Abstract
Background
Ventricular tachycardia (VT) catheter ablation success may be limited when transcutaneous epicardial access is contraindicated. Surgical ablation (SurgAbl) is an option, but ablation guidance is limited without simultaneously acquired electrophysiological data.
Objective
We describe our SurgAbl experience utilizing contemporary electroanatomic mapping (EAM) among patients with refractory VT storm.
Methods
Consecutive patients with recurrent VT despite antiarrhythmic drugs (AADs) and prior ablation, for whom percutaneous epicardial access was contraindicated, underwent open SurgAbl using intraoperative EAM guidance.
Results
Eight patients were included, among whom mean age was 63 ± 5 years, all were male, mean left ventricular ejection fraction was 39% ± 12%, and 2 (25%) had ischemic cardiomyopathy. Reasons for surgical epicardial access included dense adhesions owing to prior cardiac surgery, hemopericardium, or pericarditis (n = 6); or planned left ventricular assist device (LVAD) implantation at time of SurgAbl (n = 2). Cryoablation guided by real-time EAM was performed in all. Goals of clinical VT noninducibility or core isolation were achieved in 100%. VT burden was significantly reduced, from median 15 to 0 events in the month pre- and post-SurgAbl (P = .01). One patient underwent orthotopic heart transplantation for recurrent VT storm 2 weeks post-SurgAbl. Over mean follow-up of 3.4 ± 1.7 years, VT storm–free survival was achieved in 6 (75%); all continued AADs, although at lower dose.
Conclusion
Surgical mapping and ablation of refractory VT with use of contemporary EAM is feasible and effective, particularly among patients with contraindication to percutaneous epicardial access or with another indication for cardiac surgery.
Keywords: Epicardial ablation, Surgical ablation, Ventricular arrhythmia, Ventricular tachycardia
Key Findings.
-
▪
Open surgical access for catheter ablation is a reasonable bail-out approach for managing refractory ventricular arrhythmias in selected patients.
-
▪
Surgical ablation guided by intraoperative, contemporary electroanatomic mapping can help to refine ablation strategy and may improve outcomes in patients with limited treatment options for ventricular tachycardia management.
-
▪
Surgical ablation with electrophysiology mapping should be considered for those with refractory epicardial or midmyocardial ventricular arrhythmias and structural heart disease, and for whom transcutaneous epicardial access is limited or concurrent cardiac surgery is planned.
Introduction
Catheter ablation is an effective means for acute and long-term control of ventricular tachycardia (VT), especially when antiarrhythmic drugs (AADs) have failed.1, 2, 3 Techniques to safely achieve percutaneous epicardial access, as well as to promote deeper lesion size, have significantly augmented the ability to treat VTs that primarily involve the epicardium or midmyocardium.4, 5, 6, 7, 8, 9 However, nonsurgical epicardial access is not always feasible, particularly in cases in which significant epicardial adhesions exist, such as among those who have undergone prior cardiac surgery or have a history of pericarditis. For these cases, surgical ablation (SurgAbl) has been performed as a bailout strategy, often in the context of previously attempted endocardial catheter ablation, or in conjunction with another planned cardiac surgery, such as left ventricular assist device (LVAD) implantation, following which subsequent epicardial access for ablation would be far more challenging.10, 11, 12, 13, 14
However, in the absence of real-time electroanatomic mapping (EAM), guidance about location and extent of ablation is limited to visual identification of scarred regions by the surgeon or by preoperative data acquired in the electrophysiology (EP) laboratory or with cardiac imaging. The feasibility of performing surgical cryoablation guided by use of contemporary EAM has been demonstrated12; however, cases reported to date have been limited in number and have not incorporated a technique with which to visualize cryoprobes, which are commonly used in open SurgAbl of arrhythmias, within the EAM, at the time of ablation.
In the present study, we present our contemporary approach and associated outcomes for open surgical VT ablation, utilizing intraoperative EAM and including a method to more directly visualize ablation cryoprobe location within the EAM system, among selected patients with recurrent and refractory VT storm.
Methods
Study population
Consecutive patients with AAD-refractory, recurrent VT and contraindication to or failed transcutaneous epicardial access attempt for catheter ablation, and who underwent SurgAbl guided by EAM at the University of Colorado Hospital between 2015 and 2019 were included in the present study. All had suspected epicardial or midmyocardial free-wall substrate for VT, based on results from prior EP study and catheter ablation, 12-lead electrocardiogram (ECG) characteristics, or presence of nonischemic cardiomyopathy.15,16 All patients also had ≥1 of the following characteristics: (1) prior cardiac surgery; (2) failed attempt at percutaneous epicardial access or mapping owing to pericardial adhesions; (3) planned, concurrent LVAD implantation. Data collection and analysis were approved by the institutional review board of the University of Colorado School of Medicine. Written informed patient consent was waived owing to the use of retrospective and de-identified data. The study adhered to the guidelines set forth in the Declaration of Helsinki.
Procedural logistics
All procedures were performed with a multidisciplinary team that included cardiothoracic (CT) surgery, cardiac EP, cardiac anesthesiology, and a perfusion team. Most cases were performed in the operating room and using a portable EAM system (NavX or Precision; Abbott, St. Paul, MN); 1 case performed in the EP laboratory utilized the Carto EAM system (Biosense Webster, Diamond Bar, CA). For the latter case, percutaneous epicardial access had been attempted in the EP laboratory but failed; thus the procedure was converted to open SurgAbl. In that patient, via right femoral vein access, a quadripolar catheter and intracardiac echocardiography catheter were positioned within the right ventricle. General anesthesia was used in all cases, following which open surgical epicardial access was obtained via lateral thoracotomy or median sternotomy by and at the discretion of the CT surgeon. Cardiopulmonary bypass was instituted only in those in whom LVAD was anticipated. ECG lead and EAM as well as defibrillator patch placements were dictated by the planned surgical approach; precordial ECG leads thus often were displaced by a few centimeters inferior to standard position to accommodate the planned surgical window. Once all surgical procedures were completed, closure was performed by the CT surgeon.
Mapping and ablation
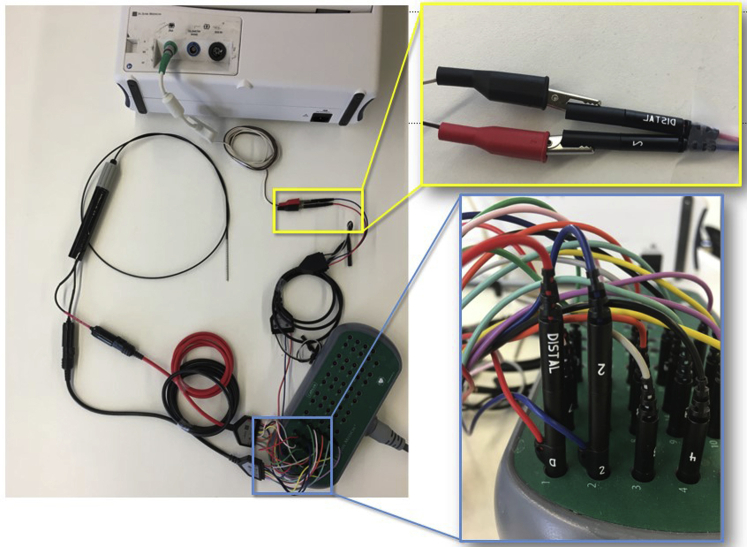
After epicardial access was achieved surgically, the opened chest cavity was filled with sterile lactated ringer’s solution in order to minimize distortion within the impedance-based EAM while mapping,12 and EAM was performed by the cardiac electrophysiologist by manually sweeping a multipolar mapping catheter (Livewire Steerable Duo-Deca or Advisor HD Grid, Abbott, or DecaNav, Biosense Webster) along the epicardial surface. Epicardial voltage maps were created in sinus rhythm, identifying low-amplitude electrograms using bipolar amplitude cut-offs of <0.5 mV for dense scar and 0.5–1.0 mV as border zone tissue;15 activation maps for late potential timing, as previously defined,17 were also performed in sinus rhythm. For all patients in whom VT did not initiate spontaneously or during mapping, ventricular programmed electrical stimulation was performed via the patient’s existing implantable cardioverter-defibrillator or, in the case of the patient for whom the procedure was performed in the EP laboratory, via the right ventricular quadripolar catheter. Pacing drives of 600 and 400 ms were used, followed by up to triple extrastimuli. Activation mapping and entrainment were performed during VT, guided by regions of abnormality noted during voltage mapping, as well as hemodynamic stability in VT. A 4-pin jumper cable and pacing system analyzer alligator-clip connector used in standard device implantation were used to connect any desired electrode pair on the mapping catheter to an Abbott device programmer, through which pacing and entrainment could be performed (Figures 1 and 2C).
Figure 1.
Displayed is the configuration created which allowed simultaneous visualization of the mapping catheter within the electroanatomic mapping system and stimulation from any electrode pair on the catheter for bipolar pacing via the Abbott (St. Paul, MN) pacing system analyzer (PSA). Modifying elements included alligator clips (yellow outlined inset) connecting the PSA to a pair of pins from a 4-pin jumper cable; the corresponding continuation of those pins were connected to desired pacing electrodes from the mapping catheter into the pin block.
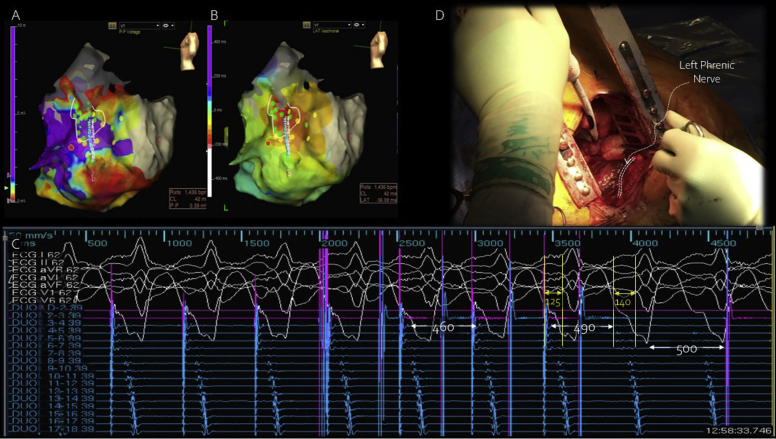
Figure 2.
Data shown are from patient 1, including sinus rhythm voltage map (A) and activation map (B) during ventricular tachycardia; with the duodecapolar mapping catheter positioned over the area of earliest activation and voltage abnormality, entrainment from the distal bipoles was performed with concealed fusion. (C) Postpacing interval 10 ms shorter than the tachycardia cycle length (490–500 ms) likely resulted from high-output pacing and increased virtual electrode size, leading to capture of tissue deeper within the circuit as well as near-field signal, as evidenced by shorter stimulus-QRS vs EGM-QRS timing (yellow lines and arrows), producing a shorter return cycle than expected but consistent with a central isthmus site.31 Cryoablation over that site (D) led to slowing and termination of tachycardia. Note that the adjacent, extraepicardial left phrenic nerve was protected from cryothermal energy with retraction and mechanical shielding.
Regions with ≥1 of the following characteristics were annotated on the EAM and incorporated into a planned ablation lesion set that would result in both substrate homogenization and core isolation:18,19 (1) low-amplitude bipolar electrograms, as defined above, with evidence for delayed conduction, including local abnormal ventricular activities,20 late potentials with fractionation (≥2 multiple-component deflections separated from surface ventricular activation by >20 ms),21 or prolonged duration (≥130 ms)22; (2) pace maps with 12/12 surface ECG vector match for induced VTs or with alternating exits, ie, different QRS morphologies produced by pacing at the same site18; (3) deceleration zones identified during sinus rhythm activation (≥3 late potential isochrone activation segments contained within a 1 cm radius);23 (4) early activation during VT, or local activation occurring anywhere within the mid-diastolic interval with respect to the surface ECG QRS complexes during VT; or (5) entrainment features consistent with central isthmus behavior.18
Ablation was performed using a linear, malleable cryoprobe in all cases (CryoICE; AtriCure, Inc, Mason, OH; or Cardioblate CryoFlex, Medtronic, Minneapolis, MN). In each case, the cryoprobe was shaped to ensure adequate contact of as much of the probe’s freezing surface as possible within the region of interest and sometimes was shaped into a circle. Fluid added to the chest cavity in order to facilitate mapping was suctioned out before cryoapplications. Lesions were applied for 2–6 minutes each, with temperatures of -80oC to -150oC, varying based on operator discretion, and they were overlapped such that the entire region of interest was targeted. In 2 cases, before cryothermal energy applications, limited radiofrequency (RF) ablation was performed using a standard 3.5-mm-tip open-irrigated RF ablation catheter (ThermoCool SmartTouch SF, Biosense Webster; or FlexAbility, Abbott). Care was made to ensure that the left phrenic nerve was protected from contact with ablation catheters during ablation (Figure 2D). Care was also made to try to avoid ablating immediately over proximal portions of the major coronary arteries, which were identified by the CT surgeon. In the majority of patients, in whom a preexisting wall motion abnormality existed in the region of substrate or in those anticipating LVAD implantation immediately following ablation, we did apply cryothermal energy within 5 mm of more distal portions of a coronary artery, when needed, in order to achieve ablation endpoints. Cryoprobe positioning in our earliest experience (n = 4) was determined anatomically, albeit in correlation with the EAM created, given challenges in direct visualization of epicardial substrate, in part because of varying distributions of epicardial fat. However, we devised a method by which the position of the cryoprobe could be better tracked and indirectly visualized within the NavX/Precision EAM system by securing it to a linear multipolar mapping catheter (LiveWire Steerable Duo-Deca, 2-2-2 spacing). Specifically, we used surgical ties to affix the cryoprobe to the proximal portion of another multipolar catheter, leaving the 3–4 most distal electrodes (total distance ∼11–14mm) free from contact with exposed metal (Figure 3). Only these distal electrodes were displayed within the EAM. In this way, impedance distortion caused by the metallic cryoprobe in direct contact with the proximal electrodes would not be displayed; therefore, the position of the distal mapping catheter, and the cryoprobe attached proximally, could be tracked on the EAM with placement of each cryoablation lesion (Supplemental Video). We compared the location of the decapolar catheter without the cryoprobe attached within the EAM to the subsequent location with the probe attached and confirmed, as best as was feasible, with visual inspection of the decapolar location in both configurations. Real-time measurement of error, approximated using the measurement tool within the EAM, was within 5 mm. We positioned the decapolar/cryoprobe assembly before each delivered lesion to ensure that the distal poles nonadjacent to the cryoprobe extended no farther than ∼13 mm beyond the substrate identified during mapping; the measuring tool within the EAM, as well as visual inspection, were used to verify accuracy of positioning. Lactated ringers solution was added to the chest cavity in between cryothermal energy applications to best confirm positioning, and then it was suctioned out just before cryoapplication to minimize dissipation of cooling away from the myocardium. This method was utilized in the last 4 patients in the series.
Figure 3.
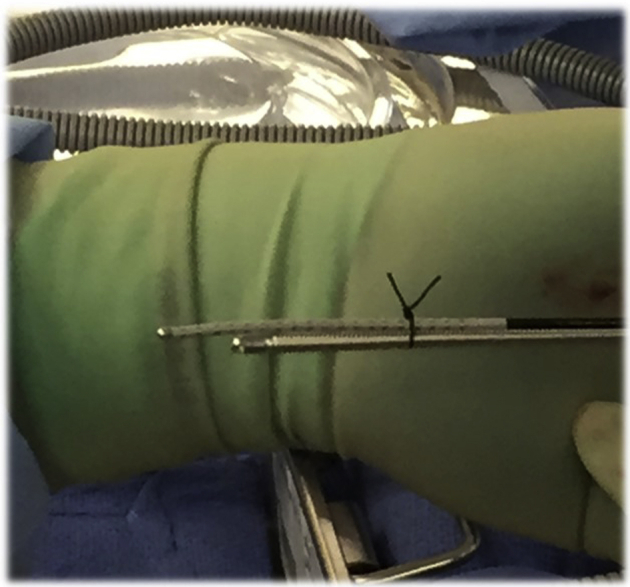
Depicted is the method devised for indirectly visualizing the cryoablation probe within the electroanatomic mapping (EAM) system. Surgical ties were used to secure the cryoprobe to the proximal portion of a multipolar mapping catheter, leaving distal poles free from contact with adjacent metal and associated local impedance and associated EAM position distortion.
Endpoints for ablation included lack of pace-capture throughout ablated regions by pacing at highest output within regions that had previously captured, which functioned as a surrogate for core isolation when local capture without propagation of stimulus within the region of attempted isolation was not definitively observed; and noninducibility of any monomorphic VT upon repeated programmed electrical stimulation, using the same or more aggressive protocol as prior to ablation, and until ventricular effective refractoriness was reached.18
Follow-up
Postoperatively, patients were hospitalized on the CT surgical service and followed by the inpatient EP consult service, until time of hospital discharge. All patients were then followed in the outpatient setting to assess for clinically significant events, including recurrent VT, transplant, and survival. Outpatient visits occurred 4–6 weeks postdischarge, and then every 3 months thereafter, either by in-person clinic visit or device interrogation. Recurrent VT was defined as spontaneous recurrence lasting ≥30 seconds, requiring any appropriate implantable cardioverter-defibrillator therapy, or as documented by telemetry, ECG, ambulatory monitoring, or device recording (for recurrent VTs that were slower than rates programmed for therapy). Dates of VT recurrence, orthotopic heart transplantation (OHT), or death were noted, in addition to the last follow-up date.
Statistical data
Continuous data are expressed as mean ± standard deviation (SD) or median (range), when not normally distributed. Categorical data are expressed as number (%). Two-sided Student t test was used to compare continuous variables. Wilcoxon rank sum test was used for nonparametric comparative testing. Calculations were performed using Microsoft Excel (version 16.32; Microsoft, Redmond, WA).
Results
Baseline characteristics
Baseline characteristics are shown in Table 1. Eight patients were included, all were male, and all presented with VT storm, 3 with incessant VT (multiple sustained episodes treated with antitachycardia pacing in 24 hours, represented by the 3 with highest number of sustained VT episodes pre-SurgAbl, Figure 4). Mean ± standard deviation age was 63 ± 5 years, and left ventricular ejection fraction was 39% ± 12%. Nonischemic etiology was present in 7 (87.5%), with mixed substrate identified on intraprocedural intracardiac echocardiogram in 1, and idiopathic VT in another (Table 1). All had previously failed multiple therapies, with a median (range) of attempted AADs of 2.5 (1–5), and with all having undergone previous catheter ablation, including 6 (75%) who also had history of percutaneous epicardial catheter ablation, 1 of whom had had perforation years prior repaired semi-surgically, with deployment of an atrial septal defect occlusion device. In all patients, recurrent VT morphologies were either identical to or similar to those previously targeted in endocardial or epicardial ablation attempts. Reasons for pursuing SurgAbl were history of cardiac surgery, including the latter patient, as well as 1 with prior open VT SurgAbl (n = 2); history of pericarditis owing to perforation and hemopericardium during previously attempted catheter ablation (n = 1), prior transcutaneous epicardial ablation (n = 2), or history of myopericarditis, with dense adhesions encountered during percutaneous epicardial access attempt (n = 1); and concomitant LVAD implantation planned (n = 2). All patients, except the 2 with prior cardiac surgery or equivalent, had failed attempt at percutaneous epicardial access just before proceeding to open ablation. Only epicardial mapping and ablation were performed in the present cases.
Table 1.
Baseline characteristics
| Patient | Sex | Age (y) | LVEF (%) | Prior AADs | Amiodarone treatment | Cardiomyopathy | Prior catheter ablation endo/epi | Reason for surgical ablation | Incessant VT | VT storm |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 65 | 57 | 4 | Yes | None known | Yes/Yes | H/o surgical ablation | No | Yes |
| 2 | M | 66 | 40 | 4 | Yes | Nonischemic | Yes/Yes | H/o hemopericardium | Yes | Yes |
| 3 | M | 54 | 19 | 5 | Yes | Nonischemic | Yes/No | LVAD planned | Yes | Yes |
| 4 | M | 62 | 40 | 2 | Yes | Mixed | Yes/Yes | H/o hemopericardium | No | Yes |
| 5 | M | 69 | 40 | 3 | Yes | Ischemic | Yes/Yes | H/o extensive epi ablation | No | Yes |
| 6 | M | 62 | 36 | 2 | Yes | Ischemic | Yes/Yes | H/o extensive epi ablation | Yes | Yes |
| 7 | M | 69 | 30 | 2 | Yes | Nonischemic | Yes/No | LVAD planned | No | Yes |
| 8 | M | 60 | 50 | 1 | Yes | Nonischemic | Yes/Yes | Extensive adhesions in prior epi attempt | No | Yes |
AAD = antiarrhythmic drug; Endo = endocardial; Epi = epicardial; H/o = history of; LVAD = left ventricular assist device; LVEF = left ventricular ejection fraction; M = male; VT = ventricular tachycardia.
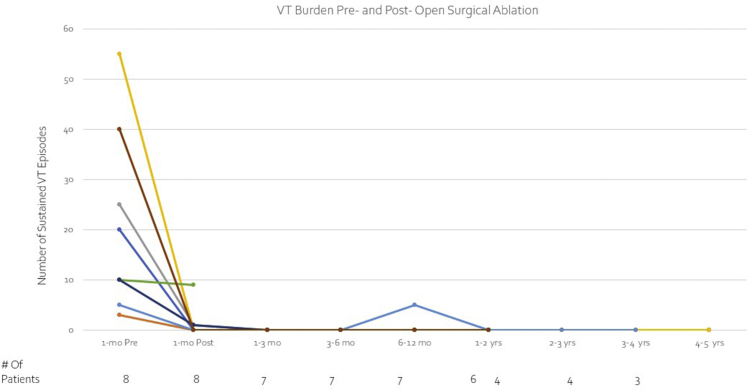
Figure 4.
Significant decrease in overall burden of ventricular tachycardia was observed for most subjects.
Procedural data
As detailed in Table 2, operative access was provided by lateral thoracotomy in 6 patients, most of whom had VT involving inferior or inferolateral left ventricular substrate, and median sternotomy in the remaining 2, both of whom underwent LVAD implantation immediately following the SurgAbl portion of the procedure. Contemporary modalities of EAM were utilized in guiding ablation, including pace mapping, voltage and activation mapping in sinus rhythm, and at least limited entrainment and activation mapping during VT, in all patients. Representative examples are shown in Figure 2, in the Supplementary Video, and in the Supplementary Figure. In all cases, substrate identified during SurgAbl correlated anatomically with sites of ablation in prior procedures.
Table 2.
Procedural data
| Patient | Surgical approach | Substrate location | N VTs targeted | Mapping time (min) | Radiofrequency ablation time (min) | Cryoablation time (min) | Cryoprobe temperature (oC) | Procedural time (min)† | Acute endpoint(s) achieved |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Lateral thoracotomy | Inferolateral LV | 1 | 20 | 0 | 30 | -80 | 240 | Any VT noninducible + exit block |
| 2 | Lateral thoracotomy | Inferior, inferolateral LV | 1 | 30 | 0 | 80 | -80 | 340 | Exit block |
| 3 | Median sternotomy | Inferolateral LV | 1 | 15 | 0 | 16 | -80 | 354 | Any VT noninducible + exit block |
| 4 | Lateral thoracotomy | Inferior, inferolateral LV | 2 | 32 | 5 | 20 | -80 | 436 | Any VT noninducible + exit block |
| 5 | Lateral thoracotomy | Inferolateral LV | 1 | 12 | 0 | 20 | -80 to -150 | 284 | Any VT noninducible + exit block |
| 6 | Lateral thoracotomy | LV summit | 1 | 10 | 2 | 15 | -150 | 313 | Clinical VT noninducible |
| 7 | Median sternotomy | LV summit | 1 | 12 | 0 | 12 | -140 | 353 | Any VT noninducible + exit block |
| 8 | Lateral thoracotomy | Inferolateral LV | 2 | 10 | 0 | 44 | -140 | 215 | Any VT noninducible + exit block |
LV = left ventricle; VT = ventricular tachycardia.
Includes total surgical time.
A median of 1 (range 1–2) VT was targeted per procedure (Table 2). Cryoablation was used in all patients; RF ablation was additionally tried briefly in 2 patients. The technique for indirectly visualizing the cryoprobe in the EAM before each cryoablation lesion (Figure 3, Supplemental Video) was applied in the last 4 patients of the series. Mean mapping and cryoablation times were 17.6 ± 8.9 minutes and 29.6 ± 22.8 minutes, respectively. Acute endpoints of both noninducibility of any VT and exit block/core isolation were achieved in 6 of 8 (75%). In 1 patient, exit block was achieved, but a nonclinical VT was still inducible. Noninducibility of the clinical VT without achieving exit block was accepted as an endpoint for the remaining patient, given length of procedure and concern for hemodynamic compromise. Mean procedure time, including surgical incision, closure, and LVAD implantation, when performed, was 304.5 ± 47.5 minutes; excluding LVAD implantation times, mean SurgAbl time was 288.2 ± 43.3 minutes.
Follow-up
Overall VT burden was significantly reduced postablation, from a median of 15 (range 3–55) events in the month preceding SurgAbl to 0 (range 0–9) in the 12 months following the procedure (P = .03; Figure 4). Table 3 provides details about postprocedural follow-up. Median postoperative length of stay was 8 (range 4–121) days, and all patients survived to discharge. Five patients (62.5%) had complete VT suppression for almost a year following SurgAbl; 1 developed recurrent VT at 332 days, underwent repeat endocardial catheter ablation at that time, and remained free of VT until he died of a noncardiac cause 2.2 years later (3.2 years after SurgAbl). The other 4 without recurrent VT within a year have remained VT-free (range 1.7–5.8 years). Of the 3 patients with acute recurrence, all recurred within a week after SurgAbl, and 2 had only isolated events, which were managed conservatively (continued AADs in both, and decreasing LVAD flow in 1, in whom recurrence was related to pump suction event). One of the latter patients went on to undergo OHT 118 days post-SurgAbl, owing to progressive heart failure. Only 1 patient with recurrence had VT storm and underwent OHT 12 days post-SurgAbl.
Table 3.
Follow-up data
| Patient | Postprocedure length of stay (days) | Recurrent VT | Recurrence with incessant VT | Time to VT recurrence (days) | Repeat catheter ablation | Time to orthotopic heart transplant (days) | Alive at last follow-up | Time to death (years) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | No | -- | -- | -- | -- | Yes | -- |
| 2 | 7 | No | -- | -- | -- | -- | Yes | -- |
| 3 | 21 | Yes | No | 7 | -- | 118 | Yes | -- |
| 4 | 4 | No | -- | -- | -- | -- | Yes | -- |
| 5 | 5 | Yes | No | 332 | Yes | -- | No | 3.2 |
| 6 | 22 | Yes | Yes | 1 | -- | 12 | Yes | -- |
| 7 | 121 | Yes | No | 1 | -- | -- | No | 1.2 |
| 8 | 9 | No | -- | -- | -- | -- | Yes | -- |
VT = ventricular tachycardia.
Excluding the patient who underwent OHT post-SurgAbl for recurrent incessant VT, AADs were continued in all at the time of discharge, including amiodarone in 6 of 7. However, overall daily amiodarone doses were significantly reduced from baseline (mean 577 ± 204 mg pre-SurgAbl, vs 286 ± 146 mg post-SurgAbl, P = .016). After mean follow-up of 3.4 ± 1.7 years, 6 of 8 patients remain alive.
Discussion
We present characteristics and outcomes from a unique cohort of patients presenting to our institution with refractory VT storm and epicardial or free-wall intramural substrate, in whom percutaneous epicardial access was limited or LVAD implantation was imminent. Using an EAM-guided open surgical mapping and ablation process, VT burden was acutely reduced by >90% overall, with durable transplant-free survival from VT storm in 6 (75%) and overall survival free from VT storm in 7 (88%) over a mean follow-up of 3.4 ± 1.7 years. Use of intraoperative, contemporary EAM to guide open SurgAbl is therefore both a feasible and beneficial bailout strategy for controlling refractory ventricular arrhythmias in this challenging cohort of patients. Notably, AADs including amiodarone were often still continued post-SurgAbl, although at significantly reduced doses.
Comparison to prior studies
VT recurrence rates reported among patients with structural heart disease undergoing nonpharmacologic treatments have ranged widely depending on underlying etiology of heart disease, duration of follow-up, and modality of treatment;24 however, the proportion of patients recurring with VT in our series compares favorably to the 30% overall recurrence observed among the International VT Center Collaborative group (>2000 patients worldwide),1 particularly when considering that the majority of the patients in this series had nonischemic substrate, which is known to be more challenging with respect to VT-free survival following ablation.
Although percutaneous epicardial access has been shown to be feasible in patients with a history of significant pericarditis and dense adhesions, including in those who have previously undergone cardiac surgery, access to critical cardiac regions may be limited, and complications that may occur in the process of dissecting adhesions can be substantial.25, 26, 27 Even in hybrid ablations, with minimally invasive (subxiphoid or thoracotomy) but surgical access planned, access to critical sites for catheter-based RF ablation using real-time EAM techniques have still been encumbered by presence of dense adhesions distant from site of surgical exposure, which can be difficult to mechanically dissect, and may also require more extensive surgical exploration, in as many as 15% of patients in whom this technique is pursued.28,29 In one series of 8 patients with refractory VT who underwent open SurgAbl via median sternotomy and maximal cardiac exposure, ablation using EAM data acquired preoperatively in the EP laboratory and cryothermal SurgAbl tools demonstrated dramatic reduction in subsequent treated VT events in almost 2 years of follow-up, although recurrences were noted in half, and the majority surviving were continued on AADs as well.10 For those patients undergoing SurgAbl at the time of LVAD implantation, our results compare favorably to those reported by Mulloy and colleagues,13 among which, in a cohort of 7 patients who underwent concomitant SurgAbl at the time of LVAD, 2 (29%) patients had recurrent VT within a month after treatment, again with ablation guided without direct EAM assistance. In our study, using real-time EAM in the operating room or at the time of SurgAbl, including to guide cryothermal probe placement and energy application, we were able to determine appropriate ablation sites with potentially greater accuracy and achieve VT-free survival in the majority, which included temporizing electrical instability in patients who might not have survived to transplant.
Limitations
Our study population was small, and selection bias was possible given the observational nature of this study. However, all patients included either failed multiple other treatments or were otherwise considered to be too high-risk for treatments alternative to those offered here. Females were not well represented in this study population, which limits generalizability. Accuracy in location of ablation performed was limited by errors of estimation using direct visualization, which were more pronounced in regions of greater epicardial fat or posterior locations, as well as errors within the EAM, including with use of a decapolar catheter to estimate location of the cryoprobe in the selected cases in which this was performed; however, prior studies have relied on direct visual inspection, as well as recall of substrate location based on previously performed EP studies or preprocedural cardiac imaging, each of which also lack accuracy, at least to the same degree or greater.
Noninvasive programmed stimulation, while typically performed within several days after nonsurgical VT ablation to inform need for repeat ablation or modified therapy,30 was not performed in this cohort, as additional immediate treatment would not have been altered, especially repeating ablation in the immediate recovery period. Instead, we mapped and ablated as comprehensively as possible during the surgical procedures and opted to allow for patient recovery, with continued AADs and close clinical follow-up. Determination about VT recurrence was through both symptoms and device interrogation, although, based on programming, VT recurrences might have been missed should asymptomatic recurrences have happened (though with overall reduced amiodarone doses, recurrent episodes would be less likely to have been underdetected).
Conclusion
With multidisciplinary coordination, open SurgAbl with intraoperative mapping including LVAD implantation is a safe treatment option in patients that have significant VT burden despite multiple AAD and prior ablations. SurgAbl is particularly helpful in patients with refractory VT of midmyocardial or epicardial origin in whom percutaneous epicardial access is not feasible.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
Drs Tzou, Nguyen, and Sauer are consultants for or have received speaker’s honoraria or research funding from Abbott, Biosense Webster, Boston Scientific, Biotronik, and Medtronic. Drs Zipse and Borne have received speaker’s honoraria from Medtronic. Mr Davies and Mr Lane are employees of Abbott.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Written informed patient consent was waived owing to the use of retrospective and de-identified data.
Ethics Statement
Data collection and analysis were approved by the institutional review board of the University of Colorado School of Medicine. The study adhered to the guidelines set forth in the Declaration of Helsinki.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2021.05.004.
Appendix. Supplementary data
References
- 1.Tung R., Vaseghi M., Frankel D.S. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: an International VT Ablation Center Collaborative Group study. Heart Rhythm. 2015;12:1997–2007. doi: 10.1016/j.hrthm.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cronin E.M., Bogun F.M., Maury P. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm. 2020;17:e2–e154. doi: 10.1016/j.hrthm.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sapp J.L., Wells G.A., Parkash R. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375:111–121. doi: 10.1056/NEJMoa1513614. [DOI] [PubMed] [Google Scholar]
- 4.Sosa E., Scanavacca M., d'Avila A., Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 5.Aryana A., d'Avila A. Epicardial approach for cardiac electrophysiology procedures. J Cardiovasc Electrophysiol. 2020;31:345–359. doi: 10.1111/jce.14282. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson W.G., Tedrow U.B., Reddy V. Infusion needle radiofrequency ablation for treatment of refractory ventricular arrhythmias. J Am Coll Cardiol. 2019;73:1413–1425. doi: 10.1016/j.jacc.2018.12.070. [DOI] [PubMed] [Google Scholar]
- 7.Tzou W.S., Rothstein P.A., Cowherd M. Repeat ablation of refractory ventricular arrhythmias in patients with nonischemic cardiomyopathy: impact of midmyocardial substrate and role of adjunctive ablation techniques. J Cardiovasc Electrophysiol. 2018;29:1403–1412. doi: 10.1111/jce.13663. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen D.T., Tzou W.S., Sandhu A. Prospective multicenter experience with cooled radiofrequency ablation using high impedance irrigant to target deep myocardial substrate refractory to standard ablation. JACC Clin Electrophysiol. 2018;4:1176–1185. doi: 10.1016/j.jacep.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S., Tedrow U.B., Stevenson W.G. Adjunctive interventional techniques when percutaneous catheter ablation for drug refractory ventricular arrhythmias fail. A contemporary review. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.003676. [DOI] [PubMed] [Google Scholar]
- 10.Anter E., Hutchinson M.D., Deo R. Surgical ablation of refractory ventricular tachycardia in patients with non-ischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:494–500. doi: 10.1161/CIRCEP.111.962555. [DOI] [PubMed] [Google Scholar]
- 11.Cantillon D.J., Bianco C., Wazni O.M. Electrophysiologic characteristics and catheter ablation of ventricular tachyarrhythmias among patients with heart failure on ventricular assist device support. Heart Rhythm. 2012;9:859–864. doi: 10.1016/j.hrthm.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Moss J.D., Oesterle A., Raiman M. Feasibility and utility of intraoperative epicardial scar characterization during left ventricular assist device implantation. J Cardiovasc Electrophysiol. 2019;30:183–192. doi: 10.1111/jce.13803. [DOI] [PubMed] [Google Scholar]
- 13.Mulloy D.P., Bhamidipati C.M., Stone M.L. Cryoablation during left ventricular assist device implantation reduces postoperative ventricular tachyarrhythmias. J Thorac Cardiovasc Surg. 2013;145:1207–1213. doi: 10.1016/j.jtcvs.2012.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang J.J., Betensky B.P., Muser D. Long-term outcome of surgical cryoablation for refractory ventricular tachycardia in patients with non-ischemic cardiomyopathy. Europace. 2018;20:e30–e41. doi: 10.1093/europace/eux029. [DOI] [PubMed] [Google Scholar]
- 15.Cano O., Hutchinson M., Lin D. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54:799–808. doi: 10.1016/j.jacc.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Tzou W.S., Tung R., Frankel D.S. Outcomes after repeat ablation of ventricular tachycardia in structural heart disease: an analysis from the International VT Ablation Center Collaborative Group. Heart Rhythm. 2017;14:991–997. doi: 10.1016/j.hrthm.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Irie T., Yu R., Bradfield J.S. Relationship between sinus rhythm late activation zones and critical sites for scar-related ventricular tachycardia: systematic analysis of isochronal late activation mapping. Circ Arrhythm Electrophysiol. 2015;8:390–399. doi: 10.1161/CIRCEP.114.002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzou W.S., Frankel D.S., Hegeman T. Core isolation of critical arrhythmia elements for treatment of multiple scar-based ventricular tachycardias. Circ Arrhythm Electrophysiol. 2015;8:353–361. doi: 10.1161/CIRCEP.114.002310. [DOI] [PubMed] [Google Scholar]
- 19.Di Biase L., Burkhardt J.D., Lakkireddy D. Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy: the VISTA randomized multicenter trial. J Am Coll Cardiol. 2015;66:2872–2882. doi: 10.1016/j.jacc.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Jaïs P., Maury P., Khairy P. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125:2184–2196. doi: 10.1161/CIRCULATIONAHA.111.043216. [DOI] [PubMed] [Google Scholar]
- 21.Bogun F., Good E., Reich S. Isolated potentials during sinus rhythm and pace-mapping within scars as guides for ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;47:2013–2019. doi: 10.1016/j.jacc.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 22.Cassidy D.M., Vassallo J.A., Miller J.M. Endocardial catheter mapping in patients in sinus rhythm: relationship to underlying heart disease and ventricular arrhythmias. Circulation. 1986;73:645–652. doi: 10.1161/01.cir.73.4.645. [DOI] [PubMed] [Google Scholar]
- 23.Aziz Z., Shatz D., Raiman M. Targeted ablation of ventricular tachycardia guided by wavefront discontinuities during sinus rhythm: a new functional substrate mapping strategy. Circulation. 2019;140:1383–1397. doi: 10.1161/CIRCULATIONAHA.119.042423. [DOI] [PubMed] [Google Scholar]
- 24.Liang J.J., Santangeli P., Callans D.J. Long-term outcomes of ventricular tachycardia ablation in different types of structural heart disease. Arrhythm Electrophysiol Rev. 2015;4:177–183. doi: 10.15420/aer.2015.4.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Killu A.M., Ebrille E., Asirvatham S.J. Percutaneous epicardial access for mapping and ablation is feasible in patients with prior cardiac surgery, including coronary bypass surgery. Circ Arrhythm Electrophysiol. 2015;8:94–101. doi: 10.1161/CIRCEP.114.002349. [DOI] [PubMed] [Google Scholar]
- 26.Tschabrunn C.M., Haqqani H.M., Cooper J.M. Percutaneous epicardial ventricular tachycardia ablation after noncoronary cardiac surgery or pericarditis. Heart Rhythm. 2013;10:165–169. doi: 10.1016/j.hrthm.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Jincun G., Faguang Z., Weibin H., Yan W., Kang D., Tung R. Outside-in subepicardial dissection during percutaneous epicardial ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2016;9:e004499. doi: 10.1161/CIRCEP.116.004499. [DOI] [PubMed] [Google Scholar]
- 28.Li A., Hayase J., Boyle N.G. Hybrid surgical ablation for ventricular arrhythmias. Card Electrophysiol Clin. 2020;12:383–390. doi: 10.1016/j.ccep.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Soejima K., Couper G., Cooper J.M., Sapp J.L., Epstein L.M., Stevenson W.G. Subxiphoid surgical approach for epicardial catheter-based mapping and ablation in patients with prior cardiac surgery or difficult pericardial access. Circulation. 2004;110:1197–1201. doi: 10.1161/01.CIR.0000140725.42845.90. [DOI] [PubMed] [Google Scholar]
- 30.Frankel D.S., Mountantonakis S.E., Zado E.S. Noninvasive programmed ventricular stimilation early after ventricular tachycardia ablation to predict risk of late recurrence. J Am Coll Cardiol. 2012;59:1529–1535. doi: 10.1016/j.jacc.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Tung R. Challenges and pitfalls of entrainment mapping of ventricular tachycardia: ten illustrative concepts. Circ Arrhythm Electrophysiol. 2017;10:e004560. doi: 10.1161/CIRCEP.116.004560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.