Summary
TP73 belongs to the TP53 family of transcription factors and has therefore been well studied in cancer research. Studies in mice, however, have revealed non-oncogenic activities related to multiciliogenesis. Utilizing whole-exome sequencing analysis in a cohort of individuals with a mucociliary clearance disorder and cortical malformation, we identified homozygous loss-of-function variants in TP73 in seven individuals from five unrelated families. All affected individuals exhibit a chronic airway disease as well as a brain malformation consistent with lissencephaly. We performed high-speed video microscopy, immunofluorescence analyses, and transmission electron microscopy in respiratory epithelial cells after spheroid or air liquid interface culture to analyze ciliary function, ciliary length, and number of multiciliated cells (MCCs). The respiratory epithelial cells studied display reduced ciliary length and basal bodies mislocalized within the cytoplasm. The number of MCCs is severely reduced, consistent with a reduced number of cells expressing the transcription factors crucial for multiciliogenesis (FOXJ1, RFX2). Our data demonstrate that autosomal-recessive deleterious variants in the TP53 family member TP73 cause a mucociliary clearance disorder due to a defect in MCC differentiation.
Keywords: primary ciliary dyskinesia, PCD, motile ciliopathy, lissencephaly, ciliogenesis, TP73, cilia, reduced generation of multiple motile cilia, RGMC
Introduction
More than 30 years after its discovery, the transcription factor TP53 is still one of the most studied proteins in the cancer field due to its powerful function as a tumor suppressor, which earned it the nickname “guardian of the genome.”1 TP53 shares remarkable structural identity with TP63 and TP73; the three proteins comprise the TP53 protein family.2 While all family members are stabilized and activated upon DNA damage and control expression of target genes involved in the regulation of cell cycle arrest and apoptosis,2 TP63 and TP73 perform distinct DNA damage-independent roles.3
As a master regulator of epidermal development and homeostasis, TP63 isoforms are expressed in a range of tissues of different germ layer origins. Germline variants in TP63 have been associated with ectodermal-related disorders manifesting with three hallmark defects (MIM: 604292): ectodermal dysplasia, limb malformation, and orofacial clefting.4
Studies in mice revealed that Trp73, in contrast to Trp53, is rarely mutated in cancer.5 In addition to its function in central nervous system neurogenesis, TRP73 acts as a master transcriptional regulator of multiciliogenesis in mice.5,6 The respiratory tract, the brain ventricles, and parts of the male and female reproductive system are covered with multiciliated cells (MCCs) that generate a cilia-powered fluid flow. These MCCs distend around 30–300 microtubule-based cilia beating in a coordinated pattern utilizing large biological motor machineries attached to the microtubules.7 Inborn dysfunctions of motile cilia are responsible for disease manifestations in various organ systems referred to as motile ciliopathies.8 Here we report a motile ciliopathy caused by autosomal-recessive loss-of-function TP73 variants resulting in a defect of MCC differentiation responsible for a severe chronic destructive airway disease and malformation of cortical development.
Material and methods
Individuals
Signed and informed consent was obtained from all affected individuals as well as relatives through approved protocols from the Institutional Ethics Review Board of the University Muenster, Freiburg and collaborating institutions.
Genetic analyses
Genomic DNA extraction from blood for sequencing was performed as described.9 Using the GeneChip Human Mapping 10K Array v.2.0 (Affymetrix), linkage analysis for family OP-1693 was performed as reported in Olbrich et al.9 Targeted-exome sequencing and/or whole-exome sequencing10 was performed as reported previously. Variant reports were analyzed as described.11 Where possible, pathogenic TP73 variants were confirmed by Sanger-based segregation analyses.
To confirm pathogenic TP73 variants (OP-3039, KI-645, 18DG0963, 19DG0120,19DG2776, and 20DG1336) or to determine the exact breakpoints (OP-1693), polymerase chain reactions (PCRs) were performed. GoTaq Flexi DNA Polymerase (Promega) was used according to the manufactureŕs protocol. Specific primers (Table S1) were used either to amplify the affected exon or to bridge the gap of exon 7–14 of TP73 for OP-1693 II1. Agarose gel electrophoresis was performed to check PCR products for correct size. Using ExoSap-IT (Affymetrix), PCR products were purified enzymatically. Following the manufacturer’s protocol, BigDye Terminator v.3.1 Cycle Sequencing kit (Applied Biosystems) was applied for bidirectional Sanger sequencing of amplicons. Sanger sequencing was performed on a 3730xl DNA Analyzer and trace files were analyzed with CodonCode aligner (v.3.7.1).
To analyze the splice-site variant TP73 c.1196+1G>A (p.?) (GenBank: NM_005427.4), RNA was extracted from air liquid interface (ALI) cultured respiratory epithelial cells originating from OP-3039 II1 and a healthy control subject (16 days after airlift) using the RNeasy Mini kit (Quiagen). RNA (100 ng) was transcribed into cDNA with SuperScript III Reverse Transcriptase using Oligo(dT)12-18 Primers and RNaseOUT Recombinant Ribonuclease Inhibitors (40 U/μL) (Invitrogen). PCR was performed with KOD Hot Start Polymerase (Merck) using specific primers (TP73_splicing_F / TP73_splicing_R; Table S1) to amplify products spanning TP73 exons 9 to 13. PCR product sizes were analyzed by agarose gel electrophoresis before Sanger sequencing analysis was performed as described.
TP73 transcript variant analysis
Respiratory epithelial cells obtained from healthy volunteers were applied for RNA extraction before or after cultivation under ALI condition. For RNA isolation, the RNeasy Mini Kit (Quiagen) was used according to the manufacturer’s instructions. RNA (150 ng) was transcribed into cDNA as described before but using a TP73 specific primer (TP73_RT primer; Table S1). Full-length TP73-transcripts were amplified by touchdown PCR using the KOD Hot Start DNA polymerase (Merck) and TP73 transcript specific primers (CCDS49: TP73_PCR_TAp73_F1 / TP73 PCR_R; CCDS44049: TP73_PCR_ΔNP73_F1 / TP73 PCR_R; Table S1). PCR products were purified using the Illustra GFX PCR DNA and gel band purification kit (GE healthcare) according to the manufacturer’s protocol, if a subsequent nested PCR was necessary. Nested PCR was performed using the KOD Hot Start DNA polymerase (Merck) with TP73_nest1_F/ TP73_nest1_R primers. To ensure complete coverage of the full transcript variants, internal primers were used (TP73_int_primers; Table S1) for additional Sanger sequencing reactions. The size of all PCR products was verified by agarose gel electrophoresis. Sanger sequencing and analyses were performed as described above.
Culture of respiratory epithelial cells
Respiratory epithelial cells were obtained from the middle turbinate by a nasal brush biopsy and suspended in RPMI medium supplemented with GIBCO 2xAntibiotic-Antimycotic (Thermo Fisher). Cells were pre-cultured in primary rat collagen-coated T25 flasks and subsequently processed as previously reported.11, 12, 13, 14 For ALI cultures, suspensions of unciliated basal cells were seeded onto primary rat collagen-coated ALI-Transwell Inserts fitted in 24-well plates (Corning). PneumaCult-Ex Medium (Stemcell) for proliferation and PneumaCult-ALI Medium (Stemcell) for differentiation were used for culture. For spheroid generation, suspensions of unciliated basal cells were transferred into uncoated T25 flasks using DMEM-Ham’s F12 from Life Technologies supplemented with GIBCO 1xAntibiotic-Antimycotic and 10% NU-Serum (Becton Dickinson) in a shaking incubator.
High-speed video microscopy
High-speed video microscopy (HVMA) was recorded after spheroid culture using a Basler acA1300-200um monochrome high-speed video camera attached to an inverted phase-contrast microscope (Zeiss Axio Vert A1) equipped with a 40× objective. Video processing was performed using SAVA as reported previously.15
Particle tracking
Tracking experiments with ALI-cultured respiratory epithelial cells were performed as reported.11 In total, six fully differentiated ALI-Transwell inserts per individual (insert #1–#6) were analyzed for tracking: day 32 after airlift, insert #1 and #2; day 39 after airlift, insert #3 and #4; day 46 after airlift, insert #5 and #6. As an addendum, FluoSpheres beads with 2.0 μm in diameter were diluted 1:1,000 in Dulbecco’s Phosphate-Buffered Saline without Mg2+/Ca2+. Utilizing the Nikon Eclips Ti-S microscope (10× objective lens) equipped with a DS-Qi2 Monochrome Microscope camera and NIS-Elements Advanced Research software, the transport of fluorescent nanoparticles by ciliary beating was recorded (20-s videos with 7.5 frames per second). Nanoparticles were excited at wavelength 546 nm for a 100 ms exposure time. The NIS-Elements Advanced Research software (v.4.51.000) and NIS Advanced 2D Tracking plug-in was applied to analyze and evaluate the particle tracking videos as described. Briefly, polar graph pictures were generated, and the velocity (μm/s) as well as the mean square displacement (μm2) per tracked particle was calculuated.11 In total, 30 videos per individual were analyzed. For statistical evaluation, the two-tailed Mann Whitney test was performed by GraphPad Prism v.5.01 for Windows. Z stack projections of tracking movies were generated using ImageJ-win64.16 Differential interference contrast (DIC) movies were recorded in parallel to each tracking video with an acA1300-200um Basler sc640-120fm monochrome high-speed video camera (recording 120 frames per second). DIC videos were evaluated with SAVA11 software.
Transmission electron microscopy
Transmission electron microscopy (TEM) was performed with freshly obtained respiratory epithelial cells by nasal brush biopsy or after ciliogenesis culture. The samples were fixed in 2.5% glutaraldehyde, washed with 1.3% osmium tetroxide, and embedded in 1,2-epoxypropan-epon mixture (1:1) at 4°C overnight. After polymerization, several sections were picked out onto copper grids. The sections were stained with Reynold’s lead citrate. TEM was performed with the Philips CM10 microscope as previously described17
High-resolution immunofluorescence microscopy
Depending on sample types, different immunofluorescence (IF) staining approaches were utilized. Cultured spheroids or freshly obtained respiratory epithelial cells were stained for rabbit polyclonal anti-GAS8 (1:500; HPA041311; Atlas Antibodies),18 mouse monoclonal anti-DNAH5,19 and mouse monoclonal anti-acetylated α-tubulin (1:10,000; T6793; Sigma) according to a recently reported immunofluorescence staining protocol.11 Immunofluorescence staining with respiratory epithelial cells cultured under ALI-condition used a modified protocol. Briefly, cell layers (including rat-collagen-coated membranes serving as growing platform) were excised from the Transwell ALI Inserts and embedded in Tissue-Tek Cryomold Cryomolds (Sakura) with Thermo Scientific Shandon Cryomatrix Frozen Embedding Medium. Using the Leica CM3050S Cryostat, embedded cell layers were sectioned into 20 μm longitudinal sections. Samples were transferred to Thermo Scientific SuperFrost Plus microscope slides. For immunofluorescence staining, excess embedding medium was removed from slides with phosphate-buffered saline (PBS). Cells were fixed for 15 min using 4% paraformaldehyde, permeabilized with 0.2% Trition X-100 (in PBS) for 10 min, and blocked with 2% bovine serum albumin (w/v) and 5% goat serum (v/v) in PBS (blocking solution) overnight at 4°C. Primary antibodies diluted in blocking solution were incubated 3 h at room temperature. The following primary antibodies were used: mouse monoclonal anti-acetylated α-tubulin (1:10,000; T6793; Sigma), rabbit polyclonal anti-FOXJ1 (1:250; HPA005714; Atlas Antibodies), and rabbit polyclonal anti-RFX2 (1:200; HPA048969; Atlas Antibodies). After washing with PBS six times (7.5 min each), secondary antibody incubation was carried out for 1 h at room temperature using Alexa Fluor 488-conjugated goat anti-mouse (1:1,000 diluted in blocking solution; A11029; Invitrogen) and Alexa Fluor 546-conjugated goat anti-rabbit (1:1,000 diluted in blocking solution; A11035; Invitrogen) antibodies. After washing with PBS six times (7.5 min each), nuclei were stained using Hoechst 33342 (1:1,000 in PBS; 14533100MG, Sigma). Slides were mounted with DAKO fluorescent Mounting medium (Dako North America).
Immunofluorescence images were taken using a Zeiss Apotome Axiovert 200 and processed with AxioVersion 4.8. software. A Zeiss LSM 880 Laser Scanning Microscope and corresponding ZEN-blue and ZEN-black software programs were utilized for generating 3D-confocal images. The Leica THUNDER Imager System and corresponding software LAS-X was utilized to create overview images. Adobe Creative Suites were used for final image processing.
Results
Whole-exome sequencing identified autosomal-recessive loss-of-function variants in TP73
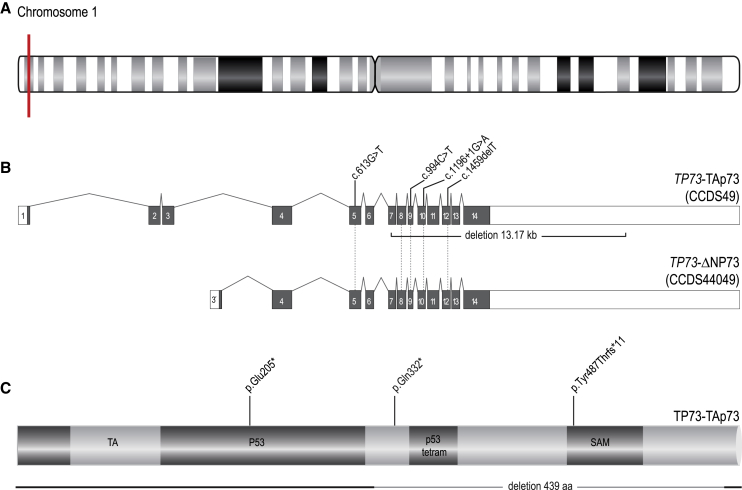
Whole-exome sequencing (WES) analyses in three individuals from two unrelated families with a mucociliary clearance disorder and malformation of cortical development (OP-1693 II1, OP-3039 II1, KI-645 II1) revealed homozygous likely deleterious variants in TP73 (Figures 1 and S1). TP73 (CCDS49) is located on chromosome 1p36 and comprises 13 coding exons and a non-coding exon 1, encoding a 5,192 bp transcript predicting a 636 amino acid protein. In OP-3039 II1 and his cousin KI-645 II1, we identified a homozygous canonical splice-site variant in TP73 (GenBank: NM_005427.4; c.1196+1G>A) (Figures 1 and S2). Using RT-PCR we were able to show that this variant indeed results in defective splicing and subsequently a retained intron which leades to a premature stop codon (Figure S2). WES analyses in OP-1693 II1 revealed a homozygous gap spanning exon 7–14 of TP73 (Figure S3). Sanger sequencing of the PCR product bridging the breakpoints corroborated these findings and confirmed the presence of a homozygous deletion with a size of 13.17 kb (Figures 1 and S3). Consistent with homozygosity by descent, all parents were heterozygous carriers for the respective variants (Figure S1). Furthermore, analyses of the WES data did not identify any other disease-causing variants in genes previously associated with malformations of cortical development or destructive lung disease. Through the use of GeneMatcher, four additional individuals originating from Saudi Arabia from three families with TP73 mutations were identified (Figures 1 and S1). Clinical exome sequencing revealed homozygous loss-of-function variants in TP73 in four unrelated individuals from three families with consanguineous parents: 19DG0120 (UPN-103720) (TP73 [GenBank: NM_005427.4], c.1459delT [p.Tyr487Thrfs∗11]), 18DG0963 II1 (UPN-136020), and 19DG2776 II1, the cousin of 18DG0963 II1 (TP73 [GenBank: NM_005427.4], c.994C>T [p.Gln332∗]) as well as 20DG1336 II1 (TP73 [GenBank: NM_005427.4], c.613G>T [p.Glu205∗]). Consistent with autosomal-recessive inheritance and homozygosity by descent, parents were heterozygous carriers of the respective variants (Figure S1).
Figure 1.
Homozygous loss-of-function variants in TP73 found in seven individuals originating from five non-related families
(A) Schematic overview of chromosome 1. TP73 is located on chromosome 1p36 (red mark).
(B) In healthy respiratory epithelial cells, two different transcripts are expressed. TAp73 (CCDS49) consists of 14 exons resulting in a 5,192 bp long transcript. The shorter variant ΔNP73 (CCDS44049) encodes for 12 exons yielding in a 5,120 bp long transcript. All LoF variants in the seven individuals from five unrelated families reported here (OP-1693, OP-3039, KI-645, 19DG2776, 18DG0963, 19DG0120, and 20DG1336) affect both transcript variants. Based on TAp73, the identified variants are marked on top. The deletion spanning exon 7–14 identified for OP-1693 II1 is marked below.
(C) Overview of TAp73 (636 amino acid). Functional domains are marked in the protein structure including the transactivation (TA-), p53-, p53 tetram-, and sterile alpha motif (SAM-) domain.
The homozygous variants affect both TP73 transcripts present in human respiratory epithelial cells
The gene structures of TP53, TP63 and TP73 are highly conserved.3 TP63 and TP73 have two promoters—P1 and P2—which produce two classes of proteins, those containing the transactivation (TA) domain and those lacking it (TAp73, ΔNP73, respectively).2 Consistently, by utilizing cDNA sequencing, we identified both major transcript variants in human respiratory epithelial cells, which have been described previously in mice.3 Immunoblot analysis corroborated these findings showing two bands corresponding to the sizes of the two transcript variants (TAp73, ΔNP73). The intensity of the band related to the ΔNP73 protein was higher, indicating that the expression of this variant is more abundant compared to the TAp73 variant in respiratory epithelial air-liquid interface (ALI) culture (Figure S4). The loss-of-function variants reported here all affect both the TAp73 as well as the ΔNP73 transcripts,5 indicating that the molecular defects reported here are consistent with complete, rather than partial, TP73 deficiency in the reported individuals (Figure 1).
Recessive TP73 variants result in cortical malformation
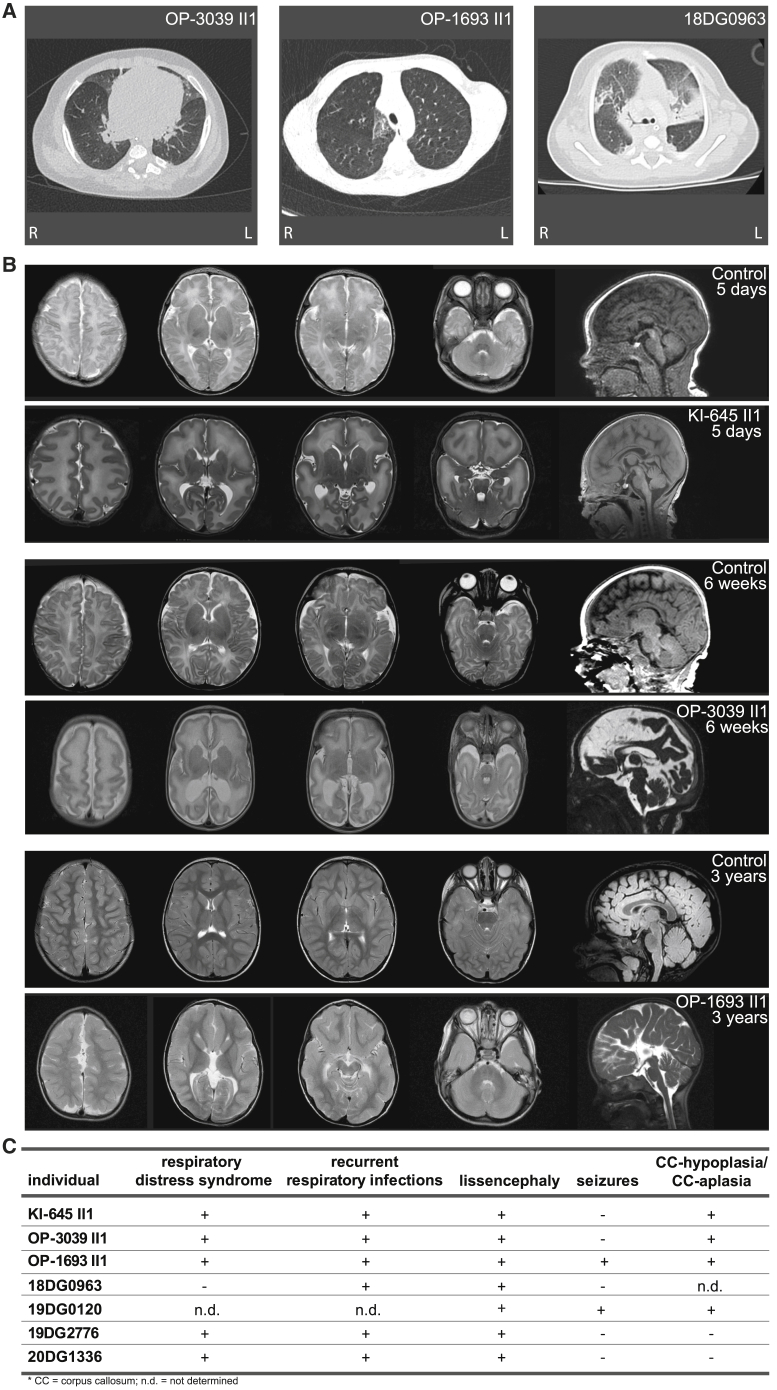
All TP73-deficient individuals exhibited cortical malformation, a group of disorders that result from disturbances of the normal developmental processes of the human cortex21 (Figure 2). Magnetic resonance imaging (MRI) of the brain showed lissencephaly in OP-1693 II1, OP-3039 II1, KI-645 II1, 18DG0963 II1, 19DG2776 II1, and 20DG1336 II1 (Figure 2). Lissencephaly (“smooth brain,” LIS) is a malformation of cortical development associated with deficient neuronal migration and abnormal formation of cerebral convolutions or gyri. The LIS spectrum (MIM: 607432, 257320, 611603, 614019, 615191, 616212, 616342, 617255, 618325, 618873, 247200) includes agyria, pachygyria, and subcortical band heterotopia (SBH). In 2017, a new LIS classification system was introduced by DiDonato et al.22 This classification system uses the (1) gradient of gyral malformation (diffuse, anterior more severe than posterior, posterior more severe than anterior, and temporal more severe than posterior); (2) grade of gyral malformation (SBH partial, SBH diffuse, LIS partial pachygyria, LIS diffuse pachygyria, LIS agyria-pachygyria, LIS diffuse agyria); (3) cortical thickness and appearance (simplified gyration overlying SBH, thin undulating, thin variable dysgyria, thin with enlarged lateral ventricles and thin mantle, thick classic); and (4) presence of non-cortical brain malformations. Based on this classification we here report an anterior (frontal and temporal) predominant “thin” LIS with or without absent or hypoplastic corpus callosum. Other non-cortical brain malformations such as basal ganglia dysgenesis, tectal hyperplasia, brainstem hypoplasia or dysgenesis, and cerebellar hypoplasia were not present. Defects of cortical malformation are common causes of developmental delay and epilepsy.23 The electroencephalograms documented susceptibility for seizures in 19DG0120 II1. All TP73-deficient individuals exhibit central muscular hypotonia and moderate to severe cognitive dysfunction.
Figure 2.
Individuals affected with mutant TP73 variants suffer from a ciliary clearance disorder and cortical malformation
(A) Chest computed tomography scans of OP-3039 II1, OP-1693 II1, and 18DG0963 II1 display chronic airway disease with atelectasis, pneumonia, mucus plugging, and bronchiectasis.
(B) Cranial magnetic resonance imaging of KI-645 II1, OP-3039 II1, and OP-1693 II1 show frontoanterior pachygyria consistent with lissencephaly and malformation of the corpus callosum. Additional dysplasia of the hippocampus but a preserved fornix and commissura anterior was observed for OP-3039 II1.
(C) Summary of clinical findings in the affected individuals.
Enlarged ventricles were described prenatally in OP-1693 II1 and sonography directly after birth confirmed slightly enlarged ventricles, the MRI at the age of 3 years did not exhibit signs of increased volume of cerebrospinal fluid in the ventricles. Neither 19DG0120 II1, 18DG096 II1, nor OP-3039 II1 are reported to suffer from hydrocephalus.
Affected individuals with TP73 variants suffer from a mucociliary clearance disorder and exhibit a reduced number of ciliated respiratory epithelial cells
Next, we carefully characterized the respiratory phenotype of the TP73 mutant individuals (Figure 2). All analyzed TP73 mutant individuals display chronic disease of the upper and lower respiratory tract characterized by recurrent infections of the airways, productive cough, and chronic rhinitis as well as otitis media. In the neonatal period, OP-1693 II1, OP-3039 II1, KI-645 II1, and 20DG1336 II1 had neonatal respiratory distress with atelectasis, and prolonged or persistent ventilation support was necessary for OP-3039 II1, KI-645 II1, and 20DG1336 II1. 20DG1336 II1 died at the age of 2 months due to respiratory failure. Chest radiographs as well as computed tomography scans show situs solitus, chronic lower airway disease with atelectasis, pneumonia, mucus plugging, and bronchiectasis in some (Figure 2).
Trp73-deficient mice have been reported to exhibit upper and lower airway infections due to a mucociliary clearance disorder.24 Severe rhinitis as well as otitis media with chronic sinusitis were reported in the TAp73−/−/Trp73−/− mouse models, respectively.6,25,26 Since the respiratory phenotype observed in TP73 mutant individuals resembled findings present in different Trp73 mutant mouse models,6,26 epithelial cells from TP73 mutant individuals were obtained by nasal brush biopsy for further analyses. Thorough analyses of respiratory epithelial cells (OP-1693 II1, OP-3039 II1, 19DG0120 II1) by high-speed video microscopy (HVMA) showed a highly reduced number of ciliated cells and shortened cilia, when compared to healthy control subjects, indicating that indeed TP73 mutant individuals suffer from a severe defect in MCC differentiation (Videos S1, S2, S3, and S4).
Respiratory cilia are reduced in length and the number of MCCs is reduced in TP73 mutant respiratory epithelia
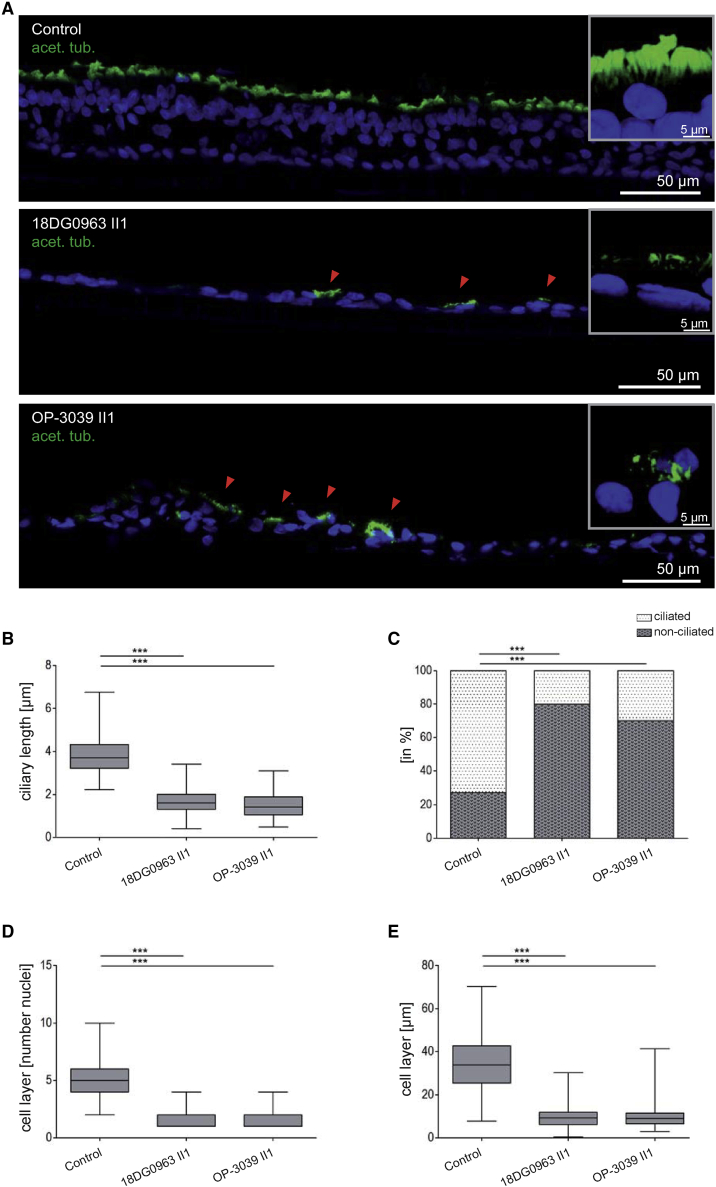
To distinguish between a primary defect of the generation of multiple motile cilia and secondary loss of cilia due to inflammation, we performed in vitro ciliogenesis experiments (spheroid cell cultures) as described previously.12 For that purpose, respiratory epithelial cells were obtained by nasal brush biopsy from TP73 mutants (OP-1693 II1, OP-3039 II1, and 18DG0963 II1) and control individuals. Consistent with a primary defect observed on HVMA, immunofluorescence microscopy (IF) using an antibody directed against acetylated α-tubulin confirmed a severe defect in MCC differentiation with a reduced number of ciliated cells and a reduction in cilia length for TP73 mutant individuals17,27 (Videos S1, S2, S3, and S4; Figures 3 and S5).
Figure 3.
The overall number of MCCs is reduced in spheroids of TP73 mutant respiratory epithelial cells
(A) Ciliary axonemes of respiratory epithelial cells cultured as spheroids (control and OP-1693 II) were stained with antibodies targeting acetylated α-tubulin (acet. tub.; green). Spheroids of OP-1693 II1 display a reduced number of multiciliated cells (MCCs) compared to healthy control subjects. Nuclei were stained with Hoechst33342.
(B) Transmission electron microscopy photographs of spheroids from a healthy control subject and OP-1693 II. TP73-affected cells show an overall reduction of the number of MCCs. While the number of cilia seems to be slightly reduced, the few cilia observed seem to be reduced in length (magnification III). Basal bodies (specialized centrioles) attached to the apical membrane anchoring the cilia in healthy control subjects are occasionally mislocalized within the cytoplasm in individuals affected by mutant TP73 (magnification IV).
To further characterize the defect of cilia generation in TP73 mutant individuals, we performed ultrastructural analyses by transmission electron microscopy (TEM) in respiratory cells from OP-1693 II1, OP-3039 II1, and 18DG0963 II1 as described previously.28 The respiratory cilia appeared stubby and reduced in length (Figure 3). To analyze the reduction in ciliary length in TP73 mutant cilia, we performed IF studies with antibodies directed against acetylated α-tubulin in fully differentiated respiratory cells cultured at the air-liquid interface. These analyses confirmed that ciliary length is significantly reduced in TP73 mutant cells, which is consistent with previous findings in mice26 (Figures 4 and S6).
Figure 4.
Ciliary length and number are reduced in ALI cultures of TP73-affected respiratory epithelia
(A) Respiratory epithelial cells were cultured under air-liquid interface (ALI) condition (control, 18DG0963 II1, OP-3039 II1). Ciliary axonemes were stained with antibodies directed against acetylated α-tubulin (acet. tub.; green) after full differentiation. Nuclei were stained with Hoechst33342. ALI cultures of individuals affected by mutant TP73 show a marked reduction in MCCs compared to healthy control subjects. MCCs are exemplary highlighted by red arrows. Grey boxes represent 3D confocal images of the ALI-cultured cells. Statistical analyses of the respiratory epithelia or cilia are performed as described in supplemental information.
(B) Cilia length was measured of ALI-cultured cells and is significantly reduced for individuals affected by mutant TP73 (18DG0963 II1: 1.8 μm; OP-3039 II1: 1.5 μm). Cilia of healthy control subjects show an average length of 3.8 μm (∗∗∗p < 0.0001; 137–145 cilia were analyzed per person).
(C) To calculate the correlation of ciliated versus non-ciliated cells, the ALI-cultured cells were counted for cilia bundles and the number of nuclei of the first apical cell layer. Under healthy condition, 72.7% of the apical cell layer is covered by MCCs. For ALI-cultured cells of individuals affected by mutant TP73, the apical cell layers represent a significant reduction in MCC-covering rate by 20.1%–29.9% (∗∗∗p < 0.0001; 266–722 cells were counted in total per person).
(D and E) To evaluate the differentiation ability for the individuals affected by mutant TP73, respiratory epithelial cell layers grown on ALI cultures were analyzed by counting the number of nuclei (D) and measuring the distance in μm (E) from basal to apical. TP73-affected cell layers represent a significant reduction in height (18DG0963 II1, OP-3039 II1: two nuclei on average or 7–10 μm, respectively) in comparison to the control. Accordingly, the six-layered control ALI cultures represent an average height of 39.9 μm (∗∗∗p < 0.0001; 66 measuring points per individual were analyzed on average). A detailed description of all statistical data described here can be found in the supplemental information.
To corroborate the finding that beside the shortened cilia also the overall number of MCCs is reduced in TP73 mutant ALI cultures, we analyzed the cell composition of differentiated ALI cultures with antibodies directed against acetylated α-tubulin. Statistical analysis of ciliated versus non-ciliated cells in ALI cultures of TP73 mutant individuals and healthy control subjects revealed significant reduction of MCCs in the ALI cultures of TP73-affected individuals (Figures 4 and S7), confirming the defect in MCC differentiation. While the overall number of basal bodies was not altered, we occasionally found basal bodies mislocalized in the cytoplasm (Figure 3), suggesting that there might be a (mild) basal body positioning defect, as reported in Trp73 −/−-mice previously.26 However, the total number of cilia per MCC was not greatly reduced indicating that there is not a severe defect in basal body amplification or positioning.
In addition, we checked for axonemal defects by TEM. The axonemal composition with nine outer doublets surrounding two single central tubules as well as outer dynein arm morphology appeared normal (Figure S8). To further corroborate the ultrastructural findings that TP73-deficient ciliary axonemes still contain motility-related protein complexes such as outer dynein arms and the nexin dynein regulatory complex, we performed IF analyses with antibodies directed against the outer dynein arm motor protein DNAH5 as well as the nexin-dynein regulatory complex component GAS8.18 DNAH5 and GAS8 were both present in the ciliary axonemes of TP73-deficient respiratory epithelial cells. Thus, axonemal assembly of analyzed motility-related proteins is not severely affected by mutations in TP73 (Figure S9).
To further analyze whether the reduction of MCCs might be due to a defect in the differentiation of respiratory epithelial cells, we analyzed the upper cell layer grown on the ALI-Transwell Inserts. Interestingly, the layer of cells (number of cells, height of cell layer) in TP73 mutant ALI cultures was severely reduced compared to healthy control subjects. This is consistent with previous findings of reduced respiratory epithelial cell growth reported in Trp73 mutant mice6 (Figures 4 and S10), indicative of a severe defect in differentiation of respiratory cells.
Previous work showed that TP73 is a direct regulator for FOXJ1, a transcription factor important for the transactivation of genes encoding proteins involved in MCC differentiation.6 To answer whether in human samples FOXJ1 expression is altered due to TP73 dysfunction, we performed immunofluorescence staining with antibodies directed against FOXJ1. Consistent with previous findings6 in mice, the number of cells expressing the nuclear transcription factor FOXJ1 was severely reduced in individuals affected by mutant TP73 confirming a defect in MCC differentiation (Figures 5 and S11). To gain further evidence to support that TP73 dysfunction results in a reduction of the FOXJ1 transcription machinery necessary to produce MCCs, we investigated RFX2 expression. RFX2 is part of the transcription factor family of RFX proteins and stabilizes the binding of FOXJ1 at the chromatin loops to enable multiciliated cell-specific gene expression.29 Like FOXJ1, the number of RFX2-positive cells was also greatly reduced in TP73 mutant cells (Figure 5). Thus, we conclude that TP73 dysfunction results in a severe defect of MCC differentiation.
Figure 5.
Key factors of the mutliciliogenesis pathway are reduced in respiratory epithelial cells of individuals affected by mutant TP73 in vitro
Air-liquid interface-cultured respiratory epithelial cells were stained for transcription factors of the NOTCH1-dependent pathway of multiciliogenesis29 using antibodies directed against FOXJ1 (A; red) and RFX2 (B; red) (control, 18DG0963 II1 and OP-3039 II1). Ciliary axonemes were marked with antibodies directed against acetylated α-tubulin (acet. tub.; green). Nuclei were stained with Hoechst33342. (A) In healthy control subjects, the transcription factor FOXJ1 locates in nuclei of multiciliated cells (MCCs). For TP73-affected a severely reduced number of FOXJ1 positive nuclei was observed. The FOXJ1 staining in OP-3039 II1 below the cell layer is an artifact staining the ALI-Transwell Insert membrane (serving as growing platform while cultivation) (B) RFX2, transcription factor and interaction partner of FOXJ1, locates in nuclei of MCCs from healthy control subjects as well. The number of RFX2-expressing cells in TP73-affected MCCs is reduced compared to healthy control subjects.
Ciliary airway clearance is severely reduced in TP73 mutant cilia
Having shown that recessive TP73 variants results in defective MCC differentiation, we aimed to study the functional impact for ciliary clearance. To mimic the process of airway clearance in vitro, we added fluorescent particles to the apical compartments of the ALI-Transwell Inserts from OP-3039 II1 as well as a healthy control subject to perform particle-tracking experiments similar to those reported previously.11 The velocity of the particle transport (μm/s) as well as the mean square displacement (μm2) were severely reduced compared to the control indicating that the cilia in the individuals with mutant TP73 were not able to generate a sufficient and directed fluid flow to propel the fluorescent particles along the surface of the differentiated airway epithelium (Figure 6). Thus, we conclude that TP73 dysfunction results in a severe defect in MCC differentiation responsible for abnormal ciliary clearance of the airways explaining the chronic destructive respiratory disease in the reported individuals.
Figure 6.
TP73 mutant respiratory epithelial cells are not able to transport particles by ciliary beating in vitro
To mimic the process of ciliary clearance function in vitro, respiratory epithelial cells from a healthy control subject and OP-3039 II1 cultured at air-liquid interface were used for particle-tracking experiments (A). Tracking videos are represented as z stack projections, while the transport direction of each particle is summarized in polar graphs (B and C). Statistical evaluation was performed for speed (μm/s) and mean square displacement (μm2). Under healthy condition, particles are transported consequently and directed along the cell layer. In contrast, a highly reduced transport of particles was observed for OP-3039 II1, indicating a reduced ciliary clearance capacity (Mann Whitney test; two-tailed: p < 0.0001). In total, thirty 20 s videos were analyzed per individual. Scale bars represent 20 μm, ∗∗∗p value < 0.0001.
Discussion
All affected individuals reported in this work suffer from a LIS variant due to recessive loss-of-function variants in TP73 (Figures 1 and S1). The detected variants probably cause complete TP73 deficiency because they are predicted to disrupt both transcripts encoding the isoforms with (TAp73) and without the transactivation domain (ΔNP73). The LIS phenotype reported here with anterior (frontal and temporal) predominant “thin” LIS appears to be distinct from other reported LIS variants. Some individuals present with absent or hypoplastic corpus callosum (Figure 2). Other non-cortical brain malformations such as basal ganglia dysgenesis, tectal hyperplasia, brainstem hypoplasia or dysgenesis, and cerebellar hypoplasia were not present.
Interestingly, the cortical malformation present in indiviudals with TP73 mutations is distinct from the findings in mouse models. While the predominant finding in mice was a severe progression of hydrocephalus ex vacuo,30 hydrocephalus is missing in most affected humans. Instead, a severe lissencephaly phenotype is present. It is important to note that rodents are lissencephalic per se and therefore it is difficult to study cortical development and especially gyrification in this model organism. Nevertheless, Trp73−/− mice exhibit cortical malformation with cortical thinning as well as hippocampal dysgenesis.26 Interestingly, mice with isoform-specific targeted mutations resulting solely in the absence of the transactivation domain containing isoform (ΔNP73) or its presence (TAp73) result in more subtle neurological defects indicating that both major TP73 isoforms perform crucial roles for neurogenesis.30 Studies in Trp73−/− mice have shown the critical role of TP73 for the maintenance of the neural stem cell pool and also for the generation as well as differentiation of mature neurons.30 Similar defects in neurogenesis with reduced cortical thickness and hippocampal defects have been observed in other mouse models such as Lis1hGFAP-Cre or Tuba1a(Jna/+) heterozygous mutants mimicking human LIS phenotypes.31 Based on our findings, we conclude that the functional role of TP73 for neurogenesis has been evolutionarily conserved from rodents to man.
Interestingly, all seven TP73 mutant individuals from the five reported families suffered from a severe destructive airway disease in addition to LIS (Figure 2). We here demonstrate that ciliary clearance of the airways in these individuals is severely hampered due to a defect in differentiation of MCCs explaining the chronic airway disease present in TP73 mutant individuals (Figures 4 and 6). We and others have characterized a heterogeneous group of inherited disorders with abnormal generation or function of motile cilia and flagella referred to as motile ciliopathies.8 Individuals with either abnormal beating of respiratory cilia (primary ciliary dyskinesia [PCD] [MIM: 244400]) or reduced generation of multiple motile cilia of the airways (reduced generation of multiple motile cilia [RGMC]) suffer from a chronic destructive airway disease consistent with respiratory findings observed in TP73 mutant individuals. Utilizing immunofluorescence as well as ciliary beat pattern analyses and/or TEM, we did not find abnormalities indicating the diagnosis of PCD such as defects of the outer or inner dynein arms, the nexin link-dynein regulatory complex (Figures S8 and S9), the radial spokes, or the central pair associated apparatus (data not shown). However, our in vitro ciliogenesis experiments using ALI and spheroid cell cultures clearly demonstrate a severe defect of cilia generation consistent with RGMC. The ciliary axonemes are reduced in length and some basal bodies appeared to be mislocalized and not correctly targeted to the apical cell region (Figures 3 and 6). This defect appeared to be less severe than the cellular RGMC defects observed in MCIDAS mutant individuals, whose respiratory cells are devoid of most or all multiple motile cilia,27 but more severe than in FOXJ1 mutant individuals who can still build reduced amounts of multiple motile cilia.11 The defect in TP73 mutant individuals also differs from RGMC due to CCNO mutations, because in those individuals mainly basal body generation and targeting to the cell surface is defective.17 The axonemal length in the few CCNO mutant cilia produced appears normal. Thus, the RGMC defect present in TP73 mutant individuals with severely hampered MCC differentiation and reduced axonemal growth is fairly unique.
Mice deficient for Trp73 exhibit sterility, hippocampal dysgenesis, hydrocephalus, and chronic infection as well as inflammation in the lungs, sinus, and ears.25 The infections of the upper and lower airways resemble the findings in TP73 mutant individuals consistent with the ciliary defects in MCCs reported in Trp73−/−, TAp73−/−, and ΔNP73−/− mutant mice. These findings indicate that the functional role of TP73 for MCC generation has been evolutionarily conserved from rodents to man.6,26 Both studies also showed a strong reduction of ciliated epithelial cells and provided evidence that TRP73 acts upstream of the master ciliogenesis transcription factor FOXJ1. Work in Xenopus shows that Foxj1-binding DNA motifs are mainly located at distal regulatory elements/enhancers, but rarely at core gene promoters to build motile cilia, while RFX2 sites are found at both enhancers and promoters. Thus, interaction of FOXJ1 with core ciliogenesis gene promoters largely depends on the presence of RFX2, which forms homo- and heterodimers with other Rfxs.29 We therefore assessed FOXJ1 and RFX2 levels in TP73 mutant ALI cultures, finding marked reduction when compared to controls, thus supporting the findings obtained in Xenopus and mice (Figure 5). Overall, our findings indicate that TP73 acts upstream of FOXJ1 and downstream of MCIDAS. Thus, we hypothesize that TP73 associated with the NOTCH1-dependent pathway is crucial for multiciliogenesis in humans.26 Mutations in MCIDAS, CCNO, FOXJ1, and TP73 can result in distinct RGMC variants responsible for severe chronic destructive airway disease.
Interestingly, multiciliogenesis and motile cilia also play a role in many cell types distinct from the respiratory epithelium.8 The epithelial cells lining the airways, the ependyma, or the female fallopian tubes are covered with multiple motile cilia moving in a coordinated beating pattern to move fluids along their surface. The sperm flagella also resemble the structure of a motile cilium. Inborn defects of motile cilia are referred to as motile ciliopathies.8 Due to impaired ciliary function in the female or male reproductive system, individuals with mutant TP73 might suffer from fertility problems as reported in Trp73−/− mice.25 However, the TP73-mutant individuals reported here are too young to answer those issues. Furthermore, in the past we and others have shown that multiciliogenesis defects can be associated with hydrocephalus.27,28,32
TP73 is a well-studied protein, which has been in focus of cancer research due to its structural similarities with the tumor suppressor TP53. In comparison to TP53, however, TP73 is rarely mutated in cancer.5 TAp73 functions also as a tumor suppressor that acts in part through induction of cell cycle arrest and apoptosis, and through regulation of genomic stability.5 Consistently, aged TAp73-deficient mice develop spontaneous lymphoma and lung cancer with increased frequency and have enhanced sensitivity to chemical carcinogenesis,2 whereas the anti-apoptotic ΔNP73 can promote cancer. Furthermore, human small cell lung cancer is characterized by recurrent somatic genomic rearrangements in TP73. Interestingly, in Trp73−/− mice missing both isoform classes, increased frequency of tumors has not been reported.5 In this study, none of the affected individuals is reported to suffer from any tumor. However, all affected individuals are still very young and therefore we cannot exclude that tumors might develop at later ages.
In summary, we here report a syndromic LIS variant associated with a severe defect of MCC differentiation responsible for a severe destructive airway disease. Our research findings highlight the importance for clinicians to be aware of the fact that a genetic defect can cause both a neurological as well as a respiratory disorder and would therefore like to emphasize that pediatric neurologists as well as pediatric pulmonologists need to consider this motile ciliopathy with complex phenotypes.8
Declaration of interests
The authors declare no competing interests.
Acknowledgment
We would kindly like to thank the affected individuals for participating in the study. We would like to thank Andreas Borgscheiper, Martina Herting, Sironi Sivalingam, Laura Schwiddessen, Kai Wohlgemut, and Anja Robbers for the technical support. H.O. received funding from the Deutsche Forschungsgemeinschaft (DFG; OM6/7, OM6/8, OM6/10, OM6/14, and DFG clinical research unit 326 project OM6/11), the Interdisziplinaeres Zentrum für Klinische Forschung (IZKF) Muenster (Om2/015/16, Om2/10/20), and the European Commission (LYSOCIL, Horizon2020 GA ID 811087 and Registry Warehouse, Horizon2020 GA ID 777295). J.W. received funding from the DFG (WA 4283/1-1), “Innovative Medical Research” of the University of Muenster Medical School (WA 1 2 14 18), and “Dekanat der Medizinischen Fakultät der WWU” and Interdisziplinären Zentrum für Klinische Forschung Muenster (IZKF) SEED/017/21.
Published: June 1, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2021.05.002.
Web resources
GraphPad Software, http://www.graphpad.com
OMIM, https://www.omim.org/
Supplemental information
References
- 1.Lane D.P. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 2.Vikhreva P., Melino G., Amelio I. Elsevier; 2018. Alternative Splicing: Exploring a Biological Role for the C-Terminal Isoforms; p. 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moll U.M., Slade N. p63 and p73: roles in development and tumor formation. Mol. Cancer Res. 2004;2:371–386. [PubMed] [Google Scholar]
- 4.Senoo M., Pinto F., Crum C.P., McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 5.Nemajerova A., Moll U.M. Tissue-specific roles of p73 in development and homeostasis. J. Cell Sci. 2019;132:132. doi: 10.1242/jcs.233338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall C.B., Mays D.J., Beeler J.S., Rosenbluth J.M., Boyd K.L., Santos Guasch G.L., Shaver T.M., Tang L.J., Liu Q., Shyr Y. p73 Is Required for Multiciliogenesis and Regulates the Foxj1-Associated Gene Network. Cell Rep. 2016;14:2289–2300. doi: 10.1016/j.celrep.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spassky N., Meunier A. The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 2017;18:423–436. doi: 10.1038/nrm.2017.21. [DOI] [PubMed] [Google Scholar]
- 8.Wallmeier J., Nielsen K.G., Kuehni C.E., Lucas J.S., Leigh M.W., Zariwala M.A., Omran H. Motile ciliopathies. Nat. Rev. Dis. Primers. 2020;6:77. doi: 10.1038/s41572-020-0209-6. [DOI] [PubMed] [Google Scholar]
- 9.Olbrich H., Schmidts M., Werner C., Onoufriadis A., Loges N.T., Raidt J., Banki N.F., Shoemark A., Burgoyne T., Al Turki S., UK10K Consortium Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am. J. Hum. Genet. 2012;91:672–684. doi: 10.1016/j.ajhg.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altmüller J., Motameny S., Becker C., Thiele H., Chatterjee S., Wollnik B., Nürnberg P. A systematic comparison of two new releases of exome sequencing products: the aim of use determines the choice of product. Biol. Chem. 2016;397:791–801. doi: 10.1515/hsz-2015-0300. [DOI] [PubMed] [Google Scholar]
- 11.Wallmeier J., Frank D., Shoemark A., Nöthe-Menchen T., Cindric S., Olbrich H., Loges N.T., Aprea I., Dougherty G.W., Pennekamp P. De Novo Mutations in FOXJ1 Result in a Motile Ciliopathy with Hydrocephalus and Randomization of Left/Right Body Asymmetry. Am. J. Hum. Genet. 2019;105:1030–1039. doi: 10.1016/j.ajhg.2019.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olbrich H., Horváth J., Fekete A., Loges N.T., Storm van’s Gravesande K., Blum A., Hörmann K., Omran H. Axonemal localization of the dynein component DNAH5 is not altered in secondary ciliary dyskinesia. Pediatr. Res. 2006;59:418–422. doi: 10.1203/01.pdr.0000200809.21364.e2. [DOI] [PubMed] [Google Scholar]
- 13.Munye M.M., Shoemark A., Hirst R.A., Delhove J.M., Sharp T.V., McKay T.R., O’Callaghan C., Baines D.L., Howe S.J., Hart S.L. BMI-1 extends proliferative potential of human bronchial epithelial cells while retaining their mucociliary differentiation capacity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;312:L258–L267. doi: 10.1152/ajplung.00471.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirst R.A., Rutman A., Williams G., O’Callaghan C. Ciliated air-liquid cultures as an aid to diagnostic testing of primary ciliary dyskinesia. Chest. 2010;138:1441–1447. doi: 10.1378/chest.10-0175. [DOI] [PubMed] [Google Scholar]
- 15.Raidt J., Wallmeier J., Hjeij R., Onnebrink J.G., Pennekamp P., Loges N.T., Olbrich H., Häffner K., Dougherty G.W., Omran H., Werner C. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur. Respir. J. 2014;44:1579–1588. doi: 10.1183/09031936.00052014. [DOI] [PubMed] [Google Scholar]
- 16.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallmeier J., Al-Mutairi D.A., Chen C.T., Loges N.T., Pennekamp P., Menchen T., Ma L., Shamseldin H.E., Olbrich H., Dougherty G.W. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Genet. 2014;46:646–651. doi: 10.1038/ng.2961. [DOI] [PubMed] [Google Scholar]
- 18.Olbrich H., Cremers C., Loges N.T., Werner C., Nielsen K.G., Marthin J.K. Loss-of-Function GAS8 Mutations Cause Primary Ciliary Dyskinesia and Disrupt the Nexin-Dynein Regulatory Complex. Am. J. Hum. Genet. 2015;97:546–554. doi: 10.1016/j.ajhg.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omran H., Kobayashi D., Olbrich H., Tsukahara T., Loges N.T., Hagiwara H., Zhang Q., Leblond G., O’Toole E., Hara C. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature. 2008;456:611–616. doi: 10.1038/nature07471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monies D., Abouelhoda M., Assoum M., Moghrabi N., Rafiullah R., Almontashiri N., Alowain M., Alzaidan H., Alsayed M., Subhani S. Lessons Learned from Large-Scale, First-Tier Clinical Exome Sequencing in a Highly Consanguineous Population. Am. J. Hum. Genet. 2019;104:1182–1201. doi: 10.1016/j.ajhg.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desikan R.S., Barkovich A.J. Malformations of cortical development. Ann. Neurol. 2016;80:797–810. doi: 10.1002/ana.24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Donato N., Chiari S., Mirzaa G.M., Aldinger K., Parrini E., Olds C., Barkovich A.J., Guerrini R., Dobyns W.B. Lissencephaly: Expanded imaging and clinical classification. Am. J. Med. Genet. A. 2017;173:1473–1488. doi: 10.1002/ajmg.a.38245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barkovich A.J., Kuzniecky R.I., Dobyns W.B., Jackson G.D., Becker L.E., Evrard P. A classification scheme for malformations of cortical development. Neuropediatrics. 1996;27:59–63. doi: 10.1055/s-2007-973750. [DOI] [PubMed] [Google Scholar]
- 24.Killick R., Niklison-Chirou M., Tomasini R., Bano D., Rufini A., Grespi F., Velletri T., Tucci P., Sayan B.S., Conforti F. p73: a multifunctional protein in neurobiology. Mol. Neurobiol. 2011;43:139–146. doi: 10.1007/s12035-011-8172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang A., Walker N., Bronson R., Kaghad M., Oosterwegel M., Bonnin J., Vagner C., Bonnet H., Dikkesk P., Sharpe A. P73-Deficient mice have neurological, Pheromonal and Inflammatory Defects but lack spontaneous tumours. Development. 1993;117:1321–1331. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 26.Nemajerova A., Kramer D., Siller S.S., Herr C., Shomroni O., Pena T., Gallinas Suazo C., Glaser K., Wildung M., Steffen H. TAp73 is a central transcriptional regulator of airway multiciliogenesis. Genes Dev. 2016;30:1300–1312. doi: 10.1101/gad.279836.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boon M., Wallmeier J., Ma L., Loges N.T., Jaspers M., Olbrich H., Dougherty G.W., Raidt J., Werner C., Amirav I. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Commun. 2014;5:4418. doi: 10.1038/ncomms5418. [DOI] [PubMed] [Google Scholar]
- 28.Ibañez-Tallon I., Pagenstecher A., Fliegauf M., Olbrich H., Kispert A., Ketelsen U.-P., North A., Heintz N., Omran H. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 2004;13:2133–2141. doi: 10.1093/hmg/ddh219. [DOI] [PubMed] [Google Scholar]
- 29.Quigley I.K., Kintner C. Rfx2 Stabilizes Foxj1 Binding at Chromatin Loops to Enable Multiciliated Cell Gene Expression. PLoS Genet. 2017;13:e1006538. doi: 10.1371/journal.pgen.1006538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niklison-Chirou M.V., Killick R., Knight R.A., Nicotera P., Melino G., Agostini M. Humana Press Inc; 2016. How Does p73 Cause Neuronal Defects? [DOI] [PubMed] [Google Scholar]
- 31.Romero D.M., Bahi-Buisson N., Francis F. Genetics and mechanisms leading to human cortical malformations. Semin. Cell Dev. Biol. 2018;76:33–75. doi: 10.1016/j.semcdb.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 32.Amirav I., Wallmeier J., Loges N.T., Menchen T., Pennekamp P., Mussaffi H., Abitbul R., Avital A., Bentur L., Dougherty G.W., Israeli PCD Consortium Investigators Systematic Analysis of CCNO Variants in a Defined Population: Implications for Clinical Phenotype and Differential Diagnosis. Hum. Mutat. 2016;37:396–405. doi: 10.1002/humu.22957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.