This randomized clinical trial examines the use of metformin with chemoradiotherapy and as consolidation treatment in patients being treated for locally advanced non–small cell lung cancer.
Key Points
Question
Could the addition of metformin to chemoradiotherapy, as a concurrent treatment as well as consolidation therapy, improve outcomes in patients without diabetes who have locally advanced non–small cell lung cancer?
Findings
In this randomized clinical trial including 54 of 96 planned patients, the addition of metformin to chemoradiotherapy was associated with worse treatment efficacy and increased toxic effects compared with chemoradiotherapy alone. The proportion of patients who experienced a failure event within 1 year (ie, locoregional disease progression, distant metastases, death, or withdrawal) was 69.2% in the metformin arm vs 42.9% in the control arm.
Meaning
Based on the results of this trial, metformin is not recommended as an adjunct to chemoradiotherapy for the treatment of unresected locally advanced non–small cell lung cancer in patients who do not have diabetes.
Abstract
Importance
Unresected locally advanced non–small cell lung cancer (LA-NSCLC) shows poor survival outcomes even after aggressive concurrent chemoradiotherapy. Whether metformin, a diabetes agent that inhibits the mitochondria oxidative phosphorylation chain, could improve radiotherapy and chemotherapy response in LA-NSCLC remains to be studied.
Objective
To examine whether metformin, given concurrently with chemoradiotherapy and as consolidation treatment, could improve outcomes in patients with LA-NSCLC.
Design, Setting, and Participants
The Ontario Clinical Oncology Group Advanced Lung Cancer Treatment With Metformin and Chemoradiotherapy (OCOG-ALMERA) study was a multicenter phase 2 randomized clinical trial. Patients were stratified for stage IIIA vs IIIB LA-NSCLC and use of consolidation chemotherapy. The trial was designed to enroll 96 patients with unresected LA-NSCLC who did not have diabetes. The trial was conducted from September 24, 2014, to March 8, 2019.
Interventions
Patients were randomized to platinum-based chemotherapy, concurrent with chest radiotherapy (60-63 Gy), with or without consolidation chemotherapy or the same treatment plus metformin, 2000 mg/d, during chemoradiotherapy and afterward for up to 12 months.
Main Outcomes and Measures
The primary outcome was the proportion of patients who experienced a failure event (ie, locoregional disease progression, distant metastases, death, and discontinuation of trial treatment or planned evaluations for any reason within 12 months). Proportions were compared using a 2-sided Fisher exact test. Conventional progression-free and overall survival were estimated using the Kaplan-Meier method. Adverse events were graded with Common Terminology Criteria for Adverse Events, version 4.03. All randomized patients were included in an intention-to-treat analysis.
Results
The trial was stopped early due to slow accrual. Between 2014 and 2019, 54 patients were randomized (26 in experimental arm and 28 in control arm). Participants included 30 women (55.6%); mean (SD) age was 65.6 (7.6) years. Treatment failure was detected in 18 patients (69.2%) receiving metformin within 1 year vs 12 (42.9%) control patients (P = .05). The 1-year progression-free survival rate was 34.8% (95% CI, 16.6%-53.7%) in the metformin arm and 63.0% (95% CI, 42.1%-78.1%) in the control arm (hazard ratio, 2.42; 95% CI, 1.14-5.10) The overall survival rates were 47.4% (95% CI, 26.3%-65.9%) in the metformin arm and 85.2% (95% CI, 65.2%-94.2%) in the control arm (hazard ratio, 3.80; 95% CI, 1.49-9.73). More patients in the experimental arm vs control arm (53.8% vs 25.0%) reported at least 1 grade 3 or higher adverse event.
Conclusions and Relevance
In this randomized clinical trial, the addition of metformin to chemoradiotherapy was associated with worse treatment efficacy and increased toxic effects compared with combined modality therapy alone. Metformin is not recommended in patients with LA-NSCLC who are candidates for chemoradiotherapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT02115464
Introduction
Non–small cell lung cancer (NSCLC) is responsible for approximately 85% of all lung cancer cases and presents frequently at an advanced stage, where the combination of platinum-based chemotherapy and conventionally fractionated thoracic radiotherapy of 60 to 66 Gy is standard of care.1 Resulting 5-year overall survival (OS) remains poor, with estimates ranging from 23% to 32%.2,3 Immune checkpoint inhibitor therapy targeting the programmed death receptor ligand 1 substantially improved progression-free survival (PFS) and OS in the PACIFIC trial.4,5
Metabolic deregulation is a central event in carcinogenesis.6,7 In lung cancer, aberrant activation of tyrosine kinase receptors, such as epidermal growth factor receptor and mutations of K-ras and phosphatidylinositol 3-kinase, are associated with growth and resistance to cytotoxic therapy mediated through the mammalian target of rapamycin pathway. This pathway is associated with a metabolic shift to glycolysis, an event described by Warburg over 100 years ago.8 However, the metabolic stress pathway of liver kinase B 1 and adenosine monophosphate–activated kinase (AMPK) counteracts these events and mediates tumor suppression through p53 and blockade of mammalian target of rapamycin and glycolytic metabolism.6,9,10
Metformin, the most widely used type 2 diabetes agent, is proposed to suppress tumor growth through metabolic stress caused by inhibition of complex I of the mitochondria oxidative phosphorylation chain. In preclinical NSCLC models, metformin activated AMPK, induced p53, suppressed mammalian target of rapamycin and tumor growth, and enhanced tumor response to radiotherapy11 and chemotherapy.12 These findings are supported by retrospective clinical evidence of improved survival in LA-NSCLC among patients with diabetes treated with metformin.6,13,14
We hypothesized that metformin might improve outcomes in patients without diabetes and offer a safe and economical adjunct to standard-of-care chemoradiotherapy. This hypothesis led to the Ontario Clinical Oncology Group Advanced Lung Cancer Treatment with Metformin and Chemoradiotherapy (OCOG-ALMERA) trial.
Methods
Study Design
ALMERA was an open label, multicenter phase 2 randomized clinical trial that investigated the potential benefit of metformin, administered concurrently with chemoradiotherapy and continuing after as consolidation therapy in patients with unresected LA-NSCLC. The trial protocol is available in Supplement 1. There were no prespecified stopping rules based on efficacy or rate of recruitment. Ethics approval was obtained by the provincial or institutional research review board of each participating center, and written informed consent was obtained from all patients. Participants did not receive financial compensation. The study was approved by Health Canada. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.
Patient Population
This study included patients without diabetes who had pathologically or cytologically proven NSCLC (adenocarcinoma, squamous cell carcinoma, large cell, or mixed histologic findings) diagnosed within 3 months of randomization; unresected stage IIIA or IIIB NSCLC (American Joint Committee on Cancer 7th edition) staged by physical examination, computed tomography (CT) of the chest and upper abdomen, brain magnetic resonance imaging or contrast-enhanced CT, and whole body fluorodeoxyglucose positron emission tomography/ CT scan, all within 2 months before randomization; and age 18 years or older. Exclusion criteria are reported in the eMethods in Supplement 2.
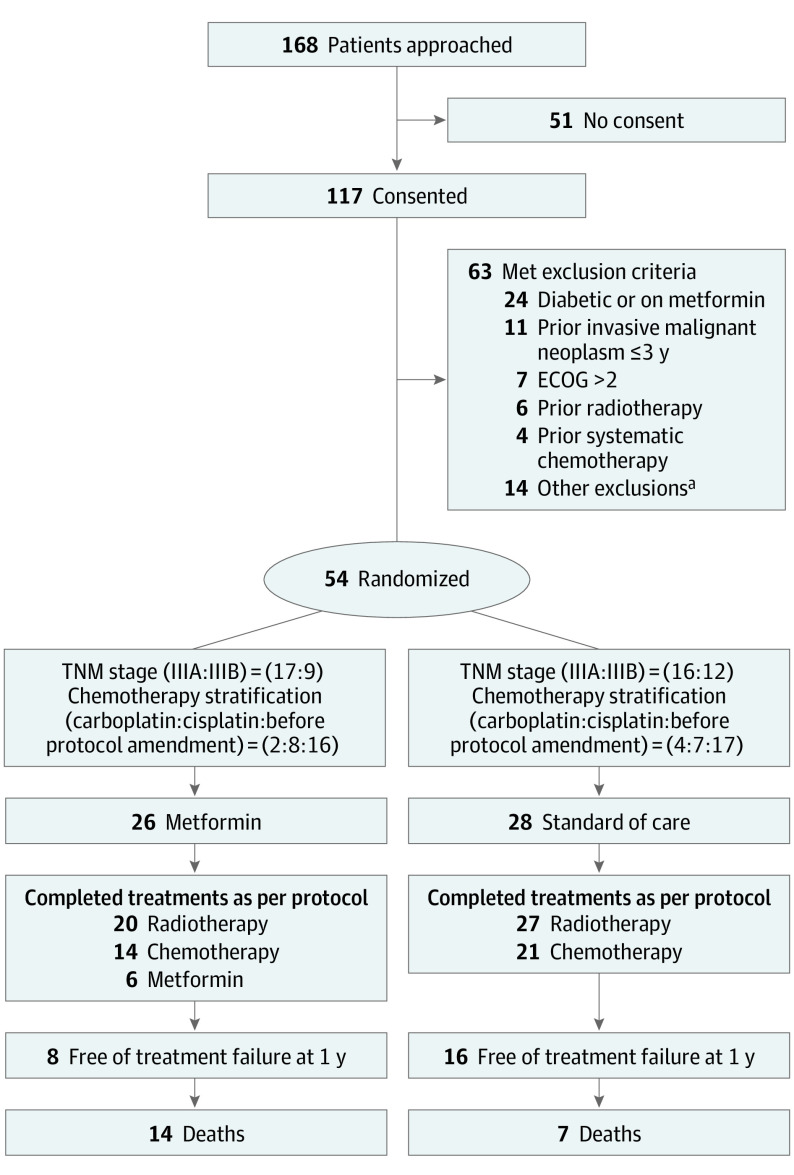
Eligible patients were randomized centrally through the OCOG coordinating center, Hamilton, Ontario, Canada, to chemoradiotherapy with or without oral metformin (Figure 1). Patients were stratified for NSCLC stage (IIIA vs IIIB). After 2 years of accrual, a protocol amendment permitted consolidation chemotherapy and its use became a stratification variable.
Figure 1. Study Flowchart.
ECOG indicates Eastern Cooperative Oncology Group.
aOther exclusion criteria met included more than 10% weight loss (n = 3), pulmonary function tests (n = 1), complete blood cell count (n = 1), glucose level (n = 2), congestive heart failure (n = 1), liver disease (n = 1), geographic inaccessibility (n = 3), and lack of consent (n = 2). Patients may have met more than 1 exclusion criterion.
Interventions
Chest radiotherapy of 60 to 63 Gy was delivered in 30 daily fractions over 6 weeks concurrent with chemotherapy. Use of intensity-modulated radiotherapy was encouraged, but 3-dimensional conformal radiotherapy or volume-modulated arc therapy were also permitted. Radiotherapy was delivered with image-guidance using cone beam CT or orthogonal kilovoltage x-rays.
Radiotherapy volume delineation was according to International Commission on Radiation Units & Measurements Report 62.15 The primary tumor and involved lymph nodes only were included in the radiotherapy gross tumor volume using guidance from staging fluorodeoxyglucose positron emission tomography images. Gross tumor volume was expanded to include respiratory tumor motion detected by 4-dimensional -CT, fluoroscopy, or estimated (1- to 1.5-cm expansion) for conventional CT simulation, respecting anatomic barriers to define internal target volume. Internal target volume was expanded by an additional 0.5 to 1.5 cm to define planned target volume. Normal tissue dose constraints followed standard Radiation Therapy Oncology Group guidelines.16
Platinum-based chemotherapy was given in 2 cycles of 3 to 4 weeks concurrently with radiotherapy. Acceptable options included cisplatin (50 mg/m2 on days of radiotherapy fractions 1, 6, 21, and 26) combined with etoposide (50 mg/m2 on days of radiotherapy fractions 1-5 and 21-25) or cisplatin (80 mg/m2 on days of radiotherapy fractions 1 and 16) plus vinorelbine (12.5-15 mg/m2 on days of radiotherapy fractions 1, 6, 11, 16, 21, and 26). Carboplatin (area under the curve = 2 weekly) in combination with etoposide or paclitaxel (50 mg/m2 weekly) was also permitted. Consolidation chemotherapy of 2 additional cycles was allowed in patients treated with the carboplatin/paclitaxel combination. Consolidation anti-programmed death receptor ligand 1 immunotherapy (durvalumab) treatment was permitted for all eligible patients after an amendment in May 2018.
Metformin was packaged as an investigational agent and shipped to centers in specified intervals. Metformin treatment started 2 weeks before the initiation of standard therapy at a dose of 1000 mg/d orally; the dose was increased to 1500 mg/d in week 2 and to 2000 mg/d at the start of chemoradiotherapy (week 3). An amendment in January 2017 permitted reducing the dose escalation period to 1 week if needed. Metformin was given concurrently with cytotoxic therapy and as a consolidation treatment at 2000 mg/d daily for up to 12 months unless dose de-escalation was required due to toxic effects or disease progression.
Assessments and Outcomes
Patients were assessed for toxic effects using (Common Terminology Criteria for Adverse Events, version 4.03) weekly during chemoradiotherapy. Assessments with physical examination, performance status (Eastern Cooperative Oncology Group), toxic effects (Common Terminology Criteria for Adverse Events, version 4.03), and CT imaging of the chest and upper abdomen were performed every 3 months in year 1. After that, there was a survival follow-up at 24 months. Beyond year 1, assessments followed institutional policies with physical examination and CT imaging of chest and upper abdomen approximately every 3 to 6 months and at disease progression.
The primary outcome was the proportion of patients who experienced a failure event (ie, locoregional disease progression, distant metastases, death, and discontinuation of trial treatment or planned evaluations for any reason within 12 months). We recognized that the condition of many patients in our trial would likely decline rapidly and patients might not be able to undergo a follow-up CT imaging scan to determine progression and that many of them would refuse other specified follow-up evaluations. Thus, using conventional PFS, a large number of patients would be censored, even though their condition was deteriorating. Furthermore, differential timing of imaging assessment could be imbalanced. Control arm patients may not be contacted by the hospital, whereas metformin patients would need to be seen to be given their next cycle of medication, potentially resulting in bias (ie, informative censoring). Thus, the primary outcome analysis was based on a binary measure at 12 months. In the study protocol, this end point was called PFS, even though it was not the usual time to event factor. All suspected events, including local or distant progression, and discontinuations were reviewed by an independent adjudication committee unaware of treatment allocation.
Secondary outcomes for efficacy were overall survival, PFS (conventional time-to-event definition), time to locoregional progression, and distant progression-free survival. These outcomes are defined in the eMethods in Supplement 2. Safety was determined based on adverse events (AEs) defined by Common Terminology Criteria for Adverse Events, version 4.03. Only grade 3 or higher AEs were recorded. Toxicity was monitored every 6 months by the standing OCOG safety committee.
Statistical Analysis
The target sample size was 94 patients (the eMethods in Supplement 2). All randomized patients were included in an intention-to-treat analysis. Patients included in the intention-to-treat analysis who received at least 1 dose of metformin were included in the safety analysis. The comparison of the proportions of patients in each group who did not respond to treatment (came off study, withdrew, progressed, or died) within 1 year was performed with the Fisher exact test. This analysis was 2-sided, and α = .05 was used to define statistical significance.
Time-to-event analyses used the Kaplan-Meier method to estimate outcomes. Cox proportional hazards regression, adjusting for baseline strata, was used for exploratory analyses of time-to-event outcomes, and 95% CIs were constructed for outcomes of interest. The frequency and proportion of patients with AEs, severe (grade 3 or higher) AEs, and attributable AEs were calculated.
Results
Patient Population
OCOG-ALMERA opened on September 24, 2014, and recruitment was closed on March 8, 2019, owing to slow accrual based on a recommendation from the safety committee. Of 168 patients who satisfied the inclusion criteria, 51 did not consent and a further 63 met at least 1 exclusion criterion (Figure 1). The main reason for exclusion was diabetes diagnosis or receiving metformin (n = 24). Twenty-six patients were allocated to chemoradiotherapy plus metformin and 28 to chemoradiotherapy alone from 7 Canadian institutions (Figure 1). Two patients (1 on each arm) withdrew before receiving treatment. The database was locked on May 4, 2020.
Treatment arms were reasonably well balanced for baseline characteristics (Table 1). Of the 54 patients randomized, 30 were women (55.6%) and 24 were men (44.4%); mean (SD) age was 65.6 (7.6) years. Most patients were treated with cisplatin plus etoposide (39 [72.2%]) (Table 2).
Table 1. Baseline Characteristics.
| Characteristic | Metformin, No. (%) (n = 26) | Standard of care, No. (%) (n = 28) |
|---|---|---|
| Age, mean (SD), y | 65.9 (8.1) | 65.3 (7.3) |
| Sex | ||
| Male | 12 (46.2) | 12 (42.9) |
| Female | 14 (53.8) | 16 (57.1) |
| ECOG | ||
| 0 | 14 (53.8) | 12 (42.9) |
| 1 | 11 (42.3) | 16 (57.1) |
| 2 | 1 (3.8) | 0 |
| BMI, mean (SD) | 26.5 (5.5) | 26.2 (4.3) |
| Histologic characteristic | ||
| Squamous cell | 10 (38.5) | 10 (35.7) |
| Adenocarcinoma | 13 (50.0) | 17 (60.7) |
| Large cell | 0 | 0 |
| Mixed | 1 (3.8) | 0 |
| Not specified | 2 (7.7) | 1 (3.6) |
| TNM stage | 17 (65.4) | 16 (57.1) |
| (IIIA/IIIB) | 9 (34.6) | 12 (42.9) |
| T stage | ||
| 1a | 3 (11.5) | 1 (3.6) |
| 1b | 4 (15.4) | 2 (7.1) |
| 2a | 4 (15.4) | 2 (7.1) |
| 2b | 3 (11.5) | 2 (7.1) |
| 3 | 5 (19.2) | 12 (42.9) |
| 4 | 7 (26.9) | 9 (32.1) |
| N stage | ||
| 0 | 1 (3.8) | 1 (3.6) |
| 1 | 2 (7.7) | 1 (3.6) |
| 2 | 18 (69.2) | 22 (78.6) |
| 3 | 5 (19.2) | 4 (14.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ECOG, Eastern Cooperative Oncology Group.
Table 2. Treatment Delivered.
| Variable | Metformin (n = 26) | Standard of care (n = 28) |
|---|---|---|
| Withdrew before receiving any drug treatment | 1 (3.8) | 1 (3.6) |
| Radiotherapy | ||
| Received radiotherapy, No. (%) | 25 (96.2) | 27 (96.4) |
| GTV, mean (SD) | 179 (171) | 116 (74) |
| PTV, mean (SD) | 536 (345) | 449 (209) |
| Duration of radiotherapy, median (range), d | 43 (6-49) | 43 (40-46) |
| Total dose, Gy | ||
| Median (range) | 60 (14.7-66) | 63 (60-63) |
| ≥60 Gy, No. (%) | 21 (80.8) | 27 (96.4) |
| Total fractions | ||
| Median (range) | 30 (7-33) | 30 (30-30) |
| ≥30 Gy, No. (%) | 20 (76.9) | 27 (96.4) |
| Discontinuation reason, No. (%) | ||
| Completed as per protocol | 20 (76.9) | 27 (96.5) |
| Toxic effects | 3 (11.5) | 0 |
| Withdrew | 2 (7.7) | 0 |
| Chemotherapy | ||
| Received chemotherapy, No. (%) | 25 (96.2) | 27 (96.4) |
| Chemotherapy completed as per protocol, No. (%) | 14 (53.8) | 21 (75.0) |
| No. of cycles | ||
| 0 | 1 (3.8) | 1 (3.6) |
| 1 | 7 (26.9) | 0 |
| 2 | 18 (69.2) | 27 (96.4) |
| Dose modifications, No. (%) | 4 (15.4) | 2 (7.1) |
| Cisplatin, etoposide | 20 (76.9) | 19 (67.8) |
| Carboplatin, etoposide | 1 (1.3) | 2 (7.1) |
| Cisplatin, vinorelbine | 2 (7.7) | 0 |
| Cisplatin, pemetrexed | 0 | 2 (7.1) |
| Carboplatin, paclitaxel | 2 (7.7) | 4 (14.3) |
| No chemotherapy | 1 (1.3) | 1 (3.6) |
| Dose omissions, No. (%) | 7 (26.9) | 4 (14.3) |
| Duration | ||
| Median (range) | 35 (4-42) | 35 (28-44) |
| ≥28 Days, No. (%) | 21 (80.8) | 27 (96.4) |
| Immunotherapy | ||
| Durvalumab immunotherapy, No. (%)a | 4 (15.4) | 7 (25.0) |
Abbreviations: GTV, gross tumor volume; PTV, planned target volume.
Given according to standard PACIFIC trial–based guidelines.4
Of 25 metformin patients who started radiotherapy, 5 (20.0%) did not complete protocol-specified treatment; 3 of these patients were hospitalized with treatment-related toxic effects. In the control arm, all 27 patients who underwent radiotherapy received 60 to 63 Gy/30 daily fractions as per protocol (Table 2).
In the metformin group, 14 of 25 (56.0%) patients completed 2 cycles of chemotherapy as per schedule. Four patients received 2 cycles but with dose modifications owing to weight loss (1), neutropenia or thrombocytopenia (2), and patient withdrawal (1). Seven other patients received only 1 cycle; the reasons for omission were tinnitus (1), dehydration (1), neutropenia or thrombocytopenia (2), esophagitis (1), patient withdrawal (1), and reason not stated (1).
In the 27 control patients, 21 (77.8%) completed 2 cycles of chemotherapy as per schedule. Four patients did not receive their last dose of weekly chemotherapy owing to chest infection (1), neutropenia (1), and low platelet counts (2). Only 6 metformin patients completed 1 year of metformin treatment as per protocol. The main reason for discontinuation was disease progression/death (8), patient request (6), toxic effects (3), intercurrent illness (1), and nonadherence (1). Seven patients in the control arm and 4 in the metformin arm received durvalumab immunotherapy (Table 2).
Outcomes
Eighteen (69.2%) patients receiving metformin experienced an event (2 had local progression first, 10 distant progression, 1 locoregional and distant progression, 3 withdrew, and 2 died before detection of a progression event). Twelve (42.9%) patients in the control arm experienced an event (2 had local progression, 7 distant metastasis, 1 new primary, and 2 withdrew) within 1 year of randomization) (P = .05). The risk difference for completing 1 year of treatment without recurrence was −26.4% (95% CI, −0.9% to −51.0%).
One-year Kaplan-Meier–estimated conventional PFS was 34.8% (95% CI, 16.6%-53.7%) in metformin patients compared with 63.0% (95% CI, 42.1%-78.1%) for control patients (hazard ratio, 2.42; 95% CI, 1.14-5.10) (Figure 2A). Similar results were observed for distant PFS (eFigure in Supplement 2). The OS was worse for metformin patients (47.4%; 95% CI, 26.3%-65.9%) vs control patients (85.2%; 95% CI, 65.2%-94.2%) (hazard ratio, 3.80; 95% CI, 1.49-9.73) (Figure 2B; eTable in Supplement 2).
Figure 2. Progression-free and Overall Survival Outcomes.
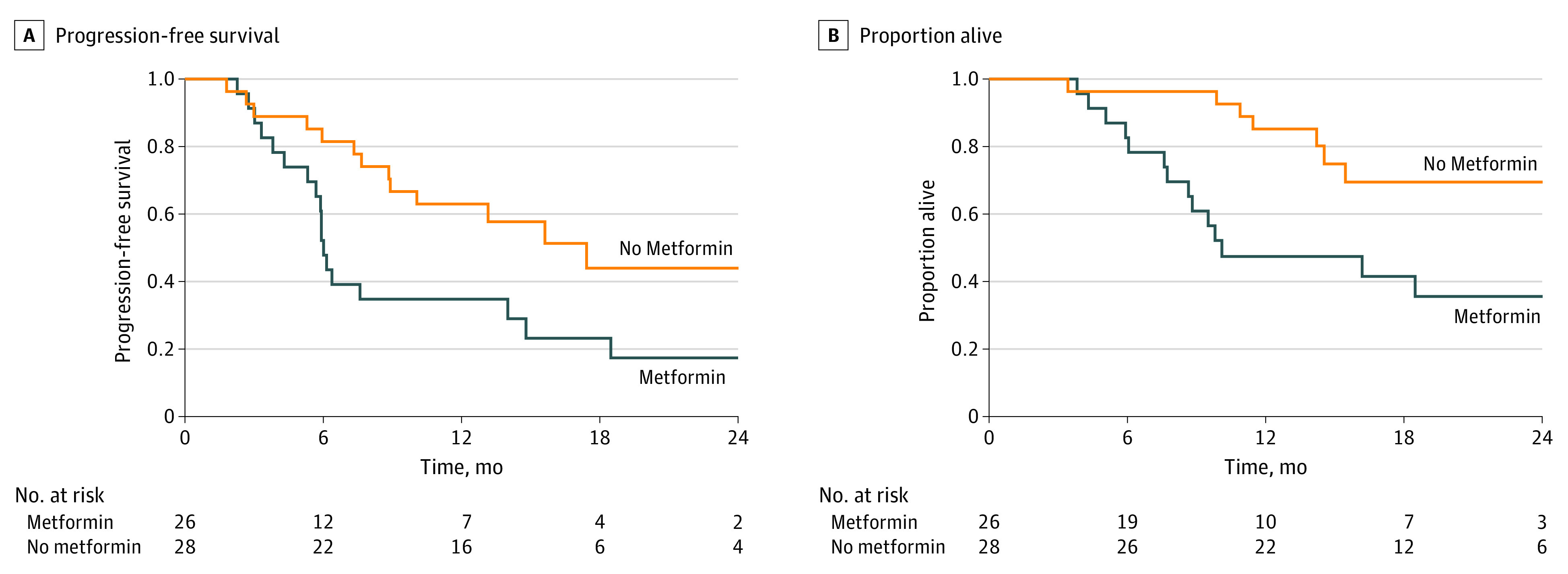
A, Progression-free survival at 1 year was 34.8% (95% CI, 16.6%-53.7%) in the metformin group and 63.0% (95% CI, 42.1%-78.1%) in the control group. B, Overall survival was 47.4% (95% CI, 26.3%-65.9%) in the metformin group and 85.2% (95% CI, 65.2%-94.2%) in the control group. Plots were generated with a time-to-event intention-to-treat analysis. Events were defined from the date of randomization to date of objective local, regional, or distant progression based on clinical examination or computed tomography, magnetic resonance imaging, or nuclear medicine imaging or to date of death.
Radiotherapy in 52 patients included intensity-modulated radiotherapy (n = 19), 3-dimensional conformal radiotherapy (n = 11), and volume-modulated arc therapy (n = 22). There was no interaction effect with treatment (metformin vs control), and there was no association between the type of radiotherapy (defined as either intensity-modulated radiotherapy or volume-modulated arc therapy vs 3-dimensional conformal radiotherapy) and any outcome, whether adjusted for treatment or univariable.
A summary of AEs experienced by patients is presented in Table 3. A total of 14 (53.8%) patients in the metformin arm and 7 (25.0%) patients in the control arm experienced at least 1 grade 3 or higher AE. Esophagitis (5 [19.2%]) and lung infections (6 [23.1%]) were the most common AEs in patients receiving metformin.
Table 3. Adverse Events.
| Variable | Metformin, No. (%) (n = 26) | Standard of care, No. (%) (n = 28) |
|---|---|---|
| All grade 3 AEs | ||
| Acute kidney injury | 1 (3.8) | 0 |
| Anemia | 2 (7.7) | 1 (3.6) |
| Body odor | 1 (3.8) | 0 |
| Dysphagia | 2 (7.7) | 0 |
| Dyspnea | 1 (3.8) | 0 |
| Edema in limbs | 1 (3.8) | 0 |
| Esophagitis | 5 (19.2) | 1 (3.6) |
| Hypokalemia | 1 (3.8) | 0 |
| Lung infection | 6 (23.1) | 0 |
| Lymphocyte count decreased | 3 (11.5) | 1 (3.6) |
| Nausea | 0 | 1 (3.6) |
| Neoplasms, benign | 1 (3.8) | 0 |
| Neutrophil count decreased | 3 (11.5) | 0 |
| Platelet count decreased | 1 (3.8) | 1 (3.6) |
| Pneumonitis | 1 (3.8) | 0 |
| Supraventricular tachycardia | 1 (3.8) | 0 |
| Syncope | 1 (3.8) | 0 |
| Thromboembolic event | 1 (3.8) | 1 (3.6) |
| Urinary tract infection | 1 (3.8) | 1 (3.6) |
| Vomiting | 0 | 1 (3.6) |
| White blood cell count decreased | 1 (3.8) | 3 (10.7) |
| All grade 4 AEs | ||
| Lymphocyte count decreased | 2 (7.7) | 0 |
| Neutrophil count decreased | 0 | 1 (3.6) |
| White blood cell count decreased | 2 (7.7) | 0 |
| All grade 5 AEs | ||
| Respiratory failure | 1 (3.8) | 0 |
| Grade 3+ AEs per patient | ||
| 0 | 12 (46.2) | 21 (75.0) |
| 1 | 5 (19.2) | 4 (14.3) |
| 2 | 4 (15.4) | 1 (3.6) |
| 3 | 2 (7.7) | 2 (7.1) |
| 6 | 1 (3.8) | 0 |
| 7 | 2 (7.7) | 0 |
| Patients with ≥1 grade 3+ AE | 14 (53.8) | 7 (25.0) |
| Attributable (possibly, probably, definitely related) AEs | ||
| All grade 3 AEs | ||
| Anemia | 1 (3.8) | 1 (3.6) |
| Body odor | 1 (3.8) | 0 |
| Dysphagia | 2 (7.7) | 0 |
| Esophagitis | 0 | 1 (3.6) |
| Lymphocyte count decreased | 3 (11.5) | 1 (3.6) |
| Nausea | 0 | 1 (3.6) |
| Neutrophil count decreased | 2 (7.7) | 0 |
| Vomiting | 0 | 1 (3.6) |
| White blood cell count decreased | 1 (3.8) | 1 (3.6) |
| All grade 4 AEs | ||
| Lymphocyte count decreased | 2 (7.7) | 0 |
| Neutrophil count decreased | 0 | 1 (3.6) |
| White blood cell count decreased | 2 (7.7) | 0 |
| All grade 5 AEs | ||
| Respiratory failure | 1 (3.8) | 0 |
| Grade 3+ attributable AEs per patient | ||
| 0 | 20 (76.9) | 24 (85.7) |
| 1 | 3 (11.5) | 2 (7.1) |
| 2 | 1 (3.8) | 1 (3.6) |
| 3 | 1 (3.8) | 1 (3.6) |
| 7 | 1 (3.8) | 0 |
| Patients with ≥1 attributable grade 3+ AE | 6 (23.1) | 4 (14.3) |
Abbreviation: AE, adverse events.
Discussion
Experimental studies suggested that metformin inhibits growth and sensitizes NSCLC cells and tumors to radiotherapy and chemotherapy.6,11,12 Based on such findings, we hypothesized that metformin could improve outcomes in patients with LA-NSCLC. In this trial, metformin was administered during cytotoxic therapy as a chemoradiotherapy sensitizer and afterward as consolidation treatment. Not only did this study fail to demonstrate improved efficacy with the addition of metformin, but the metformin arm was inferior to the control arm in terms of the primary outcome: the proportion of patients with a failure event within 12 months (labeled as PFS in the protocol). Although our primary outcome was not the conventional time-to-event end point, the robustness of this result is supported by the consistency of the inferiority with metformin for all secondary measures of efficacy, including conventional PFS and OS.
Metformin is widely used in diabetes and is generally well tolerated. The observed increased toxic effects in this trial were unexpected. Twice as many metformin patients had at least 1 grade 3 or higher AE compared with control patients. Esophagitis and lung infections were the key reported AEs. Such events are not atypical of standard chemoradiotherapy.
Another randomized phase 2 trial also investigated metformin, 2000 mg/d, in LA-NSCLC (NRG-LU001), but only during cytotoxic therapy. That study did not detect increased toxicity with metformin, which was delivered in the first 126 days of treatment.17 Notably, most AEs reported in OCOG-ALMERA took place in the first 84 days. An important difference between the 2 studies was the exclusive use of concurrent carboplatin with paclitaxel chemotherapy with consolidation in NRG-LU001,17 compared with use of mainly cisplatin-based regimens in this trial. It is possible that cisplatin combinations in particular are more toxic when combined with metformin compared with carboplatin plus paclitaxel treatment.
It is unclear why we observed increased failure events with the addition of metformin. Conceivably, the increased toxic effects observed in metformin patients limited their ability to receive the prescribed doses of chemoradiotherapy. Gross tumor volume and radiotherapy-targeted (ie, planned) tumor volume were, on average, larger in patients randomized to metformin. The difference was not statistically significant but may have contributed to the observed rates of treatment failure and toxic effects. Lactic acidosis occurs with metformin but is rare. Although no cases of overt lactic acidosis were reported in this study, it is conceivable that patients receiving metformin were at increased risk for subclinical lactic acidosis that may have contributed to decompensation. Could metformin have stimulated tumor growth? Oxidative phosphorylation chain inhibition by metformin may have altered the tumor microenvironment in support of tumor resistance to therapy, but this concept is not supported by laboratory studies. Our trial was small, and differences between groups for potential confounding factors (eg, immunotherapy) might explain in part the results. In addition, it is possible that the observed difference in outcomes may be a chance finding.
Limitations
This trial had limitations. Lack of double-blinding, placebo control, and limited accrual are weaknesses of OCOG-ALMERA. Effective blinding cannot be maintained with metformin because most patients initially develop a lingering metallic taste, bowel gas, and loose bowel movements. However, it is unlikely that bias was an issue in our trial. All events were adjudicated by a committee unaware of treatment allocation and OS is an objective outcome.
Accrual to OCOG-ALMERA was much slower than anticipated. Typical challenges of accruing good performance (Eastern Cooperative Oncology Group level 0-1) patients with stage III LA-NSCLC were exacerbated by exclusion of patients with diabetes, which limited the number of potentially eligible patients. Anticipation of the results of immunotherapy trials may have biased physicians against accrual to this study.
Conclusions
Patients in the OCOG-ALMERA trial who received metformin experienced worse treatment efficacy and increased toxic effects compared with those given combined modality therapy alone. Based on these findings, metformin is not recommended in patients with LA-NSCLC who do not have diabetes and are candidates for chemoradiotherapy.
Trial Protocol
eMethods. Detailed Methods
eReferences
eFigure 1. Distant Progression-Free Survival (DPFS)
eTable 1. Detailed Survival Outcomes
Data Sharing Statement
References
- 1.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non–small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199. doi: 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley JD, Hu C, Komaki RR, et al. Long-term results of NRG Oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non–small-cell lung cancer. J Clin Oncol. 2020;38(7):706-714. doi: 10.1200/JCO.19.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sculier JP, Lafitte JJ, Berghmans T, et al. A phase III randomised study comparing concomitant radiochemotherapy with cisplatin and docetaxel as induction versus consolidation treatment in patients with locally advanced unresectable non–small cell lung cancer. Lung Cancer. 2018;117:32-37. doi: 10.1016/j.lungcan.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 4.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. doi: 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 5.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 6.Troncone M, Cargnelli SM, Villani LA, et al. Targeting metabolism and AMP-activated kinase with metformin to sensitize non–small cell lung cancer (NSCLC) to cytotoxic therapy: translational biology and rationale for current clinical trials. Onstage. 2017;8(34):57733-57754. doi: 10.18632/oncotarget.17496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanhove K, Graulus GJ, Mesotten L, et al. The metabolic landscape of lung cancer: new insights in a disturbed glucose metabolism. Front Oncol. 2019;9:1215. doi: 10.3389/fonc.2019.01215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029-1033. doi: 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563-575. doi: 10.1038/nrc2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89(3):1025-1078. doi: 10.1152/physrev.00011.2008 [DOI] [PubMed] [Google Scholar]
- 11.Storozhuk Y, Hopmans SN, Sanli T, et al. Metformin inhibits growth and enhances radiation response of non–small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer. 2013;108(10):2021-2032. doi: 10.1038/bjc.2013.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocha GZ, Dias MM, Ropelle ER, et al. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin Cancer Res. 2011;17(12):3993-4005. doi: 10.1158/1078-0432.CCR-10-2243 [DOI] [PubMed] [Google Scholar]
- 13.Wink KC, Belderbos JS, Dieleman EM, et al. Improved progression free survival for patients with diabetes and locally advanced non–small cell lung cancer (NSCLC) using metformin during concurrent chemoradiotherapy. Radiother Oncol. 2016;118(3):453-459. doi: 10.1016/j.radonc.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 14.Tan BX, Yao WX, Ge J, et al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer. 2011;117(22):5103-5111. doi: 10.1002/cncr.26151 [DOI] [PubMed] [Google Scholar]
- 15.Landberg T, Chavaudra J, Dobbs J, et al. Absorbed doses. J ICRU. 1999;32(1):21-25. 10.1093/jicru_os32.1.21 [DOI] [Google Scholar]
- 16.Kong FM, Ritter T, Quint DJ, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys. 2011;81(5):1442-1457. doi: 10.1016/j.ijrobp.2010.07.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsakiridis T, Hu C, Skinner HD, et al. Initial reporting of NRG-LU001 (NCT02186847), randomized phase II trial of concurrent chemoradiotherapy (CRT) +/− metformin in locally advanced non–small cell lung cancer (NSCLC) [abstract]. J Clin Oncol. 2019;37(15 suppl):8502. doi: 10.1200/JCO.2019.37.15_suppl.8502 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Detailed Methods
eReferences
eFigure 1. Distant Progression-Free Survival (DPFS)
eTable 1. Detailed Survival Outcomes
Data Sharing Statement