Abstract
Background
Excessive supraventricular ectopic activity (ESVEA), defined as ≥720 premature atrial contractions (PAC) per day or any runs of ≥20 PACs, has been proposed as a surrogate marker for paroxysmal atrial fibrillation (PAF).
Objective
We aimed to estimate the prognostic impact of ESVEA on the future development of PAF in consecutive patients referred to ambulatory cardiac monitoring.
Methods
The cohort consists of a population with comorbidities referred to 48-hour ambulatory electrocardiogram aged 30–98 (n = 1316) between 2009 and 2011. After exclusion of known or current atrial fibrillation (AF) (n = 527) and patients with pacemakers (n = 7), 782 patients were included, with a median follow-up of 8.1 years. Events of incident AF and death were retrieved from patient records.
Results
Mean age was 58.6 ± 15.5 years and 56.5% were women. A total of 101 patients had ESVEA at baseline (12.9%). During follow-up, 69 (8.9%) developed incidental AF. Twenty-three patients with ESVEA developed AF (23%). Incidence rate of AF in patients with and without ESVEA was 37.1/1000 person-years and 9.1 per 1000 person-years, respectively (P < .001). ESVEA was associated with incident AF after adjustment for potential confounders in Cox regression analysis (hazard ratio [HR]: 2.39; 95% confidence interval [CI]: 1.40–4.09) and in competing risk analysis with death as competing risk (subdistribution HR: 2.35; 95% CI: 1.30–4.17).
Conclusion
ESVEA increases the risk of incident AF substantially in a population referred to ambulatory cardiac monitoring.
Keywords: Atrial fibrillation, Epidemiology, Premature atrial contractions, Risk stratification, Survival analysis
Key Findings.
-
▪
Holter finding of excessive atrial ectopy increased the risk of incident atrial fibrillation (AF) in follow-up.
-
▪
The risk of incident AF in follow-up seems to be correlated to the number of premature atrial contractions (PACs) per day or runs of PACs in a dose-response magnitude.
-
▪
Patients who develop incident AF during follow-up are equal in risk factors and extent of atrial ectopy to patients with previously diagnosed paroxysmal atrial fibrillation.
Introduction
The prevalence of atrial fibrillation (AF) is expected to increase substantially in the future.1,2 Timely detection is important in prevention of related morbidity such as thromboembolic events, heart failure, and the associated mortality.3 Frequent atrial ectopy (AE) has in numerous studies been associated with an increased risk of incident AF4, 5, 6, 7, 8 and ischemic stroke9,10 even beyond evident AF.11 However, the majority of studies investigating AE and incident AF are from community-based epidemiological cohort studies or in patients with ischemic stroke in the search of AF.12, 13, 14 The European Heart Rhythm Association (EHRA) has, in a consensus paper on device-detected subclinical arrhythmias endorsed by the Heart Rhythm Society, suggested that excessive supraventricular ectopic activity (ESVEA), defined as ≥720 premature atrial contractions (PAC) per day or any runs of ≥20 PACs, can be considered a surrogate marker of paroxysmal atrial fibrillation (PAF).15
We sought to estimate the prognostic value of having ESVEA and risk of AF in a consecutive cardiac ambulatory population referred to 48 hours of continuous electrocardiogram (ECG) recording.
Methods
Study population
The present study is a retrospective noninterventional observational study of consecutive patients (n = 1316) aged 30–98 years who had a 48-hour ambulatory continuous ECG recording between December 2009 and October 2011 at the Department of Cardiology, Bispebjerg Hospital, Copenhagen, Denmark. Bispebjerg Hospital is a secondary care facility that serves a population of approximately 460,000 citizens. The continuous ECG recording was performed at the discretion of a cardiologist, as part of the clinical work-up in patients referred from the primary sector, from other hospital departments, or from our own unit. Reasons for referral were recorded at entry. They included 4 domains: (1) palpitations; (2) syncope, dizziness, episodes of falling, dyspnea; (3) efficacy of antiarrhythmic treatment (also in conditions other than AF); and (4) unspecified suspicion of arrhythmia. Notably, this cohort does not include patients with recent ischemic stroke who were being screened for AF.
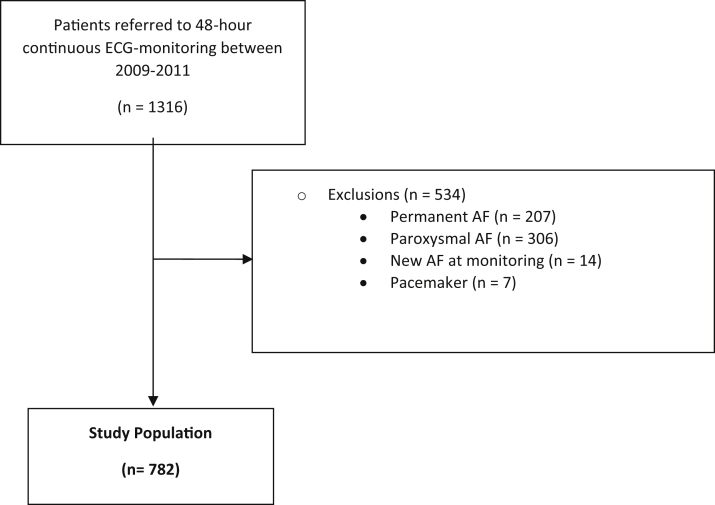
After exclusion of permanent AF, either known or during the recording (n = 207); PAF at baseline, either known or discovered during recording (n = 320); and patients with pacemakers because of missing data on pacing, pacing modus, and reason for pacemaker (n = 7), 782 patients remained for analysis (Figure 1).
Figure 1.
Flow diagram of inclusion. AF = atrial fibrillation; ECG = electrocardiogram.
In a sensitivity analysis, we reincluded the patients with known PAF (n = 306) to assess baseline similarities and differences. In the sensitivity analysis the patients were divided into 3 groups according to AF status at baseline and follow-up: (1) known PAF or PAF at baseline Holter (n = 306); (2) sinus rhythm and incidental AF at follow-up (n = 69); and (3) sinus rhythm and no AF follow-up (n = 713).
The database was approved by the local institutional review board at the Department of Cardiology at Bispebjerg Hospital, as part of internal quality assurance, and written informed consent was thus waived by the institutional review board. The research in this study was conducted according to the Helsinki Declaration guidelines on human research.
Data collection and definitions
At baseline medical history, cardiovascular risk factors, demographic data, medication, and results from any related echocardiography or blood sample during the present work-up were obtained from patient records. Continuous ECG recording was performed using Holter recorder Rozinn RZ-151Y (Scottcare Cardiovascular Solutions, Cleveland, OH). The recordings were analyzed by nurses with specialized training and were reviewed and approved by cardiologists with electrophysiological competence. Median value of acceptable recording was 44.25 hours, interquartile range (Q1–Q3) 43.2–46.3 hours. Events of incident AF and death were retrieved from patient records during follow-up in March 2019. The diagnosis of incident AF was verified with documentation in the form of ECG, telemetry, or a new ICD-10 entry of I48.x. No distinction was made between AF and atrial flutter. Median follow-up was 8.1 years. In keeping with definitions mentioned by EHRA, ESVEA was defined as ≥720 PACs per day or any supraventricular run of ≥20 PACs.
Statistical methods
Continuous variables with a normal distribution are presented as mean ± standard deviation (SD). Data not normally distributed are presented as median with interquartile range (Q1–Q3). Categorical variables are represented by frequencies and percentages. Student t test, analysis of variance (ANOVA), Pearson χ2 test, Fisher exact test, Wilcoxon rank sum test, and Kruskal-Wallis test were used for the comparison of groups as appropriate.
To estimate the cause-specific hazard of ESVEA in relation to the outcome of incidental AF with death as a censored event, we used the Cox proportional hazard model and the log-rank test to test for equality of the survivor function. Owing to the high numbers of death in our population, we used the cumulative incidence function, based on the Aalen Johansen method, to depict the cumulative incidence of AF over time, stratified according to ESVEA, PACs per day, or run of PACs, with death as the competing event. The groups were compared using the Gray test. The Fine-Gray subdistribution hazard model estimated the relative effect of ESVEA adjusted for selected covariates on the probability of AF, with death as competing risk. Confounding covariates in Cox regression models and competing risk models were selected based on their relationship with ESVEA and by risk factors associated with AF. Thus, the final models included age, sex, diabetes, hypertension, ischemic heart disease, and ischemic stroke. In a sensitivity analysis, we examined all patients who had left atrium size determined by echocardiography. This reduced the population to n = 598 patients. Schoenfeld residuals were used to assess the proportional hazard assumption in all models and were not violated. Receiver operating characteristics (ROC) curves were used to assess the cutoff that yielded the highest area under curve (AUC) based on the highest Youden index. Any 2-tailed P value < .05 was considered significant. Statistical analysis was performed using STATA version 13.1 (StataCorp, College Station, TX.)
Results
Study population
A total of 782 patients with no prior AF were included. The mean age was 58.6 ± 15.5 years and 56.5% were women. One hundred and one patients had ESVEA; 75 patients had ≥720 PACs per day, and 45 patients had at least 1 run of supraventricular tachycardia of ≥20 PACs. Nineteen patients had both abnormalities. At baseline, patients with ESVEA were older, had a higher prevalence of hypertension and use of blood pressure medication, had a higher CHA₂DS₂-VASc score, and were more likely to have had prior ischemic stroke and ischemic heart disease. There was no difference in the referral symptoms (Table 1).
Table 1.
Baseline characteristics stratified according to ESVEA
| N | All |
- ESVEA |
+ ESVEA |
P value |
|---|---|---|---|---|
| 782 | 681 | 101 | ||
| Age (years) | 58.6 ± 15.5 | 57.2 ± 15.0 | 68.0 ± 15.5 | <.001 |
| Sex (female) | 442 (56.5%) | 391 (57.4%) | 51 (50.5%) | .19 |
| CHA₂DS₂-VASc | 2 (1, 3) | 2 (1, 3) | 3 (1, 4) | <.001 |
| Hypertension | 347 (44.4%) | 287 (42.1%) | 60 (59.4%) | .001 |
| Ischemic stroke | 54 (6.9%) | 42 (6.2%) | 12 (11.9%) | .035 |
| Diabetes mellitus | 93 (11.9%) | 81 (11.9%) | 12 (11.9%) | .99 |
| Ischemic heart disease | 95 (12.1%) | 73 (10.7%) | 22 (21.8%) | .001 |
| Hypercholesterolemia | 278 (35.5%) | 236 (34.7%) | 42 (41.6%) | .17 |
| INDICATION FOR MONITORING | ||||
| Palpitations | 247 (31.6%) | 215 (31.5%) | 32 (31.6%) | .98 |
| Dizziness, syncope | 387 (49.5%) | 343 (50.4%) | 44 (43.6%) | .20 |
| Efficacy of treatment | 20 (2.6%) | 19 (2.8%) | 1 (1.0%) | .28 |
| Unspecified suspicion of arrhythmia | 128 (16.3%) | 104 (15.3%) | 24 (23.8%) | .031 |
| MEDICATIONS | ||||
| ACE/ARB | 215 (27.5%) | 180 (26.4%) | 35 (34.7%) | .084 |
| Beta-blocker | 163 (20.8%) | 144 (21.1%) | 19 (18.8%) | .59 |
| Diuretics | 171 (21.9%) | 138 (20.3%) | 33 (32.7%) | .005 |
| Calcium channel blocker | 142 (18.2%) | 116 (17.0%) | 26 (25.7%) | .034 |
| Statins | 248 (31.7%) | 205 (30.1%) | 43 (42.6%) | .012 |
| Aspirin | 235 (30.1%) | 204 (30.0%) | 31 (30.7%) | .88 |
| ADP-receptor antagonists | 63 (8.1%) | 50 (7.3%) | 13 (12.9%) | .057 |
| PACs/day | 16 (0.5, 96) | 9.5 (0, 48) | 1423 (690, 3708) | <.001 |
| Longest run of PACs | 0 (0, 6) | 0 (0, 5) | 14 (5, 28) | <.001 |
Values are mean ± SD, n (%), or median (Q1–Q3).
AF = atrial fibrillation; ACE = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; ESVEA = excessive supraventricular ectopic activity; PACs = premature atrial contractions.
Follow-up and endpoints
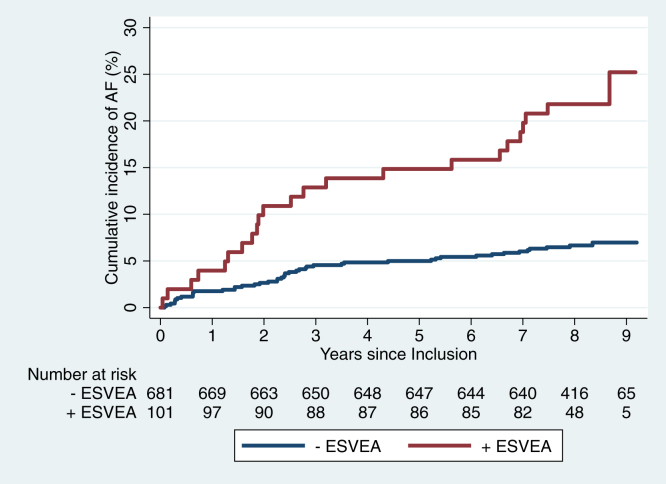
During a median follow-up of 8.1 years, 150 patients died. Sixty-nine patients developed incidental AF (8.8%; 69/782). Of the patients with ESVEA 23 developed incidental AF (23%; 23/101), compared to 46 in the group without ESVEA (6.7%; 46/681). The incidence rate of AF was 37.1 per 1000 person-years in patients with ESVEA and 9.1 per 1000 person-years in patients without ESVEA (P < .001). The crude hazard ratio (HR) was 3.94 (95% confidence interval [CI]: 2.39–6.51). After adjustment for age, sex, diabetes, hypertension, ischemic stroke, and ischemic heart disease, HR was 2.38 (95% CI: 1.39–4.07) (Table 2). Figure 2 shows the cumulative incidence of AF in patients with and without ESVEA. In the competing risk model, with death as competing event, ESVEA was significantly associated with an increased relative incidence of AF after adjustment for conventional risk factors (Table 2). The sensitivity and specificity of ESVEA in AF prediction was 33.3% (95% CI: 22.4%–45.7%) and 89.1% (95% CI: 86.5%–91.3%), respectively.
Table 2.
Cox regression and competing risk regression
| Cause-specific hazard (Cox regression) | Subdistribution hazard model (Fine & Gray) | |
|---|---|---|
| Endpoint | AF with death as censored event | AF with death as competing event |
| ESVEA univariate | 3.94 (2.39–6.51) | 3.63 (2.21–5.97) |
| ESVEA adjusted† | 2.38 (1.39–4.07) | 2.35 (1.30–4.17) |
Values are hazard ratio or subdistribution hazard (95% confidence interval).
AF = atrial fibrillation; ESVEA = excessive supraventricular ectopic activity.
Adjusted for age, sex, diabetes, hypertension, ischemic heart disease, and ischemic stroke.
Figure 2.
Cumulative incidence of atrial fibrillation (AF) according to excessive supraventricular ectopic activity (ESVEA). The risk of AF was greater in patients with ESVEA (P < .001).
ROC analysis as indicated by the highest Youden index showed an optimal cut point of 34.4 PACs per day with an AUC of 0.69 (95% CI: 0.64–0.75) and a sensitivity of 72% (95% CI: 60.4%–82.5%) and specificity of 66% (95% CI: 62.5%–69.5%).
Risk of AF associated with increasing number of PACs or runs of supraventricular tachycardia at baseline
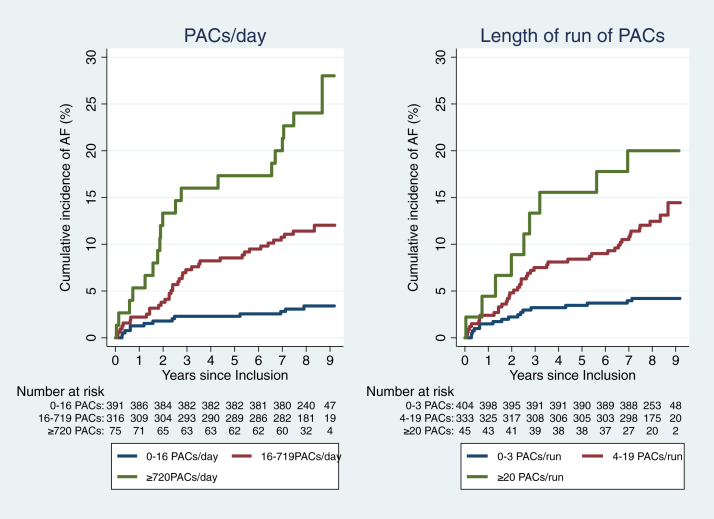
The cumulative incidence of AF increased with increasing number of PACs per day based on percentiles of 0–50th, 50th–90th, >90th (P for trend < .001.) Similarly, the cumulative incidence of AF increased according to runs of PACs based on centiles of 0–50th, 50th–95th, >95th, as shown in Figure 3 (P for trend < .001.)
Figure 3.
Cumulative incidence of atrial fibrillation (AF) according to premature atrial contractions (PACs) per day and length of run of PACs. The risk of AF increased with increasing number of PACs per day (P < .001) and length of run of PACs (P < .001).
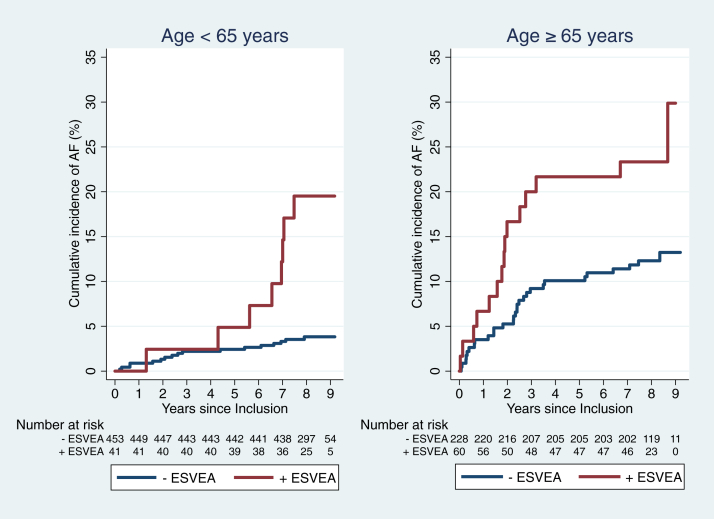
Risk of AF associated with increasing age and ESVEA
Patients with ESVEA had a significantly higher incidence rate of AF compared to those without, regardless of age. Patients younger than 65 years without ESVEA had a low risk of developing AF (Table 3). However, as displayed in Figure 4, it seems that detection of AF in patients younger than 65 years with ESVEA is delayed compared to patients older than 65 years. Of the 8 patients with ESVEA who developed incidental AF in the age group <65 years, 4 patients (50%) were aged 65 years or older at the time of AF diagnosis. The prevalence of ESVEA is correspondingly higher in patients aged ≥65 years (20.8% vs 8.3% in those <65 years, P < .001).
Table 3.
Incidence rates of atrial fibrillation stratified on age groups and ESVEA
| N | AF, n | Time at risk (years) | AF per 1000 person-years (95% CI) | P value† | |
|---|---|---|---|---|---|
| Age <65 n = 494 | |||||
| - ESVEA | 453 | 17 | 3613 | 4.7 (3.0–7.9) | Reference |
| + ESVEA | 41 | 8 | 300 | 26.7 (14.0–56.3) | <.001 |
| Age ≥65 n = 288 | |||||
| - ESVEA | 228 | 29 | 1441 | 20.1 (14.0–29.7) | Reference |
| + ESVEA | 60 | 15 | 320 | 46.9 (28.0–82.6) | .0104 |
AF = atrial fibrillation; ESVEA = excessive supraventricular ectopic activity.
P value is the log-rank test for equality of survivor function.
Figure 4.
Cumulative incidence of atrial fibrillation (AF) according to excessive supraventricular ectopic activity (ESVEA) and age. The risk of AF associated with ESVEA seemed to have an earlier onset in patients aged ≥65 years (P < .001 and P = .0164, respectively).
Sensitivity analysis
In order to assess whether left atrial enlargement (LAE) influenced the results, we did a subgroup analysis in those with available echocardiographic data and without known AF (n = 598). LAE was defined as >34 mL/m2 as a dichotomized variable. One hundred and five patients (17.5%) had LAE. Of these, 20 (19.1%) also had ESVEA. In Cox regression including LAE, ESVEA remained significantly associated with incident AF (HR: 2.49; 95% CI: 1.38–4.52) and in competing risk analyses (subdistribution hazard: 2.43; 95% CI: 1.27–4.66) after adjustment for age, sex, and hypertension in both models. There was no statistical interaction between LAE and ESVEA (P = .24). When assessing patients with both abnormalities compared to those with only LAE or ESVEA, the incidence rate and cumulative incidence of AF were comparable (Supplemental Table A1 and Supplemental Figure A1).
We also analyzed patient characteristics of patients with known PAF compared to patients who developed incidental AF. Before continuous ECG recording, 292 patients had known PAF. Of these, 48 patients (16.4%) had episodes of AF at the time of continuous ECG recording. Fourteen patients had newly discovered PAF at baseline recording, amounting to 306 patients or 28.1% of the population being defined as known PAF at baseline. Supplemental Table A2 shows the baseline characteristics of the patients with PAF at baseline, incidental AF during follow-up, and patients with no known AF at either time point. Patients with incidental AF at follow-up shared many of the same characteristics as patients with AF at baseline. A total of 28.1% of the patients with known PAF had ESVEA at baseline, compared to 33.3% in the patients who developed AF during follow-up (P = .39). Furthermore, of the 86 patients with ESVEA and known PAF, only 32 patients (37%) had episodes of AF during recording.
Discussion
Main findings
In a cardiac ambulatory population with various cardiovascular risk factors referred to 48-hour continuous ECG recording, ESVEA was associated with an increased risk of developing incident AF. In competing risk models, the association with ESVEA remained, signifying a higher relative incidence of AF accounting for the competing risk of death. The cumulative incidence of AF increased according to increasing rate of PACs per day and duration of runs of PAC, indicating a dose-response relationship. AF seemed to be detected earlier in patients with ESVEA aged ≥65 years. These results resonate with previous findings that increased AE either detected by continuous ECG recording or as a PAC present on a 12-lead ECG is a risk factor in developing AF.4, 5, 6, 7, 8
Value of excessive atrial ectopy as a diagnostic test of future risk of atrial fibrillation
When considering ESVEA as a dichotomous diagnostic test of future incident AF in this cardiac outpatient population, ESVEA indicates a high specificity but a low sensitivity. Thus, absence of ESVEA correlates with a low probability of developing incident AF, whereas presence of ESVEA does not imply certainty of future incident AF. As such, ESVEA cannot be considered a surrogate marker of incident AF. However, association to subclinical AF remains unknown; furthermore, ESVEA has been shown to increase the risk of ischemic stroke beyond incident AF and the level of risk was comparable with AF.11 Thus, there are arguments to consider ESVEA as an entity riskwise similar to AF or potentially identical to undiagnosed PAF, rather than only a precursor of AF.
Although we show that the risk of incident AF seems to increase in a dose-response-like manner with increasing burden of PAC, the cutoff value to identify patients at highest risk of AF varies significantly between different cohorts. Chong et al5 showed in 428 consecutive patients with symptoms similar to this population who were referred to elective 24-hour ECG recording, patients with more than 100 PACs per day, equivalent to the top quartile in that population, had an increased risk of future incident AF. Johnson et al8 showed in a asymptomatic screening population of 383 subjects that a doubling of PAC count was significantly associated with future AF. In ROC analysis assessing the cutoff with the highest AUC according to PACs per day in this cohort, the threshold was lower at 34.4 PACs per day, compared to the ESVEA definition of ≥720 PACs per day. This higher AUC, however, comes at the cost of lower specificity (66.1% vs 89.1%), and hence more false-positives, with an improved sensitivity, and thus fewer false-negatives (72% vs 33.3%). However, if this definition were used as the cutoff value for what constituted excessive AE, 37.2% of this population (n = 291) would be classified as such. We believe using a lower threshold of 34.4 PACs per day would compromise the diagnostic utility of what defines “excessive atrial ectopy.” In this context a more recent consensus document from EHRA on the management of asymptomatic arrhythmias ≥500 PACs per day is defined as the thresholds that should prompt further investigation for possible AF and cardiovascular risk factor modification.16 However, the threshold of ≥500 PACs per day was primarily derived from the EMBRACE study, which investigated the predictive value of excessive PACs in predicting AF in patients with cryptogenic stroke. One pragmatic approach could be the application of different cutoffs in different patient populations, depending on the background pretest probability of AF and the clinical implication of revealing such an arrhythmia. Hence, in patients with ≥500 PACs per day on initial monitoring and either a recent stroke or a CHA2DS2VASc of >1, it would seem sensible to recommend follow-up to include intensive cardiovascular risk factor modification and prolonged continuous ECG monitoring to detect possible subclinical AF. In otherwise healthy subjects, the suggested threshold of ESVEA at ≥720 PACs per day would likely entail a higher hit rate for identification of individuals at risk. Further exploration is warranted into the thresholds of ESVEA and patient characteristics for consideration of implantable loop recorder. Also, whether patients with ESVEA and CHA2DS2VASc of >1 would benefit from anticoagulative treatment as either primary or secondary stroke prevention remains to be shown.
Known PAF and prevalence of ESVEA
Interestingly, 28% of the patients with known PAF also had ESVEA by definition, and of these, only 37% (32/86) had any episode of AF during recording. Furthermore, patients with known PAF and those who developed incident AF during follow-up had a similar daily burden of PAC at baseline compared to patients who did not develop AF. To support this finding, a study by Brooks et al17 found that patients with PAF and persistent AF had a higher median PAC burden than controls without AF (48–69 PACs per day compared to 19 PACs per day). This suggests that ESVEA could be a concomitant intermediate state between episodes of PAF in a subset of patients. The association between increased AE burden and a higher risk of ischemic stroke, which we and others have previously demonstrated,9, 10, 11 could possibly be associated with nondetected subclinical AF in a subset of patients demonstrating ESVEA.
ESVEA and structural disease of the atria
Excessive AE could also be an electrocardiographic marker of an underlying fibrotic atrial cardiomyopathy where AF is a mere manifestation and not necessarily the primary driver of a diseased atrium, as suggested by Kottkamp.18 In support of this interpretation, a study by John et al19 demonstrated that subjects with frequent PAC (>100 per 24 hours) and no AF were associated with both structural and functional changes in the left atrium (LA) with an increased LA volume index and reduced LA strain compared with controls. In our subgroup analysis there was no statistical interaction between LAE and ESVEA on a multiplicative scale, and adding LAE to the model did not attenuate the results, suggesting that ESVEA increases the risk of incident AF independently of LAE. The incidence of AF was similar in patients with combined or isolated structural or electrical atrial abnormalities, but numbers are small and the analysis potentially lacks power, and therefore warrants caution in interpretation.
It seems, as is true for AF, that ESVEA and “increased atrial ectopy” represent heterogenous conditions in which some patients progress to a more advanced disease state and others do not. Further efforts to identify high-risk patients are warranted, preferably combining patient risk factors and clinical markers of electrical and structural abnormalities from different modalities (electrocardiographic recording, echocardiographic measures of atrial dysfunction, and imaging markers of left atrial late gadolinium enhancement from cardiac magnetic resonance imaging). Such indication of underlying atrial cardiomyopathy may help select patients that potentially could profit from intensified primary or secondary prevention of AF and ischemic stroke.
Limitations
Owing to the nature of this study as an internal quality assurance, selection of examinations was not done systematically, but was at the discretion of the treating physician. This results in a lack of data on risk factors associated with incident AF. Hence, a degree of residual confounding cannot be excluded, with missing data on body mass index, smoking status, sleep apnea, and ablation procedures. Moreover, blood biomarkers and measures from echocardiography were not available for all included patients. A proportion of patients were receiving beta blockers (20%) and this might have influenced the results from the 48-hour ECG recording. Incidence of AF was retrieved from patient records and is thus highly specific, but we cannot exclude cases of subclinical AF, as only AF detected in a clinical setting was determined and no systematic follow-up examinations were performed. Thus, the reported incidence rate of AF may be underestimated. This assumption, however, only strengthens the argument to use risk markers such as ESVEA to select patients in which follow-up could be relevant irrespective of symptoms. In this cohort there was a very low incidence of ischemic stroke in the follow-up (20 patients), and further analysis of this endpoint was considered futile.
The population studied is confined to the greater area of Copenhagen, and extrapolation to other populations should be done with caution. The definition of excessive supraventricular ectopic activity has varied substantially in the literature. We have applied a cutoff previously established to carry prognostic importance similar to actual AF.4,11
Conclusion
In a consecutive population referred to ambulatory continuous ECG recording between 2009 and 2011 at a single center, ESVEA increases the likelihood of incident AF substantially. One-third of all patients with incidental AF had ESVEA at index recording. Considering the projected increase in prevalence and hence morbidity of AF, detection of ESVEA should prompt further investigation, especially in patients aged >65 years. It remains unknown whether high-risk patients with ESVEA and CHA₂DS₂-VASc score >1 would benefit from anticoagulant therapy in either primary or secondary prevention of ischemic stroke.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
The database was approved as part of internal quality assurance and written informed consent was thus waived by the institutional review board.
Ethics Statement
The research in this study was conducted according to the Helsinki Declaration guidelines on human research. The database was approved by the local institutional review board at the Department of Cardiology at Bispebjerg Hospital.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2021.04.002.
Appendix. Supplementary data
References
- 1.Colilla S., Crow A., Petkun W. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Krijthe B.P., Kunst A., Benjamin E.J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGrath E.R., Kapral M.K., Fang J. Association of atrial fibrillation with mortality and disability after ischemic stroke. Neurology. 2013;81:825–832. doi: 10.1212/WNL.0b013e3182a2cc15. [DOI] [PubMed] [Google Scholar]
- 4.Binici Z., Intzilakis T., Nielsen O.W. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. doi: 10.1161/CIRCULATIONAHA.109.874982. [DOI] [PubMed] [Google Scholar]
- 5.Chong B.-H., Pong V., Lam K.-F. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace. 2012;14:942–947. doi: 10.1093/europace/eur389. [DOI] [PubMed] [Google Scholar]
- 6.Dewland T.A., Vittinghoff E., Mandyam M.C. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013;159:721–728. doi: 10.7326/0003-4819-159-11-201312030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murakoshi N., Xu D., Sairenchi T. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170–178. doi: 10.1093/eurheartj/ehu407. [DOI] [PubMed] [Google Scholar]
- 8.Johnson L.S.B., Juhlin T., Juul-Möller S. A prospective study of supraventricular activity and incidence of atrial fibrillation. Heart Rhythm. 2015;12:1898–1904. doi: 10.1016/j.hrthm.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 9.O’Neal W.T., Kamel H., Kleindorfer D. Premature atrial contractions on the screening electrocardiogram and risk of ischemic stroke: the Reasons for Geographic and Racial Differences in Stroke Study. Neuroepidemiology. 2016;47:53–58. doi: 10.1159/000448619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada S., Lin C.Y., Chang S.L. Risk of stroke in patients with short-run atrial tachyarrhythmia. Stroke. 2017;48:3232–3238. doi: 10.1161/STROKEAHA.117.018475. [DOI] [PubMed] [Google Scholar]
- 11.Larsen B.S., Kumarathurai P., Falkenberg J. Excessive atrial ectopy and short atrial runs increase the risk of stroke beyond incident atrial fibrillation. J Am Coll Cardiol. 2015;66:232–241. doi: 10.1016/j.jacc.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Weber-Krüger M., Gröschel K., Mende M. Excessive supraventricular ectopic activity is indicative of paroxysmal atrial fibrillation in patients with cerebral ischemia. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochhäuser S., Dechering D.G., Dittrich R. Supraventricular premature beats and short atrial runs predict atrial fibrillation in continuously monitored patients with cryptogenic stroke. Stroke. 2014;45:884–886. doi: 10.1161/STROKEAHA.113.003788. [DOI] [PubMed] [Google Scholar]
- 14.Gladstone D.J., Dorian P., Spring M. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke. 2015;46:936–941. doi: 10.1161/STROKEAHA.115.008714. [DOI] [PubMed] [Google Scholar]
- 15.Gorenek B., Bax J., Boriani G. Device-detected subclinical atrial tachyarrhythmias: definition, implications and management - An European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad L. Europace. 2017;19:1556–1578. doi: 10.1093/europace/eux163. [DOI] [PubMed] [Google Scholar]
- 16.Arnar D.O., Mairesse G.H., Boriani G. Management of asymptomatic arrhythmias: a European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of So. Europace. 2019;1–32 doi: 10.1093/europace/euz046. [DOI] [PubMed] [Google Scholar]
- 17.Brooks A.G., Rangnekar G., Ganesan A.N. Characteristics of ectopic triggers associated with paroxysmal and persistent atrial fibrillation: evidence for a changing role. Heart Rhythm. 2012;9:1367–1374. doi: 10.1016/j.hrthm.2012.03.062. [DOI] [PubMed] [Google Scholar]
- 18.Kottkamp H. Fibrotic atrial cardiomyopathy: a specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications. J Cardiovasc Electrophysiol. 2012;23:797–799. doi: 10.1111/j.1540-8167.2012.02341.x. [DOI] [PubMed] [Google Scholar]
- 19.John A.G., Hirsch G.A., Stoddard M.F. Frequent premature atrial contractions impair left atrial contractile function and promote adverse left atrial remodeling. Echocardiography. 2018;35:1310–1317. doi: 10.1111/echo.14026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.