to a cascade of downstream inflammatory signaling, resulting in the increase of IL-6. An increase in high-sensitivity C-reactive protein (hs-CRP), a biomarker of inflammation, has also been shown to occur with inflammasome activation. IL-6 and hs-CRP have been shown to independently predict future cardiovascular events4,15 (Figure 1).
Figure 1.
NLRP3 activation leads to downstream electrical remodeling within cardiomyocytes that result in increased ectopic firing and shortened atrial refractory period, thereby creating a reentry substrate. In addition, it leads to downstream cytokine release that promotes atrial remodeling necessary to maintain atrial fibrillation.
The study by Yao and colleagues demonstrated that NLRP3 inflammasomes are enhanced in atrial cardiomyocytes of experimental models of AF, which promotes both the ectopic firing necessary to initiate AF and the remodeling of substrate needed to maintain and promote AF.4,13 In mouse models with constitutively active NLRP3 within cardiomyocytes, NLRP3 activation resulted in increased ectopic firing as a result of enhanced sarcoplasmic reticulum calcium release. It also led to electrical remodeling via transcriptional modifications that promoted a shortened atrial effective refractory period, thereby creating a reentry substrate. In addition, the enhanced inflammatory signaling resulted in activation of inflammatory cells that promote atrial fibrosis, thereby leading to the development of AF-maintaining substrate. The use of novel NLRP3 inhibitors or ablation of NLRP3 in the mouse models prevented the development of AF, supporting a causal link between NLRP3 activation within cardiomyocytes and AF. This study is consistent with prior results that have shown that inflammatory markers such as IL-6 and IL-1β, which are downstream signaling cytokines of NLRP3 inflammasome activation, correlate with progression of AF and also freedom from recurrence after AF ablation4 (Figure 1).
Recent clinical trials have demonstrated the importance of targeting the NLRP3 inflammasome pathway in reducing future atherosclerotic cardiovascular disease (ASCVD) events. The Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) demonstrated that canakinumab, a monoclonal antibody against IL-1β, when given to patients with elevated hsCRP (≥2 mg/L) following a myocardial infarction, resulted in a 7%–15% reduction of cardiovascular events.16 However, the high cost and adverse side effect profile of canakinumab resulted in exploration of alternative anti-inflammatory agents such as colchicine.
Colchicine is an inexpensive anti-inflammatory medication that is commonly used for the treatment of gout and pericarditis.17 Colchicine works by inhibiting tubulin polymerization and has also been shown to interfere with the NLRP3/ IL-1β signaling pathway.17 The 2013 Low Dose Colchicine (LoDoCo) trial, the Colchicine Cardiovascular Outcomes (COLCOT) trial, and the LoDoCo 2 trial have demonstrated that treatment with colchicine for secondary prevention resulted in a reduction in future ASCVD events.17, 18, 19 Given the role of the NLRP3 inflammasome in both ASCVD and AF, we review the current evidence evaluating the role of anti-inflammatory therapies for treatment and prevention of AF.
Anti-inflammatory therapies in AF
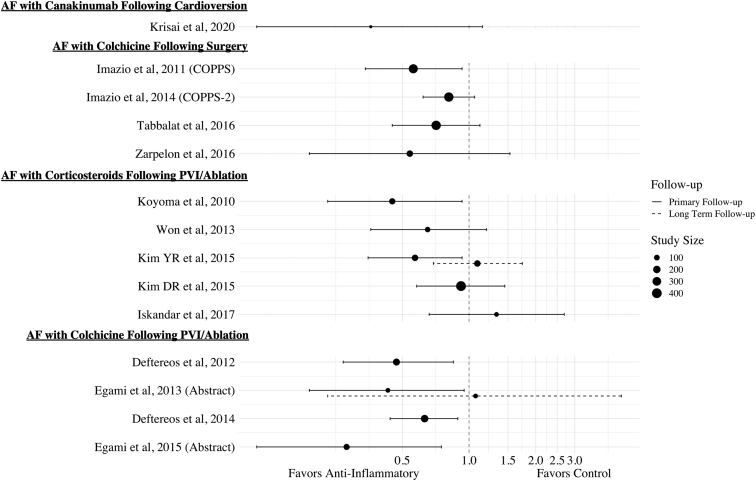
Currently, therapeutic strategies seek to minimize symptoms and avoid the complications associated with AF. Even procedural options such as ablation and cardioversion are complicated by high recurrence rates.3,4 However, current approaches do not target the potential key inflammatory mediators in the pathogenesis of AF. Although anti-inflammatory therapies such as canakinumab, colchicine, and steroids have shown ability to attenuate NLRP3-mediated inflammation, studies evaluating their role in the treatment and prevention of AF are limited20 (Table 1, Figure 2).
Table 1.
Outcomes of atrial fibrillation recurrence with anti-inflammatory therapies
| Study | Study type | Sample size | Intervention | Primary endpoint | Median follow-up | Effect estimate (95% CI) | Deduction |
|---|---|---|---|---|---|---|---|
| AF with canakinumab following cardioversion | |||||||
| Krisai et al, 2020 | Randomized controlled trial | 24 | Canakinumab | AF recurrence at 6 months | 6 months | HR: 0.36 (0.11; 1.15) | Neutral |
| AF with colchicine following surgery | |||||||
| Imazio et al, 2011 (COPPS) | Randomized controlled trial | 336 | Colchicine | Post-op AF at 1 month | 1 month | RR: 0.56 (0.34; 0.93) | Positive |
| Imazio et al, 2014 (COPPS-2) | Randomized controlled trial | 360 | Colchicine | Post-op AF within 3 months | 3 months | RR: 0.81 (0.62; 1.06) | Neutral |
| Tabbalat et al, 2016 | Randomized controlled trial | 360 | Colchicine | Post-op AF after 1 week | 8 days | RR: 0.71 (0.45; 1.12) | Neutral |
| Zarpelon et al, 2016 | Randomized controlled trial | 140 | Colchicine | Post-op AF after 2 weeks | 14 days | RR: 0.54 (0.19; 1.53) | Neutral |
| AF with corticosteroids following PVI/ablation | |||||||
| Koyoma et al, 2010 | Randomized controlled trial | 125 | Hydrocortisone and prednisolone | AF recurrence at 14 months | 14 months | HR: 0.45 (0.23; 0.93) | Positive |
| Won et al, 2013 | Prospective cohort study | 89 | Low-dose hydrocortisone | AF recurrence at 12 months | 12 months | RR: 0.65 (0.36; 1.20) | Neutral |
| Kim YR et al, 2015 | Randomized controlled trial | 138 | Methylprednisolone | AF recurrence at 3 months AF recurrence at 24 months |
3 months 24 months |
RR: 0.57 (0.35; 0.93) RR: 1.09 (0.69; 1.74) |
Positive Neutral |
| Kim DR et al, 2015 | Randomized controlled trial | 407 | Hydrocortisone and methylprednisolone | AF recurrence at 12 months | 12 months | RR: 0.92 (0.58; 1.45) | Neutral |
| Iskandar et al, 2017 | Randomized controlled trial | 60 | Prednisone | AF recurrence at 12 months | 12 months | RR: 1.33 (0.66; 2.69) | Neutral |
| AF with colchicine following PVI/ablation | |||||||
| Deftereos et al, 2012 | Randomized controlled trial | 170 | Colchicine | AF recurrence at 3 months | 3 months | RR: 0.47 (0.27; 0.85) | Positive |
| Egami et al, 2013 (Abstract) | Randomized controlled trial | 62 | Colchicine | AF recurrence at 2 weeks AF recurrence at 2 months |
2 weeks 2 months |
RR: 0.43 (0.19; 0.95) RR: 1.07 (0.23; 4.87) |
Positive Neutral |
| Deftereos et al, 2014 | Randomized controlled trial | 206 | Colchicine | AF recurrence at 12 months | 12 months | RR: 0.63 (0.44; 0.89) | Positive |
| Egami et al, 2015 (Abstract) | Prospective cohort study | 122 | Colchicine | AF recurrence at 12 months | 12 months | RR: 0.28 (0.11; 0.75) | Positive |
AF = atrial fibrillation; CI = confidence interval; HR = hazard ratio; Post-op = postoperative; PVI = pulmonary vein isolation; RR = relative risk.
Figure 2.
There appears to be a trend towards benefit of steroid therapy to treat atrial fibrillation (AF) after cardiac surgery. Although the results for steroid treatment after AF ablation to prevent recurrence appear to be equivocal, there is a clearer trend toward benefit with colchicine therapy. Further study is required to evaluate the benefit of canakinumab after cardioversion.
Krisai and colleagues21 evaluated the use of canakinumab after electrical cardioversion in 24 patients with persistent AF. After achieving sinus rhythm, patients treated with canakinumab vs placebo were followed to determine recurrence rate of AF after 6 months. Although there was a lower incidence of AF recurrence, the results were not statistically significant; the small sample size, however, was a significant limitation.21
Several RCTs have evaluated the role of colchicine for prevention of post–cardiothoracic surgery AF.10 The Colchicine for the Prevention of the Postcardiotomy Syndrome (COPPS) Atrial Fibrillation Substudy showed a reduced incidence of pAF in patients treated with colchicine for 1 month after pericardiotomy. A follow-up trial (COPPS-2) was conducted to evaluate patients undergoing any cardiac surgery except cardiac transplantation. Although the on-treatment subanalysis revealed a beneficial effect of colchicine for AF, this was not statistically significant based on an intention-to-treat analysis. Other studies have noted no benefit with colchicine in post-revascularization surgeries or in other open heart surgery patients.22,23
Patients who undergo AF ablation are known to have high levels of endocardial inflammation, including elevated IL-6 and hsCRP, in the first 3 months following the procedure, which may be associated with the 40%–50% rate of early and late AF recurrence.24,25 As such, several studies have evaluated the role of anti-inflammatory therapies such as colchicine or steroids after pulmonary vein isolation (PVI) and AF ablation to prevent recurrence of AF.10,25
The results with steroids have been inconsistent. In a small RCT by Koyama and colleagues26 including 125 patients undergoing PVI and ablation, the use of hydrocortisone on the day of AF ablation and prednisolone for 3 days after resulted in the decrease in immediate and late recurrence of AF. However, a similar study by Kim and colleagues27 using methylprednisolone showed there was a decrease in early recurrence rates, but no difference in late recurrence rates at the 24-month follow-up.
In the prospective, randomized, double-blinded Steroid-AF Study, 60 patients who failed antiarrhythmic therapy were randomized to prednisone or placebo after ablation for pAF.28 Prednisone did not reduce early or late recurrence of AF, and in fact, there was a trend towards higher rates of early AF recurrence with prednisone use. Two studies that evaluated the efficacy of a single-dose steroid injection after AF ablations did not show any benefit in preventing AF recurrence.29,30
In 2012, Deftereos and colleagues31 conducted the first study evaluating the efficacy of colchicine therapy after PVI and AF ablation in preventing AF recurrence. In this double-blinded placebo-controlled RCT, 161 patients undergoing ablation for pAF were treated with colchicine for 3 months after the procedure. Treatment with colchicine significantly reduced incidence of AF recurrence (16% vs 33.5%, P = .01).31
A follow up study by Deftereos and colleagues32 including patients who underwent ablation and treatment with colchicine for 3 months showed similar long-term reduction in AF recurrence rates at the 15-month follow-up (31% vs 50%, P = .01). In a 2013 prospective study, colchicine was effective at preventing AF recurrences within 2 weeks after ablation, but the effect was no longer present between 2 weeks and 2 months postablation.33 Several combined meta-analyses including postsurgical and postablation patients have evaluated the role of colchicine for preventing recurrence of AF after ablation, with 5 out of 6 meta-analyses suggesting a protective role of colchicine, whereas only 1 study was neutral.34, 35, 36, 37, 38, 39
To better understand the variable effect of colchicine for AF recurrence prevention, 122 patients with preprocedure computed tomography imaging undergoing AF ablation and treatment with colchicine were evaluated.40 The evaluation identified that patients with larger left atrial epicardial adipose tissue (LA-EAT) volumes were more likely to benefit from colchicine therapy than those who had smaller LA-EAT.40, 41, 42 In addition, LA-EAT has been linked to LA arrhythmogenicity and may be helpful in distinguishing who may benefit from anti-inflammatory therapy after AF ablation.41,42
Limitations
In the studies evaluating the use of anti-inflammatory therapies for postprocedure AF, the role of the NLRP3 inflammasome pathway remains unclear. Although the inflammatory markers that are elevated in postprocedure AF (IL-1β, IL-6, and CRP) are similar to those after NLRP3 inflammasome activation, future studies that demonstrate the role of the NLRP3 inflammasome in postprocedure AF are necessary. In addition, studies that evaluate the role of the NLRP3 inflammasome in nonsurgical AF are also necessary.
Future Implications
Currently, the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart and Rhythm Society (HRS) Guideline for the Management of AF gives a class IIb recommendation to treat individuals with colchicine after cardiac surgery to prevent AF.43,44 Following data from Yao and colleagues13 linking the NLRP3 inflammasome to AF, and multiple cardiovascular outcome trials demonstrating a benefit with anti-inflammatory therapies for the secondary prevention of ASCVD, further investigation is required to determine a role for anti-inflammatory therapies for prevention and treatment of AF.
Given the recent evidence from LoDoCo, COLCOT, and LoDoCo 2, colchicine could be considered for primary and secondary prevention of ASCVD.45 High-quality RCTs are necessary to determine the efficacy of colchicine therapy for prevention and treatment of AF before it can receive a strong recommendation in future ACC/AHA and HRS guidelines. Colchicine and other anti-inflammatory agents that target the NLRP3 inflammasome pathway may prove to be a valuable tool in reducing overall AF burden and preventing progression of AF. However, given the overlap in risk factors for ASCVD and AF, addressing the modifiable risk factors will remain the cornerstone of therapy for prevention of AF.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
H.C. is a consultant for Medtronic Inc and St. Jude Medical/Abbott. H.C. receives research support from Boston Scientific Corp.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
References
- 1.Chugh S.S., Rasmus H., Kumar N. Worldwide epidemiology of atrial fibrillation. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnabel R.B., Yin X., Gona P. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staerk L., Sherer J.A., Ko D., Benjamin E.J., Helm R.H. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. 2017;120:1501–1517. doi: 10.1161/CIRCRESAHA.117.309732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li N., Brundel B.J.J.M. Inflammasomes and proteostasis novel molecular mechanisms associated with atrial fibrillation. Circ Res. 2020;127:73–90. doi: 10.1161/CIRCRESAHA.119.316364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allessie M., Ausma J., Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 6.Nattel S., Burstein B., Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 7.Nattel S., Heijman J., Zhou L., Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res. 2020;127:51–72. doi: 10.1161/CIRCRESAHA.120.316363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nattel S., Dobrev D. Controversies about atrial fibrillation mechanisms. Circ Res. 2017;120:1396–1398. doi: 10.1161/CIRCRESAHA.116.310489. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y.-F., Chen Y.-J., Lin Y.-J., Chen S.-A. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–243. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 10.Deftereos S.G., Vrachatis D.A., Angelidis C. The role of colchicine in treating postoperative and post-catheter ablation atrial fibrillation. Clin Ther. 2019;41:21–29. doi: 10.1016/j.clinthera.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Ho K.M., Tan J.A. Benefits and risks of corticosteroid prophylaxis in adult cardiac surgery: a dose-response meta-analysis. Circulation. 2009;119:1853–1866. doi: 10.1161/CIRCULATIONAHA.108.848218. [DOI] [PubMed] [Google Scholar]
- 12.Marott S.C.W., Nordestgaard B.G., Zacho J. Does elevated C-reactive protein increase atrial fibrillation risk? A Mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol. 2010;56:789–795. doi: 10.1016/j.jacc.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 13.Yao C., Veleva T., Scott L. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–2242. doi: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swanson K.V., Deng M., Ting J.P.-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbate A., Toldo S., Marchetti C., Kron J., Van Tassell B.W., Dinarello C.A. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020;126:1260–1280. doi: 10.1161/CIRCRESAHA.120.315937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 17.Nidorf S.M., Fiolet A.T.L., Mosterd A. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 18.Nidorf S.M., Eikelboom J.W., Budgeon C.A., Thompson P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Tardif J.-C., Kouz S., Waters D.D. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 20.Martínez G.J., Celermajer D.S., Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 2018;269:262–271. doi: 10.1016/j.atherosclerosis.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Krisai P., Blum S., Schnabel R.B. Canakinumab after electrical cardioversion in patients with persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.008197. [DOI] [PubMed] [Google Scholar]
- 22.Zarpelon C.S., Netto M.C., Jorge J.C.M. Colchicine to reduce atrial fibrillation in the postoperative period of myocardial revascularization. Arq Bras Cardiol. 2016;107:4–9. doi: 10.5935/abc.20160082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabbalat R.A., Hamad N.M., Alhaddad I.A., Hammoudeh A., Akasheh B.F., Khader Y. Effect of ColchiciNe on the InciDence of Atrial Fibrillation in Open Heart Surgery Patients: END-AF Trial. Am Heart J. 2016;178:102–107. doi: 10.1016/j.ahj.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Issac T.T., Dokainish H., Lakkis N.M. Role of inflammation in initiation and perpetuation of atrial fibrillation. J Am Coll Cardiol. 2007;50:2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 25.De Vecchis R. Promising effects of moderate-dose corticosteroid therapy in the blanking period for prevention of atrial fibrillation (AF) recurrences in patients undergoing AF ablation. Eur J Clin Pharmacol. 2019;75:1179–1180. doi: 10.1007/s00228-019-02683-4. [DOI] [PubMed] [Google Scholar]
- 26.Koyama T., Tada H., Sekiguchi Y. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol. 2010;56:1463–1472. doi: 10.1016/j.jacc.2010.04.057. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y.R., Nam G.-B., Han S. Effect of short-term steroid therapy on early recurrence during the blanking period after catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8:1366–1372. doi: 10.1161/CIRCEP.115.002957. [DOI] [PubMed] [Google Scholar]
- 28.Iskandar S., Reddy M., Afzal M.R. Use of oral steroid and its effects on atrial fibrillation recurrence and inflammatory cytokines post ablation - The Steroid AF Study. J Atr Fibrillation. 2017;9:1604. doi: 10.4022/jafib.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D.-R., Won H., Uhm J.-S. Comparison of two different doses of single bolus steroid injection to prevent atrial fibrillation recurrence after radiofrequency catheter ablation. Yonsei Med J. 2015;56:324–331. doi: 10.3349/ymj.2015.56.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Won H., Kim J.-Y., Shim J. Effect of a single bolus injection of low-dose hydrocortisone for prevention of atrial fibrillation recurrence after radiofrequency catheter ablation. Circ J. 2013;77:53–59. doi: 10.1253/circj.cj-12-0728. [DOI] [PubMed] [Google Scholar]
- 31.Deftereos S., Giannopoulos G., Kossyvakis C. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol. 2012;60:1790–1796. doi: 10.1016/j.jacc.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 32.Deftereos S., Giannopoulos G., Efremidis M. Colchicine for prevention of atrial fibrillation recurrence after pulmonary vein isolation: mid-term efficacy and effect on quality of life. Heart Rhythm. 2014;11:620–628. doi: 10.1016/j.hrthm.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Egami Y., Nishino M., Kato T. Impact of short duration colchicine use on reduction of immediate atrial fibrillation after catheter ablation: prospective study. J Am Coll Cardiol. 2013;61 E408–E408. [Google Scholar]
- 34.Salih M., Smer A., Charnigo R. Colchicine for prevention of post-cardiac procedure atrial fibrillation: meta-analysis of randomized controlled trials. Int J Cardiol. 2017;243:258–262. doi: 10.1016/j.ijcard.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Papageorgiou N., Briasoulis A., Lazaros G., Imazio M., Tousoulis D. Colchicine for prevention and treatment of cardiac diseases: a meta-analysis. Cardiovasc Ther. 2017;35:10–18. doi: 10.1111/1755-5922.12226. [DOI] [PubMed] [Google Scholar]
- 36.Verma S., Eikelboom J.W., Nidorf S.M. Colchicine in cardiac disease: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2015;15:96. doi: 10.1186/s12872-015-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lennerz C., Barman M., Tantawy M., Sopher M., Whittaker P. Colchicine for primary prevention of atrial fibrillation after open-heart surgery: systematic review and meta-analysis. Int J Cardiol. 2017;249:127–137. doi: 10.1016/j.ijcard.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 38.Trivedi C., Sadadia M. Colchicine in prevention of atrial fibrillation following cardiac surgery: systematic review and meta-analysis. Indian J Pharmacol. 2014;46:590–595. doi: 10.4103/0253-7613.144905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M.-X., Deng X.-L., Mu B.-Y. Effect of colchicine in prevention of pericardial effusion and atrial fibrillation: a meta-analysis. Intern Emerg Med. 2016;11:867–876. doi: 10.1007/s11739-016-1496-5. [DOI] [PubMed] [Google Scholar]
- 40.Egami Y., Nishino M., Shutta R., Makino N., Tanouchi J. Abstract 12012: relation between colchicine and epicardial adipose tissue volume surrounding left atrium in atrial fibrillation recurrence after ablation. Circulation. 2015;132 A12012–A12012. [Google Scholar]
- 41.Ciuffo L., Nguyen H., Marques M.D. Periatrial fat quality predicts atrial fibrillation ablation outcome. Circ Cardiovasc Imaging. 2019;12 doi: 10.1161/CIRCIMAGING.118.008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sepehri Shamloo A., Dagres N., Dinov B. Is epicardial fat tissue associated with atrial fibrillation recurrence after ablation? A systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2019;22:132–138. doi: 10.1016/j.ijcha.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.January C.T., Samuel W.L., Hugh Calkins. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 44.January C.T., Wann L.S., Alpert J.S. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 45.Samuel M., Waters D.D. Will colchicine soon be part of primary and secondary cardiovascular prevention? Can J Cardiol. 2020;36:1697–1699. doi: 10.1016/j.cjca.2020.06.012. [DOI] [PubMed] [Google Scholar]