Abstract
Background:
Chronic pain is an important complication of breast surgery, estimated to affect 20–30% of patients. We prospectively examined surgical, demographic, and psychosocial factors associated with chronic pain 6 months after breast surgery.
Methods:
Patients undergoing breast surgery for benign and malignant disease preoperatively completed validated questionnaires to assess baseline pain and psychosocial characteristics. Pain at 6 months was quantified as the Pain Burden Index (PBI), which encompasses pain locations, severity, and frequency. Surgical type was categorized as breast conserving surgery (BCS), mastectomy, and mastectomy with reconstruction; axillary procedure was categorized as no axillary surgery, sentinel lymph node biopsy (SLNB), and axillary dissection. PBI was compared between groups using one-way analysis of variance (ANOVA) or Kruskal-Wallis ANOVA, and relationship between baseline demographic and psychosocial factors and PBI was assessed using Spearman’s Rank Correlation. P<0.05 was considered significant.
Results:
PBI was variable amongst patients reporting this endpoint (n=216) at 6 months, but no difference was found between primary breast surgical types (BCS, mastectomy, and mastectomy with reconstruction) or with surgical duration. However, axillary dissection was associated with higher PBI than SLNB and no axillary procedure (p<0.001). Younger age (<0.001) and higher BMI (p=0.010), as well as higher preoperative anxiety (p=0.017), depression (p<0.001), and catastrophizing scores (p=0.005) correlated with higher 6-month PBI.
Conclusions:
Amongst surgical variables, only axillary dissection was associated with greater pain at 6 months after surgery. Patient characteristics that were associated with higher PBI included lower age and higher BMI, as well as higher baseline anxiety, depression, and catastrophizing.
Introduction:
Breast surgery is one of the most commonly performed surgical procedures. While the pain associated with breast surgery resolves in the majority of patients, chronic pain, defined as pain lasting greater than 3–6 months after surgery, affects a significant minority of patients, with an estimated incidence of 20–30%.1 Chronic postsurgical pain is an increasingly recognized problem, and a growing potential source of chronic opioid use.2 The burden of chronic pain is multi-faceted, impacting both individuals and society in the form of increased healthcare time and costs, along with loss in productivity. The negative effects on patients are extensive, affecting sleep, causing anxiety and depression, placing strain on interpersonal relationships, and decreasing overall quality of life.3 Because chronic pain may be a cause of psychosocial stress, the association between these factors and pain in previous retrospective and cross sectional studies raises a question of directionality of effect: are depression and anxiety risk factors for chronic pain, or the result of it? At the same time, choices about the type of surgical management, including the surgical type (BCS vs mastectomy), the presence and type of reconstruction, and extent of the axillary procedure are known to be related to more severe acute postoperative pain.4 However, the relationship of these surgical factors with the development or persistence of chronic pain is somewhat less clear.5,6 We sought to investigate the relative contribution of relevant surgical variables and prospectively identify the relationship of baseline patient psychosocial characteristics to persistent postoperative pain at 6 months.
Methods:
Study Design
This prospective, observational study was approved by the Partner’s/Brigham and Women’s Institutional Review Board. Between 2014 and 2017, women aged 18–80 scheduled to undergo breast surgery with or without known malignancy were recruited in the preoperative anesthesia clinic. Patients completed validated questionnaires assessing psychosocial phenotype, demographics, and baseline pain in surgical sites and other body areas via secure link to the REDCap data entry system before undergoing surgery. Chronic pain at 6 months after breast surgery (primary outcome of this study) was similarly assessed via electronic survey.
Surgery
Data regarding type, laterality, and duration of surgery, as well as type of reconstruction and axillary procedure was collected. Additional clinical and pathologic factors were abstracted from the medical record. Surgical type was categorized as: (1) breast conservation surgery including partial mastectomy and excisional biopsy, (2) mastectomy, and (3) mastectomy with reconstruction. Axillary surgery was evaluated independently and categorized as: (1) no axillary surgery, (2) sentinel lymph node biopsy, and (3) axillary lymph node dissection. Surgeries were performed by 11 breast surgical oncologists and 11 breast reconstructive surgeons.
Anesthesia
The majority (96%) of patients received general anesthesia, and 4% received local anesthesia with sedation. As per institutional clinical practice, regional anesthesia including thoracic paravertebral block or proximal intercostal block, was offered preoperatively to those undergoing total mastectomy, and additional pectoralis nerve blocks to those undergoing tissue expander or immediate implant. Additional intraoperative and postoperative analgesia was provided using opioids, celecoxib, ketamine and acetaminophen administration according to the preference of the anesthesia providers.
Psychosocial Evaluation
Questionnaires assessing psychosocial factors were chosen based upon strong psychometric validation characteristics and brevity. The Pain Catastrophizing Scale (PCS), which has been validated in pain patients and controls was used to measure catastrophic thinking associated with pain.7,8 Depressive symptoms and anxiety were assessed using short-form instruments from the Patient Reported Outcome Measurement Information System (PROMIS). The PROMIS instruments have been extensively validated in studies comparing results with established scales, and have been calibrated on over 20,000 persons.9,10
Pain Assessment
The primary outcome of this study was chronic pain at 6 months, collected using the breast cancer pain questionnaire first developed by Gartner et al.11 This questionnaire queries specifically about pain in 4 surgically-related body areas, and allows calculation of a Pain Burden Index (PBI). The PBI is calculated by summing the pain severity scale (0–10) at each of four locations (breast, axilla, chest wall, arm) multiplied by the frequency of the pain at each site (constantly – 5 points, daily – 4 points, occasionally – 3 points, weekly – 2 points, monthly – 1 point, and never – 0 points), thus Pain Burden Index (PBI) = ∑ Pain scale at each site x frequency.
Statistical Analysis
Baseline characteristics and Pain Burden Index (PBI) at 6 months were compared between surgical groups and between pain groups using one-way analysis of variance (ANOVA) or Kruskal-Wallis ANOVA, Mann Whitney U-test or Chi square/Fisher’s Exact analysis, as appropriate. Association between continuous baseline demographic and psychosocial factors and PBI was assessed using Spearman’s Rank Correlation. P<0.05 was considered significant.
Results:
Study Participants
Of patients approached preoperatively, 337 patients initially consented to the study and 259 completed the baseline questionnaires. At the 6-month time point, 216 patients completed the breast cancer pain questionnaire, allowing calculation of the pain burden index (PBI). Table 1 shows the baseline clinical characteristics of this sample,. Subjects were female, predominantly Caucasian (86.4%), with a mean age of 55.6 years old. Patients underwent surgery for invasive cancer (76.9%), DCIS (14.8%), prophylactic mastectomy (4.6%), and benign lesions (3.7%). The eight cases for benign diseases included 2 fibroadenomas, 1 intraductal papilloma, 1 radial scar, 3 atypical ductal hyperplasia, and 1 case of benign calcifications. Table 2 compares baseline characteristics of surgical groups entering the study. The most common surgery performed in this study was breast conservation surgery (BCS, 54.2% of cases), with 10.2% receiving mastectomy alone, and 35.6% mastectomy with reconstruction. Of the reconstructive cases, 79.2% were tissue expander or implant reconstruction. In patients undergoing axillary procedures (n=169) 63.4% were sentinel lymph node biopsies and 14.8% were axillary lymph node dissections. No axillary surgery was performed in 21.8% of cases.
TABLE 1:
Clinical characteristics
| Age (mean) | 55.6 years |
| BMI (mean) | 27.3 kg/m2 |
| Indications for surgery, N (%) | |
| Invasive cancer | 166 (76.9%) |
| DCIS | 32 (14.8%) |
| Prophylactic surgery | 10 (4.6%) |
| Benign | 8 (3.7%) |
| Stages for Invasive cancer | |
| Stage I | 95 (57.2%) |
| Stage II | 50 (30.1%) |
| Stage III | 20 (12.0%) |
| Stage IV | 1 (0.6%) |
| Surgical Category | |
| Breast Conservation Surgery | 117 (54.2%) |
| Mastectomy | 22 (10.2%) |
| Mastectomy with Reconstruction | 77 (35.6%) |
| Types of Reconstruction | |
| Tissue expander | 61 (79.2%) |
| Direct to implant | 1 (1.3%) |
| DIEP flap | 9 (11.7%) |
| Latissimus dorsi flap | 3 (3.9%) |
| TRAM flap | 3 (3.9%) |
| Axillary Surgery | |
| No axillary surgery | 47 (21.8%) |
| Sentinel lymph node biopsy | 137 (63.4%) |
| Axillary dissection | 32 (14.8%) |
Table 2:
Surgical group characteristics
| Breast Conservation | Mastectomy | Mastectomy with immediate reconstruction | P-value | |
|---|---|---|---|---|
| Age (mean ± SD) | 59.8 (±10.9) | 55.3 (±15.0) | 48.8 (±10.2) | <0.0001 |
| BMI median, (25–75%) | 26.9 (23.6–32.5) | 27.2 (24.0–34.4) | 23.7 (21.9–27.6) | 0.395 |
| Race, n (%) | ||||
| African American | 6 (4.4) | 0 (0) | 1 (1.2) | 0.010 |
| Caucasian | 117 (85.4) | 28 (73.7) | 78 (94.0) | |
| Asian | 3 (2.2) | 1 (2.6) | 1 (1.2) | |
| Hispanic/Latina | 7 (5.1) | 4 (10.5) | 0 (0.0) | |
| Mixed Race | 2 (1.5) | 3 (7.9) | 3 (3.6) | |
| Other | 2 (1.5) | 2 (5.3) | 0 (0.0) | |
| Menopausal status, n (%) | ||||
| Premenopausal | 26 (19.1) | 12 (31.6) | 40 (48.2) | <0.0001 |
| Perimenopausal | 11 (8.1) | 5 (13.2) | 15 (18.1) | |
| Postmenopausal | 99 (72.8) | 21 (55.3) | 28 (33.7) | |
| Baseline pain median, (25–75%) | ||||
| Baseline average pain | 0.5 (0–1.3) | 1.0 (0.6–2.6) | 0.4 (0–1.5) | 0.458 |
| Other chronic pain (%) | 53 (38.7) | 18 (47.4) | 32 (38.6) | 0.597 |
| Take opioid painkiller (%) | 5 (3.7) | 5 (13.2) | 3 (3.6) | 0.075 |
| Baseline psychosocial traits, median (25–75%) | ||||
| Catastrophizing | 3 (0–8) | 3 (1–10) | 5 (1–9) | 0.123 |
| Anxiety | 16 (13–19) | 17 (15–20) | 18 (14–20) | 0.866 |
| Depression | 11 (9–14) | 11 (10–14) | 12 (9–15) | 0.563 |
| Surgical variables (%) | ||||
| Previous breast surgery | 43 (31.6) | 20 (52.6) | 35 (41.7) | 0.043 |
| Bilateral procedure | 3 (2.2) | 9 (23.7) | 35 (42.2) | <0.0001 |
| Surgical duration (min) | 79 (54–121) | 113 (81–146) | 204 (157–341) | 0.004 |
| Sentinel node procedure | 92 (67.2) | 14 (36.8) | 29 (34.5) | <0.0001 |
| Axillary dissection | 14 (10.2) | 8 (21.1) | 10 (11.9) | 0.215 |
| Anesthetic variables median (25–75%) | ||||
| Intraoperative MMEa | 12.7 (10.0–19.2) | 20.0(12.9–25.0) | 22.0 (15.3–26.7) | <0.0001 |
| PACU MMEa | 2.5 (0.0–7.6) | 2.7 (0.0–6.3) | 5.3 (1.7–8.0) | <0.0001 |
MME=morphine milligram equivalents
Association of demographic factors with pain at 6 months
At 6 months, 20.4% of patients reported moderate-severe pain (≥4/10) in at least one of the following areas (breast, arm, chest wall or axilla). The remaining 79.6% of patients reported no pain or mild pain (<4/10) in the involved surgical sites. Pain burden (PBI) was quite variable amongst patients in any group, as shown in Figure 1. Younger age (ρ=−.249, p<0.001) and higher BMI (ρ=0.176, p=0.010) correlated with higher PBI at 6 months, and differed between groups with and without moderate-severe pain at 6 months (Table 3). PBI differed according to menopausal status (F=7.5, p=0.001), with lower PBI amongst postmenopausal patients (8.1 ± 14.4) than perimenopausal (17.9 ± 21.8) or premenopausal (18.6 ± 26.6) patients.
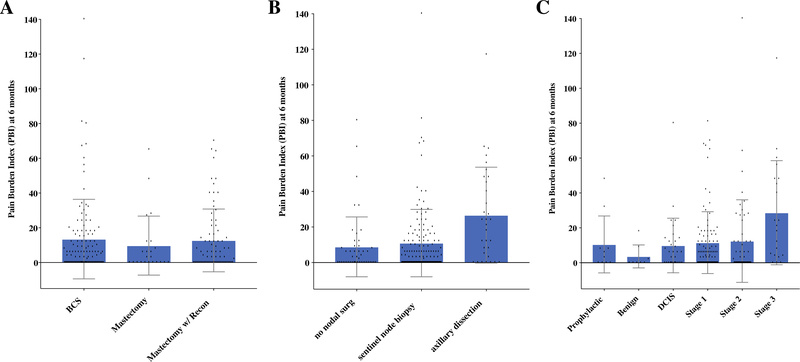
FIGURE 1:
A Pain Burden Index (PBI) at 6 months after surgery in 3 surgical categories (breast conservation, mastectomy, mastectomy with reconstruction) (F=0.173, p=0.841). B PBI at 6 months according to axillary surgery (no axillary surgery, sentinel lymph node biopsy, axillary lymph node dissection) to Pain Burden Index at 6 months (F= 9.302, p<0.001). C PBI at 6 months according to cancer stage or indication for surgery (F=3.168, p=0.009).
Table 3:
Characteristics of patients with no/mild pain vs moderate/severe pain at 6 months
| Patients with NO/MILD (pain <4/10 on pain scale) at 6 months N=172 | Patients with MODERATE/SEVERE (pain >4/10 on pain scale) at 6 months N=44 | p-value | |
|---|---|---|---|
| Mean Age | 56.5 ± 12.5 years | 51.5 ± 11.6 years | 0.014 |
| Mean BMI | 26.6 ± 5.5 kg/m2 | 29.0 ± 7.3 kg/m2 | 0.055 |
| Baseline chronic pain | 64/172 (37.2%) | 19/43 (44.1%) | 0.484 |
| Baseline opioid usage | 4/172 (2.3%) | 4/44 (9.1%) | 0.056 |
| Baseline Depression – PROMIS mean scores | 12.05 | 14.09 | 0.040 |
| Baseline Anxiety – PROMIS mean scores | 16.44 | 18.8 | 0.044 |
| Baseline PCS – PCS mean scores | 5.08 | 7.52 | 0.008 |
Baseline characteristics of surgical subgroups
Baseline pain and psychosocial traits including anxiety, depression, or pain catastrophizing did not differ between breast conservation surgery, mastectomy, and mastectomy with reconstruction groups. There was a significantly younger mean age and lower BMI amongst those undergoing mastectomy with reconstruction. Previous breast surgery, bilateral procedure, and total surgical duration were greater amongst those undergoing mastectomy, with those undergoing reconstruction having the greatest surgical duration. Importantly, there were no significant differences between the three surgical categories in terms of frequency of concurrent axillary dissection.
Comparison of surgical subgroups and evaluation those with higher pain at 6 months
The Pain Burden Index (PBI) at 6 months, though variable between patients, did not significantly differ according to primary surgical groups of BCS, mastectomy, and mastectomy with reconstruction (Figure 1A, p=0.841). Similarly, longer duration of surgery was not significantly correlated with PBI (p=0.437). However, PBI at 6 months was significantly different according to type of axillary nodal procedure (Figure 1B, p<0.001), with those having axillary dissection reporting higher PBI. PBI also differed between breast cancer stages, with those with stage III reporting higher PBI (p=0.009). For those who developed moderate/severe pain (≥4/10) at 6 months, the rate of preoperative baseline opioid usage was low at 9.1% (n=4), but was higher than the 2.3% (n=4) baseline opioid usage of those who went on to have no/mild pain (<4/10) at 6 months (Table 3). Patients reporting lymphedema symptoms at 6 months (swelling or heaviness in the arm or hand, n=17) reported higher PBI (39.6 ± 32.8) than those not reporting these symptoms (9.3 ± 17.3) (p<0.001).
Psychosocial factors associated with pain at 6 months
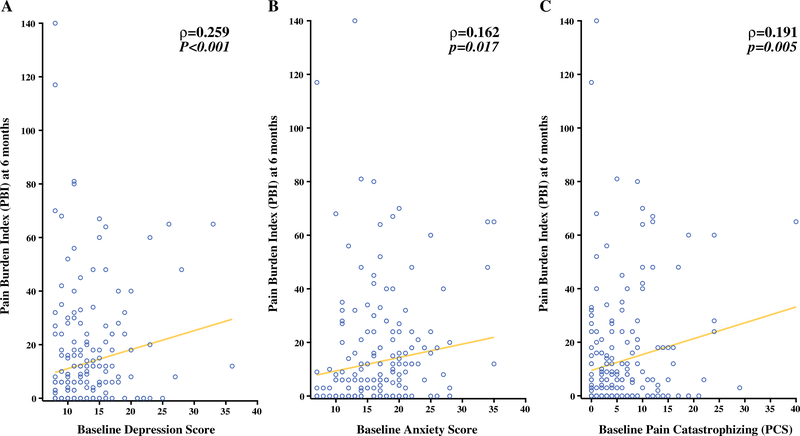
Baseline depression, measured using the PROMIS short form, was significantly correlated with higher PBI at 6 months (Figure 2A, Table 3). Baseline anxiety, also assessed using the PROMIS short form, was significantly correlated with higher PBI at 6 months (Figure 2B, Table 3). Catastrophizing was measured at baseline using the Pain Catastrophizing Scale (PCS), and greater baseline PCS was also correlated with higher PBI at 6 months (Figure 2C, Table 3).
FIGURE 2:
A Correlation of baseline PROMIS depression score to Pain Burden Index (PBI) at 6 months (Spearman ρ=0.259, p<0.001). B Correlation of baseline PROMIS anxiety score to PBI at 6 months (Spearman ρ=0.162, p=0.017). C Correlation of baseline pain catastrophizing (PCS) to PBI at 6 months (Spearman ρ=0.191, p=0.005).
Discussion:
Advances in the surgical management of breast cancer continue to achieve decreased locoregional recurrence, increased overall survival, decreased rates of lymphedema, and improved cosmesis, collectively minimizing the mortality and morbidity associated with breast surgery. In the same vein, efforts to understand and potentially prevent chronic postoperative pain in survivors should be pursued with the same vigor and enthusiasm. Although the majority of patients do not develop significant chronic pain, when present, it is rated as one of the most troubling symptoms after breast surgery, leading to disability and suffering, and is notably resistant to management.12 This study prospectively investigated factors associated with chronic pain 6 months after breast surgery, with a specific focus on evaluating the impact of both surgical and individual patient factors. Although previous reports indicate that more extensive surgery (i.e. bilateral procedures, receipt of reconstruction) translated into more severe acute postoperative pain at 1 week,4 it is less consistently associated with chronic pain. In the current prospective study, we also did not observe a significant relationship between surgical type (breast conservation surgery, mastectomy, and mastectomy with reconstruction) and pain at 6 months. Patients undergoing mastectomy with reconstruction comprised 35.6% of our cohort, with the majority undergoing tissue expander reconstruction (79.2%), but these patients did not report increased pain at 6 months.
On the other hand, we did find a significant prospective association of axillary dissection with pain burden at 6 months. It is well-established that the most morbid procedure performed in breast cancer surgery is the axillary lymph node dissection. The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial, the AMAROS Trial (After Mapping of the Axilla: Radiotherapy or Surgery) and the International Breast Cancer Study Group Trial 23–01 among other trials, have provided evidence regarding the safety to forego axillary dissection for early disease stages which had previously required axillary lymph node dissection.13,14,15 These major advances translate into significant reduction in the risk of lymphedema and its concomitant associated morbidity, without impacting regional recurrence or survival. Retrospective studies have demonstrated the association of axillary dissection and the development of chronic pain.16,17 Wilson et al retrospectively evaluated 470 patients after breast surgery and univariate analysis found that concomitant axillary surgery and axillary lymph node dissection were both associated with chronic postoperative neuropathic pain.16 While we also found that axillary dissection was associated with greater pain burden at 6 months, the findings of this study did not implicate sentinel lymph node biopsy as a risk factor for the development of persistent postoperative pain. We found that incidence of patient-reported lymphedema at 6 months corresponded to a higher pain score. Thus, the efforts made to limit the use of axillary dissection to its more confined indications may decrease not only the risk of lymphedema, but also potentially the risk of chronic pain.
Quantification of the severity, frequency, and number of body areas, or pain burden index (PBI) revealed a relatively large variation of pain experienced amongst individual patients at 6 months (Figure 1), underscoring the dramatically different experience patients may have with regard to pain. We treated pain as a continuous variable, including not only pain severity, but also frequency and number of affected body areas, in order allow a more sensitive resolution of individual patient factors that may predict risk. Similar to previous retrospective and cross sectional studies, we found that younger age and higher BMI are associated with greater pain.18,19,20
Importantly, patients’ degree of anxiety and depression before surgery were prospectively correlated to the amount of pain they would experience at 6 months. Using the PROMIS validated questionnaires for these measures allowed a relatively brief assessment, but also a comparison to larger sample means. For example, patients were somewhat more anxious than average (mean t-score of our sample was 55.7) and had slightly lower depressive symptoms reporting mean t-score of 48.2. Pain catastrophizing, which includes aspects of rumination, amplification, and helplessness8, has been associated with many types of chronic pain. Interestingly, the baseline PCS score, although lower compared to most samples of patients with persistent pain,21 was also prospectively associated with a higher PBI at 6 months. The fact that these scores, collected before surgery, were associated with later pain, suggests that individual differences in these relevant modifiers of pain processing may in fact increase the risk of persistent pain, rather than simply being the result of it. Notably, a clinical diagnosis of anxiety or depression was not associated with the outcome of more severe pain at 6 months, although these were relatively frequent amongst subjects in the study.
There are some important limitations to this study. The relatively limited sample precludes investigation of more fine surgical subcategorization (e.g. analysis of each specific type of surgery or reconstruction in combination with each type of axillary nodal procedure). Another potential confounding factor may be intervening adjuvant treatments, which were somewhat variable in presence and timing, although primarily not occurring within the window of assessment, and not systematically varying between groups. The impact of chemotherapy and radiation therapy as it relates to chronic pain warrants further study. Additionally, although the baseline PBI was relatively low, if patients reported preoperative baseline pain, we did not capture whether pain was specifically from previous breast surgery or axillary surgeries or recent biopsy. Furthermore, with 86.4% of the total number of patients electing to participate in this study being Caucasian, this study cannot state with certainty the experience of different ethnic groups.
Patients are commonly offered surgical options with their diagnosis, including breast conservation or mastectomy with or without the option to include reconstruction. These surgical options span a range, with the more extensive surgery involving more acute pain and recovery time. This study adds to previous investigations to help inform whether or not these surgical options portend differing risks of long term chronic pain, suggesting that reconstruction is not necessarily associated with a greater risk, and breast conservation is not necessarily with a lesser risk. Furthermore, for those patients who require axillary dissection as a part of their surgical treatment, this study agrees with previous evidence that this procedure does carry a significant risk of chronic pain and should be consented accordingly. More fundamentally, patients bring differing degrees of inherent risk for persistent pain to their preoperative surgical consultation in terms of age, BMI, and psychosocial characteristics. The identification of these risks may facilitate proper counseling and perioperative planning, with the intent to minimize the risk of developing chronic pain. Preventive perioperative management, consisting of multimodal analgesic techniques including regional anesthesia, analgesic adjuvants, and even coping strategies training or rational expectation setting, may be particularly important for those individuals that are at higher risk of persistent pain.
Synopsis:
Chronic pain affects 20–30% of patients after breast surgery. Patient characteristics associated with a higher risk of chronic postoperative pain include: younger age, higher BMI, higher baseline anxiety, depression, and pain catastrophizing, as well as undergoing axillary lymph node dissection.
Acknowledgements:
This research is supported by a K23 grant from the National Institutes of Health (NIGMS).
Disclosure: No conflicts of interest. This research is supported by a grant from the NIH (NIGMS)/ K23 GM110540.
References:
- 1.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 367(9522):1618–25 (2006) [DOI] [PubMed] [Google Scholar]
- 2.Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, Bohnert ASB, Kheterpal S and Nallamothu BK (2017). "New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults." JAMA Surg 152(6): e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duenas M, Ojeda B, Salazar A, Mico JA, Failide I. “A review of chronic pain impact on patients, their social environment and the health care system.” J Pain Res 2016; 9: 457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni AR, Pusic AL, Hamill JB, Kim HM, Qi J, Wilkins EG and Roth RS (2017). "Factors Associated with Acute Postoperative Pain Following Breast Reconstruction." JPRAS Open 11: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belfer I, Schreiber KL, Shaffer JR, Shnol H, Blaney K, Morando A, Englert D, Greco C, Brufsky A, Ahrendt G, Kehlet H, Edwards RR and Bovbjerg DH (2013). "Persistent postmastectomy pain in breast cancer survivors: analysis of clinical, demographic, and psychosocial factors." J Pain 14(10): 1185–1195. [DOI] [PubMed] [Google Scholar]
- 6.Fillingim RB (2017). "Individual differences in pain: understanding the mosaic that makes pain personal." Pain 158 Suppl 1: S11–S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Damme S, Crombez G, Bijttebier P, Goubert L and Van Houdenhove B (2002). "A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations." Pain 96(3): 319–324. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan MJ, Bishop SR, Pivik J. “The Pain Catastrophizing Scale: Development and Validation.” Psychol Assess. 7:524–32. (1995). [Google Scholar]
- 9.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K and Hays R (2010). "The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008." J Clin Epidemiol 63(11): 1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi SW, Reise SP, Pilkonis PA, Hays RD, Cella D: Efficiency of static and computer adaptive short forms compared to full length measures of depressive symptoms. Qual Life Res;19:125–135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H: Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 302(18):1985–1992 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Lauridsen MC, Overgaard M, Overgaard J, Hessov IB and Cristiansen P (2008). "Shoulder disability and late symptoms following surgery for early breast cancer." Acta Oncol 47(4): 569–575. [DOI] [PubMed] [Google Scholar]
- 13.Guilliano AE, McCall L, Peitsch P, Whitworth PW, Blumencranz P, Leitch M, Saha S, Hunt KK, Morrow M, Ballman K. “Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without Axillary Dissection in Patients With Sentinel Lymph Node Metastases.” Annals of Surgery Vol 252 September 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donker et al. “Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomized, multicenter, open-label, phase 3 non-inferiority trial.” Lancet Oncol. 2014. Nov, 15 (12): 1303–10. Epub 2014 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galimberti V, Cole BF, Zurrida S et al. “Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 radnomised controlled trial.” Lancet Oncol. 2013. Apr; 14(4):297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson et al. “Incidence and Predictors of Neuropathic Pain Following Breast Surgery.” Ann Surg Oncol (2013). 20:3330–3334. [DOI] [PubMed] [Google Scholar]
- 17.Hack TF, Winkel BK, Thomas-MacLean RL, Towers A, Miedema B, Tilley A, Chateau D. “Predictors of arm morbidity following breast cancer surgery.” Psycho-oncology 19: 1205–1212. (2010) [DOI] [PubMed] [Google Scholar]
- 18.Andersen KG, Kehlet H: Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain 12:725–746(2011). [DOI] [PubMed] [Google Scholar]
- 19.Schreiber KL, Kehlet H, Belfer I and Edwards RR (2014). "Predicting, preventing and managing persistent pain after breast cancer surgery: the importance of psychosocial factors." Pain Manag 4(6): 445–459. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber KL, Martel MO, Shnol H, Shaffer JR, Greco C, Viray N, Taylor LN, McLaughlin M, Brufsky A, Ahrendt G, Bovbjerg D, Edwards RR and Belfer I (2013). "Persistent pain in postmastectomy patients: comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain." Pain 154(5): 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards RR, Calahan C, Mensing G, Smith M, Haythornthwaite JA: Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol 7:216–24 (2011). [DOI] [PubMed] [Google Scholar]