Key Points
Question
Do prescribers in China follow established guidelines for prescription of oral anticoagulants (OACs) to patients with ischemic stroke and atrial fibrillation, and has adherence to prescribing guidelines changed over time?
Findings
In this multicenter quality improvement study, among 35 767 eligible patients with ischemic stroke and atrial fibrillation at admission, fewer than 1 in 5 were taking OACs at admission, and of 49 531 eligible patients at discharge, only 41% were prescribed OACs at discharge. Although adherence to OACs has significantly improved over time, it remains suboptimal; the increase was mainly associated with warfarin, not non–vitamin K antagonist OACs.
Meaning
This study suggests that more specific programs educating physicians and patients are needed to ensure that OACs, especially non–vitamin K OACs, are prescribed to eligible patients.
Abstract
Importance
Adherence to oral anticoagulants (OACs) per guideline recommendations is crucial in reducing ischemic stroke and systemic thromboembolism in high-risk patients with ischemic stroke and atrial fibrillation. However, data on OAC use are underreported in China.
Objective
To assess adherence to the Chinese Stroke Association or the American Heart Association/American Stroke Association’s clinical management guideline–recommended prescription of OACs, the temporal improvement in adherence, and the risk factors associated with OAC prescriptions.
Design, Setting, and Participants
This quality improvement study was conducted at 1430 participating hospitals in the Chinese Stroke Center Alliance (CSCA) among patients with ischemic stroke and atrial fibrillation enrolled in the CSCA between August 1, 2015, and July 31, 2019.
Exposure
Calendar year.
Main Outcomes and Measures
Adherence to the Chinese Stroke Association or the American Heart Association/American Stroke Association’s clinical management guideline–recommended prescribing of OACs (warfarin and non–vitamin K OACs, including dabigatran, rivaroxaban, apixaban, and edoxaban) at discharge.
Results
Among 35 767 patients (18 785 women [52.5%]; mean [SD] age, 75.5 [9.2] years) with previous atrial fibrillation at admission, the median CHA2DS2-VASc (cardiac failure or dysfunction, hypertension, age 65-74 [1 point] or ≥75 years [2 points], diabetes, and stroke, transient ischemic attack or thromboembolism [2 points]–vascular disease, and sex category [female]) score was 4.0 (interquartile range, 3.0-5.0); 6303 (17.6%) were taking OACs prior to hospitalization for stroke, a rate that increased from 14.3% (20 of 140) in the third quarter of 2015 to 21.1% (118 of 560) in the third quarter of 2019 (P < .001 for trend). Of 49 531 eligible patients (26 028 men [52.5%]; mean [SD] age, 73.4 [10.4] years), 20 390 (41.2%) had an OAC prescription at discharge, an increase from 23.2% (36 of 155) in the third quarter of 2015 to 47.1% (403 of 856) in the third quarter of 2019 (P < .001 for trend). Warfarin was the most commonly prescribed OAC (11 956 [24.2%]) and had the largest temporal increase (from 5.8% [9 of 155] to 20.7% [177 of 856]). Older age (adjusted odds ratio [aOR] per 5 year increase, 0.89;95% CI, 0.89-0.90), lower levels of education (aOR for below elementary school, 0.84; 95% CI, 0.74-0.95 ), lower income (aOR for ≤¥1000 [$154], 0.66; 95% CI, 0.59-0.73), having new rural cooperative medical scheme insurance (aOR, 0.92; 95% CI, 0.87-0.96), prior antiplatelet use (aOR, 0.70; 95% CI, 0.66-0.74), having several cardiovascular comorbid conditions (including stroke or transient ischemic attack [aOR, 0.78; 95% CI, 0.75-0.82], hypertension [aOR, 0.84; 95% CI, 0.80-0.89], diabetes [aOR, 0.91; 95% CI, 0.83-0.99], dyslipidemia [aOR, 0.87; 95% CI, 0.80-0.94], carotid stenosis [aOR, 0.83; 95% CI, 0.69-0.98], and peripheral vascular disease [aOR, 0.80; 95% CI, 0.71-0.90]), and admission to secondary hospitals (aOR, 0.71; 95% CI, 0.68-0.74) or hospitals located in the central region of China (aOR, 0.80; 95% CI, 0.75–0.84) were associated with not being prescribed an OAC at discharge.
Conclusions and Relevance
This quality improvement study suggests that, despite significant improvement over time, OAC prescriptions remained low. Efforts to increase OAC prescriptions, especially non–vitamin K OACs, are needed for vulnerable subgroups by age, socioeconomic status, and presence of comorbid conditions.
This quality improvement study assesses adherence to guideline-recommended oral anticoagulants, the temporal improvement in adherence, and the risk factors associated with oral anticoagulant prescriptions.
Introduction
Atrial fibrillation (AF) is a known cardiac risk factor for ischemic stroke.1,2,3 Findings from contemporary registry-based observational studies from various geographical regions have consistently shown that patients with ischemic stroke and AF have worse prognoses, higher rates of medical and neurologic complications, and higher case fatality rates than those without AF.4,5,6,7 Nevertheless, stroke associated with AF is mostly preventable, and the use of oral anticoagulants (OACs), including warfarin and non–vitamin K oral anticoagulants (NOACs), was associated with effectively reducing ischemic stroke and systemic thromboembolism in high-risk patients with AF.8,9,10 However, the prescribing of OACs has not been well characterized for patients in China with ischemic stroke and AF, as previous studies have been limited in terms of sample size, geographical regions studied, or types of OACs prescribed.11,12,13,14
The Chinese Stroke Center Alliance (CSCA) is a national stroke care quality assessment and improvement program with the primary goal of improving the quality of care and outcomes for patients with stroke.15 Using the nationwide and rigorously assessed data on consecutively recruited patients from CSCA, we aimed to assess adherence to guideline-directed prescribing of OAC therapy for stroke prevention. We hypothesized that adherence to prescribing of OACs per guideline recommendations for patients with AF would improve over time.
Methods
The data that support the findings from this study are available from the corresponding author upon reasonable request and after clearance by the ethics committee. This report follows the revised Standards for Quality Improvement Reporting Excellence (SQUIRE) reporting guideline for quality improvement studies.
CSCA Program
The CSCA program is a national, hospital-based, multicenter, multifaceted quality improvement initiative for stroke and transient ischemic attack. This program was made available to all secondary and tertiary hospitals in China. The ethics committee of Beijing Tiantan Hospital approved the CSCA program. Each participating hospital received institutional review board approval to enroll participants without individual patient consent under a waiver of authorization and exemption because only deidentified data were collected from routine clinical practice. The protocol for case identification and data collection has been reported in detail elsewhere.15
Patients were enrolled if they had a primary diagnosis of stroke confirmed by brain computed tomography or magnetic resonance imaging. Data were collected by trained hospital personnel using the web-based Patient Management Tool (Medicine Innovation Research Center). The China National Clinical Research Center for Neurological Diseases served as the data analysis center and had an agreement to analyze the aggregate deidentified data for feedback on quality of care and for research purposes.
Multifaceted Quality Improvement Initiative
The multifaceted quality improvement initiative included 2 parts, which were developed using the Plan-Do-Check-Act cycle. The first part consists of a web-based data collection and feedback system,16 which collects concurrent data via predefined logic features, range checks, and user alerts; provides feedback on key performance measures that were proven to be effective in a randomized clinical trial of multifaceted quality improvement intervention17; and allows participating hospitals to compare their performance with the past and current standards of other hospitals. The second part is the construction of stroke centers by collaborative workshops and webinars. More details can be found in the protocol of the CSCA.15
Study Population
The CSCA enrolled 70 365 patients with ischemic stroke and AF from 1430 participating hospitals from August 1, 2015, to July 31, 2019. The presence of AF was ascertained by medical history taking, in-hospital diagnosis by electrocardiography, or 24-hour Holter monitoring. A total of 4319 patients were excluded owing to discharge against medical advice, missing discharge information, or transfer to a tertiary hospital.
For the remaining 66 046 patients with ischemic stroke and AF, we created 2 study populations for at-admission analysis and at-discharge analysis. We included 35 767 participants for the at-admission analysis after excluding those with AF diagnosed at discharge (n = 23 949), a CHA2DS2-VASc (cardiac failure or dysfunction, hypertension, age 65-74 [1 point] or ≥75 years [2 points], diabetes mellitus, and stroke, transient ischemic attack or thromboembolism [2 points]–vascular disease, and sex category [female]) score less than 2 (n = 4241), and missing OAC information prior to admission (n = 2089). For the at-discharge analysis, we included 49 531 patients after excluding in-hospital deaths (n = 1297), those with contraindications to OACs (n = 8689), those with a prescription for heparin or low-molecular-weight heparin (n = 3886), those missing information on OACs at discharge (n = 1935), and those with a prescription for 2 or more OACs at discharge (n = 708). A flowchart of study population identification is presented in eFigure 1 in the Supplement.
Study Variables and Definitions
The OACs in this study included warfarin and NOACs; the latter included dabigatran, rivaroxaban, apixaban, and edoxaban. Adherence to guideline-recommended prescribing of OACs was assessed based on clinical management guidelines for patients with ischemic stroke from the Chinese Stroke Association or the American Heart Association/American Stroke Association.18,19 We collected data on sociodemographic characteristics, including age, sex, educational level, insurance type (urban employee basic medical insurance, urban resident basic medical insurance, new rural cooperative medical scheme, self-pay, and others),20 and per capita family income (calculated by dividing total family income by the number of family members). Data were also collected on whether patients had a history of AF, stroke and transient ischemic attack, carotid stenosis, coronary heart disease, myocardial infarction, hypertension, diabetes, dyslipidemia, or peripheral vascular disease. Information on medication use in the 6 months prior to stroke hospitalization was also collected. Stroke severity was measured by the National Institutes of Health Stroke Scale score (range, 0-42, with a higher score indicating greater stroke severity), with moderate or severe stroke defined by a score of 16 or more.21,22 Geographical regions were classified into eastern, central, and western, according to the China Health and Family Planning Statistics Yearbook 2017.23 Additional information on data collection and variable definition were described in the CSCA protocol.15
Statistical Analysis
Patients for at-admission analysis were described overall and by OAC use on admission using counts with percentages for categorical variables and mean (SD) values or median values with interquartile ranges for continuous variables. Given the large sample size, comparisons between groups using traditional methods such as the Pearson χ2 test, independent t test, or Wilcoxon rank sum tests would have been susceptible to false-positive findings. Therefore, we used the absolute standardized difference (ASD) to compare patient characteristics between groups, with an ASD greater than 10% considered clinically significant.24 Cochran-Armitage trend tests were used to assess temporal trends of OAC use and contraindication to OACs from the third quarter of 2015 to the third quarter of 2019.
All P values were 2-sided, with P < .05 considered statistically significant. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc). An SAS macro called %ggBaseline was used to analyze and report baseline characteristics automatically.25
Results
Baseline Characteristics
Among 35 767 patients with ischemic stroke and previous AF on admission, the mean (SD) age was 75.5 (9.2) years, and 18 785 patients (52.5%) were female. The median CHA2DS2-VASc score was 4.0 (interquartile range, 3.0-5.0).
OAC Use Prior to Stroke Hospitalization
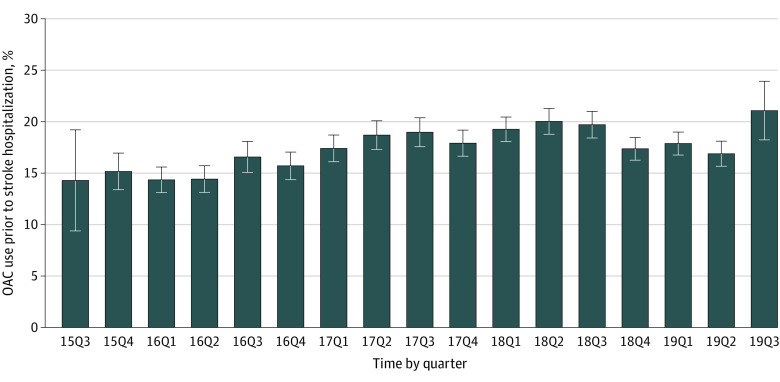
At admission, 6303 of 35 767 (17.6%) patients with ischemic stroke and previous AF and with a CHA2DS2-VASc score of 2 or greater were taking OACs. The rate of OAC use prior to stroke hospitalization was 14.3% (20 of 140) in the third quarter of 2015 and increased to 21.1% (118 of 560) in the third quarter of 2019 (P < .001 for trend) (Figure 1). Compared with patients not taking OACs (n = 29 464), those taking OACs prior to stroke hospitalization were younger (mean [SD] age, 73.0 [10.0] vs 76.0 [8.9] years; ASD = 31.7%), had higher levels of education (high school, 1732 [27.5%] vs 6797 [23.1%]; ASD = 10.1%), were less likely to be covered by the new rural cooperative medical scheme (1956 [31.0%] vs 10 894 [37.0%]; ASD = 12.7%), and were more likely to be admitted to a tertiary hospital (4585 [72.7] vs 19 837 [67.3%]; ASD = 11.8%); a higher proportion had experienced prior stroke and transient ischemic attack (4061 [64.4%] vs 12 835 [43.6%]; ASD = 42.7%), carotid stenosis (225 [3.6%] vs 517 [1.8%]; ASD = 11.1%), coronary heart disease or myocardial infarction (1848 [29.3%] vs 6915 [23.5%]; ASD = 13.2%), dyslipidemia (1024 [16.2%] vs 2644 [9.0%]; ASD = 21.8%), and peripheral vascular disease (490 [7.8%] vs 1084 [3.7%]; ASD = 17.7%); and a higher proportion had a history of taking antiplatelet agents (2815 [44.7%] vs 9909 [33.6%]; ASD = 22.9%), antihypertensive agents (3811 [60.5%] vs 16 126 [54.7%]; ASD = 11.8%), glucose-lowering medications (1309 [20.8%] vs 4661 [15.8%]; ASD = 13.0%), and lipid-lowering medications (3123 [49.5%] vs 6202 [21.0%]; ASD = 62.5%) (Table 1).
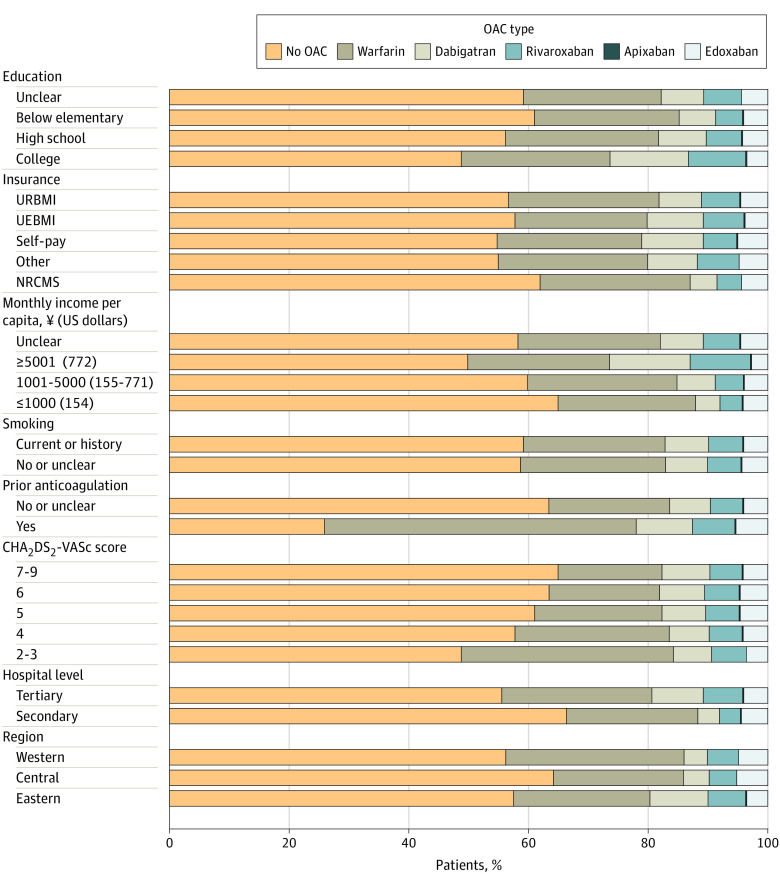
Figure 1. Patterns of Oral Anticoagulant (OAC) Prescription at Discharge in Selected Subgroups of Eligible Patients.
CHA2DS2-VASc indicates cardiac failure or dysfunction, hypertension, age 65-74 (1 point) or ≥75 years (2 points), diabetes, and stroke, transient ischemic attack or thromboembolism (2 points)–vascular disease, and sex category (female); NRCMS, new rural cooperative medical scheme; UEBMI, urban employee basic medical insurance; and URBMI, urban resident basic medical insurance.
Table 1. Baseline Characteristics of Patients With Prior AF and a CHA2DS2-VASc Score of 2 or Higher.
| Characteristic | Patients, No. (%) | ASD, % | ||
|---|---|---|---|---|
| Total (N = 35 767) | OAC (n = 6303) | No OAC (n = 29 464) | ||
| Age, mean (SD), y | 75.5 (9.2) | 73.0 (10.0) | 76.0 (8.9) | 31.7 |
| Male | 16 982 (47.5) | 3102 (49.2) | 13 880 (47.1) | 4.2 |
| Female | 18 785 (52.5) | 3201 (50.8) | 15 584 (52.9) | 4.2 |
| Educational level | ||||
| College | 977 (2.7) | 231 (3.7) | 746 (2.5) | 6.9 |
| High school | 8529 (23.8) | 1732 (27.5) | 6797 (23.1) | 10.1 |
| Below elementary school | 13 465 (37.6) | 2020 (32.0) | 11 445 (38.8) | 14.3 |
| Unclear | 12 796 (35.8) | 2320 (36.8) | 10 476 (35.6) | 2.5 |
| Insurance | ||||
| UEBMI | 12 420 (34.7) | 2333 (37.0) | 10 087 (34.2) | 5.9 |
| URBMI | 7249 (20.3) | 1312 (20.8) | 5937 (20.2) | 1.5 |
| NRCMS | 12 850 (35.9) | 1956 (31.0) | 10 894 (37.0) | 12.7 |
| Self-pay | 1703 (4.8) | 343 (5.4) | 1360 (4.6) | 3.7 |
| Other | 1545 (4.3) | 359 (5.7) | 1186 (4.0) | 7.9 |
| Monthly income per capita, ¥ (US dollars)a | ||||
| ≤1000 (154) | 2568 (7.2) | 433 (6.9) | 2135 (7.2) | 1.2 |
| 1001-5000 (155-771) | 12 497 (34.9) | 2140 (34.0) | 10 357 (35.2) | 2.5 |
| ≥5001 (772) | 2155 (6.0) | 464 (7.4) | 1691 (5.7) | 6.9 |
| Unclear | 18 547 (51.9) | 3266 (51.8) | 15 281 (51.9) | 0.2 |
| Current smoker or history of smoking | 8874 (24.8) | 1566 (24.8) | 7308 (24.8) | 0.0 |
| Drinking | 5545 (15.5) | 1047 (16.6) | 4498 (15.3) | 3.6 |
| Risk factors known before admission | ||||
| Prior stroke or TIA | 16 896 (47.2) | 4061 (64.4) | 12 835 (43.6) | 42.7 |
| CHD or MI | 8763 (24.5) | 1848 (29.3) | 6915 (23.5) | 13.2 |
| Hypertension | 24 852 (69.5) | 4193 (66.5) | 20 659 (70.1) | 7.7 |
| Diabetes | 7460 (20.9) | 1467 (23.3) | 5993 (20.3) | 7.3 |
| Dyslipidemia | 3668 (10.3) | 1024 (16.2) | 2644 (9.0) | 21.8 |
| Heart failure | 3274 (9.2) | 672 (10.7) | 2602 (8.8) | 6.4 |
| Carotid stenosis | 742 (2.1) | 225 (3.6) | 517 (1.8) | 11.1 |
| PVD | 1574 (4.4) | 490 (7.8) | 1084 (3.7) | 17.7 |
| Medication before admission | ||||
| Antiplatelet medication | 12 724 (35.6) | 2815 (44.7) | 9909 (33.6) | 22.9 |
| Antihypertension medication | 19 937 (55.7) | 3811 (60.5) | 16 126 (54.7) | 11.8 |
| Glucose-lowering medication | 5970 (16.7) | 1309 (20.8) | 4661 (15.8) | 13.0 |
| Lipid-lowering medication | 9325 (26.1) | 3123 (49.5) | 6202 (21.0) | 62.5 |
| CHA2DS2-VASc score, median (IQR) | 4.0 (3.0-5.0) | 4.0 (3.0-5.0) | 4.0 (3.0-5.0) | |
| Hospital level | ||||
| Secondary | 11 345 (31.7) | 1718 (27.3) | 9627 (32.7) | 11.8 |
| Tertiary | 24 422 (68.3) | 4585 (72.7) | 19 837 (67.3) | 11.8 |
| Region | ||||
| Eastern | 19 589 (54.8) | 3341 (53.0) | 16 248 (55.1) | 4.2 |
| Central | 8982 (25.1) | 1504 (23.9) | 7478 (25.4) | 3.5 |
| Western | 7196 (20.1) | 1458 (23.1) | 5738 (19.5) | 8.8 |
Abbreviations: AF, atrial fibrillation; ASD, absolute standardized difference; CHA2DS2-VASc, cardiac failure or dysfunction, hypertension, age 65-74 (1 point) or ≥75 years (2 points), diabetes, and stroke, transient ischemic attack or thromboembolism (2 points)–vascular disease, and sex category (female); CHD, coronary heart disease; IQR, interquartile range; MI, myocardial infarction; NRCMS, new rural cooperative medical scheme; OAC, oral anticoagulation; PVD, peripheral vascular disease; TIA, transient ischemic attack; UEBMI, urban employee basic medical insurance; URBMI, urban resident basic medical insurance.
Conversion rate, $1 = ¥6.41.
Contraindications to OACs at Discharge
Among 66 046 patients with ischemic stroke and AF, 1297 in-hospital deaths were excluded and 64 749 patients were analyzed for contraindications to OACs. A total of 8689 patients (13.4%) had at least 1 contraindication to OACs, and the 3 most common contraindications were bleeding risk (5452 [8.4%]), family member or patient preference (2943 [4.5%]), and terminal illness (443 [0.7%]) (Table 2). The rate of contraindications decreased from 16.7% (329 of 1973) in 2015 to 12.7% (1487 of 11 749) in 2019 (24% relative reduction; P < .001 for trend). Bleeding risk as a contraindication decreased over time from 11.1% (219 of 1973) in 2015 to 8.1% (949 of 11 749) in 2019 (27% relative reduction; P = .001 for trend), and family member or patient preference as a contraindication also decreased over time, from 5.0% (99 of 1973) in 2015 to 4.2% (494 of 11 749) in 2019 (16% relative reduction; P < .001 for trend).
Table 2. Contraindications to OACs Among Discharged Patients With a CHA2DS2-VASc Score of 2 or Higher.
| OAC contraindication | Patients, No. (%) | P value for trend | |||||
|---|---|---|---|---|---|---|---|
| Total (N = 64 749) | 2015 (n = 1973) | 2016 (n = 13 857) | 2017 (n = 16 391) | 2018 (n = 20 779) | 2019 (n = 11 749) | ||
| Contraindication | 8689 (13.4) | 329 (16.7) | 2044 (14.8) | 2247(13.7) | 2582 (12.4) | 1487 (12.7) | <.001 |
| Bleeding risk | 5452 (8.4) | 219 (11.1) | 1185 (8.6) | 1402 (8.6) | 1697 (8.2) | 949 (8.1) | .001 |
| Patient refusal | 2943 (4.5) | 99 (5.0) | 755 (5.4) | 767 (4.7) | 828 (4.0) | 494 (4.2) | <.001 |
| Allergy or comorbid illness | 300 (0.5) | 7 (0.4) | 90 (0.6) | 73 (0.4) | 83 (0.4) | 47 (0.4) | .009 |
| Falls | 176 (0.3) | 4 (0.2) | 49 (0.4) | 46 (0.3) | 48 (0.2) | 29 (0.2) | .12 |
| Mental health | 103 (0.2) | 4 (0.2) | 26 (0.2) | 26 (0.2) | 34 (0.2) | 13 (0.1) | .15 |
| Severe adverse effect | 145 (0.2) | 5 (0.3) | 32 (0.2) | 38 (0.2) | 47 (0.2) | 23 (0.2) | .53 |
| Terminal illness | 443 (0.7) | 14 (0.7) | 102 (0.7) | 108 (0.7) | 134 (0.6) | 85 (0.7) | .76 |
Abbreviations: CHA2DS2-VASc, cardiac failure or dysfunction, hypertension, age 65-74 (1 point) or ≥75 years (2 points), diabetes, and stroke, transient ischemic attack or thromboembolism (2 points)–vascular disease, and sex category (female); OAC, oral anticoagulant.
OAC Prescription at Discharge
At discharge, 20 390 of 49 531 eligible patients (41.2%) without contraindications and with a CHA2DS2-VASc score of 2 or more were prescribed an OAC. The most commonly prescribed OAC was warfarin (11 956 [24.2%]), followed by dabigatran (3514 [7.1%]), rivaroxaban (2796 [5.6%]), and edoxaban (2091 [4.2%]). Detailed OAC prescription patterns are shown in Figure 2. Patients with a college education, higher income, and prior use of anticoagulants, as well as those admitted to tertiary hospitals, were more likely to be prescribed OACs. Higher CHA2DS2-VASc scores were not associated with a higher rate of OAC prescription.
Figure 2. Trends of Oral Anticoagulant (OAC) Use Prior to Stroke Hospitalization.
P < .001 for trend. Error bars indicate 95% CIs. 15Q3 to 19Q3 indicate the third quarter of 2015 to the third quarter of 2019.
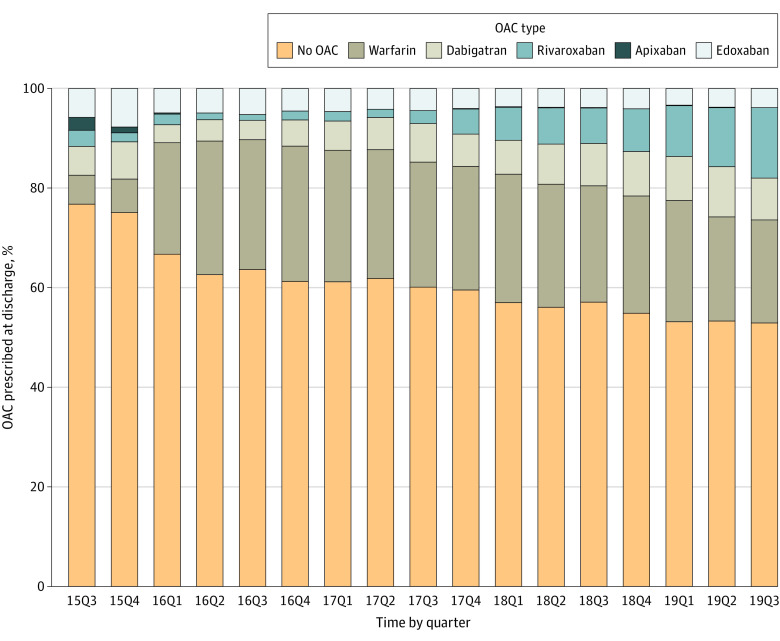
To assess OAC prescription changes at discharge from 2015 to 2019, we assessed the quarterly temporal trend (Figure 3). Oral anticoagulant prescriptions at discharge increased from 23.2% (36 of 155) in the third quarter of 2015 to 47.1% (403 of 856) in the third quarter of 2019 (P < .001 for trend). The largest increase was observed for warfarin (from 5.8% [9 of 155] to 20.7% [177 of 856]), followed by rivaroxaban (from 3.2% [5 of 155] to 14.1% [121 of 856]) and dabigatran (from 5.8% [9 of 155] to 8.4% [72 of 856]).
Figure 3. Trends of Oral Anticoagulant (OAC) Prescription at Discharge Among Eligible Patients.
15Q3 to 19Q3 indicate the third quarter of 2015 to the third quarter of 2019.
In addition, we assessed the variation in OAC prescriptions across 604 hospitals with at least 100 patients. The rates of OAC prescription at hospitals ranged from 0% to 95%, with a median of 38.8% (interquartile range, 23.1%-52.9%) (eFigure 2 in the Supplement).
Factors Associated With OAC Prescription at Discharge
Patients prescribed OACs at discharge (n = 20 390) were younger than those not prescribed OACs at discharge (n = 29 141) (mean [SD] age, 71.7 [10.6] vs 74.6 [10.2] years; ASD = 27.9%), less likely to be covered by the new rural cooperative medical scheme (7013 [34.4%] vs 11 443 [39.3%]; ASD = 10.2%), had a lower rate of hypertension (11 919 [58.5%] vs 18 633 [63.9%]; ASD = 11.1%), had a lower rate of prior antiplatelet agent use (4616 [22.6%] vs 8063 [27.7%]; ASD = 11.8%), had a higher rate of prior anticoagulant agent use (4483 [22.0%] vs 1563 [5.4%]; ASD = 49.7%), were more likely to be from tertiary hospitals (15 373 [75.4%] vs 19 247 [66.0%]; ASD = 20.8%), and were less likely to be from hospitals located in the central region of China (4266 [20.9%] vs 7652 [26.3%]; ASD = 12.7%) (eTable 1 in the Supplement). Multivariable logistic models showed that older age (adjusted odds ratio [aOR] per 5 year increase, 0.89; 95% CI, 0.89-0.90), lower levels of education (aOR for below elementary school, 0.84; 95% CI, 0.74-0.95), being covered by the new rural cooperative medical scheme (aOR, 0.92; 95% CI, 0.87-0.96), lower levels of family income (aOR for ≤¥1000 [$154], 0.66; 95% CI, 0.59-0.73), having certain comorbid conditions (including stroke or transient ischemic attack [aOR, 0.78; 95% CI, 0.75-0.82], hypertension [aOR, 0.84; 95% CI, 0.80-0.89], diabetes [aOR, 0.91; 95% CI, 0.83-0.99], dyslipidemia [aOR, 0.87; 95% CI, 0.80-0.94], carotid stenosis [aOR, 0.83; 95% CI, 0.69-0.98], and peripheral vascular disease [aOR, 0.80; 95% CI, 0.71-0.90]), having had a previous prescription of antiplatelet agents (aOR, 0.70; 95% CI, 0.66-0.74), and being from a secondary hospital (aOR, 0.71; 95% CI, 0.68-0.74) or a hospitals located in the central region of China (aOR, 0.80; 95% CI, 0.75-0.84) were associated with a lower rate of OAC prescription at discharge. Conversely, previous AF diagnosis (aOR, 1.08; 95% CI, 1.04-1.13) as well as prior use of anticoagulant agents (aOR, 5.17; 95% CI, 4.84-5.52), antihypertensive agents (aOR,1.08; 95% CI, 1.02-1.14), and lipid-lowering medications (aOR, 1.10; 95% CI, 1.03-1.17) were associated with higher odds of OAC prescription at discharge (eTable 2 in the Supplement).
Discussion
In this multicenter quality improvement study, we used data on patients with ischemic stroke and prior AF in China to assess adherence to guideline-recommended prescribing of OAC therapy at admission and OAC prescription at discharge. Our analysis yielded several findings. First, adherence to guidelines for prescribing OAC therapy improved over time but remains low, with warfarin as the most commonly prescribed OAC and the fastest growing in use. Furthermore, bleeding risk was the most common concern among documented contraindications to OACs. Finally, we identified risk factors associated with being less likely to be prescribed an OAC at discharge.
Atrial fibrillation is a well-recognized risk factor for stroke, and previous studies from both randomized clinical trials and registry studies showed that OAC use prior to admission was associated with more favorable outcomes.10,26,27 However, OACs, particularly NOACs, are underprescribed in China. Data from the China National Stroke Registry showed that only 21% of patients with acute ischemic stroke and AF were prescribed anticoagulants at discharge from 2012 to 201328 compared with more than 90% in the Get With the Guidelines–Stroke program in the United States from 2003 to 2009.29,30 The community-based data from the China National Stroke Screening Survey during 2013 and 2014 showed that, among patients with ischemic stroke and AF, only 2.2% were taking OACs, and of them, 98.2% were taking warfarin.13 Another community-based survey of 47 841 adults aged 45 years or older in 7 geographical regions of China between 2014 and 2016 showed that AF prevalence was 1.8% (95% CI, 1.7%-1.9%), with an estimation of 7.9 million patients (95% CI, 7.4 million to 8.4 million patients) with AF in China. However, only 6.0% of patients with high-risk AF received anticoagulation therapy.31 Therefore, more efforts at the community level are needed for stroke prevention. Considerable efforts were made to improve adherence to evidence-based performance measures among patients with acute ischemic stroke in China. A multifaceted quality improvement intervention improved the proportion of patients with ischemic stroke and AF receiving anticoagulation to 40.6%.17 In the present analysis, the proportion of eligible patients receiving anticoagulation was 47.1% in 2019, thus showing further improvement. However, large gaps were still present compared with the rates observed in developed countries.29,30
The increase in OAC prescriptions was mainly associated with warfarin, not NOACs. NOACs offer a more favorable risk-benefit profile, with significant reductions in intracranial hemorrhage.10,32 Although the medical community in China has been making efforts to increase OAC and NOAC prescriptions for patients with AF, the shift from antiplatelet agents to anticoagulant agents remains slow, according to the Chinese Stroke Association guidelines for the clinical management of cerebrovascular disorders.18 Higher cost and lack of insurance coverage for NOACs for most Chinese patients prohibited the use of NOACs. To provide financial resources, the Ministry of Human Resources and Social Security of the People’s Republic of China released the 2017 Medicine Catalogue for National Basic Medical Insurance, Injury Insurance, and Maternity Insurance in February 2017. This initiative covered the fees for dabigatran and rivaroxaban for patients with AF. Rivaroxaban prescriptions subsequently increased from 3.2% (5 of 155) in the first quarter of 2017 to 14.1% (121 of 856) in the third quarter of 2019.
Although we observed improvements, we also found wide variation in the rates of OAC prescription among hospitals, ranging from 0% to 95%. Future studies should focus on improving the rates of OAC prescription for hospitals at the lower end of the distribution. Because one of the most common reasons for not prescribing OACs is concern about hemorrhage, patient and family education on the benefits and risks associated with using OACs should be made available and widely adopted by health care professionals.
Limitations
This study has several limitations. First, this analysis was based on the voluntary enrollment of participating hospitals and does not have an elaborately designed sampling frame, which may have biased our study to include hospitals with better stroke care than the national average. Second, data on detailed OAC types and the quality control of OAC use prior to stroke hospitalization were not collected; therefore, the percentages of different OAC types and of patients with well-controlled anticoagulation therapy could not be calculated. Third, postdischarge OAC compliance and clinical outcomes were not assessed because no follow-up data were collected. Fourth, the results of this study may not be generalizable to other countries because participants were restricted to Chinese patients, and many of the issues studied are specific to the Chinese context. Despite these limitations, the present analysis provides a novel contribution by characterizing OAC prescriptions among patients with ischemic stroke and AF, providing insights into stroke prevention and control at a national level.
Conclusions
Although adherence to guideline-recommended prescribing of OAC therapy to eligible patients with indications of ischemic stroke and AF improved over time, the prescription rate remains suboptimal. Programs and interventions to increase the rate of prescription for OACs, in particular NOACs, are needed to improve the quality of care, particularly for vulnerable subgroups by age, socioeconomic status, and presence of comorbid conditions.
eFigure 1. Flowchart of Study Population Identification
eFigure 2. Variation in OAC Prescription at Discharge
eTable 1. Baseline Characteristics by OAC Prescription at Discharge
eTable 2. Factors Associated With OAC Prescription at Discharge
References
- 1.Pandian JD, Gall SL, Kate MP, et al. Prevention of stroke: a global perspective. Lancet. 2018;392(10154):1269-1278. doi: 10.1016/S0140-6736(18)31269-8 [DOI] [PubMed] [Google Scholar]
- 2.Freedman B, Potpara TS, Lip GYH. Stroke prevention in atrial fibrillation. Lancet. 2016;388(10046):806-817. doi: 10.1016/S0140-6736(16)31257-0 [DOI] [PubMed] [Google Scholar]
- 3.Lip GYH, Lim HS. Atrial fibrillation and stroke prevention. Lancet Neurol. 2007;6(11):981-993. doi: 10.1016/S1474-4422(07)70264-8 [DOI] [PubMed] [Google Scholar]
- 4.Steger C, Pratter A, Martinek-Bregel M, et al. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke registry. Eur Heart J. 2004;25(19):1734-1740. doi: 10.1016/j.ehj.2004.06.030 [DOI] [PubMed] [Google Scholar]
- 5.Lamassa M, Di Carlo A, Pracucci G, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project). Stroke. 2001;32(2):392-398. doi: 10.1161/01.STR.32.2.392 [DOI] [PubMed] [Google Scholar]
- 6.Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36(6):1115-1119. doi: 10.1161/01.STR.0000166053.83476.4a [DOI] [PubMed] [Google Scholar]
- 7.Hannon N, Sheehan O, Kelly L, et al. Stroke associated with atrial fibrillation—incidence and early outcomes in the North Dublin Population Stroke Study. Cerebrovasc Dis. 2010;29(1):43-49. doi: 10.1159/000255973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e531S-e575S. doi: 10.1378/chest.11-2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857-867. doi: 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 10.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi: 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 11.Gao Q, Fu X, Wei JW, et al. ; China QUEST Study Investigators . Use of oral anticoagulation among stroke patients with atrial fibrillation in China: the China QUEST (Quality Evaluation of Stroke Care and Treatment) registry study. Int J Stroke. 2013;8(3):150-154. doi: 10.1111/j.1747-4949.2011.00716.x [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Yang Z, Wang C, et al. Significant underuse of warfarin in patients with nonvalvular atrial fibrillation: results from the China National Stroke Registry. J Stroke Cerebrovasc Dis. 2014;23(5):1157-1163. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 13.Guo J, Guan T, Fan S, Chao B, Wang L, Liu Y. Underuse of oral anticoagulants in patients with ischemic stroke and atrial fibrillation in China. Am J Cardiol. 2018;122(12):2055-2061. doi: 10.1016/j.amjcard.2018.08.057 [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Yang XA, Zhang Y, et al. Oral anticoagulant use in atrial fibrillation–associated ischemic stroke: a retrospective, multicenter survey in northwestern China. J Stroke Cerebrovasc Dis. 2017;26(1):125-131. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.042 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Li Z, Wang Y, et al. Chinese Stroke Center Alliance: a national effort to improve healthcare quality for acute stroke and transient ischaemic attack: rationale, design and preliminary findings. Stroke Vasc Neurol. 2018;3(4):256-262. doi: 10.1136/svn-2018-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.China Stroke Center Alliance . China Stroke Center Alliance data management cloud platform. Accessed June 16, 2021. https://csca.chinastroke.net/cuzhong.html
- 17.Wang Y, Li Z, Zhao X, et al. ; GOLDEN BRIDGE–AIS Investigators . Effect of a multifaceted quality improvement intervention on hospital personnel adherence to performance measures in patients with acute ischemic stroke in China: a randomized clinical trial. JAMA. 2018;320(3):245-254. doi: 10.1001/jama.2018.8802 [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Chen W, Zhou H, et al. ; Chinese Stroke Association Stroke Council Guideline Writing Committee . Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol. 2020;5(2):159-176. doi: 10.1136/svn-2020-000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Gregersen H, Yuan W. Chinese health care system and clinical epidemiology. Clin Epidemiol. 2017;9:167-178. doi: 10.2147/CLEP.S106258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864-870. doi: 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- 22.Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53(1):126-131. doi: 10.1212/WNL.53.1.126 [DOI] [PubMed] [Google Scholar]
- 23.National Health and Family Planning Commission of the People's Republic of China . China Health and Family Planning Statistics Yearbook 2017. Peking Union Medical College Press; 2018. [Google Scholar]
- 24.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Statistics Simulation Computation. 2009;38(6):1228-1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 25.Gu HQ, Li DJ, Liu C, Rao ZZ. %ggBaseline: a SAS macro for analyzing and reporting baseline characteristics automatically in medical research. Ann Transl Med. 2018;6(16):326. doi: 10.21037/atm.2018.08.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meinel TR, Branca M, De Marchis GM, et al. ; Investigators of the Swiss Stroke Registry . Prior anticoagulation in patients with ischemic stroke and atrial fibrillation. Ann Neurol. 2021;89(1):42-53. doi: 10.1002/ana.25917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xian Y, O’Brien EC, Liang L, et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in-hospital outcomes among patients with atrial fibrillation. JAMA. 2017;317(10):1057-1067. doi: 10.1001/jama.2017.1371 [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Wang C, Zhao X, et al. ; China National Stroke Registries . Substantial progress yet significant opportunity for improvement in stroke care in China. Stroke. 2016;47(11):2843-2849. doi: 10.1161/STROKEAHA.116.014143 [DOI] [PubMed] [Google Scholar]
- 29.Schwamm LH, Fonarow GC, Reeves MJ, et al. Get With the Guidelines–Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119(1):107-115. doi: 10.1161/CIRCULATIONAHA.108.783688 [DOI] [PubMed] [Google Scholar]
- 30.Fonarow GC, Reeves MJ, Smith EE, et al. ; GWTG-Stroke Steering Committee and Investigators . Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in Get With the Guidelines–Stroke. Circ Cardiovasc Qual Outcomes. 2010;3(3):291-302. doi: 10.1161/CIRCOUTCOMES.109.921858 [DOI] [PubMed] [Google Scholar]
- 31.Du X, Guo L, Xia S, et al. Atrial fibrillation prevalence, awareness and management in a nationwide survey of adults in China. Heart. 2021;107(7):535-541. doi: 10.1136/heartjnl-2020-317915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan YH, Lee HF, See LC, et al. Effectiveness and safety of four direct oral anticoagulants in Asian patients with nonvalvular atrial fibrillation. Chest. 2019;156(3):529-543. doi: 10.1016/j.chest.2019.04.108 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart of Study Population Identification
eFigure 2. Variation in OAC Prescription at Discharge
eTable 1. Baseline Characteristics by OAC Prescription at Discharge
eTable 2. Factors Associated With OAC Prescription at Discharge