Abstract
Background
Accumulating evidence demonstrates that certain microRNAs play critical roles in epileptogenesis. Our previous studies found microRNA (miR)‐129–2‐3p was induced in patients with refractory temporal lobe epilepsy (TLE). In this study, we aimed to explore the role of miR‐129–2‐3p in TLE pathogenesis.
Method
By bioinformatics, we predicted miR‐129–2‐3p may target the gene GABRA1 encoding the GABA type A receptor subunit alpha 1. Luciferase assay was used to investigate the regulation of miR‐129–2‐3p on GABRA1 3’UTR. The dynamic expression of miR‐129–2‐3p and GABRA1 mRNA and protein levels were measured in primary hippocampal neurons and a rat kainic acid (KA)‐induced seizure model by quantitative reverse transcription‐polymerase chain reaction (qPCR), Western blotting, and immunostaining. MiR‐129–2‐3p agomir and antagomir were utilized to explore their role in determining GABRA1 expression. The effects of targeting miR‐129–2‐3p and GABRA1 on epilepsy were assessed by electroencephalography (EEG) and immunostaining.
Results
Luciferase assay, qPCR, and Western blot results suggested GABRA1 as a direct target of miR‐129–2‐3p. MiR‐129–2‐3p level was significantly upregulated, whereas GABRA1 expression downregulated in KA‐treated rat primary hippocampal neurons and KA‐induced seizure model. In vivo knockdown of miR‐129–2‐3p by antagomir alleviated the seizure‐like EEG findings in accordance with the upregulation of GABRA1. Furthermore, the seizure‐suppressing effect of the antagomir was partly GABRA1 dependent.
Conclusions
The results suggested GABRA1 as a target of miR‐129–2‐3p in rat primary hippocampal neurons and a rat kainic acid (KA) seizure model. Silencing of miR‐129–2‐3p exerted a seizure‐suppressing effect in rats. MiR‐129–2‐3p/GABRA1 pathway may represent a potential target for the prevention and treatment of refractory epilepsy.
Keywords: epilepsy, GABAA receptor subunit α1, GABRA1, hippocampus neuron, kainic acid (KA), miR‐129–2‐3p
Accumulating evidence demonstrates that certain microRNAs play critical roles in epileptogenesis. In this study, we found GABA type A receptor subunit alpha 1 (GABRA1) as a direct target of miR‐129–2‐3p, resulting in the downregulation of GABRA1 expression. Silencing of miR‐129–2‐3p exerted a seizure‐suppressing effect in KA‐induced seizure model, and miR‐129–2‐3p/GABRA1 pathway may represent a potential target of the prevention and treatment of refractory epilepsy.
1. INTRODUCTION
As a common and devastating neurologic disorder, epilepsy is typical of recurring unprovoked seizures induced by abnormal firing of functional neurons in the central nervous system (CNS) (Jimenez‐Mateos & Henshall, 2013). Nowadays, about 50 million people around the world were suffering from active epilepsy with persistent seizures in a requirement for treatment, and nearly 30% of these patients are drug‐refractory (Pitkanen & Lukasiuk, 2011).
The physiological function of the CNS is manipulated by a balance of excitatory and inhibitory signaling. Experimental and clinical practice indicate several accredited pathogenetic mechanisms of epilepsy, including unbalance between excitatory (glutamate) and inhibitory (γ‐aminobutyric acid, GABA) neuronal stimulations. GABA is the major inhibitory neurotransmitter in CNS and functions via two kinds of receptors, GABAA and GABAB receptors. In humans, there are 19 isoforms of GABAA subunits, six α, three β, three γ, and one of δ, ε, π, θ, known to form heteromeric GABAA receptors and three ρ subunits that were reclassified by the Nomenclature Committee of IUPHAR (Olsen & Sieghart, 2009) to GABAA from a distinct class of receptors known as GABAC receptors. Genetic mutations or acquired functional alterations result in dysfunction of GABAergic neurotransmission (Simonato, 2018). Several epilepsy‐causing mutations and risk single nucleotide polymorphisms (SNPs) in GABA receptor subunits and associated proteins are related to disordered neuronal discharge, linking defective GABA inhibition with neuronal over‐excitation (Nutt & Malizia, 2001). As the major inhibitory neurotransmitter receptor in the mammalian brain, GABAA receptors exist in at 20% to 50% of neuronal synapses (Nutt & Malizia, 2001). Particularly, mutations in the gene GABRA1 which encodes GABAA receptor subunit α1 have been identified as a causative factor for juvenile myoclonic epilepsies and idiopathic generalized epilepsies (Cossette et al., 2002; Hirose, 2014).
MicroRNAs are small non‐coding RNAs in the regulation of post‐transcriptional gene expression. MicroRNAs negatively regulate gene expression by targeting the 3’ untranslated region (UTR) of target mRNAs for transcriptional degradation or translational repression. These microRNAs represent a crucial layer of gene expression control in epilepsy and have a potential as therapeutic biomarkers (Shazadi et al., 2014).
Our previous study in human with temporal lobe epilepsy (TLE) indicated that miR‐129–2‐3p level was upregulated in cortical brain tissue and plasma samples from patients with refractory TLE (Sun et al., 2016). However, the specific role of miR‐129–2‐3p in temporal lobe epilepsy remains to be defined. In the present study, we aimed to further explore the underlying mechanism of miR‐129–2‐3p in refractory temporal lobe epilepsy.
2. METHODS AND MATERIALS
2.1. Cell Culture
Rat primary hippocampal neurons were cultured as previously described (Lu et al., 2016). Briefly, postnatal day 1 Sprague–Dawley (SD) rats were used for cell culture preparation. After meninges were removed, cerebral hippocampi were separated from the brains of 8 rats in each group. Tissue fragments were covered with dissociation medium (DMEM) with 10% FBS and dissociated by repeated aspirations via pipetting after 10‐min mild trypsinization in 0.25% Trypsin‐EDTA (Gibco, Canada) at 37 ℃. Suspension was pelleted by mild centrifugation and then seeded in culture dishes with the dissociation medium. Culture dishes were pretreated with 0.1% poly‐D‐lysine at room temperature for at least 2 hr. Cells were incubated under 5% CO2 at 37 ℃. After 4 hr, the medium was changed to a serum‐free medium, Neurobasal medium (Gibco, Canada) with 2% B27 supplement (Gibco, Canada), Penicillin‐Streptomycin, and 0.25% Glumax 0.5 ml. For treatment, cells were treated with a medium containing 100 μM KA for 24 hr. The human neuroblastoma cell line SH‐SY5Y was cultured and passaged in DMEM supplemented with 10% FBS, and maintained under 5% CO2 at 37 ℃.
2.2. Transfection
Transfection of cultured rat primary hippocampal neurons was performed with Nucleofector (Lonza, Basel, Switzerland) following the supplier's protocol. Briefly, cells were transfected with miR‐129–2‐3p agomir (Ago‐129), miR‐129–2‐3p agomir scrambled control microRNA (Con‐129), miR‐129–2‐3p antagomir (Anta‐129), or miR‐129–2‐3p antagomir negative control (Anta‐129 NC) (GenePharma, Shanghai, China) and seeded in a 6‐well plate (60%–80% confluency). Cells were harvested 48 hr after transfection for qPCR or Western blot analysis. GABRA1 siRNAs were transfected in SH‐SY5Y cells by Lipofectamine® 3,000 (Thermo Fisher, MA, USA).
2.3. Animal Procedures
All animal care and experiments were approved by the Ethical Committee of the Dalian Medical University and in strict accordance with the National Institutes of Health guidelines for animal usage in research. Adult male Sprague–Dawley rats weighing 200–240 g (6–8 weeks) were obtained from the Medical Experimental Animal Center of Dalian Medical University. The rats were housed in a controlled temperature (21 ± 1◦C) and humidity (50%–60%) biomedical research room with a 12‐hr light/dark cycle and allowed free access to food and water available ad libitum. Before starting the experiments, the rats were treated to adapt to the laboratory environment for at least a week.
2.4. KA‐induced Seizure Model
Rats were i.c.v. injected with a microsyringe into the right lateral ventricle (Bregma coordinates: AP, 0.8 mm; L, 1.5 mm; V, 4.5 mm, based on rat brain stereotaxic atlas) of KA (40 μg/kg). Behavioral observations and seizure scoring according to the Racine scale were used to assess the activity of epilepsy. Rats with a score of 4–5 were included in the experiment. The beginning of status epilepticus (SE) was defined as the onset of continuous generalized seizure activity (stage 4 or 5 based on Racine's scale) without regaining normal behavior between seizures. Electroencephalography (EEG) was recorded at baseline and post KA injection. All the rats were monitored by EEG for up to 90 min after the successful modeling. Rats in the control group were injected with the same amount of normal saline in the right ventricle. Spontaneous recurrent seizures were monitored during the study period (6 hr, 1 day, 3 days, 7 days, and 14 days after KA treatment).
2.5. Intracerebroventricular Injections
The rats were anesthetized with isoflurane (5% induction and 1%–2% maintenance). MiR‐129–2‐3p antagomir (Anta) and miR‐129–2‐3p antagomir negative control (Anta NC) were, respectively, dissolved in artificial cerebrospinal fluid (Harvard Apparatus Holliston, MA, U.S.A.) at concentrations of 200 nmol/ml (1 nmol/5 μl per rat, infusion rate 0.5 μl/minute) and injected into the lateral ventricle of rats (bregma coordinates: AP, 0.8 mm; L, 1.5 mm; V, 4.5 mm, based on rat brain stereotaxic atlas) with a microsyringe. For knockdown of GABRA1 in vivo, we designed two GABRA1 siRNAs and the effects for these siRNAs were tested (Figure S1). We selected the most effective GABRA1 siRNA (GR siRNA: sense sequence‐5’ GCC AGA AAU UCC CUC CCA ATT 3’ and antisense sequence‐5’ UUG GGA GGG AAU UUC UGG CTT 3’) for the following experiments. The GR siRNA was diluted in 5% glucose and then complexed with in vivo jetPEI transfection reagent (Polyplus, France) at a final concentration of 200 ng/ul. 500 ng mixture of GR siRNA and transfection reagent was injected into the lateral ventricle of rats (bregma coordinates: AP, 0.8 mm; L, 1.5 mm; V, 4.5 mm, based on rat brain stereotaxic atlas) at a rate of 0.5 μl/minute. Rats were subjected to KA treatment 24 hr after antagomir and siRNA injection. EEG was recorded 7 days after KA treatment, and the small stainless‐steel spiral electrodes were fixed in the same place for the injection. The rats were then euthanized for further molecular analysis.
2.6. EEG Recordings and Behavioral Observations
EEG recordings were applied as described previously (Brandt et al., 2003). Respectively, rats were implanted with three small stainless‐steel screw electrodes (diameter: 1.2 mm) into the bilateral temporal lobe and the right frontal lobe (as a reference electrode) under anesthesia with isoflurane (5% induction, 1%–2% maintenance). EEG was analyzed using Nicolet 1.0 software (Natus, USA). Seizures were defined as high‐amplitude (>29 baseline), high‐frequency (>5 Hz) poly spike discharges lasting>5s.
2.7. Sample preparation
Rats were euthanized by intraperitoneal injection of pentobarbital sodium and perfused with ice‐cold saline through the ascending aorta to remove intravascular blood components. Hippocampus was microdissected on wet ice for further molecular and biochemical processes. For immunostaining, deeply anesthetized rats were perfusion‐fixed through the ascending aorta with paraformaldehyde (4%).
2.8. Luciferase Reporter Assay
The miR‐129–2‐3p target gene was predicted by the miRDB database (http://www.mirdb.org/cgi‐bin/search.cgi). The wild‐type GABRA1 3’UTR sequence predicted bound by miR‐129–2‐3p were cloned into the downstream of the luciferase reporter in the pGL3‐basic luciferase vector (Promega, Madison, WI, USA). The resultant construct was designated as GABRA1‐WT 3’UTR Luc reporter and sequenced to validate the fragment orientation and sequence. The wild‐type 3’UTR sequence of the GABRA1‐WT 3’UTR Luc reporter was then mutant to construct the GABRA1‐MUT 3’UTR Luc reporter. The SH‐SY5Y cells were cultured and transfected with the GABRA1‐WT 3’UTR or GABRA1‐MUT 3’UTR Luc reporters for 24 hr using the Lipofectamine® 3,000 Transfection Reagent. The fluorescence was examined by using the Dual Luciferase Reporter Assay Kit (Promega, Madison, WI, USA) and a luminometer (Turner BioSystems, USA) according to the manufacturer's protocols. The results were normalized with renilla luciferase activity. The experiments of transfection were done in triplicate.
2.9. microRNA Expression
Total RNA was extracted using the miRcute microRNA isolation kit (Tiangen, Beijing, China), and 250 ng RNA was reverse‐transcribed using stem‐loop Multiplex primer pools (Applied Biosystems, Foster City, CA, U.S.A.). Reverse transcription (RT)‐specific primers for miR‐129–2‐3p were used for all microRNA RT. The reaction mixtures were sequentially incubated at 42 ℃ for 15 min and 85 ℃ for 5 s. Quantitative polymerase chain reaction (qPCR) was performed using a Roche Light Cycler 96 Real‐Time PCR system (Roche, Switzerland) with the following cycle: 94 ℃ for 30 s, followed by 40 cycles of 95 ℃ for 12 s and 62 ℃ for 40 s. Endogenous RNA U6 small nuclear 2 (RNU6B) was used for normalization. The relative fold change in expression of the target gene transcript was determined using the comparative cycle threshold method (2‐△△CT). The PCR primers for miR‐129–2‐3p were listed in Table 1 .
TABLE 1.
Primers used for qPCR in this study
| Gene | Sequence (5’ to 3’) | |
|---|---|---|
| GABRA1 | Forward | GAGGGTATGCGTGGGATG |
| Reverse | GCTTGACTTCTTTCGGTTCTAT | |
| β‐actin | Forward | AGCCATGTACGTAGCCATCC |
| Reverse | CTCTCAGCTGTGGTGGTGAA | |
| miR−129–2−3p | Forward | TTCCAAGCCCTTACCCCA |
| Reverse | CACTTCCTCAGCACTTGTTCCTAT | |
| U6 | Forward | GCTTCGGCAGCACATATACTAAAAT |
| Reverse | CGCTTCACGAATTTGCGTGTCAT | |
2.10. Western Blot Analysis
Cells and tissues were lysed in a solution containing 20 mM Tris pH 7.5, 150 mM NaCl, 1% TritonX‐100, 2.5 mM sodium pyrophosphate, 1 mM EDTA, 1% Na3VO4, 0.5 μg/ml leupeptin, and 1 mM phenylmethanesulfonyl fluoride. Protein concentrations were determined using a BCA method (Thermo Fisher Scientific, MA, U.S.A.). Equal amount of protein from each sample was added to 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transfected into nitrocellulose filter membrane (NC) (Millipore, Billerica, MA, USA). Primary antibodies were incubated overnight at 4°C. Primary antibodies were as follows: GABAA receptor subunit α1 (Abcam; ab33299, Cambridge, MA, USA), GAPDH (Beyotime; AF0009, Shanghai, China). After incubation, the membrane and the second anti‐incubation solution were incubated for 1 hr at room temperature. Tris‐buffered saline and Tween 20 (TBST) (PBS with 0.05% Tween 20) were used, and the membrane was washed 6 times; protein bands were analyzed by Tanon (Tanon, Shanghai, China). The images were analyzed using a Chemiluminescent Imaging System (Tanon 5,200, china).
2.11. Immunofluorescence Staining
GABAA receptor subunit α1 immunofluorescence staining was applied by using a previous protocol (Andreska et al., 2014). Cells were washed by PBS and immobilized by 4% paraformaldehyde (PFA) followed by permeabilization with 0.2% Triton X‐100. Cells were blocked with PBS containing 10% goat serum and 1% BSA, and then incubated overnight at 4°C with primary antibody. Cells were incubated with secondary antibodies labeled with Alexa Red fluorescent dye for 1 hr, and then dyed with 4–6‐diamidino‐2‐phenylindole‐dihydrochloride (DAPI, 1:500, SLBR3299 V, Sigma, Shanghai, China) for 1 hr. Cells were observed under a Leica TCS SP8 (Leica Microsystems, Heidelberg, Germany) laser scanning fluorescence confocal microscope. Representative areas were imaged with a digital camera.
2.12. TUNEL Staining
TdT‐mediated dUTP Nick‐End Labeling (TUNEL) staining was performed on brain sections on Day 7 after KA treatment by using an In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. Briefly, after washing with 0.85% NaCl and PBS, the sections were fixed with 4% formaldehyde for 15 min. Following washing with PBS, the sections were covered with proteinase K solution for 15 min. After another PBS wash, the tissue sections were covered with the TUNEL reaction mixture and incubated for 1 hr. After terminating the reaction by three washes with PBS, the sections were examined and photographed using a bright field/fluorescence microscope (Leica, Wetzlar, Germany). Apoptotic cells were quantified by Image J (Rawak Software Inc., Stuttgart, Germany).
2.13. Statistical Analysis
Statistical analysis was performed with Prism GraphPad version 7.0 (GraphPad Software Inc., La Jolla, CA, USA) software presented as mean ± SD. Student's t test was used to access the statistical significance of the data between two groups, and the variations between the groups were examined by means of one‐way ANOVA. The staining quantitative analysis was performed by Image J software (Rawak Software Inc., Stuttgart, Germany). p <.05 was considered statistically significant.
3. RESULTS
3.1. miR‐129–2‐3p targets GABRA1
Our previous studies identified microRNAs differentially expressed in the temporal lobe of refractory TLE patients by a microRNA microarray (Sun et al., 2016), which indicated that miR‐129–2‐3p level was upregulated in cortical brain tissue and plasma of the patients (Figure 1a). To define the difference of miR‐129–2‐3p expression between the normal controls and refractory TLE patients, the miR‐129–2‐3p level was verified in a repeated sample‐set containing 9 controls and 11 TLE patients, which showed significant induction of miR‐129–2‐3p in the TLE group (Figure 1b).
FIGURE 1.
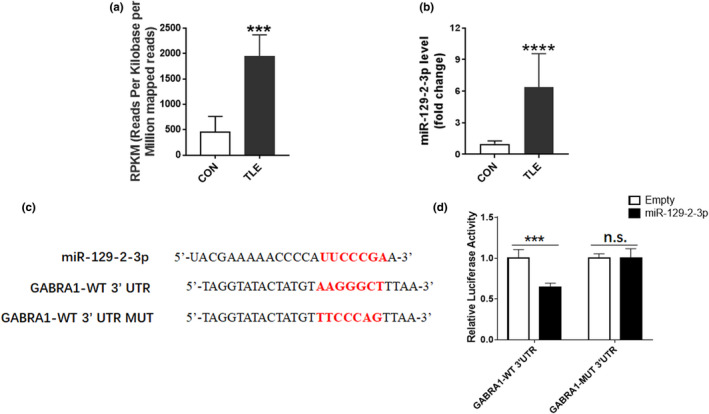
Direct target of GABRA1 by miR‐129–2‐3p (a) Quantitative measurement of miR‐129–2‐3p as assessed by RNA microarray analysis (***p <.001 compared to control group, n = 3 per group). (b) qPCR analysis showing the induction of miR‐129–2‐3p in refractory TLE patients (****p <.0001 compared to control group, n = 9 in control group and n = 11 in TLE group). (c) Sequences of miR‐129–2‐3p and predicted target of miR‐129–2‐3p on GABRA1 3’UTR. (d) Measured luciferase activity showing the inhibitory effect on GABRA1 3’UTR (***p <.001 compared to empty pGL3‐basic luciferase vector, n = 5–6 per group)
To investigate the underlying mechanism of miR‐129–2‐3p in refractory TLE, miRDB (http://www.mirdb.org/cgi‐bin/search.cgi) program was referred to search for target genes of miR‐129–2‐3p. MiR‐129–2‐3p may bind to GABRA1 3’ UTR (Figure 1c). To confirm the regulation of miR‐129–2‐3p on the expression of GABRA1,a dual‐luciferase assay was applied. Consistent with the prediction, 3’UTR luciferase activity of the wild‐type GABRA1 was markedly inhibited by miR‐129–2‐3p. However, the mutant GABRA1 3’UTR region has no response for miR‐129–2‐3p (Figure 1d).
Transfection of mimic miR‐129–2‐3p (Ago‐129) downregulated GABRA1 mRNA and protein levels in primary hippocampal neurons, whereas its inhibitor (Anta‐129) increased GABRA1 mRNA and protein levels (Figure 2a‐d). In order to further determine the effect of miR‐129–2‐3p on GABAA receptor subunit α1, the sublocalization of GABAA receptor subunit α1 in neurons was examined by cellular immunostaining. The results showed that GABAA receptor subunit α1 was broadly distributed in neurons which were upregulated by Anta‐129 (Figure 2e‐f).
FIGURE 2.
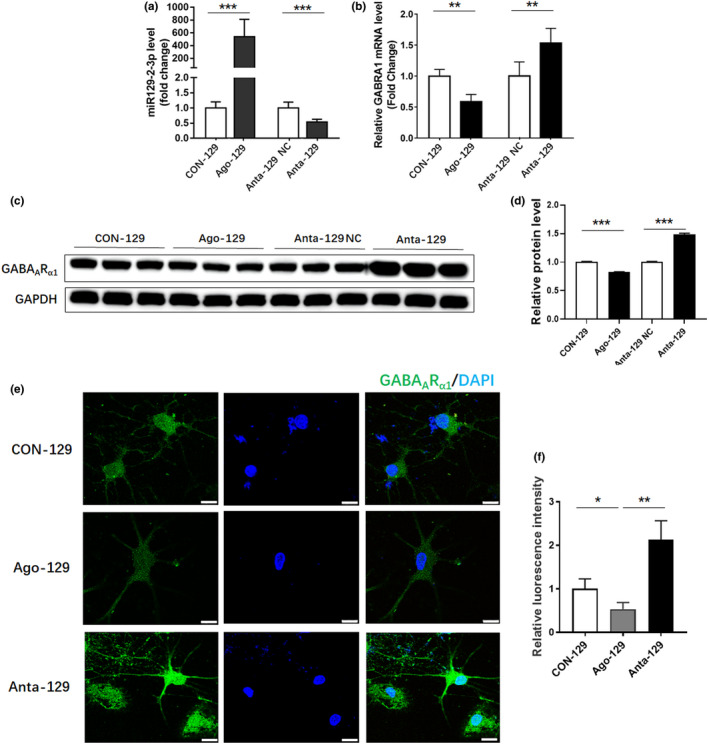
miR‐129–2‐3p regulated the expression of GABAR1 in cultured hippocampal neurons. (a‐b). qPCR analysis showing miR‐129–2‐3p (a) and GABRA1 (b) level after treatment with miR‐129–2‐3p agomir (Ago‐129), miR‐129–2‐3p agomir scrambled control miRNA (Con‐129), miR‐129–2‐3p antagomir (Anta‐129), or miR‐129–2‐3p antagomir negative control (Anta‐129 NC) (**p <.01, ***p <.001, n = 4–5). (c) Western blot analysis demonstrating the GABAARα1 protein level after treatment with Ago‐129, Con‐129, Anta‐129, or Anta‐129 NC. (d) Quantitative analysis of GABAARα1 protein level in (c) (***p <.001 compared to the CON‐129 group, n = 3). (e) Immunofluorescent staining showing the sublocalization of GABAARα1 in neuron. Bar=10 μm. (f) Quantitative analysis of the immunofluorescent signal in (e) (*p <.05, **p <.01 compared to the CON‐129 group, n = 3)
3.2. miR‐129–2‐3p and GABAA receptor subunit α1 are modulated in KA‐treated primary hippocampal neurons
After 7‐day growth of successfully cultured rat primary hippocampal neurons, 100 μM KA was added to 6‐well culture plates for 24 hr. Then, the mRNA expression levels of miR‐129–2‐3p and GABRA1 were detected by qPCR. Western blot was used to detect the protein level of GABAA receptor subunit α1. The results were as follows: miR‐129–2‐3p level was increased, and GABRA1 mRNA expression decreased and its protein expression level was consistent with the mRNA level (Figure 3a‐d). To further determine the regulation of miR‐129–2‐3p on GABRA1, miR‐129–2‐3p inhibitor (Anta‐129) was transfected to the KA‐treated rat primary hippocampal neurons. The mRNA expression and protein level of GABAA receptor subunit α1 were reversed after miR‐129–2‐3p inhibition (Figure 3e‐h).
FIGURE 3.
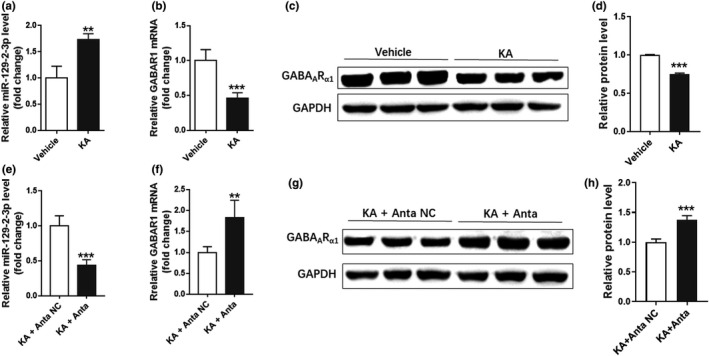
miR‐129–2‐3p was increased in KA‐treated primary hippocampal neurons. (a‐b). qPCR analysis indicating miR‐129–2‐3p (a) and GABRA1 (b) level after KA treatment in primary hippocampal neurons (**p <.01, ***p <.001 compared to the vehicle group, n = 3–4). (c‐d) Western blot analysis demonstrating the GABAARα1 protein level after KA treatment (***p <.001 compared to the Vehicle group, n = 3). (e‐f) qPCR analysis indicating miR‐129–2‐3p (e) and GABRA1 (f) level after treatment of Anta‐129 together with KA (**p <.01, ***p <.001 compared to the vehicle group, n = 3–4). (g‐h) Western blot analysis demonstrating the GABAARα1 protein level after treatment of Anta‐129 together with KA (***p <.001 compared to the KA+Anta NC group, n = 3)
3.3. In vivo KA‐induced seizure upregulated miR‐129–2‐3p and decreased GABAA receptor subunit α1 in hippocampus
To study the potential involvement of miR‐129–2‐3p and GABRA1 in epilepsy, we generated an animal model of epilepsy by unilateral i.c.v. injection of KA into the SD rat brain. We found that rats exhibited generalized tonic‐clonic seizures after KA treatment (Figure 4a). EEG recordings at 24 hr after KA treatment identified high‐amplitude and high‐frequency discharges in rats (Figure 4b‐c). More TUNEL‐positive cells were observed after KA treatment (Figure 4d‐e), indicating KA‐induced seizure‐induced neuronal death in the hippocampus.
FIGURE 4.
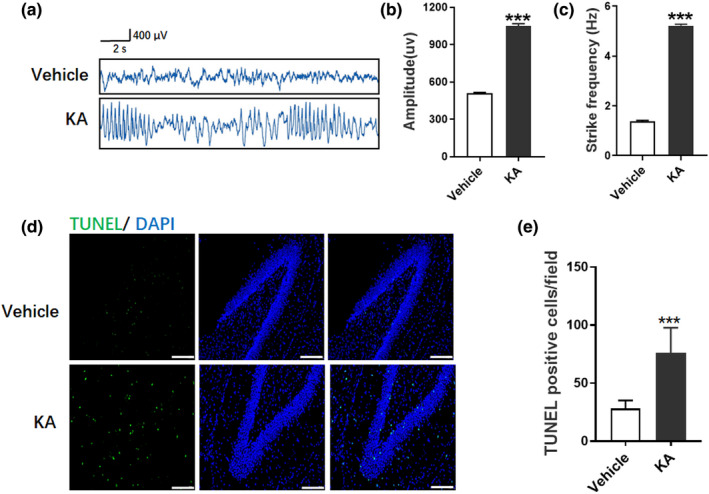
Seizure‐like EEG in KA‐induced seizure model. (a) Rats treated with vehicle and KA were analyzed by EEG and representative images were shown. (b‐c) Amplitude (b) and spike frequency (c) of seizure EEG were quantified for comparison between vehicle and KA group (***p <.001 compared to the vehicle group, n = 5). (d) TUNEL staining demonstrated the neuronal apoptosis in vehicle and KA group. Bar=25 μm. E. Quantitative analysis of TUNEL‐positive cells in (d) (***p <.001 compared to the Vehicle group, n = 3)
Rats in KA and control groups were sacrificed on Day 1, 3, 7, 10, or 30 after treatments, and the EEG recordings were examined to confirm the KA‐induced seizure. Levels of miR‐129–2‐3p in hippocampal regions of these rats were detected by qPCR, which showed that KA treatment resulted in an increase of miR‐129–2‐3p level and a decrease of GABRA1 mRNA in the hippocampus on Day 1, 3, 7, and14 after treatment (Figure 5a‐b). The induction of miR‐129–2‐3p level recovered over time, and no significant difference of miR‐129–2‐3p level between KA‐treated and control rats after 14 days. Protein levels of the miR‐129–2‐3p targeted GABAA receptor subunit α1 followed an opposite trend, which were remarkably lower in rat hippocampi after KA treatment. These results of GABAA receptor subunit α1 were confirmed by Western blotting, which showed opposing dynamic changes to miR‐129–2‐3p expression on Day 1, 3, 7, 14, and 30 after KA treatment (Figure 5c‐d, Figure S2).
FIGURE 5.
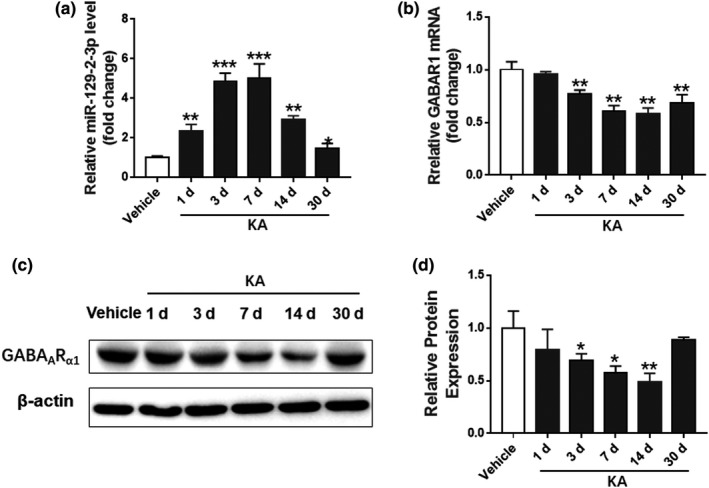
Regulation of miR‐129–2‐3p and GABRA1 in KA‐induced seizure model. (a‐b) qPCR analysis showing the miR‐129–2‐3p (a) and GABRA1 (b) level at different time points in KA‐induced seizure model (**p <.01, ***p <.001 compared to the vehicle group, n = 3–4). (c‐d) Western blot analysis demonstrating the GABAARα1 protein level at different time points in KA‐induced seizure model (*p <.05, **p <.01 compared to the vehicle group, n = 3–4)
3.4. Inhibition of miR‐129–2‐3p alleviated seizure‐like EEG
We further explored the function of miR‐129–2‐3p in vivo by inhibiting miR‐129–2‐3p expression in KA‐induced seizure model. We intracerebroventricularly injected miR‐129–2‐3p inhibitor (antagomir, Anta‐129) or its control (antagomir negative control, Anta‐129 NC) into the brains of rats, and 24 hr later the rats were treated with KA. 72 hr later, miR‐129–2‐3p level was measured which showed a significant reduction in the Anta‐129 group compared to the Anta‐129 NC group (Figure 6a), while GABRA1 mRNA level showed a significant increase in the Anta‐129 group compared with the Anta‐129 NC group, but no compelling difference compared with the normal rats (Figure 6b). Since the level of miR‐129–2‐3p was significantly increased in KA‐induced seizure models and TLE patients, it is reasonable to speculate that its depletion might reduce seizure in KA‐induced rats. After Anta‐129 injection into the rat brain, it is worth mentioning that the severity of KA‐induced seizures in rats was significantly reduced, and abnormal EEG records were associated with high amplitude and frequency (Figure 6c‐e).
FIGURE 6.
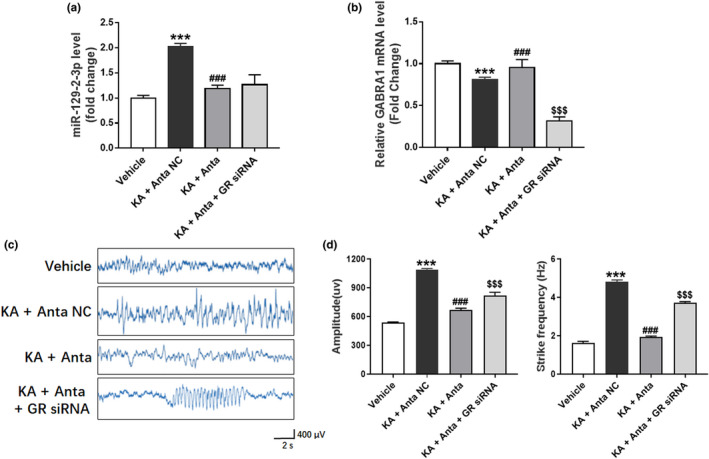
Reduced seizure‐like EEG by miR‐129–2‐3p Inhibition and counteraction of this effect by silencing of GABRA1. (a‐b) qPCR analysis showing miR‐129–2‐3p (a) and GABRA1 (b) level after treatment of miR‐129–2‐3p antagomir (Anta) with or without GABRA1 siRNA in KA‐induced seizure model (***p <.001 KA +Anta NC compared to Vehicle, ### p <.001 KA+Anta compared to KA +Anta NC, $$$ p <.001 KA +Anta + GABRA1 siRNA compared to KA +Anta, n = 5–10). (c) EEG and representative images for rats treated with miR‐129–2‐3p antagomir (Anta) with or without GABRA1 siRNA in KA‐induced seizure model. (d‐e) Amplitude (d) and spike frequency (e) of seizure EEG were quantified for comparison between different grouops (***p <.001 KA +Anta NC compared to Vehicle, ### p <.001 KA+Anta compared to KA +Anta NC, $$$ p <.001 KA +Anta + GABRA1 siRNA compared to KA +Anta, n = 5–10)
3.5. Silencing of GABRA1 diminished the seizure‐suppressing effects of miR‐129–2‐3p inhibition
Meanwhile, we investigated whether epilepsy can be suppressed by the inhibition of miR‐129–2‐3p together with the silencing of GABRA1. Rats received GABRA1 siRNA immediately after intracerebroventricular injection of Anta‐129 or Anta‐129 NC, followed by KA treatment 24 hr later. We measured GABRA1 expression (72 hr after GABRA1 siRNA treatment), and the GABRA1 level markedly decreased (Figure 6b). The seizure‐suppressive phenotype of Anta‐129 was partly reversed in GABRA1 siRNA cotreated rats, compared with control rats (Figure 6c‐e). Also, the apoptosis in hippocampus which was recovered by Anta‐129 disappeared after GABRA1 silencing (Figure S3).
4. DISCUSSION
MicroRNAs play a major regulatory effect in gene expression. Single microRNA influences various proteins involved in diverse molecular pathways and networks. Thus, changes in level or activity of a certain microRNA may have significant effects on cellular function, indicating aberrant microRNA‐induced silencing as a striking potential disease mechanism in complex disorders. MicroRNAs have been indicated as post‐transcriptional regulators of the pathogenesis of epilepsy. Alternant microRNA level has been detected in brain and blood from patients with various epilepsy disorders. It has been reported that pilocarpine‐induced seizures induce differential regulation of microRNA‐stability related genes in rat hippocampal neurons (Kinjo et al., 2016). MiR‐146a and miR‐132 were both significantly upregulated in patients with TLE (Aronica et al., 2010; Jimenez‐Mateos et al., 2011). MiR‐324‐5p and miR‐124 were significantly reduced in an animal epilepsy model and patients with TLE (Bot et al., 2013; Brennan et al., 2016; McArdle et al., 2017). MiR‐324‐5p functions in seizure onset by targeting voltage‐gated potassium channel Kv4.2, a regulator of neuronal excitability (Gross et al., 2016). MiR‐124 attenuated the severity of epilepsy and extended the latency of epilepsy by inhibiting CREB, indicating its neuroprotective effect in epilepsy (Wang et al., 2016). In addition, epileptogenesis induced by altered microRNA signaling might lead to subsequent reorganization of hippocampal networks (Xiang et al., 2016). These data suggested that microRNAs might be effective targets for epilepsy therapy.
Our previous microRNA microarray study in patients with TLE indicated that miR‐129–2‐3p level was upregulated in cortical brain tissue and plasma samples from patients with refractory TLE (Sun et al., 2016). However, the specific role of miR‐129–2‐3p in TLE remains to be defined. Previous studies on miR‐129 mainly focused on cancer. MiR‐129–1, as a negative regulator of IGF2BP3 and MAPK1, participates in cell cycle arrest in glioblastoma and acts as a potential tumor suppressor (Kouhkan et al., 2016). The downregulation of miR‐129–1‐3p and miR‐129–2‐3p in succus gastricus of gastric cancer patients may be used as a biomarker for gastric carcinoma (Yu et al., 2013). By directly inhibiting E26 transformation specific‐1 (ETS1), miR‐129 controlled the survival, proliferation, migration, and invasion of prostate cancer cells (Xu et al., 2017). MiR‐129‐5p inhibited the development of epilepsy by inhibiting MAPK signaling and c‐Fos expression (Wu et al., 2018).
To further explore the underlying mechanism of miR‐129–2‐3p in refractory temporal lobe epilepsy, miRDB (http://www.mirdb.org/cgi‐bin/search.cgi) program was applied to search for target genes of miR‐129–2‐3p. We found that GABRA1 3’ UTR may be directly anchored by miR‐129–2‐3p. In common, microRNAs negatively regulate the expression of a gene by binding to its 3′‐UTR. We proved that the regulation of GABRA1 by miR‐129–2‐3p by dual‐luciferase reporter assay, indicating that GABRA1 3’ UTR may be directly anchored by miR‐129–2‐3p. As the main inhibitory neurotransmitter in mammal, γ‐aminobutyric acid (GABA) is widely distributed in the whole nervous system (Brooks et al., 2015). It has been acknowledged that epilepsy is related to the decline of inhibitory synaptic function, and stimulating GABA‐mediated inhibitory synapse can effectively alleviate the epileptic attack (Schipper et al., 2016). The major inhibitory ligand‐gated channels in mammalian brain are GABAA receptors, and approximately 60% of GABAA receptors are α1‐containing GABAA receptors (Benarroch, 2007). The gene encoding GABAA receptor subunit α1 isoform is GABRA1 on chromosome 5q34 in human. Heterozygous GABRA1 knockout mice and GABRA1 A322D knockin mice experienced absence‐like seizures accompanied with EEG spike‐wave discharges and developed myoclonic seizures late in life (Arain et al., 2012). In clinical research, a mutation of GABRA1 was observed in children with absence seizure (Maljevic et al., 2006). Mutation in GABRA1 has also been found in affected individuals of a large French Canadian family with juvenile myoclonic epilepsy (Cossette et al., 2002). Accumulated evidence indicated that mutations in GABRA1 contribute to the genetic etiology of both benign and severe epilepsy syndromes (Johannesen et al., 2016). Many of the currently used antiepileptic drugs act through enhancing GABAergic functions that include positive allosteric modulators of GABAA receptors, blockers of GABA uptake, and blockers of GABA degradation (Khazipov et al., 2015).
In recent years, cholesterol‐binding antagomirs and agomirs were used to inhibit or enhance the targeted microRNAs (Krutzfeldt et al., 2005). We found that miR‐129–2‐3p was significantly higher in Ago‐129 (agomir) group than that in Con‐129 (agomir scrambled control microRNA) group, while miR‐129–2‐3p significantly downregulated in Anta‐129 (antagomir) group than that in Anta‐129 NC (antagomir negative control) group. However, the alteration of GABRA1 expression level was contrary to the miR‐129–2‐3p level. KA‐induced seizure model is a widely used and recognized model for refractory TLE (Lauren et al., 2010). We found that, after treated with KA, miR‐129–2‐3p increased significantly in primary hippocampal neurons, and the levels of GABRA1 decreased markedly. However, Anta‐129 reversed the alteration of miR‐129–2‐3p level and GABRA1 expression, which further demonstrated that miR‐129–2‐3p may regulate GABRA1 and act as a therapeutic target. In addition, we found that the mRNA and protein expression of GABRA1 decreased gradually within 14 days after KA induction in rats, which is consistent with previous research (Lauren et al., 2005). We also compared the EEG and immunostaining of the KA‐treated rats at different time points, which showed a negative correlation with the GABRA1 expression, especially on Day 7. Based on the above results, we cotreated rats with KA and Anta‐129 (antagomir) and observed the GABRA1 level and EEG, which showed an over‐expression effect on GABRA1 and effective inhibition on EEG. However, when GABRA1 interfered by siRNA in hippocampus, the protective function of Anta‐129 was eliminated, which further indicated the involvement of miR‐129–2‐3p in epilepsy was related to its regulation on GABRA1.
Neuronal cell death is a direct pathophysiological consequence of various brain injuries caused by epilepsy. Epileptic attack is associated with acute and delayed neuronal death, gliosis, changes in synaptic and circuit functions, neuroinflammation, neurodysplasia, and extracellular matrix remodeling (Henshall & Engel, 2013). Loss of neurons in the hippocampus is a common pathological marker for cerebral ischemia in human and aminol models. Neuronal death may promote hyperactivity by reactive gliosis and inflammation accompanied by neuronal damage (Maroso et al., 2010). To determine whether miR‐129–2‐3p participates in the process of neuron apoptosis, we investigated neuronal apoptosis by TUNEL staining. The apoptotic effect of KA for hippocampal neurons was reversed by Anta‐129 (antagomir), while the anti‐apoptotic role of Anta‐129 (antagomir) was weakened after GABRA1 knockdown by siRNA. These data indicated that miR‐129–2‐3p may be involved in the regulation of apoptosis. Inhibition of miR‐129–2‐3p has a certain neuroprotective effect. However, in the absence of GABRA1, the anti‐apoptotic role of miR‐129–2‐3p was counteracted.
The results of the current study demonstrated that miR‐129–2‐3p may be involved in the mechanism of refractory epilepsy by regulating GABRA1, and a miR‐129–2‐3p antagomir alleviated seizures and abnormal EEG findings. We therefore identified miR‐129–2‐3p as a novel seizure regulator and characterized the miR‐129–2‐3p/GABRA1 pathway as a potential target in the prevention and treatment of epilepsy.
CONFLICT OF INTEREST
All authors claim that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Guan‐Yu Wang: Methodology, Software, Formal analysis, and Writing—original draft. Zhi‐Lin Luan: Conceptualization, Methodology, Data curation, Validation, Writing—review & editing, and Funding acquisition. Ning‐Wei Che: Methodology and Formal analysis. De‐Bin Yan: Formal analysis and Software. Jian Yin: Conceptualization, Supervision, Writing—review & editing, and Funding acquisition.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.2195.
Supporting information
Figure S1‐S3
ACKNOWLEDGMENT
This work was supported by grants from the National Natural Science Foundation of China (81671298 and 81601174), Education Department of Liaoning Province, China (507123), and the Dalian Young Star of Science and Technology (2019RQ116). We are also grateful for the support from Liaoning BaiQianWan Talents Program.
Wang G‐Y, Luan Z‐L, Che N‐W, et al. Inhibition of microRNA‐129–2‐3p protects against refractory temporal lobe epilepsy by regulating GABRA1. Brain Behav. 2021;11:e02195. 10.1002/brb3.2195
Contributor Information
Zhi‐Lin Luan, Email: luanzl@dmu.edu.cn.
Jian Yin, Email: yin0815@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Andreska, T. , Aufmkolk, S. , Sauer, M. , & Blum, R. (2014). High abundance of BDNF within glutamatergic presynapses of cultured hippocampal neurons. Frontiers in Cellular Neuroscience, 8, 107. 10.3389/fncel.2014.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arain, F. M. , Boyd, K. L. , & Gallagher, M. J. (2012). Decreased viability and absence‐like epilepsy in mice lacking or deficient in the GABAA receptor alpha1 subunit. Epilepsia, 53(8), e161–165. 10.1111/j.1528-1167.2012.03596.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica, E. , Fluiter, K. , Iyer, A. , Zurolo, E. , Vreijling, J. , van Vliet, E. A. , Baayen, J. C. , & Gorter, J. A. (2010). Expression pattern of miR‐146a, an inflammation‐associated microRNA, in experimental and human temporal lobe epilepsy. European Journal of Neuroscience, 31(6), 1100–1107. 10.1111/j.1460-9568.2010.07122.x [DOI] [PubMed] [Google Scholar]
- Benarroch, E. E. (2007). GABAA receptor heterogeneity, function, and implications for epilepsy. Neurology, 68(8), 612–614. 10.1212/01.wnl.0000255669.83468.dd [DOI] [PubMed] [Google Scholar]
- Bot, A. M. , Debski, K. J. , & Lukasiuk, K. (2013). Alterations in miRNA levels in the dentate gyrus in epileptic rats. PLoS One, 8(10), e76051. 10.1371/journal.pone.0076051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, C. , Potschka, H. , Loscher, W. , & Ebert, U. (2003). N‐methyl‐D‐aspartate receptor blockade after status epilepticus protects against limbic brain damage but not against epilepsy in the kainate model of temporal lobe epilepsy. Neuroscience, 118(3), 727–740. 10.1016/s0306-4522(03)00027-7 [DOI] [PubMed] [Google Scholar]
- Brennan, G. P. , Dey, D. , Chen, Y. , Patterson, K. P. , Magnetta, E. J. , Hall, A. M. , Dube, C. M. , Mei, Y.‐T. , & Baram, T. Z. (2016). Dual and Opposing Roles of MicroRNA‐124 in Epilepsy Are Mediated through Inflammatory and NRSF‐Dependent Gene Networks. Cell Reports, 14(10), 2402–2412. 10.1016/j.celrep.2016.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, J. M. , Carrillo, G. L. , Su, J. , Lindsay, D. S. , Fox, M. A. , & Blader, I. J. (2015). Toxoplasma gondii Infections Alter GABAergic Synapses and Signaling in the Central Nervous System. mBio, 6(6), 10.1128/mBio.01428-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossette, P. , Liu, L. , Brisebois, K. , Dong, H. , Lortie, A. , Vanasse, M. , Saint‐Hilaire, J.‐M. , Carmant, L. , Verner, A. , Lu, W.‐Y. , Tian Wang, Y. U. , & Rouleau, G. A. (2002). Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nature Genetics, 31(2), 184–189. 10.1038/ng885 [DOI] [PubMed] [Google Scholar]
- Gross, C. , Yao, X. , Engel, T. , Tiwari, D. , Xing, L. , Rowley, S. , Danielson, S. W. , Thomas, K. T. , Jimenez‐Mateos, E. M. , Schroeder, L. M. , Pun, R. Y. K. , Danzer, S. C. , Henshall, D. C. , & Bassell, G. J. (2016). MicroRNA‐mediated downregulation of the potassium channel Kv4.2 contributes to seizure onset. Cell Reports, 17(1), 37–45. 10.1016/j.celrep.2016.08.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshall, D. C. , & Engel, T. (2013). Contribution of apoptosis‐associated signaling pathways to epileptogenesis: Lessons from Bcl‐2 family knockouts. Frontiers in Cellular Neuroscience, 7, 110. 10.3389/fncel.2013.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose, S. (2014). Mutant GABA(A) receptor subunits in genetic (idiopathic) epilepsy. Progress in Brain Research, 213, 55–85. 10.1016/B978-0-444-63326-2.00003-X [DOI] [PubMed] [Google Scholar]
- Jimenez‐Mateos, E. M. , Bray, I. , Sanz‐Rodriguez, A. , Engel, T. , McKiernan, R. C. , Mouri, G. , Tanaka, K. , Sano, T. , Saugstad, J. A. , Simon, R. P. , Stallings, R. L. , & Henshall, D. C. (2011). miRNA Expression profile after status epilepticus and hippocampal neuroprotection by targeting miR‐132. American Journal of Pathology, 179(5), 2519–2532. 10.1016/j.ajpath.2011.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez‐Mateos, E. M. , & Henshall, D. C. (2013). Epilepsy and microRNA. Neuroscience, 238, 218–229. 10.1016/j.neuroscience.2013.02.027 [DOI] [PubMed] [Google Scholar]
- Johannesen, K. , Marini, C. , Pfeffer, S. , Moller, R. S. , Dorn, T. , Niturad, C. E. , & Maljevic, S. (2016). Phenotypic spectrum of GABRA1: From generalized epilepsies to severe epileptic encephalopathies. Neurology, 87(11), 1140–1151. 10.1212/WNL.0000000000003087 [DOI] [PubMed] [Google Scholar]
- Khazipov, R. , Valeeva, G. , & Khalilov, I. (2015). Depolarizing GABA and developmental epilepsies. CNS Neuroscience & Therapeutics, 21(2), 83–91. 10.1111/cns.12353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjo, E. R. , Higa, G. S. V. , Santos, B. A. , de Sousa, E. , Damico, M. V. , Walter, L. T. , Morya, E. , Valle, A. C. , Britto, L. R. G. , & Kihara, A. H. (2016). Pilocarpine‐induced seizures trigger differential regulation of microRNA‐stability related genes in rat hippocampal neurons. Scientific Reports, 6, 20969. 10.1038/srep20969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouhkan, F. , Mobarra, N. , Soufi‐Zomorrod, M. , Keramati, F. , Hosseini Rad, S. M. A. , Fathi‐Roudsari, M. , Tavakoli, R. , Hajarizadeh, A. , Ziaei, S. , Lahmi, R. , Hanif, H. , & Soleimani, M. (2016). MicroRNA‐129‐1 acts as tumour suppressor and induces cell cycle arrest of GBM cancer cells through targeting IGF2BP3 and MAPK1. Journal of Medical Genetics, 53(1), 24–33. 10.1136/jmedgenet-2015-103225 [DOI] [PubMed] [Google Scholar]
- Krutzfeldt, J. , Rajewsky, N. , Braich, R. , Rajeev, K. G. , Tuschl, T. , Manoharan, M. , & Stoffel, M. (2005). Silencing of microRNAs in vivo with 'antagomirs'. Nature, 438(7068), 685–689. 10.1038/nature04303 [DOI] [PubMed] [Google Scholar]
- Lauren, H. B. , Lopez‐Picon, F. R. , Brandt, A. M. , Rios‐Rojas, C. J. , & Holopainen, I. E. (2010). Transcriptome analysis of the hippocampal CA1 pyramidal cell region after kainic acid‐induced status epilepticus in juvenile rats. PLoS One, 5(5), e10733. 10.1371/journal.pone.0010733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren, H. B. , Lopez‐Picon, F. R. , Korpi, E. R. , & Holopainen, I. E. (2005). Kainic acid‐induced status epilepticus alters GABA receptor subunit mRNA and protein expression in the developing rat hippocampus. Journal of Neurochemistry, 94(5), 1384–1394. 10.1111/j.1471-4159.2005.03274.x [DOI] [PubMed] [Google Scholar]
- Lu, Z. , Piechowicz, M. , & Qiu, S. (2016). A simplified method for ultra‐low density, long‐term primary hippocampal neuron culture. Journal of Visualized Experiments, (109), 53797. 10.3791/53797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maljevic, S. , Krampfl, K. , Cobilanschi, J. , Tilgen, N. , Beyer, S. , Weber, Y. G. , & Heils, A. (2006). A mutation in the GABA(A) receptor alpha(1)‐subunit is associated with absence epilepsy. Annals of Neurology, 59(6), 983–987. 10.1002/ana.20874 [DOI] [PubMed] [Google Scholar]
- Maroso, M. , Balosso, S. , Ravizza, T. , Liu, J. , Aronica, E. , Iyer, A. M. , Rossetti, C. , Molteni, M. , Casalgrandi, M. , Manfredi, A. A. , Bianchi, M. E. , & Vezzani, A. (2010). Toll‐like receptor 4 and high‐mobility group box‐1 are involved in ictogenesis and can be targeted to reduce seizures. Nature Medicine, 16(4), 413–419. 10.1038/nm.2127 [DOI] [PubMed] [Google Scholar]
- McArdle, H. , Jimenez‐Mateos, E. M. , Raoof, R. , Carthy, E. , Boyle, D. , ElNaggar, H. , & Spain, E. (2017). "TORNADO" ‐ Theranostic One‐Step RNA Detector; microfluidic disc for the direct detection of microRNA‐134 in plasma and cerebrospinal fluid. Scientific Reports, 7(1), 1750. 10.1038/s41598-017-01947-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt, D. J. , & Malizia, A. L. (2001). New insights into the role of the GABA(A)‐benzodiazepine receptor in psychiatric disorder. British Journal of Psychiatry, 179, 390–396. 10.1192/bjp.179.5.390 [DOI] [PubMed] [Google Scholar]
- Olsen, R. W. , & Sieghart, W. (2009). GABA A receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology, 56(1), 141–148. 10.1016/j.neuropharm.2008.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen, A. , & Lukasiuk, K. (2011). Mechanisms of epileptogenesis and potential treatment targets. The Lancet Neurology, 10(2), 173–186. 10.1016/S1474-4422(10)70310-0 [DOI] [PubMed] [Google Scholar]
- Schipper, S. , Aalbers, M. W. , Rijkers, K. , Swijsen, A. , Rigo, J. M. , Hoogland, G. , & Vles, J. S. (2016). Tonic GABAA receptors as potential target for the treatment of temporal lobe epilepsy. Molecular Neurobiology, 53(8), 5252–5265. 10.1007/s12035-015-9423-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shazadi, K. , Petrovski, S. , Roten, A. , Miller, H. , Huggins, R. M. , Brodie, M. J. , & Sills, G. J. (2014). Validation of a multigenic model to predict seizure control in newly treated epilepsy. Epilepsy Research, 108(10), 1797–1805. 10.1016/j.eplepsyres.2014.08.022 [DOI] [PubMed] [Google Scholar]
- Simonato, M. (2018). Epilepsy an update on disease mechanisms: The potential role of microRNAs. Frontiers in Neurology, 9, 176. 10.3389/fneur.2018.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , Wang, X. , Wang, Z. , Zhang, Y. , Che, N. , Luo, X. , & Yin, J. (2016). Expression of microRNA‐129‐2‐3p and microRNA‐935 in plasma and brain tissue of human refractory epilepsy. Epilepsy Research, 127, 276–283. 10.1016/j.eplepsyres.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Wang, W. , Wang, X. , Chen, L. , Zhang, Y. , Xu, Z. , Liu, J. , & Luo, J. (2016). The microRNA miR‐124 suppresses seizure activity and regulates CREB1 activity. Expert Reviews in Molecular Medicine, 18, e4. 10.1017/erm.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, D. M. , Zhang, Y. T. , Lu, J. , & Zheng, Y. L. (2018). Effects of microRNA‐129 and its target gene c‐Fos on proliferation and apoptosis of hippocampal neurons in rats with epilepsy via the MAPK signaling pathway. Journal of Cellular Physiology, 233(9), 6632–6643. 10.1002/jcp.26297 [DOI] [PubMed] [Google Scholar]
- Xiang, L. , Ren, Y. , Li, X. , Zhao, W. , & Song, Y. (2016). MicroRNA‐204 suppresses epileptiform discharges through regulating TrkB‐ERK1/2‐CREB signaling in cultured hippocampal neurons. Brain Research, 1639, 99–107. 10.1016/j.brainres.2016.02.045 [DOI] [PubMed] [Google Scholar]
- Xu, S. , Ge, J. , Zhang, Z. , & Zhou, W. (2017). MiR‐129 inhibits cell proliferation and metastasis by targeting ETS1 via PI3K/AKT/mTOR pathway in prostate cancer. Biomedicine & Pharmacotherapy, 96, 634–641. 10.1016/j.biopha.2017.10.037 [DOI] [PubMed] [Google Scholar]
- Yu, X. , Song, H. , Xia, T. , Han, S. , Xiao, B. , Luo, L. , & Guo, J. (2013). Growth inhibitory effects of three miR‐129 family members on gastric cancer. Gene, 532(1), 87–93. 10.1016/j.gene.2013.09.048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1‐S3
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


