Abstract

High-throughput screening of mechanical properties can transform materials science research by both aiding in materials discovery and developing predictive models. However, only a few such assays have been reported, requiring custom or expensive equipment, while the mounting demand for enormous data sets of materials properties for predictive models is unfulfilled by the current characterization throughput. We address this problem by developing a high-throughput colorimetric adhesion screening method using a common laboratory centrifuge, multiwell plates, and microparticles. The technique uses centrifugation to apply a homogeneous mechanical detachment force across individual formulations in a multiwell plate. We also develop a high-throughput sample deposition method to prepare films with uniform thickness in each well, minimizing well-to-well variability. After establishing excellent agreement with the well-known probe tack adhesion test, we demonstrate the consistency of our method by performing the test on a multiwell plate with two different formulations in an easily discernible pattern. The throughput is limited only by the number of wells in the plates, easily reaching 103 samples/run. With its simplicity, low cost, and large dynamic range, this high-throughput method has the potential to change the landscape of adhesive material characterization.
Short abstract
A simple, inexpensive, and high-throughput colorimetric screening method for adhesive strength in soft materials using centrifugation and microparticles, expediting novel adhesive materials discovery.
Introduction
Advances in combinatorial chemistry, synthetic biology, and sequence-specific polymers have drastically increased the sizes of material libraries for applications ranging from nanomedicines to catalysts.1−3 Advanced computational tools and machine learning have matched this expanded capability by training models for predictive design using these large data sets.1,4,5 However, characterization techniques with the potential to provide data at a commensurate throughput have mostly been limited to the biological community. For example, enzyme activity and antibody binding are routinely tested at a rate of 105–106 per day by utilizing colorimetric or fluorescence assays.6,7 However, techniques for characterizing properties outside the biological realm have yet to match the required throughput for the exponentially increasing demand for new experimental data, particularly for mechanical properties such as adhesion. A key challenge to reaching a similar throughput with mechanical testing is the lack of techniques for both high-throughput sample preparation and measurement. A successful rapid, cost-effective, and straightforward high-throughput characterization pipeline would expedite both the development of predictive models and the discovery of novel materials. The ability to implement such a pipeline with standard lab equipment would ensure the accessibility of high-throughput mechanical testing to the materials community at large.
Many high-throughput techniques for mechanical testing have been developed to enable combinatorial material design.8 Existing methods can measure properties such as elastic modulus, hardness, and residual stress at throughputs of hundreds or thousands of formulations per run. These methods use techniques such as scanning nanoindentation, micromachined cantilever beams, and micro-electro-mechanical systems.9−11 However, these tests require custom instrumentation or fabrication of samples into a specific form.12,13 As a result, the low throughput of the specific fabrication or instrumentation requirements significantly hinder the accessibility and the overall throughput of these techniques. Therefore, a high-throughput mechanical testing pipeline that combines simple sample fabrication/preparation steps with easy characterization techniques would provide the materials science community broad access to large mechanical property data sets that would enable transformative advances.
Adhesion phenomena are essential in materials with low elastic moduli, a category widely encompassing biological soft tissues, elastomers, hydrogels, etc.14 Specifically, soft matter adhesion phenomena are crucial in various medical and industrial applications, such as bioimplants, sealing agents, and ship fouling prevention.15−17 In the case of medical adhesives, such as tissue sealants, exceptional underwater adhesion is required to prevent blood loss and possible infections.18,19 Adhesives inspired by biological organisms such as geckos and mussels are promising candidates that offer excellent reversible adhesive properties in both dry and wet environments.20−24 Achieving these desired adhesive properties depends on the ability to finely tune adhesive strength via several iterations of formulation and characterization, which can be costly and time-consuming. Thus, a characterization method that can quantitatively measure adhesive strength in a high-throughput and cost-effective manner is clearly necessary to accelerate the development of novel adhesive materials.
High-throughput adhesion testing often requires preparing samples in a specific form of gradients or arrays, which frequently involves building complex custom-made instrumentation. For example, Potyrailo et al. developed a high-throughput characterization method that can characterize the coating materials’ resistance to adhesion loss at a 10-fold increase in throughput from 5 coatings per day up to 48 coatings per day, but this method requires a complex automated system that complicates widespread adoption.25 Other characterization methods have also been repurposed to measure adhesion at relatively high throughput. For example, atomic force microscopy (AFM) can determine the adhesion force on a surface by measuring the deformation of the cantilever, but it can only measure one sample per run and requires expensive instrumentation. Although the automation of AFM measurements potentially gives a higher throughput,26,27 the expensive instrumentation prevents the method from being widely accessible.
We identified four key requirements for a high-throughput adhesion characterization pipeline to be widely adopted: (1) It uses commonly available laboratory equipment. (2) It applies the same force to many samples simultaneously. (3) It efficiently converts a mechanical response into an optical signal (4) Its sample preparation throughput matches the throughput of characterization. A common laboratory centrifuge satisfies the first two criteria and is used in assessments of cell adhesion and biophysical properties. Reyes et al. reported using centrifugation in quantitative measurements of cell adhesion, allowing for the fast screening of biomaterial surface modifications and tissue scaffolds.28 Centrifugation has also been used to mechanically manipulate single molecules and enable the measurement of the rupture and unlooping force of DNA nanoswitches at high throughput with high precision.29,30 To use centrifugation for high-throughput mechanical testing, we were inspired by the well-known probe tack apparatus that measures the adhesion energy of material between a spherical probe and a flat surface.31−33 By replacing the spherical probe with colored or fluorescent spherical particles and centrifuging, we can correlate adhesive strength to particle retention at a known centrifugal force, which is easily visualized and thus satisfies requirement 3 (Figure 1). Finally, the uniform forces applied during centrifugation also enable the preparation of samples at the same throughput as the adhesion testing, integrating perfectly into the pipeline and satisfying requirement 4.
Figure 1.
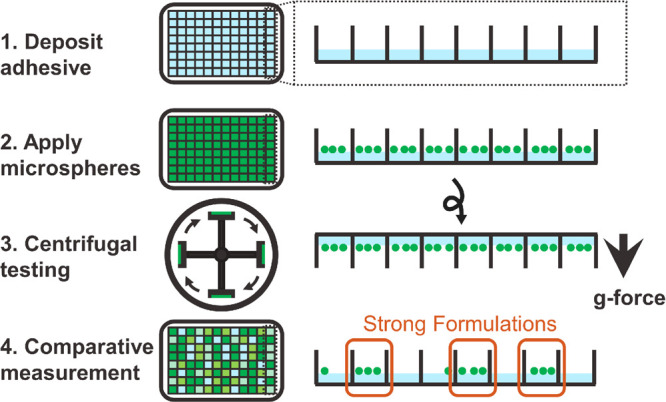
Schematic of the centrifugal adhesion testing procedure. After casting adhesive films in a multiwell plate, colored or fluorescent particles are evenly distributed in each of the wells; then, the plate is centrifuged with the particles facing outward, causing the particles to detach from the films with weaker adhesive properties. The arrow indicates the direction of centrifugal acceleration (up to 4700g).
In this Article, we describe a novel pipeline that can prepare uniform-thickness films of individual formulations and measure adhesive properties at high throughput with a common benchtop centrifuge and commercially available microparticles and multiwell plates. The method can characterize thousands of adhesive formulations simultaneously, and it can characterize a wide range of materials with easily controlled and tunable testing environments (dry/wet, aqueous/organic solvent, basic/acidic, etc.). First, we introduce a high-throughput sample preparation method to prepare adhesive films with an even thickness in a multiwell plate. We then demonstrate the method with a model pressure-sensitive adhesive (PSA) polymer and calibrate using measurements made by a standard probe tack test, showing excellent agreement. Finally, we illustrate the throughput of the method by displaying a pattern on a 384-well plate and conduct a consistency analysis by calculating the Z-factor. The simplicity of our method can speed up the characterization process of new adhesive materials to a throughput of 1536 samples per run in our specific setup. However, the throughput is limited only by the number of wells in each plate and the number of plates a rotor can hold, meaning that our method has a throughput in the range 103–104, which is a drastic improvement compared to the traditional one-sample-per-run method.
Experimental Section
PSA Film Preparation
We demonstrated our method using a waterborne acrylic PSA (Figure S1) that is a copolymer of n-butyl acrylate with 3% of methacrylic acid (Dow Chemical). The polymer is provided as a water-based emulsion with a solid content of ∼40 wt %. To reduce the viscosity of the solution, we prepared a 6.25 wt % working stock PSA solution by diluting with ddH2O to facilitate pipetting. Additionally, we prepared a 6.25 wt % solution of an inherently nonadhesive polymer, polyethylene glycol (PEG, Mn = 300 g/mol), in water. By mixing the two solutions at different ratios, we made working stocks of the polymer mixture at varying concentrations (70–100% of PSA). We then pipetted 5 μL total of the polymer solution with different formulations into corresponding wells of a 384-well plate (Nunc 384-well, nontreated, flat-bottom microplate, Thermo Scientific). Using a benchtop centrifuge (Sorvall Legend XFR centrifuge with a TX-750 swinging bucket rotor, Thermo Scientific), the plate was spun at 1000g and 40 °C for 6 h to evaporate the water in the polymer solution. To ensure that the films were thoroughly dried, we placed the 384-well plate in a vacuum oven at 65 °C for 1 h to further evaporate the water. We then characterized the morphology and the thickness of the films using a Contour X-100 3D optical profilometer (Bruker).
In Situ Bottom Layer Curing
To cast a bottom layer in each well of the multiwell plate, we adapted the formulation and the procedure from Rapp et al.34 We chose poly(methyl methacrylate) (PMMA) as it is fast curing and is glassy at room temperature.35,36 We made a prepolymer of PMMA by dissolving PMMA (purchased from Sigma-Aldrich, average MW ∼15 000 g/mol by GPC) in methyl methacrylate (Fisher Chemical) at a 1:2 mass ratio. In an amber vial, we added 2-hydroxy-2-methylpropiophenone as the initiator (5%), ethylene glycol dimethacrylate as the cross-linker (5%), and prepolymer (90%), and we sparged the solution with nitrogen for 1 h. We then pipetted 30 μL of the mixed solution into each well of a 384-well plate composed of polyproylene (384PP 2.0, Labcyte). To cure the polymers while centrifuging, we purchased a UV LED light panel (UV intensity 0.1 mW/cm3, LED Cool Lights) and constructed a custom setup in the centrifuge rotor (Figure S2). The setup was then centrifuged (3000g, 10 h), while the prepolymers were UV irradiated, and then placed in a vacuum oven at 65 °C for 1 h to remove any residual uncured monomer. The morphology and the thickness profile of the cured polymers were measured by a Contour X-100 3D optical profilometer.
Centrifugal Adhesion Test
We purchased green silica microparticles with size ranges 600–710 μm (geometric average radius, 325 μm) and 710–850 μm (geometric average radius, 390 μm) in diameter from Corpuscular, Inc. Microparticles were further sieved through three U.S. standard stainless steel sieves purchased from Fisher Scientific (pore sizes: 600, 710, and 850 μm) to ensure that their sizes were correct. Each of the PSA films was then covered with a layer of microparticles of a specific size range. We then centrifuged the 384-well plate containing the PSA films with the particles facing inward at 4700g for 5 min to ensure good particle contact with the film. A picture of the plate was taken as a reference. The plate was then flipped so that the particles faced outward, and the plate was centrifuged at various angular speeds for 1 min. After each spin, we took a picture of the plate and counted the number of particles in each well. For the high-throughput assay, we instead used red fluorescent silica microparticles with a size range 600–710 μm in diameter from Cospheric Inc. (exc., 575 nm; em., 607 nm). We followed the same procedure for the green silica particles to perform the centrifugal adhesion test. For characterization, we measured the fluorescence reading with a plate reader (Synergy HTX, Biotek) prior to and after spinning at each speed.
Axisymmetric Probe Tack Test
We performed a probe tack test to measure the force vs displacement history for each formulation under the same strain rate. The setup was previously described by Wang et al.37 We used the same microparticle from the centrifugal test to replace the hemispherical glass indenter described in the paper. We first measured the contact area between the particle and the adhesive film during the centrifugal adhesion test for each formulation. To ensure consistency, we steadily increased the compressive load at an approach velocity of 1 μm/s until the same contact area as the particle–film interface from the centrifugal method was achieved. The contact area of the particle–film interfaces for both methods was monitored using a microscope that was installed below the transparent substrate of the probe tack apparatus. We allowed a 1 s dwell time to improve the reproducibility of the measurement.38 The indenter was then detached at a velocity of 1 μm/s while measuring the detachment force. We obtained Favg from the force vs displacement profile by integrating the force–displacement curve and normalizing it by the total displacement during the detachment process, δ (Figure S3):
| 1 |
Plate Image Analysis and Z-Factor Calculation
To quantify the statistics of particle detachment and enable a high-throughput analysis for a measurement with colored particles, we developed a Python-based image analysis code to automate the particle counting. Images of each 384-well plate were converted to grayscale and then split into a 24 by 16 grid, with each grid box representing each well. For each grid box, a threshold value was then calculated to distinguish the darker particles from the lighter-colored background of the well. The code then counts the number of pixels registered as particles and divides that value by the total pixel count of the grid box. The area ratios were calculated for all of the wells in the control photo (0g), and the area ratios of each of the wells at different centrifugal speeds were divided by the ratios calculated in the control photo. The final ratios calculated represent the detachment of particles in each well. A screening window coefficient for high-throughput screening assays, Z-factor, was used to quantify the assay signal dynamic range and the data measurement variation. A Z-factor of 0.5–1.0 is usually considered excellent. The Z-factor of the centrifugal adhesion method was calculated as previously described, with the 70% PSA as the negative control and the 100% PSA as the positive control.39
Safety Statement
No unexpected or unusually high safety hazards were encountered during the experiments.
Results and Discussion
Centrifugation Enables Film Deposition with Uniform Thickness
One of the significant challenges for realizing high-throughput mechanical testing is preparing samples consistently and combinatorially in a simple manner. To achieve this goal, we selected an emulsion-based acrylic pressure-sensitive adhesive (PSA) polymer as the model material to demonstrate our sample preparation method (Figure S1). An accurate adhesion measurement relies heavily upon sample preparation, specifically ensuring that the sample films have a known and uniform thickness.40 Though drop-casting on a substrate is commonly used, this causes an uneven surface of the deposited film because of the “coffee-ring” effect from surface tension in which capillary flow drives the solute to the outer edge of a drying droplet (Figure 2A).41 To solve this issue, we deposited films while applying centrifugal force, by spinning a 384-well plate in a centrifuge while drying the PSA solution in the plate for 6 h. The water removal process was performed under ambient ventilation at 40 °C, but the drying process can be significantly accelerated if the polymer is in a more volatile solvent, or the centrifuge can be heated to higher temperatures. The resulting films were transparent and smooth upon visual inspection. We later measured the morphology of the films using a 3D optical profilometer. The images showed that the deposited films have a consistent thickness with a much smoother surface compared to films prepared by drop-casting (Figure 2B). The measured thickness of 20 μm matched the expected thickness calculated from mass and density (Table S1).
Figure 2.
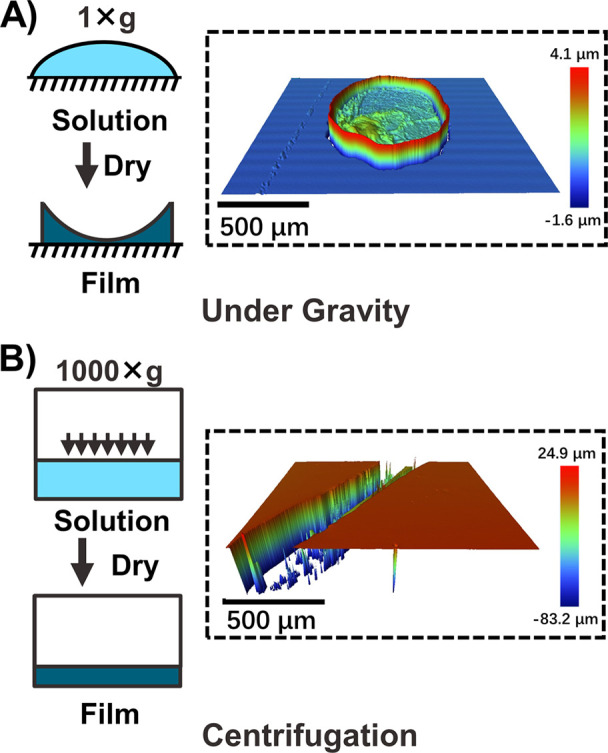
3D optical profilometry of adhesive polymer films dried (A) under gravity and (B) under centrifugation. The film that was dried under centrifugation showed a significantly smoother surface than the one under gravity. The groove was purposely created before the measurement to obtain the thickness of the film (20 μm). Scratches into the substrate inside the groove resulted in the maximum measured depth being higher than the film thickness.
The centrifugal film casting method introduces another challenge that arises from the finite size of the multiwell plate relative to the spinning radius of the centrifuge. Specifically, wells that are farther away from the center of the plate experience centrifugal forces that are misaligned relative to the bottom of the plate, resulting in slanted films of nonuniform thickness (Figure 3A). To overcome this limitation, a hard bottom layer that is perpendicular to the centrifugal force at each well position can be cast prior to the film deposition step, using a similar centrifugal film casting method as discussed above. Thus, as the deposited films slant at the same angle as the hard bottom layer, the relative thickness of the film deposited on the hard layer is uniform (Figure 3B). We cast this hard bottom layer from poly(methyl methacrylate) by UV curing in situ while the plate was centrifuging.34 We designed a custom-made in situ UV-curing system (Figure S2) and found an optimal liquid PMMA formulation (2:1 liquid MMA to PMMA) for fast curing purposes. We then developed a casting procedure for the bottom layer (Figure 3B) and demonstrated the feasibility of this approach. The observed angles of the PMMA layers ranged from 0° to 13.2° from the center well to the edge as measured by 3D optical profilometry (Figure 3C), closely matching the theoretical range from 0° to 11.9° calculated based on a 384-well plate of size 3.2 cm, spinning in our rotor of radius 15.2 cm.
Figure 3.
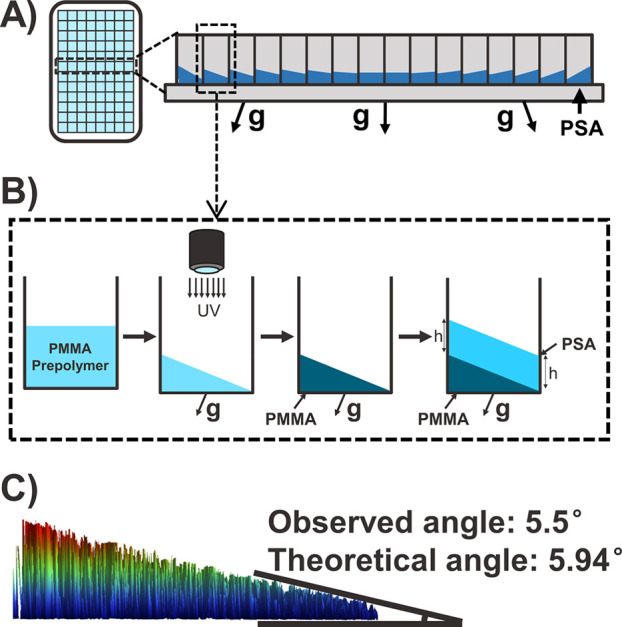
(A) Schematic of the off-alignment effect due to the geometry of the centrifuge rotor, which causes slanted films if polymers are cast directly on the bottom of the multiwell plate. The off-aligned angle of the centrifugal force increases as the wells are farther away from the center. The angles are exaggerated to illustrate the effect. (B) Schematic of the in situ UV curing procedure, shown by a selected well (boxed in part A). The prepolymer of PMMA with photoinitiator and cross-linker is added to each well. The plate is then centrifuged while the prepolymer is UV cured, such that the surface of the liquid becomes perpendicular to the off-aligned centrifugal force, and the angle is preserved once the liquid solidifies upon curing. Thus, when we cast the adhesive layer under the same off-aligned centrifugal force, the relative thickness will be the same throughout the well. (C) 3D optical profilometer image of the bottom layer in the selected well (4th well from the center). Measurements show that the observed angle matches the geometrically calculated angle.
Centrifugal Adhesion Test Can Differentiate Formulations with Various Adhesive Strengths
With the centrifugal deposition technique established, we developed a adhesion screen with groundbreaking throughput, by capitalizing on the unparalleled capabilities of the centrifugation to apply homogeneous force. We reasoned that we could use colored or fluorescent microparticle detachment from films at varying centrifugal speeds to measure adhesion, converting adhesive properties to optical signals for high-throughput measurement. To test this idea, we selected polymer formulations with different adhesive strengths. We used the same PSA polymer as in the previous section and modulated its adhesion by mixing it at varying ratios with nonadhesive polyethylene glycol (PEG). After depositing the polymer films using the centrifugal method and covering them with colored 325 μm radius particles, we used the centrifuge to apply a pressing force on the particles to ensure good film–particle contact. We then inverted the plate and centrifuged with the particles facing outward such that they experienced a centrifugal pulling force. We ramped up the centrifugal acceleration from 0g to 4700g with a 250g increment after each run. At each centrifugal speed, we took a picture of the multiwell plate to record the detachment of particles and compared it to the picture of the original plate. We observed that, for formulations with higher adhesion, particles were retained at higher angular speeds. For example, particles detached from a film composed of 70% PSA at 500g, while the 90% PSA film was able to retain particles all the way up to 2750g (Figure 4).
Figure 4.

Pictures of the 384-well taken at different centrifugal accelerations. Each row depicts the same plate centrifuged at different speeds, with a picture taken at the end of each spin.
Our method can be used for many different applications depending on the specific characterization need. Materials such as solution-based or photocurable adhesives that are compatible with the testing instruments can be easily measured using our approach. Moreover, the testing environment is adaptable, as each of the individual wells can be kept empty or filled with different testing environment solutions. For example, our method can potentially characterize underwater adhesion by simply filling each well with the solution of interest coupled with plate seals.
Comparison to the Probe Tack Adhesion Test Validates the Centrifugal Adhesion Test
Next, we validated our method by comparing it to a standard, state-of-the-art measurement technique. We selected the probe tack adhesion test as the standard technique for comparison, as it is widely used to characterize adhesion phenomena in soft materials with great accuracy and sensitivity.42,43 Specifically, the probe tack test has previously been used to measure the adhesive properties of acrylic PSAs, providing a direct comparison to our study.44,45 While this test is not designed for high-throughput applications, as a single measurement can take several minutes, the probe tack test can record a detailed force vs displacement profile while taking a video of the contact area during debonding. It also involves using a spherical indenter in contact with an adhesive layer, so it is mechanistically similar to our centrifugal test. Accordingly, we used the same microparticles from the centrifugal test as the probe for the measurement. We next obtained the average adhesion force (Favg) by analyzing the load–displacement profile for each of the formulations. We use this as the key point of comparison with the centrifugal test results.
To verify the accuracy of our method, we compared the adhesive properties of 74–100% PSA measured by both the centrifugal adhesion test and the probe tack test. For the centrifugal adhesion test, the number of particles was counted in each well using the pictures taken. In Figure 5, we denote the status of each well as dots of varying colors as the plate is centrifuged at successively higher speeds. Each row of dots in the figure is one centrifugation of several PSA formulations, at an angular speed that was converted to a detachment force experienced by the particle during the spin (Figure 5, left axes). This conversion is given by eq 2 below, and a table of values for the particles used in this study is shown in Table S2. While the status of each well was determined manually through visual inspection, further automation can be achieved using fluorescent particles and measuring the optical signal with a plate reader or using an image analysis code. We repeated this procedure with red fluorescent particles (Figure S4), and we observed a similar trend as in Figure 4, demonstrating that the use of these probes enables improved automation in the workflow without any sacrifice in accuracy.
Figure 5.
Comparison of measurements made by the centrifugal adhesion test and the probe tack test. The detachment of the microparticles is shown by colors. A blue dot means that >60% of the particles remained on the film a yellow dot that 40–60% of the particles remained on the film, and a red dot that <40% of the particles remained on the film. Favg measured using the probe tack test (after adjusting by a factor of a = 1.6) was also plotted on the same figures. (A) Measurements are done by using particles of a 325 μm radius with the actual image of the centrifugal measurement at 750g. The colored boxes and arrows indicate the detachment status of each condition. (B) Measurements are done by using particles of a 390 μm radius.
Although all of the particles would ideally detach at the same centrifugal speed for a given formulation, our experiments were more stochastic than expected. This variability is likely due to the inhomogeneity of the particle size and film defects. For example, in our size range of particles (710–850 μm), the largest particles should experience a 71% higher centrifugal force than the smallest particles. To mitigate this variability, we define the centrifugal force at which 40–60% of the particles detach as the critical adhesive force measured for the corresponding formulation. We expect this quantitative metric to be less affected by the variability, as it is obtained by averaging the detachment status of ∼20 particles in each well. It also provides a quantitative value for the adhesion force that can be compared between different formulations and with other characterization methods, such as the probe tack test. In fact, our method may have even lower variability compared to a traditional adhesion test, as its results are an average of over as many particles as can fit in a well, each of which is analogous to an individual probe tack test.
Results from our method are in agreement with probe tack test results on the same formulations, proving that our method is capable of quantitative measurement (Figure 5A). The best match occurs when comparing to Favg from the axisymmetric probe test with an order 1 linear factor, which was empirically determined to be 1.6. Measurements done using larger microparticles (390 μm radius) demonstrated the robustness of this method when using particles of different sizes (Figure 5B). The dynamic range of the probing adhesive strength can be easily tuned by simply changing the particle size in the experiment, as the centrifugal force is a function of particle radius:
| 2 |
where G is the centrifugal acceleration controlled by the rotor’s rotational speed and radius, and ρparticle and rparticle are the particle density and radius, respectively. Common commercially available microparticles have the size range ∼10–1000 μm in diameter, and centrifuges can usually go from 0g to 5000g with a plate rotor. This corresponds to a tunable force range of at least 8 orders of magnitude. For example, the centrifugal force could go as low as 1.23 nN by using polyethylene particles (ρ = 0.96 g/cm3) with a radius of 5 μm at 250g and as high as 0.188 N by using stainless steel particles (ρ = 7.8 g/cm3) with a radius of 500 μm at 4700g. The large dynamic range enables the application of the method to a wide range of soft materials.
We hypothesize that the empirical linear factor of 1.6 arises when bridging the difference between our method as a stress-controlled experiment versus the probe tack test as a strain-controlled method. Though we keep the experimental parameters as identical as possible, the strain rate dependence of soft matter adhesion37,46 makes direct comparison between the two methods difficult because measuring and controlling the strain rate during the centrifugation is inherently challenging. In fact, our method is only able to achieve such high throughputs as a stress-controlled method, by applying the same force to each particle using centrifugation. The linear factor is introduced to correlate the two methods with potentially different strain rates, and the factor may be closer to 1 if we could perfectly match these strain rates. While our method is powerful in its simple and quick screening of the adhesive material candidates, measuring more detailed adhesion phenomena is always possible on a smaller set of material candidates after screening.
Simultaneous Measurements of 384 Samples Shows Both the Throughput and the Robustness of Our Method
For our method to be useful as a high-throughput screen, we needed to ensure that there was minimal well-to-well variability across a multiwell plate. For example, not all of the wells are perfectly normal to the radius of centrifugation as previously discussed, causing centrifugal forces to be misaligned relative to the bottom of the plate (Figure 3). The calculated maximum misalignment angle during film preparation is 11.9°, and the multiwell plate is inverted meaning that the centrifugal force is misaligned by another 11.9°, resulting in a total misalignment angle of 23.8° relative to the plane of the film. Moreover, the radius of centrifugation at the edge wells is larger by a factor of 1/cos(θmax) compared to the center wells, where θmax is the maximum misalignment angle. Taking both factors into account, this corresponds to a maximum difference between the centrifugal force at the center and the centrifugal force at the furthest well of only 6.5%, as calculated by the following equation (see Figure S5 for details):
| 3 |
Taking advantage of this minimal variability in forces across the entire plate, we further demonstrated both the consistency of the measurement and the throughput of the method. To do this, we performed an all-plate demonstration by depositing two different PSA formulations on a well plate, prearranged in a pattern. The two formulations were chosen based on their adhesive strength difference, which offers enough contrast to reveal the pattern while demonstrating the sensitivity of the screen. This pattern was shown as the particles in wells containing a weaker formulation (80% PSA) detached simultaneously at a relatively low angular speed, while wells with a stronger formulation (92% PSA) showed retention until much higher speeds (Figure 6B). Furthermore, we conducted a consistency analysis on our method by calculating the Z-factor, a coefficient that measures the screening assay quality and measurement data variation,39 with negative controls (70% PSA) and positive controls (100% PSA) deposited in alternating columns (Figure S6). The Z-factor increases as we ramp up the centrifugal speed, with a peak score of 0.92 at 2500g, when almost all particles were detached from the negative controls and retained on the positive controls. The results again indicate the robustness of the method. The high Z-factor score also suggests that the 6.5% difference in centrifugal force from the center to the edge wells can be considered negligible.
Figure 6.
(A) Schematic of the all-plate experiment to demonstrate the throughput. A pattern (dark blue) was displayed by depositing a relatively strong formulation (92% PSA), and a weaker formulation (80% PSA) was deposited in the background (light blue). (B) Here, particles of a 325 μm radius were used for the all-plate experiment. Boxed in red are two wells deposited with 100% PSA that served as the negative control. The particles in the background detached first as those films are less adhesive, revealing the “NU ROCKS” pattern. The particles in the pattern eventually detached at 4700g. The experiment was done following the same spin adhesion testing procedure.
For a centrifuge rotor that holds four plates at the same time, our method has a throughput of 1536 samples/run if using a 384-well plate. The potential throughput can be further improved by using a centrifuge rotor that can hold additional plates or doing the centrifugal test with 1536-well plates. The high throughput of this method can significantly expedite the characterization of adhesive materials, enabling large adhesive property data sets to be generated.
Although our method has many advantages over conventional methods, it is designed to complement traditional mechanical testing. For example, as our method is a stress-controlled experiment, it is challenging to control the strain rate during the detachment, as previously discussed. It also might be hard to identify the failure mode (adhesive/cohesive failure) by our method, though the problem can potentially be tackled using a built-in camera inside the centrifuge or detailed ex situ analysis of the adhesive films after testing. In this case, classic adhesive testing methods would be the perfect follow-up to our method by providing a more detailed characterization of material candidates selected with our screening approach. We expect that the integration of our technique into the material development pipeline can substantially benefit the community.
Conclusion
We developed a high-throughput, fast, and cost-effective centrifugal adhesion mechanical testing pipeline that can prepare and characterize adhesive materials with easily accessible laboratory equipment and consumables. The unique centrifugal deposition method overcomes the drawbacks of drop-casting, making it possible to deposit films with a smooth surface and an even thickness in all wells of a multiwell plate. Furthermore, the centrifugal adhesion testing method provides a quantitative measurement with the results validated by a more standard adhesion test. We envision expanding our centrifugal force-based method to measure other mechanical properties at high throughput, expanding our capability of advanced materials characterization. Our work enables the high-throughput discovery of adhesives and brings a novel tool to the materials community. Moreover, we hope that the idea of converting mechanical properties to an optical signal can inspire additional new processes for high-throughput mechanical testing.
Acknowledgments
The authors would like to thank the members of Wang lab and Tullman-Ercek lab for insightful discussions and support. This work was sponsored by the MRSEC at Northwestern University under NSF Award Number DMR-1720139. This work also made use of the Keck-II facility of Northwestern’s NUANCE Center, which has received support from the SHyNE Resource (NSF ECCS-2025633), the IIN, and Northwestern’s MRSEC program. The adhesive material was provided by The Dow Chemical Company.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.1c00414.
Supplemental figures and tables including chemical structures of the adhesive materials, in situ UV curing setup, probe-tack test illustration, fluorescent measurement of the centrifugal test, Z-factor analysis, film thickness measurements, conversion between centrifugal forces and centrifugal accelerations, and detailed calculations for the misaligned angle effect and a conversion table (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ong S. P. Accelerating Materials Science with High-Throughput Computations and Machine Learning. Comput. Mater. Sci. 2019, 161, 143–150. 10.1016/j.commatsci.2019.01.013. [DOI] [Google Scholar]
- Williams T.; McCullough K.; Lauterbach J. A. Enabling Catalyst Discovery through Machine Learning and High-Throughput Experimentation. Chem. Mater. 2020, 32 (1), 157–165. 10.1021/acs.chemmater.9b03043. [DOI] [Google Scholar]
- Yamankurt G.; Berns E. J.; Xue A.; Lee A.; Bagheri N.; Mrksich M.; Mirkin C. A. Exploration of the Nanomedicine-Design Space with High-Throughput Screening and Machine Learning. Nat. Biomed. Eng. 2019, 3 (4), 318–327. 10.1038/s41551-019-0351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew K.; Montoya J. H.; Faghaninia A.; Dwarakanath S.; Aykol M.; Tang H.; Chu I. heng; Smidt T.; Bocklund B.; Horton M.; et al. Atomate: A High-Level Interface to Generate, Execute, and Analyze Computational Materials Science Workflows. Comput. Mater. Sci. 2017, 139, 140–152. 10.1016/j.commatsci.2017.07.030. [DOI] [Google Scholar]
- Malhotra R.Combinatorial Materials Development; American Chemical Society, 2002.
- Major J. Challenges and Opportunities in High Throughput Screening: Implications for New Technologies. J. Biomol. Screening 1998, 3 (1), 13–17. 10.1177/108705719800300102. [DOI] [Google Scholar]
- Udenfriend S.Fluorescence Assay in Biology and Medicine; Academic Press, 2014; Vol. 2.
- Zhang X.; Xiang Y. Combinatorial Approaches for High-Throughput Characterization of Mechanical Properties. J. Mater. 2017, 3 (3), 209–220. 10.1016/j.jmat.2017.07.002. [DOI] [Google Scholar]
- Zhao J.-C.; Jackson M. R.; Peluso L. A.; Brewer L. N. A Diffusion Multiple Approach for the Accelerated Design of Structural Materials. MRS Bull. 2002, 27 (4), 324–329. 10.1557/mrs2002.100. [DOI] [Google Scholar]
- Ludwig A.; Cao J.; Brugger J.; Takeuchi I. MEMS Tools for Combinatorial Materials Processing and High-Throughput Characterization. Meas. Sci. Technol. 2005, 16 (1), 111–118. 10.1088/0957-0233/16/1/015. [DOI] [Google Scholar]
- Kim H. J.; Han J. H.; Kaiser R.; Oh K. H.; Vlassak J. J. High-Throughput Analysis of Thin-Film Stresses Using Arrays of Micromachined Cantilever Beams. Rev. Sci. Instrum. 2008, 79 (4), 045112. 10.1063/1.2912826. [DOI] [PubMed] [Google Scholar]
- Oellers T.; Arigela V. G.; Kirchlechner C.; Dehm G.; Ludwig A. Thin-Film Microtensile-Test Structures for High-Throughput Characterization of Mechanical Properties. ACS Comb. Sci. 2020, 22 (3), 142–149. 10.1021/acscombsci.9b00182. [DOI] [PubMed] [Google Scholar]
- Salzbrenner B. C.; Rodelas J. M.; Madison J. D.; Jared B. H.; Swiler L. P.; Shen Y. L.; Boyce B. L. High-Throughput Stochastic Tensile Performance of Additively Manufactured Stainless Steel. J. Mater. Process. Technol. 2017, 241, 1–12. 10.1016/j.jmatprotec.2016.10.023. [DOI] [Google Scholar]
- Shull K. R. Contact Mechanics and the Adhesion of Soft Solids. Mater. Sci. Eng., R 2002, 36 (1), 1–45. 10.1016/S0927-796X(01)00039-0. [DOI] [Google Scholar]
- Wilke P.; Helfricht N.; Mark A.; Papastavrou G.; Faivre D.; Börner H. G. A Direct Biocombinatorial Strategy toward next Generation, Mussel-Glue Inspired Saltwater Adhesives. J. Am. Chem. Soc. 2014, 136 (36), 12667–12674. 10.1021/ja505413e. [DOI] [PubMed] [Google Scholar]
- Petrie E. M.An Introduction to Adhesive and Sealants. In Handbook of Adhesives and Sealants, 1st ed.; McGraw-Hill Education: New York, 1999; pp 2–48. [Google Scholar]
- Ge L.; Sethi S.; Ci L.; Ajayan P. M.; Dhinojwala A. Carbon Nanotube-Based Synthetic Gecko Tapes. Proc. Natl. Acad. Sci. U. S. A. 2007, 104 (26), 10792–10795. 10.1073/pnas.0703505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumville J. C.; Gray T. A.; Walter C. J.; Sharp C. A.; Page T.; Macefield R.; Blencowe N.; Milne T. K. G.; Reeves B. C.; Blazeby J. Dressings for the Prevention of Surgical Site Infection. Cochrane Database Syst. Rev. 2016, (12), 3091. 10.1002/14651858.CD003091.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A.; Kaur S.; Peng C.; Debnath D.; Mishra K.; Liu Q.; Dhinojwala A.; Joy A. Viscosity Attunes the Adhesion of Bioinspired Low Modulus Polyester Adhesive Sealants to Wet Tissues. Biomacromolecules 2019, 20 (7), 2577–2586. 10.1021/acs.biomac.9b00383. [DOI] [PubMed] [Google Scholar]
- Lee H.; Lee B. P.; Messersmith P. B. A Reversible Wet/Dry Adhesive Inspired by Mussels and Geckos. Nature 2007, 448 (7151), 338–341. 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- Autumn K.; Liang Y. A.; Hsieh S. T.; Zesch W.; Chan W. P.; Kenny T. W.; Fearing R.; Full R. J. Adhesive Force of a Single Gecko Foot-Hair. Nature 2000, 405 (6787), 681–685. 10.1038/35015073. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Liu Q.; Narayanan A.; Jain D.; Dhinojwala A.; Joy A. Mussel-Inspired Polyesters with Aliphatic Pendant Groups Demonstrate the Importance of Hydrophobicity in Underwater Adhesion. Adv. Mater. Interfaces 2017, 4 (22), 1700506. 10.1002/admi.201700506. [DOI] [Google Scholar]
- Mahdavi A.; Ferreira L.; Sundback C.; Nichol J. W.; Chan E. P.; Carter D. J. D.; Bettinger C. J.; Patanavanich S.; Chignozha L.; Ben-Joseph E.; et al. A Biodegradable and Biocompatible Gecko-Inspired Tissue Adhesive. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (7), 2307–2312. 10.1073/pnas.0712117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurdumakan B.; Raravikar N. R.; Ajayan P. M.; Dhinojwala A. Synthetic Gecko Foot-Hairs from Multiwalled Carbon Nanotubes. Chem. Commun. 2005, (30), 3799–3801. 10.1039/b506047h. [DOI] [PubMed] [Google Scholar]
- Potyrailo R. A.; Chisholm B. J.; Morris W. G.; Cawse J. N.; Flanagan W. P.; Hassib L.; Molaison C. A.; Ezbiansky K.; Medford G.; Reitz H. Development of Combinatorial Chemistry Methods for Coatings: High-Throughput Adhesion Evaluation and Scale-up of Combinatorial Leads. J. Comb. Chem. 2003, 5 (4), 472–478. 10.1021/cc030022s. [DOI] [PubMed] [Google Scholar]
- Lin D. C.; Dimitriadis E. K.; Horkay F. Robust Strategies for Automated AFM Force Curve Analysis. I. Non-Adhesive Indentation of Soft, Inhomogeneous Materials. J. Biomech. Eng. 2007, 129, 430. 10.1115/1.2720924. [DOI] [PubMed] [Google Scholar]
- Lin D. C.; Dimitriadis E. K.; Horkay F. Robust Strategies for Automated AFM Force Curve Analysis. II: Adhesion-Influenced Indentation of Soft, Elastic Materials. J. Biomech. Eng. 2007, 129, 904. 10.1115/1.2800826. [DOI] [PubMed] [Google Scholar]
- Reyes C. D.; Garcia J. A Centrifugation Cell Adhesion Assay for High-Throughput Screening of Biomaterial Surfaces. J. Biomed. Mater. Res., Part A 2003, 67A, 328. 10.1002/jbm.a.10122. [DOI] [PubMed] [Google Scholar]
- Yang D.; Ward A.; Halvorsen K.; Wong W. P. Multiplexed Single-Molecule Force Spectroscopy Using a Centrifuge. Nat. Commun. 2016, 11026. 10.1038/ncomms11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen K.; Wong W. P. Massively Parallel Single-Molecule Manipulation Using Centrifugal Force. Biophys. J. 2010, 98 (11), L53–L55. 10.1016/j.bpj.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn D.; Shull K. R. JKR Studies of Acrylic Elastomer Adhesion to Glassy Polymer Substrates. Macromolecules 1996, 29 (12), 4381–4390. 10.1021/ma9518924. [DOI] [Google Scholar]
- Crosby A. J.; Shull K. R. Adhesive Failure Analysis of Pressure-Sensitive Adhesives. J. Polym. Sci., Part B: Polym. Phys. 1999, 37 (24), 3455–3472. . [DOI] [Google Scholar]
- Shull K. R.; Ahn D.; Chen W. L.; Flanigan C. M.; Crosby A. J. Axisymmetric Adhesion Tests of Soft Materials. Macromol. Chem. Phys. 1998, 199 (4), 489–511. . [DOI] [Google Scholar]
- Nargang T. M.; Brockmann L.; Nikolov M.; Schild D.; Helmer D.; Keller N.; Sachsenheimer K.; Wilhelm E.; Pires L.; Dirschka M. Liquid Polystyrene: A Room-Temperature Photocurable Soft Lithography Compatible Pour-and-Cure-Type Polystyrene. Lab Chip 2014, 14, 2698–2708. 10.1039/C4LC00045E. [DOI] [PubMed] [Google Scholar]
- Silvaroli A. J.; Heyl T. R.; Qiang Z.; Beebe J. M.; Ahn D.; Mangold S.; Shull K. R.; Wang M. Tough, Transparent, Photocurable Hybrid Elastomers. ACS Appl. Mater. Interfaces 2020, 12 (39), 44125–44136. 10.1021/acsami.0c11643. [DOI] [PubMed] [Google Scholar]
- Kotz F.; Arnold K.; Wagner S.; Bauer W.; Keller N.; Nargang T. M.; Helmer D.; Rapp B. E. Liquid PMMA: A High Resolution Polymethylmethacrylate Negative Photoresist as Enabling Material for Direct Printing of Microfluidic Chips. Adv. Eng. Mater. 2018, 20 (2), 1700699. 10.1002/adem.201700699. [DOI] [Google Scholar]
- Wang Q.; Griffith W. B.; Einsla M.; Zhang S.; Pacholski M. L.; Shull K. R. Bulk and Interfacial Contributions to the Adhesion of Acrylic Emulsion-Based Pressure-Sensitive Adhesives. Macromolecules 2020, 53 (16), 6975–6983. 10.1021/acs.macromol.0c01354. [DOI] [Google Scholar]
- Davis C. S.; Lemoine F.; Darnige T.; Martina D.; Creton C.; Lindner A. Debonding Mechanisms of Soft Materials at Short Contact Times. Langmuir 2014, 30 (35), 10626–10636. 10.1021/la5023592. [DOI] [PubMed] [Google Scholar]
- Zhang J.-H.; Chung T. D. Y.; Oldenburg K. R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screening 1999, 4 (2), 67–73. 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Creton C.; Ciccotti M. Fracture and Adhesion of Soft Materials: A Review. Rep. Prog. Phys. 2016, 79 (4), 046601. 10.1088/0034-4885/79/4/046601. [DOI] [PubMed] [Google Scholar]
- Sun J.; Bao B.; He M.; Zhou H.; Song Y. Recent Advances in Controlling the Depositing Morphologies of Inkjet Droplets. ACS Appl. Mater. Interfaces 2015, 7 (51), 28086–28099. 10.1021/acsami.5b07006. [DOI] [PubMed] [Google Scholar]
- Zosel A. Adhesion and Tack of Polymers: Influence of Mechanical Properties and Surface Tensions. Colloid Polym. Sci. 1985, 263 (7), 541–553. 10.1007/BF01421887. [DOI] [Google Scholar]
- Creton C.; Ciccotti M. Fracture and Adhesion of Soft Materials: A Review. Rep. Prog. Phys. 2016, 79 (4), 046601. 10.1088/0034-4885/79/4/046601. [DOI] [PubMed] [Google Scholar]
- Lakrout H.; Sergot P.; Creton C. Direct Observation of Cavitation and Fibrillation in a Probe Tack Experiment on Model Acrylic Pressure-Sensitive-Adhesives. J. Adhes. 1999, 69 (3–4), 307–359. 10.1080/00218469908017233. [DOI] [Google Scholar]
- Karnal P.; Roberts P.; Gryska S.; King C.; Barrios C.; Frechette J. Importance of Substrate Functionality on the Adhesion and Debonding of a Pressure-Sensitive Adhesive under Water. ACS Appl. Mater. Interfaces 2017, 9 (48), 42344–42353. 10.1021/acsami.7b13984. [DOI] [PubMed] [Google Scholar]
- Shull K. R.; Creton C. Deformation Behavior of Thin, Compliant Layers under Tensile Loading Conditions. J. Polym. Sci., Part B: Polym. Phys. 2004, 42 (22), 4023–4043. 10.1002/polb.20258. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




