Elevated low-density lipoprotein cholesterol (LDL-C) is associated with an increased risk of adverse ischemic outcomes in the early post-acute coronary syndrome (ACS) period.1 Achieving AHA/ACC and ESC recommendations for secondary prevention in high-risk patients, such as those with ACS, however, is not typically reached until four weeks following initiation of high-dose statins.2 Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition offers an option for additional and more rapid LDL-C lowering during the peri-and early post-ACS period.3
The EVACS (Evolocumab in Acute Coronary Syndrome; ClinicalTrials.gov, Unique identifier: NCT03515304) trial enrolled non-ST-elevation myocardial infarction (NSTEMI) patients with a troponin I of ≥5ng/mL and randomly assigned them in a 1:1 ratio to a one-time dose of evolocumab SQ 420mg or matching placebo within 24-hours of presentation. We present here the LDL-C, the primary, and other atherogenic lipid outcomes, and interpret them in the clinical context by reporting the proportion of patients in each group achieving LDL-C AHA/ACC (<70mg/dL) and ESC (<55mg/dL for very high risk) targets at hospital discharge and 30-day follow-up. All patients received high intensity statins unless contraindicated and were treated in accordance with current ACS guidelines. An independent Data Safety and Monitoring Board monitored the progress of the study, which was approved by the Johns Hopkins Institutional Review Board. All participants gave informed consent.
Of 272 patients with type 1 NSTEMI, 57 (21%) met eligibility criteria and were randomly assigned to receive one dose of evolocumab SQ 420mg or placebo. Lipid values were not an inclusion criterion and were obtained at baseline, throughout hospitalization, and at 30 days. The principal reason for exclusion was a troponin of <5ng/mL.
Mean(SD) age was 55±13 years, 26% were African American, 42% were women, 60% were on prior statin, and the only significant difference was the gender distribution (male: evolocumab 22(73%), placebo 11(41%); p=0.03), which was adjusted for in the analysis. Lipid lowering therapy and intensity at baseline and changes throughout the study did not differ between the two groups and did not confound the study results. Results of fasting lipoproteins are presented in Figure 1A. Mean LDL-C levels at baseline were 91.5±35mg/dL in the evolocumab and 89.6±41mg/dL in the placebo group (p=0.85). We used a linear mixed-effects model with participant-ID as the random effect. After adjusting for baseline LDL-C, gender, lipid lowering therapies, time, and site, evolocumab lowered LDL-C (χ2=37.8, p<0.0001), on average by 28.4±4mg/dL. LDL-C decreased from baseline by day one in the evolocumab group (70.4±27mg/dL; p<0.01 vs baseline), and was lower than that in the placebo group by day three (p=0.02 vs placebo). The group difference continued throughout the hospitalization and at the 30-day follow-up (p<0.01). Linear regression analysis revealed that after adjusting for baseline LDL-C and statin use, change in statin, and ezetimibe use, LDL-C in the evolocumab participants was an average 28.6mg/dL lower than in the placebo participants at 30 days (p<0.0001). The non-HDL-C and apolipoprotein B (ApoB) levels were also significantly lower in the evolocumab, than in the placebo, group at hospital discharge and 30 days. There were no significant triglyceride or HDL-C group differences.
Figure. Atherogenic lipoprotein values and percent of patients achieving LDL-C, ApoB, and non-HDL-C AHA/ACC/ESC targets at hospital discharge and at 30-day follow-up in the evolocumab and placebo groups.
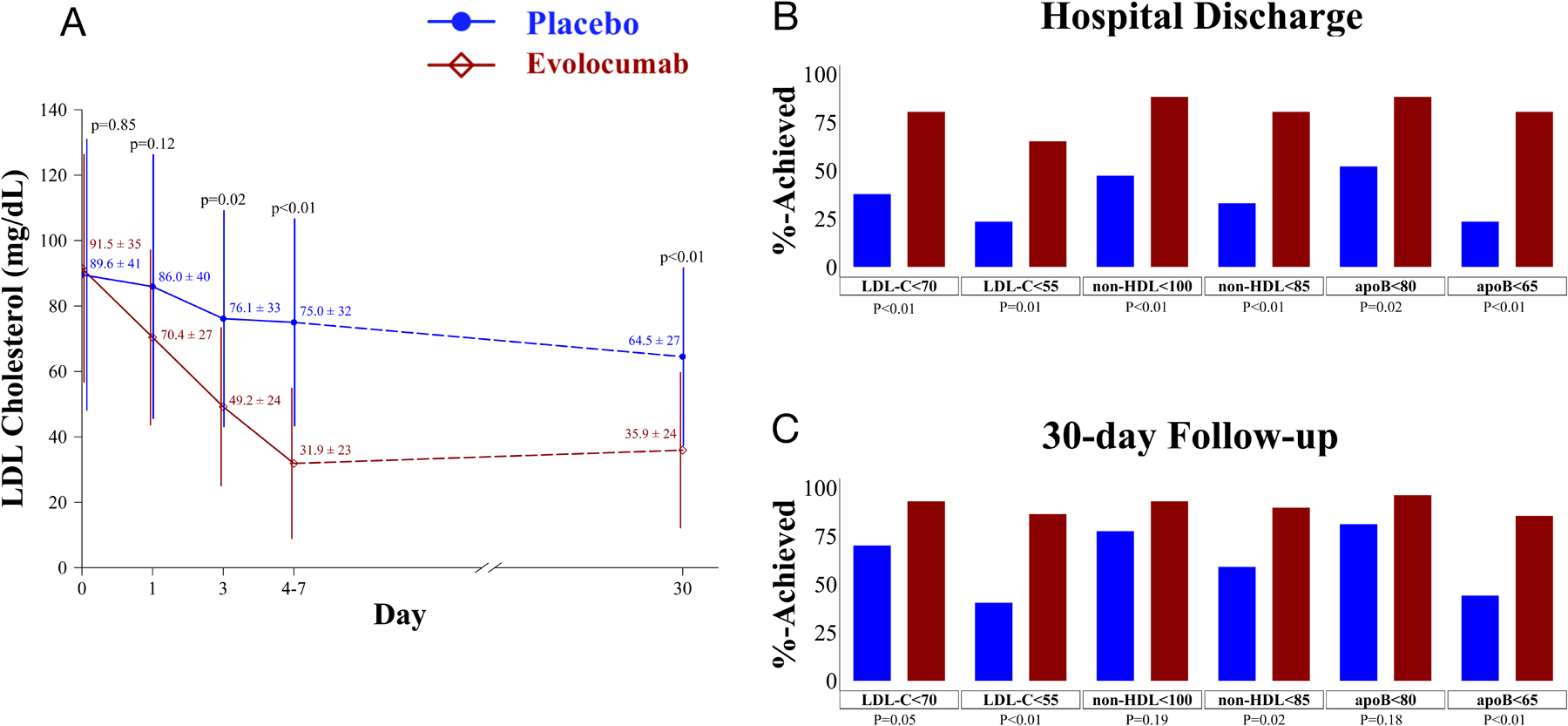
(A) Shown is a graph of the mean and standard deviation (SD) values for LDL-C at the studied time points in the two study groups. The number of participants assessed at the different time points are as follows: Baseline= 57 (evolocumab=30; placebo=27); Day 1=51 (evolocumab=26; placebo=25); Day 3=30 (evolocumab=16; placebo=14); Day 4–7=23 (evolocumab=15; placebo=8); 30-days=57 (evolocumab=30; placebo=27). Panels B and C: Displayed are the proportions of participants whose LDL-C, non-HDL-C, and ApoB levels were at or below 2018 AHA/ACC and 2019 ESC secondary prevention targets at hospital discharge (B) and at 30-day follow-up (C) in the evolocumab and placebo groups. The mean discharge day was 4±2 days, similar to that of the national average. Discharge values were obtained within 24-hours of discharge (evolocumab n=26; placebo n=21). We excluded the values of 10 participants; two who were discharged <24 hrs after randomization, six who were discharged >7 days after study drug administration and two who declined follow up blood drawing during the hospitalization but presented for the 30-day follow-up lipid measurements. For panel A the p-values were obtained using two-sample t-tests and for panels B and C, p-values were obtained using the Fisher’s exact test.
The proportions of the evolocumab participants whose LDL-C levels were at or below the AHA/ACC and ESC targets at hospital discharge, 80.8% and 65.4% respectively, were higher than in those randomized to placebo, 38.1% and 23.8%, (p=0.01 for both the AHA/ACC and ESC comparisons). These data and the non-HDL-C and ApoB results are presented in Figure panels 1B&C. The number of evolocumab and placebo participants with any adverse event, 10 and 12, and a serious adverse event, two and six, respectively did not differ.
The increased risk of recurrent ASCVD events early after ACS warrants intensive lipid lowering.4 Our placebo group data indicate that atherogenic lipoproteins remain significantly above secondary prevention targets during the early post-infarct period despite high-intensity statin therapy, and the results in our treatment group indicate that early guideline achievement is superior with added evolocumab. The ability to administer a hospital-based intervention with a PCSK9 antibody reduces concern for non-compliance with therapy during the first 30 days and provides assurance that desirable lipid levels will likely still be present until the usual follow-up evaluation.
To our knowledge, this is the first study of evolocumab’s impact on early post-infarct atherogenic lipoprotein trajectories in ACS patients. The EVOPACS protocol also randomized ACS participants to evolocumab or placebo and reported lipid results at four and eight weeks.5 Our results demonstrate that evolocumab initiated in the hospital early after an ACS rapidly and significantly reduces LDL-C in just 24-hours. With this approach, AHA/ACC and ESC secondary prevention guideline recommended LDL-C, non-HDL-C and ApoB targets applicable to ACS can be achieved in most patients at hospital discharge and are still present at 30 days. However, the clinical benefits of this early, aggressive lipid lowering approach are uncertain and a randomized controlled trial in ACS is needed.
Acknowledgment Section
Conflict of Interests Disclosures:
| Thorsten M. Leucker: | Grants: American Heart Association Career Development Award, NIH, Amgen |
| Michael Blaha: | Grants: NIH, FDA, AHA, Amgen, Aetna; Advisory Board: Novo Nordisk, Bayer, Novartis, Amgen, Sanofi, Regeneron, Akcea |
| Steven R. Jones: | No disclosures |
| Michael Vavuranakis: | Grants: NIH |
| Marlene S. Williams: | Scientific Advisory Board: Haemonetics; Steering Committee for CME on DAPT – University of North Texas and Rockpointe; Corporation, supported by educational grant from Astra Zeneca |
| Hong Lai: | No disclosures |
| Thomas H. Schindler: | Grants: GE Healthcare Research study (F-Flurpirdaz Perfusion PET study - phase 3 clinical trial) |
| Jacqueline Latina: | No disclosures |
| Steven P. Schulman: | No disclosures |
| Gary Gerstenblith: | Grants: NIH |
Funding/Support:
The study was funded by Amgen Inc.
Role of the Funder/Sponsor:
Amgen, the funder of the study, had no role in the design of the study, the collection, management, or interpretation of the data, or the statistical analysis; the funder reviewed the first submitted version of the manuscript but was not involved in the writing or approval of the manuscript or the decision to submit the manuscript for publication.
Footnotes
The data and analytical methods that support the findings of this study are available from the corresponding author on reasonable request.
References
- 1.Wiviott SD, Cannon CP, Morrow DA, Ray KK, Pfeffer MA, Braunwald E and PROVE IT-TIMI 22 Investigators. Can low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy: a PROVE IT-TIMI 22 substudy. J Am Coll Cardiol. 2005;46:1411–1416. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T and Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering Study I. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: A randomized controlled trial. JAMA. 2001;285:1711–1718. [DOI] [PubMed] [Google Scholar]
- 5.Koskinas KC, Windecker S, Pedrazzini G, Mueller C, Cook S, Matter CM, Muller O, Haner J, Gencer B, Crljenica C, et al. Evolocumab for Early Reduction of LDL Cholesterol Levels in Patients With Acute Coronary Syndromes (EVOPACS). J Am Coll Cardiol. 2019;74:2452–2462. [DOI] [PubMed] [Google Scholar]


