This multicenter collaborative cohort study of 550 patients assesses whether the development of pharyngocutaneous fistula after salvage laryngectomy is associated with locoregional and distant control, disease-free survival, and overall survival.
Key Points
Question
Is the development of pharyngocutaneous fistula (PCF) after salvage laryngectomy associated with locoregional or distant control?
Findings
This multicenter collaborative cohort study of 550 patients found that the rate of distant metastatic disease was 13% higher in patients who developed PCF after salvage laryngectomy. Multivariable analysis, controlling for chemotherapy, lymphovascular invasion, extranodal extension, primary T category, and recurrence N category showed that PCF was independently associated with a 2-fold increase in distant metastases; however, no association was observed between PCF and locoregional control, overall survival, or disease-free survival.
Meaning
Pharyngocutaneous fistula, a common complication following salvage laryngectomy, is associated with an increased risk of developing distant metastases but not locoregional failure.
Abstract
Importance
Pharyngocutaneous fistula (PCF) results in an inflammatory reaction, but its association with the rate of locoregional and distant control, disease-free survival, and overall survival in laryngeal cancer remains uncertain.
Objective
To determine if pharyngocutaneous fistula after salvage laryngectomy is associated with locoregional and distant control, disease-free survival, and/or overall survival.
Design, Setting, and Participants
A multicenter collaborative retrospective cohort study conducted at 5 centers in Canada and the US of 550 patients who underwent salvage laryngectomy for recurrent laryngeal cancer from January 1, 2000, to December 31, 2014. The median follow-up time was 5.7 years (range, 0-18 years).
Main Outcomes and Measures
Outcomes examined included locoregional and distant control, disease-free survival, and overall survival. Fine and Gray competing risk regression and Cox-proportional hazard regression models were used for outcomes. Competing risks and the Kaplan-Meier methods were used to estimate outcomes at 3 years and 5 years.
Results
In all, 550 patients (mean [SD] age, 64 [10.4] years; men, 465 [85%]) met inclusion criteria. Pharyngocutaneous fistula occurred in 127 patients (23%). The difference in locoregional control between the group of patients with PCF (75%) and the non–PCF (72%) group was 3% (95% CI, −6% to 12%). The difference in overall survival between the group with PCF (44%) and the non–PCF group (52%) was 8% (95% CI, −2% to 20%). The difference in disease-free survival between PCF and non–PCF groups was 6% (95% CI, −4% to 16%). In the multivariable model, patients with PCF were at a 2-fold higher rate of distant metastases (hazard ratio, 2.00; 95% CI, 1.22 to 3.27). Distant control was reduced in those with PCF, a 13% (95% CI, 3% to 21%) difference in 5-year distant control.
Conclusions and Relevance
This multicenter retrospective cohort study found that development of PCF after salvage laryngectomy is associated with an increased risk for the development of distant metastases.
Introduction
The effect of postoperative wound infection on cancer recurrence and survival is not well defined. This relationship has been studied across various disease sites, including breast,1 colorectal2 and esophageal,3 with some studies reporting improved outcomes with wound infections, and others, poorer outcomes.2,3,4,5,6 To our knowledge, only a limited number of studies in the head and neck oncology field have investigated this relationship. Previous work in oral cavity cancer4 found that postoperative wound infection was not a predictor of survival or locoregional control (LRC); however, there was a trend toward increased distant metastases.
Pharyngocutaneous fistula (PCF), a complication that occurs in 8% to 35% of patients after a total laryngectomy, is associated with a robust local inflammatory reaction and frequent infection7,8,9; therefore, it provides an opportunity for studying the relationship between surgical wound inflammation and/or infection and oncologic outcomes. Small retrospective case series studies of oncologic outcomes of PCF in patients who underwent laryngectomy have found conflicting results in terms of survival and local recurrence.10,11,12,13 Limitations in terms of small sample sizes and the confounding factor of postoperative radiotherapy, along with inclusion of patients who underwent primary laryngectomy, makes the literature difficult to interpret. To address these gaps in the literature, we performed a multicenter study to evaluate the relationship between PCF after salvage laryngectomy and oncologic outcomes.
Methods
Study Design
This was a multicenter retrospective cohort study of patients who had undergone laryngectomy from January 1, 2000, to December 31, 2014, for local cancer recurrence after radiation or chemoradiation at any of the 5 participating study sites: Princess Margaret Cancer Center (University Health Network, Toronto, Ontario, Canada), Odette Cancer Center (Sunnybrook Health Science Center, Toronto), Mount Sinai Hospital (Toronto), Memorial Sloan Kettering Cancer Center (New York, NY), and the MD Anderson Cancer Center (The University of Texas, Houston). Ethics approvals were obtained through the Institutional Review Board or Research Ethics Board at each of the participating centers prior to study commencement. Informed consent was waived because the study used only retrospective deidentified data.
Patients with distant metastatic disease on initial presentation were excluded, as were those patients treated with partial laryngectomy or laryngectomy performed for a primary site other than the larynx (ie, hypopharynx cancer) and those with chondronecrosis. Patients who had a primary laryngectomy were excluded to avoid the confounder of postoperative radiation on LRC, as well as the lower rates of PCF in patients undergoing primary laryngectomy compared with those undergoing salvage laryngectomy.3
Variables collected included demographic information, medical comorbidities, details of primary laryngeal cancer, primary treatment modality, and outcome data (postoperative complications, recurrence, and survival). Postoperative PCF was defined as any identified communication between the pharynx and the skin.
Those patients with radiographic findings suggestive of an anastomotic leak, but without clinical evidence of PCF, were included in the non–PCF group because radiographic leaks have been demonstrated to have low positive predictive value14—a number of patients deemed to have leaks on radiographic studies have had false positive results. Leak studies are often ordered based on clinician preference prior to oral feeding. Variations in practice regarding ordering postoperative swallow studies exist both within and across institutions. Lastly, if a leak is present without clinical manifestations, it presumably would not be associated with a significant inflammatory response. When a PCF was identified, the time to fistula (from date of the laryngectomy) and management strategies were recorded.
Statistical Analysis
Clinicopathologic and treatment-related data were summarized using descriptive statistics for both PCF and non–PCF groups. Univariate Cox proportional hazards regression was applied on time to PCF development to identify variables that are associated with PCF.
Outcomes were LRC, distant control (DC), disease-free survival, and overall survival, and were defined as follows: LRC, from date of salvage laryngectomy to date of local, regional, or locoregional failure, as determined by the results of biopsy testing or diagnostic imaging; DC, from date of salvage laryngectomy to date of distant failure, as determined by the results of biopsy testing or diagnostic imaging; disease-free survival, from date of salvage laryngectomy to date of recurrence (local, regional, or distant) or death; and overall survival, from date of salvage laryngectomy to date of death from any cause. Competing risks method was applied for LRC and DC, while death without specific events were treated as competing risk. Fine and Gray competing risk regression was used for univariable and multivariable analysis of LRC and DC. Cox proportional hazards regression models were used for disease-free survival and overall survival. Base models were built by backward selection for each outcome. The effect of PCF, controlling for factors in the base model, was reported for each outcome.
Variables that may have influenced rates of distant metastases (ie, concurrent chemotherapy, recurrent N category, and extranodal extension) were added to the multivariable analysis of DC to further confirm the effect of PCF. The effect size for rates was calculated as the difference in rates using 95% CIs. Data analyses were performed using SAS, version 9.5 (SAS Institute Inc) and R, version 3.1.2 (The R Foundation for Statistical Computing). P values were 2-tailed and statistical significance was defined as P < .05.
Results
Cohort Description
From the 5 centers, there were 550 patients (mean [SD; range] age, 64 [10.4; 32-90] years; 465 [85%] men and 84 [15%] women) eligible for inclusion in the study. Demographic information and clinicopathologic data at initial presentation and details regarding primary laryngeal cancer treatment are summarized in Table 1. The median event time from initial treatment of laryngeal cancer and recurrence was 1 year (range, 1 month-27 years). All patients underwent salvage total laryngectomy, with 8% of patients requiring total circumferential pharyngectomy. A bilateral neck dissection was performed in 71% of participants and a subtotal thyroidectomy in 53%. The overall incidence of PCF was 23% (n = 127), with a median time to development of PCF after salvage laryngectomy of 2.9 weeks (interquartile range [IQR], 0.6-3.5 weeks). Of the 127 patients who developed PCF, 73 were treated with local wound care and the remainder with surgical management. The difference in the mean number of days spent in hospital for the group with PCF (24.7 days) compared with the non–PCF group (13.8 days) was 10.7 days (95% CI, 10.3-11.1 days).
Table 1. Baseline Characteristics of Patients, Stratified by Presence or Absence of PCF.
| Characteristic | No. (%) | Hazard ratio (95% CI) | ||
|---|---|---|---|---|
| Overall | No PCF | PCF | ||
| No. (%) | 550 (100) | 423 (77) | 127 (23) | NA |
| Age at diagnosis, median (IQR), y | 64 (32-90) | 64 (39-90) | 63 (32-88) | 0.99 (0.97-1.00) |
| Men | 465 (85) | 361 (85) | 104 (83) | 0.84 (0.52-1.34) |
| Women | 85 (15) | 62 (15) | 23 (17) | 1 [Reference] |
| Primary site | ||||
| Glottis | 350 (64) | 276 (66) | 74 (59) | 1 [Reference] |
| Subglottis | 9 (2) | 8 (2) | 1 (1) | 0.51 (0.07-3.85) |
| Supraglottis | 188 (34) | 137 (33) | 51 (40) | 1.29 (0.9-1.85) |
| Primary cT category | ||||
| T1/T2 | 346 (68) | 275 (70) | 71 (60) | 1 [Reference] |
| T3/T4 | 164 (32) | 116 (30) | 48 (40) | 1.51 (1.05-2.19) |
| Primary cN category | ||||
| N0/N1 | 456 (89) | 354 (90) | 102 (86) | 1 [Reference] |
| N2/N3 | 56 (11) | 39 (10) | 17 (14) | 1.3 (0.78- 2.15) |
| Primary treatment | ||||
| Radiation alone | 383 (70) | 302 (71) | 81 (64) | 1 [Reference] |
| Concurrent chemoradiation | 167 (30) | 121 (29) | 46 (36) | 1.32 (0.91-1.93) |
| Radiation dosage, median (IQR), Gy | 66 (20-85) | 66 (20-85) | 68 (24-79) | 1.01 (0.98-1.03) |
| Radiation fractions, median (IQR) | 33 (3-72) | 33 (5-72) | 35 (3-66) | 1.00 (0.98-1.02) |
| Recurrence cT category | ||||
| T1/T2 | 133 (34) | 102 (35) | 31 (32) | 1 [Reference] |
| T3/T4 | 258 (66) | 193 (65) | 65 (68) | 1.07 (0.7-1.65) |
| Recurrence cN category | ||||
| N0/N1 | 475 (89) | 367 (90) | 108 (86) | 1 [Reference] |
| N2/N3 | 60 (11) | 43 (10) | 17 (14) | 1.3 (0.78-2.15) |
| Extent of salvage surgery | ||||
| Total laryngectomy/partial pharyngectomy | 502 (92) | 385 (92) | 117 (92) | 1 [Reference] |
| Bilateral neck dissection | 365 (71) | 274 (70) | 91 (74) | 1.21 (0.81-1.81) |
| Total thyroidectomy | 132 (24) | 97 (23) | 35 (28) | 1.23 (0.84-1.8) |
| Total laryngopharyngectomy | 44 (8) | 34 (8) | 10 (8) | 1.01 (0.53-1.93) |
| Reconstruction | ||||
| No flap | 335 (45) | 254 (60) | 81 (64) | 1 [Reference] |
| Regional flap | 74 (30) | 54 (13) | 20 (16) | 0.82 (0.65-1.05) |
| Free flap (inlay/onlay) | 139 (25) | 113 (27) | 26 (20) | 1.01 (0.99-1.03) |
| Surgical margins | ||||
| Clear | 453 (84) | 358 (86) | 95 (75) | 1 [Reference] |
| Close (<5 mm) | 46 (8) | 30 (7) | 16 (13) | 1.85 (1.09-3.13) |
| Positive (>5 mm) | 43 (8) | 28 (7) | 15 (12) | 1.91 (1.11-3.29) |
| Pathology | ||||
| Extranodal extension | 51 (10) | 37 (9) | 14 (11) | 1.23 (0.71-2.14) |
| Perineural invasion | 223 (43) | 163 (41) | 60 (50) | 1.37 (0.96-1.97) |
| Lymphovascular invasion | 125 (24) | 87 (22) | 38 (32) | 1.51 (1.02-2.23) |
| Follow-up postlaryngectomy, median (range), y | 5.7 (0-18) | 6 (0-18) | 3.5 (0-15) | 1.00 (0.9-1.11) |
Abbreviations: cN, primary nodule category; cT, primary clinical tumor category; IQR, interquartile range; NA, not applicable; PCF, pharyngocutaneous fistula.
On pathologic evaluation, negative margins were achieved in 84% (n = 453) of patients, with 8% having close margins and 8% having positive margins. Perineural spread and lymphovascular invasion were present in 43% and 24% of biopsy specimen specimens, respectively. Most (62%) recurrences were moderately differentiated, 23% were poorly differentiated, and the remainder well differentiated. Nodal metastases were present in 100 patients (18%), of which 51% had extranodal extension. On pathologic staging, 133 tumors were classified as recurrent pathological tumor (rpT) 1 or rpT2, and 258 tumors as rpT3 or rpT4. Overall staging was almost equally divided among localized (stage I or II, n = 264) and advanced (stage II or III, n = 242) recurrent disease.
Survival Analysis and Disease Control
The median follow-up time among participants was 5.7 years (range, 0-18 years). Locoregional recurrence was observed in 132 patients (24%). The most frequent sites of recurrence were the neck (n = 72; 54%), the stoma (n = 32; 24%), the neopharynx (n = 20; 15%), the base of the tongue (n = 13; 10%), the nasopharynx (n = 2; 2%), and the esophagus (n = 1; 1%). The difference (95% CI) in LRC between the group of patients who developed PCF (75%) and non–PCF (72%) group was 3% (−6% to 12%; Figure, A). Predictors of decreased LRC on multivariable analysis included low preoperative hemoglobin (hazard ratio [HR], 0.99; 95% CI, 0.98 to 0.99), positive margins (HR, 2.56; 95% CI, 1.50 to 4.39), and advanced initial T category (HR, 1.59; 95% CI, 1.11 to 2.29).
Figure. Cancer Outcomes in Patients by Presence or Absence of Pharyngocutaneous Fistula After Salvage Laryngectomy.
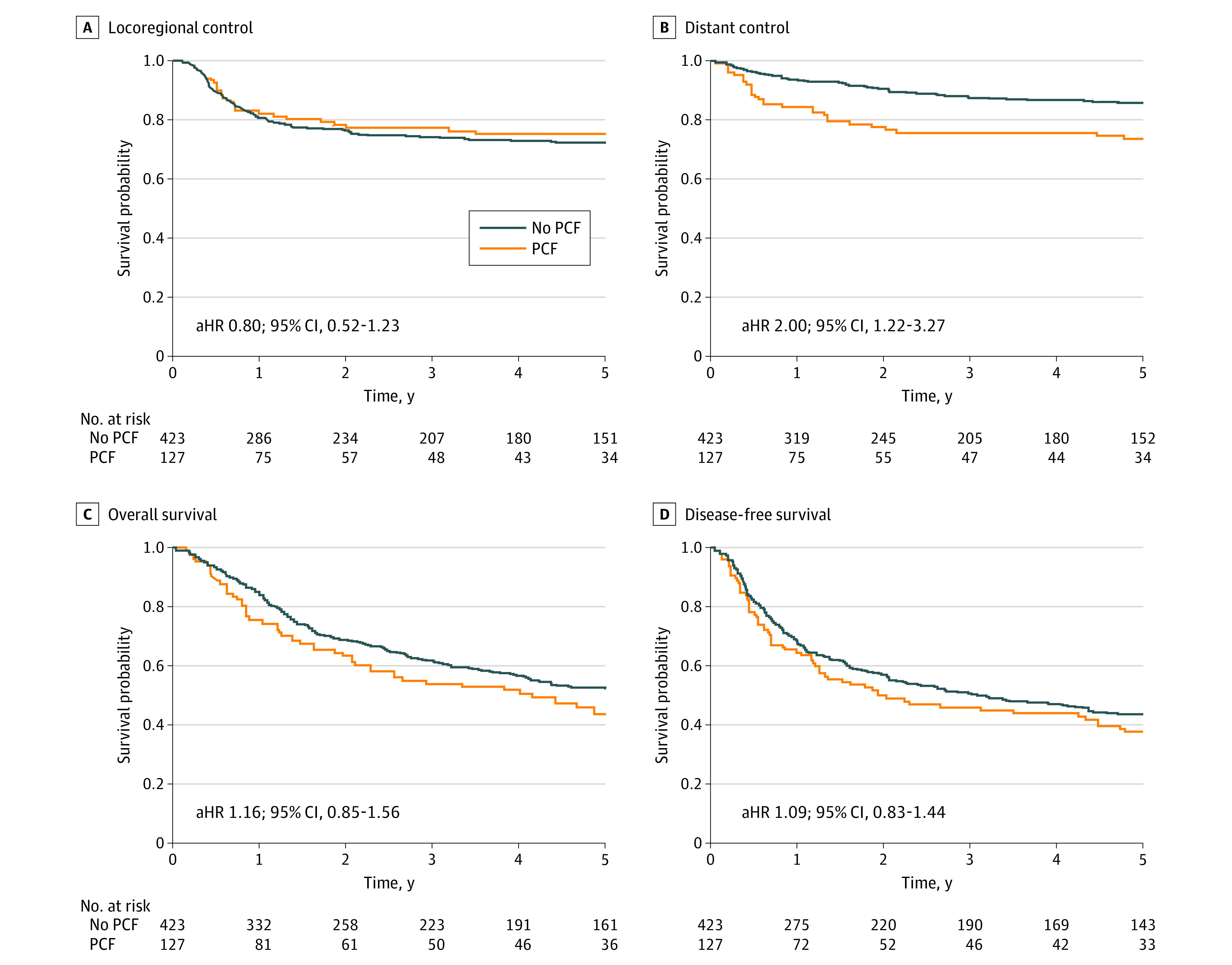
A, Locoregional control; B, distant control; C, overall survival; and D, disease-free survival among the group of patients with PCF and the non–PCF group. aHR indicates adjusted hazard ratio; PCF, pharyngocutaneous fistula.
A total of 90 patients (16%) developed distant recurrence: 60 patients (67%) to the lung; 14 (15%), bone; 6 (7%), brain; 5 (6%), liver; 3 (3%), mediastinum; and 2 (2%), adrenal glands. Among the 127 patients with PCF, 34 patients (26%) developed distant failure compared with 56 of 423 patients (13%) in the non–PCF group, for a difference of 13% (range, 3%-21%; Figure, B). On univariable analysis, predictors of DC included PCF, advanced recurrent N category, extranodal extension, perineural invasion, and lymphovascular invasion (Table 2). On multivariable analysis, the presence of PCF was an independent predictor for reduced DC (HR, 2.00; 95% CI, 1.22- 3.27; P = .005) while controlling for chemotherapy, lymphovascular invasion, extranodal extension, primary T category, and recurrence N category.
Table 2. Univariable Analysis of Distant Metastatic Control and Associated Hazard Ratios.
| Covariate | Hazard ratio (95% CI) |
|---|---|
| Pharyngocutaneous fistula | |
| No | 1 [Reference] |
| Yes | 2.34 (1.53-3.58) |
| Sex | |
| Women | 1 [Reference] |
| Men | 1.16 (0.63-2.12) |
| Diabetes | |
| No | 1 [Reference] |
| Yes | 0.83 (0.44-1.57) |
| Tracheoesophageal puncture | |
| No | 1 [Reference] |
| Primary | 0.47 (0.3-0.74) |
| Secondary | 0.65 (0.34-1.24) |
| Preoperative hemoglobin | 0.99 (0.98-0.99) |
| Surgical margins | |
| Clear | 1 [Reference] |
| Close | 1.32 (0.68-2.56) |
| Positive | 2.09 (1.04-4.19) |
| Primary cT category | |
| T1/T2 | 1 [Reference] |
| T3/T4 | 2.15 (1.41-3.27) |
| Primary cN category | |
| N0/N1 | 1 [Reference] |
| N2/N3 | 1.65 (0.94-2.9) |
| Recurrence cT category | |
| T1/T2 | 1 [Reference] |
| T3/T4 | 1.51 (0.9-2.54) |
| Recurrence cN category | |
| N0/N1 | 1 [Reference] |
| N2/N3 | 2.26 (1.28-3.97) |
| Total radiation dosage | 1 (0.96-1.04) |
| Extranodal extension | |
| No | 1 [Reference] |
| Yes | 2.92 (1.67-5.12) |
| Perineural invasion | |
| No | 1 [Reference] |
| Yes | 1.64 (1.07-2.51) |
| Lymphovascular invasion | |
| No | 1 [Reference] |
| Yes | 2.34 (1.51-3.62) |
| Concurrent chemoradiation | |
| No | 1 [Reference] |
| Yes | 1.47 (0.95-2.27) |
Abbreviations: cN, primary nodule category; cT, primary clinical tumor category.
There were only 5 patients who had radiographic leaks without a PCF. When these 5 patients were included in the PCF group rather than the non–PCF group, reanalysis failed to demonstrate any differences in rates of DC (13%; 95% CI, 3% to 21%), disease-free survival (6%; 95% CI, −4% to 16%), and overall survival (8%; 95% CI, −2% to 20%).
The 5-year overall survival and disease-free survival of the entire cohort was 50% and 42%, respectively. The difference in 5-year overall survival was not significantly different between the PCF (44%) and the non–PCF (52%) groups (rate difference, 8%; 95% CI, −2% to 20%; Figure, C). Multivariable analysis determined the predictors of reduced overall survival to be low preoperative hemoglobin (HR, 0.99; 95% CI, 0.98 to 0.99), advanced initial T category (HR, 1.43; 95% CI, 1.09 to 1.86), extranodal extension (HR, 1.89; 95% CI, 1.89 to 2.87), perineural invasion (HR, 1.52; 95% CI, 1.16 to 1. 99), and lymphovascular invasion (HR, 1. 43; 95% CI, 1.06 to 1.93). Five-year disease-free survival was not significantly different between the PCF (38%) and non–PCF groups (44%; rate difference, 6%; 95% CI, −4% to 16%; Figure, D). Multivariable analysis determined the predictors of reduced disease-free survival to be low preoperative hemoglobin (HR, 0.99; 95% CI, 0.98 to 0.99), positive margins (HR, 1.88; 95% CI, 1.25 to 2.85), advanced initial T category (HR, 1.33; 95% CI, 1.04 to 1.71), extranodal extension (HR, 2.30; 95% CI, 1.58 to 3.35), perineural invasion (HR, 1.46; 95% CI, 1.14 to 1.87), and lymphovascular invasion (HR, 1.43; 95% CI, 1.07 to 1.90).
Discussion
Pharyngocutaneous fistula after salvage laryngectomy is a well-recognized complication with a reported rate of 8% to 35%.5,13,15,16,17,18 Patients with PCF can develop an intense inflammatory reaction when saliva bathes the local tissues, which is often associated with a superimposed bacterial infection. While complications associated with PCF, such as nutritional derangements, soft tissue infections, pneumonia, carotid blowout, and prolonged hospitalization, are well known, there is little understanding of how the intense inflammatory reaction and frequent accompanying infection may affect oncologic outcomes (eg, locoregional recurrence, distant metastases, and survival).
The relationship between local inflammation and cancer recurrence or progression has been evaluated across many different cancer types, and various theories have been proposed to account for the association.1,19,20,21,22,23 In the breast cancer literature, it has been hypothesized that alterations in the local tissue and tumor microenvironment may potentiate the growth of breast cancer cells.1 As hypothesized by Boudreau and colleagues,24 the interrelation between the inflammatory stimulus of surgery and the additional insult of a postoperative infection may lead to the release of cytokines and growth factors that may potentiate the growth of tumor cells. Endotoxins released from bacteria have been shown to activate the toll-like receptor pathway leading to the release of more proinflammatory cytokines.25 These cytokines are associated with tumor cell migration and invasion seen in distant metastases.24 At a cellular level, Wu and Zhou15 found that inflammatory microenvironments can increase IL-6 and vascular endothelial growth factor secretion, which are associated with immune suppression and the generation of metastatic tumor cells. The role of inflammatory mediators, including C-reactive protein and neutrophil-to-lymphocyte ratio, on cancer recurrence has also been evaluated in patients with esophageal cancer.26 Powell and colleagues26 found that inflammatory mediators were independently associated with worse overall and disease-free survival for patients undergoing esophagectomy with curative intent.
In a recent systematic review of 17 569 patients who had developed recurrence following breast cancer surgery, 2077 patients were found to have a concomitant surgical site infection.1 Through this analysis, both local and distant recurrence were found to be associated with surgical site infection.1 However, it was noted that many of the included studies had not specified the proportion of patients who developed local, rather than distant, recurrence.1 An association between anastomotic leakage after resection of colon cancers and local recurrence has been reported in several studies.27,28,29,30 It has been hypothesized that cancer cells with metastatic potential may remain in the bowel lumen and that leakage can be associated with implantation leading to recurrence.31 Similar findings have been demonstrated in patients undergoing surgery for gastric cancer.20 Specifically, infectious complications were identified as an independent prognostic factor because both local and distant recurrence rates were higher in patients with such complications.20
In contrast to these disease sites, an association between local infection or inflammation and locoregional recurrence or survival was not found in this cohort of patients with larynx cancer undergoing salvage laryngectomy. While the association of local infection with locoregional recurrence could be confounded by the addition of adjuvant radiation, the present study included patients who had surgery for recurrent disease after radiation therapy, thus controlling for radiation therapy. Recently, de Almeida and colleagues4 reported on a retrospective analysis of 551 patients who had developed postoperative local wound infections after surgery for oral cavity cancers. Similarly, they did not find an association with locoregional control, recurrence-free or overall survival, but noted that there was a trend toward reduced DC.4 Grandis and colleagues32 and González-Márquez and colleagues13 examined the effects of postoperative wound infection on survival and recurrence in 23 patients and 80 patients with laryngeal cancer, respectively. Higher rates of recurrence and worse disease-specific survival were observed in those patients who had developed postoperative infections. Jackson and colleagues12 and Penel and colleagues33,34 were unable to detect a difference in overall survival and local control in patients who developed postoperative infections (27 of 60 patients and 23 of 53 patients, respectively) postlaryngectomy. These findings are in contrast with the findings of Schantz and colleagues10 and Ramadan and colleagues11 who reported improved survival and lower rates of recurrence, respectively, after total laryngectomy in patients who developed postoperative surgical site infections.
The role of local tissue inflammation and/or infection on the development of distant recurrence of cancer has been noted in the breast, colorectal, lung, and esophageal cancer literature.1,19,21,26 We found that the rate of DC was significantly reduced in those that developed PCF and was an independent predictor in multivariable analysis.
One theory that has been proposed to explain the relationship between wound infection following oncologic surgery and DC revolves around the effect of neutrophil extracellular traps (NETs) within sites of active infection or inflammation.35 It has been demonstrated that activated neutrophils can form an extracellular network within sites of infection as a means of trapping bacterial, viral, and fungal particles to prevent the spread of infection.36 However, NETs have also been demonstrated to be expressed in many malignant tumors.36 Studies have shown an association between NETs and tumor proliferation, activation of angiogenesis and ultimately distant metastases.37 It is hypothesized that the NETs capture circulating tumor cells and assist in evading the immune system.37 The development of a PCF may result in a robust inflammatory response even prior to an overt fistula being detected. This scenario would ultimately lead to the patient having longstanding exposure to an inflammatory milieu and set the stage for possible capture or transport of remnant tumor cells via NETs to distant metastatic sites.
Another potential explanation is that the alteration in inflammatory microenvironments can lead to propagation of tumor cells that are prone to metastasis.15,21 Finally, there may be patient-related factors not accounted for in this study that could have contributed to the development of distant metastases. However, we did perform an univariable analysis to examine any preexisting immunodeficiency, including diabetes mellitus, and no association was observed.
Limitations
This study has a few limitations to consider. First, the retrospective nature of the study means that we could not account for confounding variables that were not captured through data collection. Specifically, we may not have captured a patient-related factor (eg, a gene variation) that is common to all patients who developed PCF that also increased the risk of developing distant metastases. Second, although this study, to our knowledge, represents the largest to date, the limited number of patients who developed distant metastases and other competing risks for death may have led to the inability to demonstrate a significant difference in overall survival and disease-free survival, despite increased distant metastases. To observe changes in survival, a larger overall sample size may be required to capture more patients who developed distant metastases. Lastly, although not significant, there was a trend toward reduced median follow-up postlaryngectomy for patients who had developed PCF. This may have underestimated the number of patients who had developed distant metastases within the PCF group and may not have captured deaths secondary to distant metastases. If follow-up for the PCF group was longer, we may have observed enough deaths to reach significance on overall survival and disease-free survival.
Conclusions
This multicenter collaborative retrospective cohort study found that PCF after salvage laryngectomy was independently associated with a 2-fold increased risk of developing distant metastases. This exploratory study found results similar to those seen for other cancers; however, further research is needed to better understand the potential mechanism for development of distant metastases after developing PCF.
References
- 1.O’Connor RÍ, Kiely PA, Dunne CP. The relationship between post-surgery infection and breast cancer recurrence. J Hosp Infect. 2020;106(3):522-535. doi: 10.1016/j.jhin.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 2.Song A, Eo W, Lee S. Comparison of selected inflammation-based prognostic markers in relapsed or refractory metastatic colorectal cancer patients. World J Gastroenterol. 2015;21(43):12410-12420. doi: 10.3748/wjg.v21.i43.12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosumi K, Baba Y, Ishimoto T, et al. Neutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patients. Surg Today. 2016;46(4):405-413. doi: 10.1007/s00595-015-1197-0 [DOI] [PubMed] [Google Scholar]
- 4.de Almeida JR, Yao CMKL, Ziai H, et al. Postoperative wound infections, neutrophil-to-lymphocyte ratio, and cancer recurrence in patients with oral cavity cancer undergoing surgical resection. Oral Oncol. 2019;97(March):23-30. doi: 10.1016/j.oraloncology.2019.07.023 [DOI] [PubMed] [Google Scholar]
- 5.Matsubara D, Arita T, Nakanishi M, et al. The impact of postoperative inflammation on recurrence in patients with colorectal cancer. Int J Clin Oncol. 2020;25(4):602-613. doi: 10.1007/s10147-019-01580-1 [DOI] [PubMed] [Google Scholar]
- 6.Chen Z-Y, Raghav K, Lieu CH, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. 2015;112(6):1088-1097. doi: 10.1038/bjc.2015.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hier M, Black MJ, Lafond G. Pharyngo-cutaneous fistulas after total laryngectomy: incidence, etiology and outcome analysis. J Otolaryngol. 1993;22(3):164-166. [PubMed] [Google Scholar]
- 8.Dedivitis RA, Aires FT, Cernea CR, Brandão LG. Pharyngocutaneous fistula after total laryngectomy: systematic review of risk factors. Head Neck. 2015;37(11):1691-1697. doi: 10.1002/hed.23804 [DOI] [PubMed] [Google Scholar]
- 9.Silverman DA, Puram SV, Rocco JW, Old MO, Kang SY. Salvage laryngectomy following organ-preservation therapy: an evidence-based review. Oral Oncol. 2019;88:137-144. doi: 10.1016/j.oraloncology.2018.11.022 [DOI] [PubMed] [Google Scholar]
- 10.Schantz SP, Skolnik EM, O’Neill JV. Improved survival associated with postoperative wound infection in laryngeal cancer: an analysis of its therapeutic implications. Otolaryngol Head Neck Surg. 1980;88(4):412-417. doi: 10.1177/019459988008800417 [DOI] [PubMed] [Google Scholar]
- 11.Ramadan HH, Wetmore SJ. Effect of wound infections on head and neck cancer. Arch Otolaryngol Head Neck Surg. 1992;118(5):486-487. doi: 10.1001/archotol.1992.01880050032007 [DOI] [PubMed] [Google Scholar]
- 12.Jackson RM, Rice DH. Wound infections and recurrence in head and neck cancer. Otolaryngol Head Neck Surg. 1990;102(4):331-333. doi: 10.1177/019459989010200405 [DOI] [PubMed] [Google Scholar]
- 13.González-Márquez R, Rodrigo JP, Suárez Nieto C. Prognostic significance of postoperative wound infections after total laryngectomy. Head Neck. 2012;34(7):1023-1027. doi: 10.1002/hed.21866 [DOI] [PubMed] [Google Scholar]
- 14.Amin J, Ortlip TE, Cohen D, Vakharia K, Lubek JE. The utility of barium swallow studies for evaluation of pharyngocutaneous fistula after total laryngectomy. Arch Otolaryngol Head Neck Surg. 2020;4(2):2-7. doi: 10.24983/scitemed.aohns.2020.00133 [DOI] [Google Scholar]
- 15.Wu Y, Zhou BP. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;8(20):3267-3273. doi: 10.4161/cc.8.20.9699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sessions DG, Lenox J, Spector GJ, et al. Management of T3N0M0 glottic carcinoma: therapeutic outcomes. Laryngoscope. 2002;112(7 Pt 1):1281-1288. doi: 10.1097/00005537-200207000-00026 [DOI] [PubMed] [Google Scholar]
- 17.Grenader T, Plotkin Y, Mohammadi B, et al. Predictive value of the neutrophil/lymphocyte ratio in peritoneal and/or metastatic disease at staging laparoscopy for gastric and esophageal adenocarcinoma. J Gastrointest Cancer. 2015;46(3):267-271. doi: 10.1007/s12029-015-9727-y [DOI] [PubMed] [Google Scholar]
- 18.Sandulache VC, Vandelaar LJ, Skinner HD, et al. Salvage total laryngectomy after external-beam radiotherapy: a 20-year experience. Head Neck. 2016;38(suppl 1):E1962-E1968. doi: 10.1002/hed.24355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueda K, Murakami J, Tanaka T, Nakamura T, Yoshimine S, Hamano K. Postoperative complications and cancer recurrence: impact on poor prognosis of lower lobe cancer. Ann Thorac Surg. 2020;109(6):1750-1756. doi: 10.1016/j.athoracsur.2019.12.061 [DOI] [PubMed] [Google Scholar]
- 20.Han WH, Oh YJ, Eom BW, Yoon HM, Kim YW, Ryu KW. Prognostic impact of infectious complications after curative gastric cancer surgery. Eur J Surg Oncol. 2020;46(7):1233-1238. doi: 10.1016/j.ejso.2020.04.032 [DOI] [PubMed] [Google Scholar]
- 21.Akabane S, Egi H, Takakura Y, et al. The prognostic value of organ/space surgical site infection in stage I colorectal cancer recurrence. Int J Colorectal Dis. 2020;35(9):1689-1694. doi: 10.1007/s00384-020-03643-6 [DOI] [PubMed] [Google Scholar]
- 22.Katoh H, Yamashita K, Wang G, Sato T, Nakamura T, Watanabe M. Anastomotic leakage contributes to the risk for systemic recurrence in stage II colorectal cancer. J Gastrointest Surg. 2011;15(1):120-129. doi: 10.1007/s11605-010-1379-4 [DOI] [PubMed] [Google Scholar]
- 23.Belt EJT, Stockmann HBAC, Abis GSA, et al. Peri-operative bowel perforation in early stage colon cancer is associated with an adverse oncological outcome. J Gastrointest Surg. 2012;16(12):2260-2266. doi: 10.1007/s11605-012-2053-9 [DOI] [PubMed] [Google Scholar]
- 24.Boudreau A, van’t Veer LJ, Bissell MJ. An “elite hacker”: breast tumors exploit the normal microenvironment program to instruct their progression and biological diversity. Cell Adh Migr. 2012;6(3):236-248. doi: 10.4161/cam.20880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui P, Fang X. Pathogenesis of infection in surgical patients. Curr Opin Crit Care. 2015;21(4):343-350. doi: 10.1097/MCC.0000000000000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell AGMT, Eley C, Chin C, Coxon AH, Christian A, Lewis WG; South East Wales Oesophagogastric Cancer Collaborative . Prognostic significance of serum inflammatory markers in esophageal cancer. Esophagus. 2021;18(2):267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha GW, Kim JH, Lee MR. Oncologic impact of anastomotic leakage following colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg Oncol. 2017;24(11):3289-3299. doi: 10.1245/s10434-017-5881-8 [DOI] [PubMed] [Google Scholar]
- 28.Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg. 2015;261(3):497-505. doi: 10.1097/SLA.0000000000000854 [DOI] [PubMed] [Google Scholar]
- 29.Voron T, Bruzzi M, Ragot E, et al. Anastomotic location predicts anastomotic leakage after elective colonic resection for cancer. J Gastrointest Surg. 2019;23(2):339-347. doi: 10.1007/s11605-018-3891-x [DOI] [PubMed] [Google Scholar]
- 30.Goto S, Hasegawa S, Hida K, et al. ; Study Group for Nomogram of the Japanese Society for Cancer of the Colon and Rectum . Multicenter analysis of impact of anastomotic leakage on long-term oncologic outcomes after curative resection of colon cancer. Surgery. 2017;162(2):317-324. doi: 10.1016/j.surg.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 31.Gertsch P, Baer HU, Kraft R, Maddern GJ, Altermatt HJ. Malignant cells are collected on circular staplers. Dis Colon Rectum. 1992;35(3):238-241. doi: 10.1007/BF02051014 [DOI] [PubMed] [Google Scholar]
- 32.Grandis JR, Snyderman CH, Johnson JT, Yu VL, D’Amico F. Postoperative wound infection: a poor prognostic sign for patients with head and neck cancer. Cancer. 1992;70(8):2166-2170. doi: [DOI] [PubMed] [Google Scholar]
- 33.Penel N, Fournier C, Lefebvre D, Lefebvre JL. Multivariate analysis of risk factors for wound infection in head and neck squamous cell carcinoma surgery with opening of mucosa: study of 260 surgical procedures. Oral Oncol. 2005;41(3):294-303. doi: 10.1016/j.oraloncology.2004.08.011 [DOI] [PubMed] [Google Scholar]
- 34.Penel N, Fournier C, Roussel-Delvallez M, et al. Prognostic significance of wound infections following major head and neck cancer surgery: an open non-comparative prospective study. Support Care Cancer. 2004;12(9):634-639. doi: 10.1007/s00520-004-0600-y [DOI] [PubMed] [Google Scholar]
- 35.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532-1535. doi: 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 36.Zhang LM, Chen JH. Progression of NETs correlating with tumor-related diseases. Asian Pac J Cancer Prev. 2015;16(17):7431-7434. doi: 10.7314/APJCP.2015.16.17.7431 [DOI] [PubMed] [Google Scholar]
- 37.Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70(14):6071-6082. doi: 10.1158/0008-5472.CAN-09-4442 [DOI] [PMC free article] [PubMed] [Google Scholar]


