ABSTRACT
Background
Acute kidney injury (AKI) caused by cast nephropathy is associated with increased morbidity and mortality among patients with multiple myeloma (MM). High cut-off haemodialysis (HCO-HD) has proven to be effective in the removal of serum light chains but the effect on clinical outcomes, especially renal recovery, remains uncertain.
Methods
A systematic review and meta-analysis were performed examining all randomized controlled trials (RCTs) and observational studies (OBSs) assessing the effect of HCO-HD on clinical outcomes of patients with MM complicated by cast nephropathy–induced severe AKI. The primary outcome was all-cause mortality at the end of the study. The secondary outcomes included all-cause mortality at 12 months, HD independence and serum kappa and lambda light chain reduction. Pooled analysis was performed using random effects models.
Results
We identified five studies, comprising two RCTs and three retrospective cohort studies, including 276 patients with a mean follow-up of 18.7 months. The majority of the studies were of suboptimal quality and underpowered. Compared with patients treated with conventional HD, HCO-HD was not associated with a survival benefit at 12 months {five studies, 276 patients, relative risk [RR] 1.02 [95% confidence interval (CI) 0.76–1.35], I2 = 33.9%} or at the end of the studies at an average of 34 months [five studies, 276 patients, RR 1.32 (95% CI 0.71–2.45), I2 = 62.0%]. There was no difference in HD independence at 90 days [two trials, 78 patients, RR 2.23 (95% CI 1.09–4.55)], 6 months [two studies, 188 patients, RR 1.19 (95% CI 0.68–2.06)] or 12 months [two studies, 188 patients, RR 1.14 (95% CI 0.58–2.26)]. Patients receiving HCO dialysis, however, had a greater reduction in serum kappa [two studies, 188 patients, weighted mean difference (WMD) 46.7 (95% CI 38.6–54.7), I2 = 52.0%] and lambda [two studies, 188 patients, WMD 50.3 (95% CI 21.4–79.3), I2 = 95.1%] light chain levels.
Conclusion
Current evidence from RCTs and OBSs suggests HCO dialysis is able to reduce serum free light chains but makes no significant improvement in all-cause mortality and renal outcomes compared with conventional HD for patients with myeloma cast nephropathy. However, there is a trend towards better renal outcomes with the use of HCO dialysis. The lack of long-term data and the small sample sizes of the included studies limit this analysis. Therefore further large-scale RCTs with longer follow-up are needed to assess the effect of HCO dialysis on clinical outcomes in patients with myeloma cast nephropathy.
Keywords: acute kidney injury, high-cutoff hemodialysis, meta-analysis, myeloma cast nephropathy, systematic review
INTRODUCTION
Multiple myeloma (MM) constitutes the second most common haematological malignancy and is associated with significant mortality and morbidity [1–5]. Its incidence has been increasing worldwide and renal impairment occurs in up to 50% of patients, with 10% of patients developing severe renal failure requiring dialysis [6].
Cast nephropathy is the most common cause of renal impairment among myeloma patients [7]. It occurs when the pathologically high levels of serum free light chains (sFLCs) produced by plasma cells are precipitated in the renal tubules after being filtered through the glomeruli [8, 9]. Cast nephropathy and overall renal impairment are associated with increased mortality in myeloma patients [3, 10].
Treatment of cast nephropathy is directed at reducing sFLC levels, using treatments such as bortezomib (BTZ) [11]. In addition, mechanical removal of sFLC via haemodialysis (HD) or plasmapheresis [12–14] has been considered as another biologically plausible therapeutic path for management of patients with cast nephropathy. Compared with standard HD membranes, high cut-off haemodialysis (HCO-HD) membranes have increased pore size, allowing the removal of larger molecules such as immunoglobulin light chains [15, 16]. While in vitro studies showed sustained reductions in sFLC with extended HCO dialysis [17, 18], two randomized controlled trials (RCTs) demonstrated discrepant results upon recovery of renal function [19, 20].
We set out to systematically review the literature to summarize the current evidence on the effect of HCO dialysis on clinical outcomes in patients with MM complicated by cast nephropathy.
MATERIALS AND METHODS
Data sources and searches
The following databases were searched from their inception to September 2019: MEDLINE via OvidSP, Embase via OvidSP and the Cochrane Central Register of Controlled Trials. In addition, hand searches of eligible studies were completed and unpublished studies were sought in references of all selected studies, relevant conference abstracts and from the ClinicalTrials.gov website. There were no language or publication period restrictions. Relevant text words and Medical Subject Headings were included in the search strategy (Supplementary data, Figure S1).
Study outcomes
The primary outcome was all-cause mortality at the end of the study. Secondary outcomes were all-cause mortality at 3 and 12 months, HD independence at 3, 6 and 12 months, sFLC (kappa and lambda) post-dialysis reduction percentages and haematological response at 90 days. Haematological response was defined according to the International Myeloma Working Group criteria [21] as the achievement of at least a partial response, including one of the following criteria: 50% reduction of serum M-protein and reduction in 24-h urinary M-protein by ≥90% or to 200 mg/24 h, ≥50% decrease in the difference between involved and uninvolved FLC levels and ≥50% reduction in plasma cells in bone marrow biopsy, provided there was a baseline percentage of ≥30%; in addition to this criteria, a ≥50% reduction in the size of soft tissue plasmacytomas is also required, if present at baseline.
Study selection
Titles and abstracts of all citations were independently evaluated by two reviewers (B.T. and N.Y.) and all potentially relevant articles were retrieved for full-text review. Any disagreements on the eligibility of a study were resolved by consensus or with the help of a third reviewer (A.Y.W.). We included RCTs as well as observational studies (OBSs) with a cohort or case–control design assessing the effect of HCO dialysis as an adjuvant treatment for patients with cast nephropathy–related acute kidney injury (AKI).
Data extraction and quality assessment
Data were extracted using a standardized data extraction form in Excel (Microsoft, Redmond, WA, USA) and verified independently by two reviewers (B.T. and N.Y.). The information gathered from each study included the following: title, first author, year of publication, study design, location, characteristics of study participants, follow-up duration, description of intervention (chosen dialyzer, dialysis schedule) and adjuvant treatments (chemotherapy), as well as the primary and secondary outcomes.
The Cochrane risk-of-bias tool was used to assess the risk of bias for RCTs and the Newcastle-Ottawa Scale [22] was used to assess the risk of bias for OBSs. Two reviewers (B.T. and N.Y.) independently assessed each included study for the risk of bias. Any persistent disagreements were resolved by consensus or discussion with a third reviewer (A.Y.W.).
Data synthesis and analysis
The pooled analysis was performed using a random effects model. Pre-specified subgroup analyses were performed separating OBSs and RCTs. Relative risks (RRs) and 95% confidence intervals (CIs) were calculated for dichotomous variables. Weighted mean differences (WMDs) and 95% CIs were calculated for continuous variables. Statistical heterogeneity was assessed using I2 for pooled results. Statistical analyses were performed using Stata version 14.1 (StataCorp, College Station, TX, USA) and Review Manager version 5.3 (Cochrane Collaboration, London, UK), with P-values <0.05 considered statistically significant for all analyses.
RESULTS
Study selection and characteristics
The search identified five studies, two RCTs and three retrospective cohort studies with a total of 276 patients. The flow chart of the study selection was shown in Figure 1 and the key characteristics of the included studies are presented in Table 1. End-of-study timepoints varied from 12 to 72.7 months with a mean follow-up of 18.7 months (Table 1). The mean age of the participants ranged from 63.1 to 71 years. There was a higher proportion of male participants, ranging from 50 to 73.7%. All five studies included patients with a diagnosis of cast nephropathy secondary to MM, and histological proof of cast nephropathy reached 100% of patients in four of the five studies [19, 20, 23, 24]. Three studies only included patients with de novo MM [19, 20, 23], while two of the OBSs also included patients with relapsed MM [24, 25]. The immunoglobulin heavy chain types of immunoglobulin G (IgG) and IgA were the predominant types studied [19, 20, 24, 25]. All patients included had severe AKI, defined as an estimated glomerular filtration rate <15 mL/min/1.73 m2 or clinical indication for dialysis. In the interventional group, HCO-HD was performed using a Gambro HCO 1100 dialyser [20, 23–25] or a Theralite dialyser [19, 24, 25], while control groups received high-flux HD (HF-HD) [19, 20, 25] or haemodiafiltration [24]. The chemotherapy treatments used included a BTZ alone regimen [19, 20, 24] in three studies and BTZ in combination with other chemotherapy in two studies [23, 25]. Pre-existing chronic kidney disease (CKD) was reported in three studies [19, 20, 25], ranging from 6 to 10% in the intervention group and from 2 to 17% in the control group.
FIGURE 1:
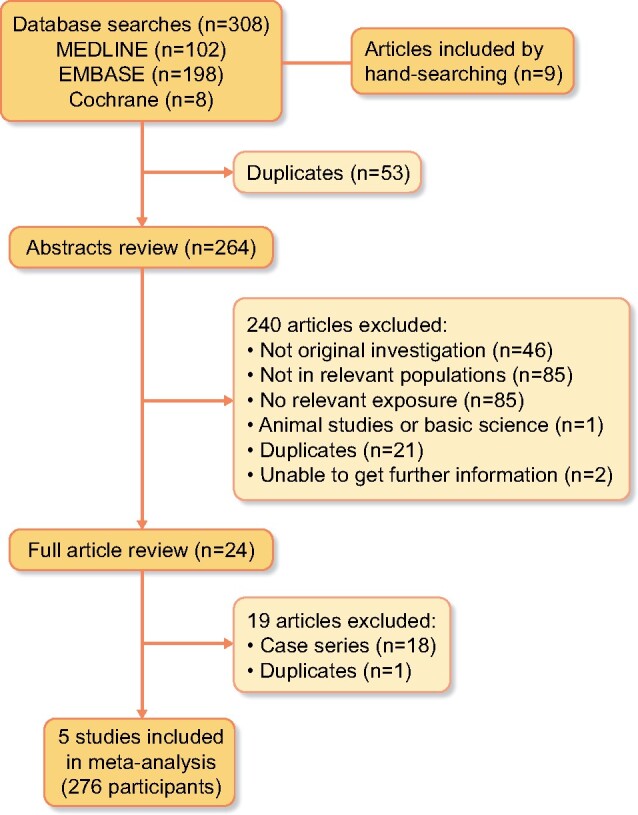
Flow chart of studies considered for inclusion.
Table 1.
Summary of included studies
| Studya | N | Intervention versus control | Study design | Follow-up (months) | Age (years) | Male sex (%) | De novo MM (%) | Previous CKDb (%) | Chemotherapy regimen intervention/ control |
|---|---|---|---|---|---|---|---|---|---|
| Hutchison et al. [20] (2019, UK, Germany) | 90 | HCO-HD versus HF-HD | RCT | 24 | 65.5 | 57 | 100 | 4.4 | BTZ + DOX + DEX/ BTZ + DOX + DEX |
| Bridoux et al. [19] (2017, France) | 98 | HCO-HD versus HF-HD | RCT | 48 | 68.8 | 55 | 100 | 11.7 | BTZ + DEX/BTZ + DEX |
| Gerth et al. [25] (2016, Germany) | 59 | HCO-HD versus HF-HD | Retrospective cohort | 12 | 63.1 | 50.8 | 54 | 27.1 | Novel agentsc 76.2%/novel agents 23.5% |
| Curti et al. [24] (2016, Switzerland) | 19 | HCO-HD versus HF-HD | Retrospective cohort | 72.7 | 63.0 | 73,7 | 83 | NR | BTZ + DEX/BTZ + DEXd |
| Peters et al. [23] (2011, France) | 10 | HCO-HD versus HD | Retrospective cohort | 12.9 | 71 | 50 | 100 | NR | BTZ + DEX/BTZ + DEX or DEX + MPT or DEX |
DOX, doxorubicin; DEX, dexamethasone; MPT, melphalan; NR, not reported.
Year of publication and setting (country or region) provided in parentheses.
CKD is defined as an estimated glomerular filtration rate ≤30 mL/min/1.73 m2.
BTZ, thalidomide or lenalidomide.
Other additional chemotherapy agents were administered to some patients.
All-cause mortality
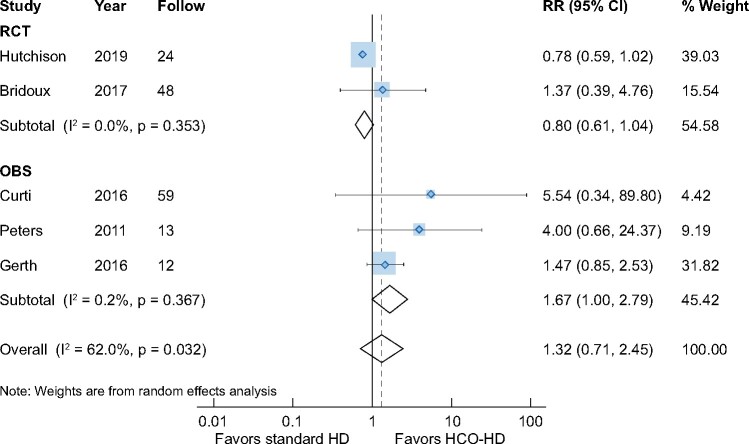
All-cause mortality was reported in all five studies. Compared with patients treated with conventional HD, patients receiving HCO dialysis did not show a survival benefit at the end-of-study timepoint at the average of 34 months [five studies, 276 patients, RR 1.32 (95% CI 0.71–2.45), I2 = 62.0%] (Figure 2). In the subgroup analysis, although survival benefits at the end of the study were seen in the OBSs [three studies, 88 patients, RR 1.67 (95% CI 1.00–2.79), I2 = 0.2%] [23–25], no differences in all-cause mortality were seen in the RCTs [two studies, 188 patients, RR 0.80 (95% CI 0.61–1.04), I2 = 0.0%] [19, 20].
FIGURE 2:
Impact of HCO-HD on all-cause mortality at end-of-study time point.
Patients treated with HCO-HD did not show survival benefits at 3 months [four studies, 266 patients, RR 1.02 (95% CI 10.95–1.09), I2 = 14.8%] or at 12 months [five studies, 276 patients, RR 1.02 (95% CI 0.76–1.35), I2 = 33.9%]. There was no difference in all-cause mortality at 3 and 12 months between HCO dialysis and conventional HD in both the OBSs and RCTs (Supplementary data, Figures S2 and S3).
Renal outcomes: HD independence
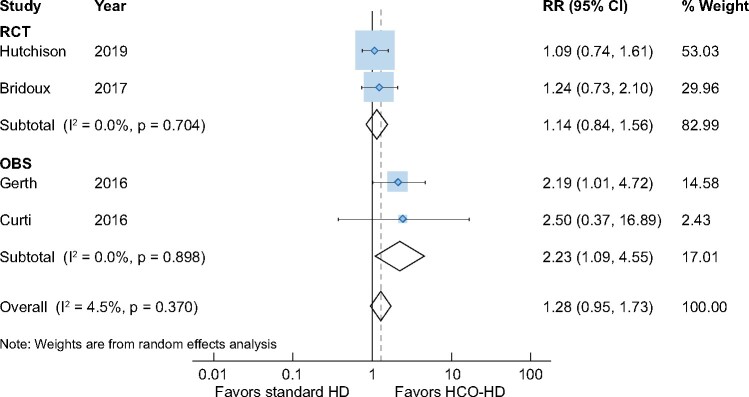
There was no difference in the rates of HD independence at 90 days between HCO-HD and standard-HD patients [four studies, 266 patients, RR 1.28 (95% CI 0.95–1.73), I2 = 4.5%]. Although the pooled data from the OBSs demonstrated significantly higher rates of HD independence at 90 days [two trials, 78 patients, RR 2.23 (95% CI 1.09–4.55), I2 = 0.0%] [24, 25], subgroup analysis including only RCTs showed no differences [two studies, 188 patients, RR 1.14 (95% CI 0.84–1.56), I2 = 0%) [19, 20] (Figure 3).
FIGURE 3:
Impact of HCO-HD on HD independence at 90 days.
Only the two RCTs reported renal outcomes at 6 and 12 months; there was no difference in HD independence at 6 months [two studies, 188 patients, RR 1.19 (95% CI 0.68–2.06), I2 = 71.8%] [19, 20] nor at 12 months [two studies, 188 patients, RR 1.14 (95% CI 0.58–2.26), I2 = 81.3%) [19, 20] between these two therapies, although significant heterogeneity was also found (Supplementary data, Figures S4 and S5).
sFLC reduction and haematological response
Only the included RCTs reported changes in sFLCs post-dialysis. Patients receiving HCO-HD had significant reductions in post-dialysis sFLC for both kappa [two studies, 188 patients, WMD 46.7 (95% CI 38.6–54.7), I2 = 52.0%] (Supplementary data, Figure S6) and lambda [two studies, 188 patients, WMD 50.3 (95% CI 21.4–79.3), I2 = 95.1%] (Supplementary data, Figure S7) light chains, only assessed in the included RCTs [19, 20].
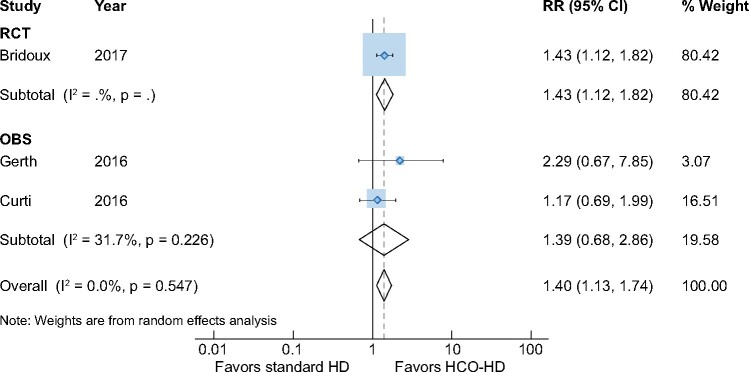
Likewise, a better haematological response was seen at 90 days with HCO dialysis [three studies, 176 patients, RR 1.40 (95% CI 1.13–1.74), I2 = 3.3%] (Figure 4). Subgroup analysis of the OBSs did not show the difference in haematological response between the two dialysis treatments [two studies, 62 patients, RR 1.39 (95% CI 0.68–2.86), I2 = 0%], but the result from the included RCTs demonstrated that HCO dialysis was associated with improved haematological response [one study, 94 patients, RR 1.43 (95% CI 1.12–1.82), I2 = 0%].
FIGURE 4:
Impact of HCO-HD on haematological outcomes at 90 days.
Study quality
Quality assessment is summarized in Supplementary data, Figure S8. Only two of the studies were RCTs. Blinding of the participants and personnel was not deemed possible due to the nature of the intervention. Overall, the majority of the studies were of suboptimal quality, small sample size and/or underpowered.
DISCUSSION
In this systematic review assessing the effect of different dialysis membranes on clinical outcomes in patients with MM complicated by severe AKI due to cast nephropathy, we found that HCO-HD did not confer survival or renal benefits over conventional HD, although a trend towards higher HD independence rates was seen in the HCO-HD group. HCO-HD as an adjuvant treatment, however, yielded better FLC reductions.
Strategies for improving renal function in patients with MM are necessary. As renal impairment is associated with an increased risk of mortality in patients with MM, exploration of different strategies to improve renal outcomes in this population is paramount [3, 26]. In 2007 and 2008, Hutchison et al. [27] proved HCO-HD to be efficient in the removal of sFLC in both in vitro and in vivo studies, suggesting that optimization of the clearance of kappa and lambda sFLC could be a key factor in reversing or preventing renal damage in this population [12, 13]. In this meta-analysis we found a higher reduction of both kappa and lambda light chains in the HCO-HD group. Furthermore, a reduction in FLC levels was also an independent factor for renal and survival benefits, which was shown in three studies [19, 24, 25]. This is consistent with previous publications that postulated an association between sFLC levels and both survival [28–31] and renal recovery [12, 13, 31]. sFLC levels >500 mg/L are currently considered a threshold for cast formation [9, 32], although some authors establish that threshold at up to 1500 mg/L [33–35]. These findings pose the hypothesis of a targeted and tailored use of HCO-HD to achieve renal and patient survival benefits and have already been used as such in one of the included studies [25].
Outcomes in patients with MM have improved in the last decade with proteasome inhibitor–based therapies [36, 37]. Our review found that the addition of HCO-HD in patients with myeloma cast nephropathy–related severe renal impairment was associated with a better haematological response at Day 90 compared with conventional HD [19, 24, 25]. It is important to point out that the better haematological response associated with HCO dialysis is via increased sFLC reduction rather than disease response.
In our review, additional use of HCO-HD in novel chemotherapy regimens also demonstrated a trend towards better renal response in the form of HD independence rates at 3, 6 and 12 months, although the differences did not achieve statistical significance. There are discrepant results regarding renal recovery among the two included RCTs, which is partially due to the fact that the MYRE study did not include patients that recovered renal function after initial measures like hydration, high-dose steroids and correction of hypercalcaemia, resulting in selection of patients with worse renal function.
In our study, HCO-HD was not associated with survival benefits in the short and medium term compared with conventional high flux HD. The longer-term survival outcomes were not able to be assessed due to a lack of data. Again, the inconsistent result on mortality was seen by two RCTs. The EuLITE trial reported a trend towards increased mortality in the HCO-HD group at 3 months and at the end of the study (24 months), while the MYRE study demonstrated a trend towards better survival outcomes at 3 months in the HCO-HD group, although this benefit disappeared in all the longer-term analyses at 12 and 48 months [19, 20]. On the other hand, the pooled data from the three OBSs included in our meta-analysis showed a survival benefit at the end-of-study timepoint [23–25]. This was probably due to the small sample size of the studies and the presence of potential confounding factors in the OBSs, which can have impacts on all-cause mortality. Previous literature demonstrated the association between sFLC levels with mortality and renal impairment. Renal impairment itself is also a well-known independent factor for poorer survival [3, 10]. Therefore better sFLC clearance provided by HCO-HD could potentially have an impact on patient survival, which needs further large RCTs to prove.
The strength of our study is that, to our knowledge, this is the first systematic review assessing the effect of HCO dialysis in patients with severe AKI due to myeloma cast nephropathy. Other strengths include methodological rigor and consideration of multiple relevant outcomes. The findings from our systematic review clearly indicate a need for high-quality RCTs to assess the impact of HCO dialysis in this population.
This study has a few limitations. First, most included studies were observational, therefore the presence of potential bias and confounders may impact interpretation of the results. Second, studies were overall underpowered. In particular, there were limited data on long-term HD independence and kappa/lambda sFLC reduction. We could only analyse mortality at 3 and 12 months, which we consider not long enough to assess long-term renal and patient survival. Third, current RCTs were open-labelled, which could potentially produce selection bias. Conducting a double-blind study assessing the effect of different types of HD membranes is feasible based on the previous studies investigating the efficacy of HCO-HD in septic patients complicated by AKI, although albumin monitoring might be necessary due to higher clearance of albumin associated with HCO-HD and could unmask treatment allocation [38]. Lastly, a notable heterogeneity was found in the characteristics of the patients, specifically the inclusion of relapsed myeloma and unconfirmed cast nephropathy, as well as a substantial difference in treatment received, both chemotherapy and HD regimens. These can have an impact on interpretation of the overall findings.
In conclusion, current evidence from RCTs and OBSs suggests better clearance of sFLCs with HCO dialysis, but no significant differences in mortality or renal outcomes are seen between HCO dialysis and conventional HD for patients with myeloma cast nephropathy–induced AKI. However, there is a trend towards better renal outcomes with HCO dialysis. The lack of long-term data and the small sample sizes of the included studies limit this analysis. Therefore further large-scale double-blinded RCTs with longer follow-up are needed to assess the effect of HCO dialysis on clinical outcomes in patients with MM complicated by cast nephropathy.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Ma Rosario Llópez at the Nephrology Department in Hospital Universitario Puerta de Hierro Majadahonda for her general support and coordination of Dr Blanca Tarragón’s stay at the George Institute for Global Health and Concord Repatriation Geneal Hospital. Dr Amanda Y Wang was supported by National Heart Foundation Post-Doctorate Fellowship.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Cancer Research UK. Myeloma incidence statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/myeloma/incidence#heading-One (20 September 2019, date last accessed)
- 2. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30 [DOI] [PubMed] [Google Scholar]
- 3. Kleber M, Ihorst G, Terhorst M. et al. Comorbidity as a prognostic variable in multiple myeloma: comparative evaluation of common comorbidity scores and use of a novel MM-comorbidity score. Blood Cancer J 2011; 1: e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haynes RJ, Read S, Collins GP. et al. Presentation and survival of patients with severe acute kidney injury and multiple myeloma: a 20-year experience from a single center. Nephrol Dial Transplant 2010; 25: 419–426 [DOI] [PubMed] [Google Scholar]
- 5. Kazandjian D. Multiple myeloma epidemiology and survival: a unique malignancy. Semin Oncol 2016; 43: 676–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Korbet SM, Schwartz MM.. Multiple myeloma. J Am Soc Nephrol 2006; 17: 2533–2545 [DOI] [PubMed] [Google Scholar]
- 7. Dimopoulos MA, Terpos E, Chanan-Khan A. et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol 2010; 28: 4976–4984 [DOI] [PubMed] [Google Scholar]
- 8. Sanders PW. Mechanisms of light chain injury along the tubular nephron. J Am Soc Nephrol 2012; 23: 1777–1781 [DOI] [PubMed] [Google Scholar]
- 9. Hutchison CA, Batuman V, Behrens J. et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol 2012; 8: 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evison F, Sangha J, Yadav P. et al. A population-based study of the impact of dialysis on mortality in multiple myeloma. Br J Haematol 2018; 180: 588–591 [DOI] [PubMed] [Google Scholar]
- 11. Dimopoulos MA, Sonneveld P, Leung N. et al. International Myeloma Working Group recommendations for the diagnosis and management of Myeloma-related renal impairment. J Clin Oncol 2016; 34: 1544–1557 [DOI] [PubMed] [Google Scholar]
- 12. Hutchison CA, Heyne N, Airia P. et al. Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol Dial Transplant 2012; 27: 3823–3828 [DOI] [PubMed] [Google Scholar]
- 13. Leung N, Gertz MA, Zeldenrust SR. et al. Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int 2008; 73: 1282–1288 [DOI] [PubMed] [Google Scholar]
- 14. Heyne N, Denecke B, Guthoff M. et al. Extracorporeal light chain elimination: high cut-off (HCO) hemodialysis parallel to chemotherapy allows for a high proportion of renal recovery in multiple myeloma patients with dialysis-dependent acute kidney injury. Ann Hematol 2012; 91: 729–735 [DOI] [PubMed] [Google Scholar]
- 15. Gondouin B, Hutchison CA.. High cut-off dialysis membranes: current uses and future potential. Adv Chronic Kidney Dis 2011; 18: 180–187 [DOI] [PubMed] [Google Scholar]
- 16. Ward RA. Protein-leaking membranes for hemodialysis: a new class of membranes in search of an application? J Am Soc Nephrol 2005; 16: 2421–2430 [DOI] [PubMed] [Google Scholar]
- 17. Hutchison CA, Cockwell P, Reid S. et al. Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: in vitro and in vivo studies. J Am Soc Nephrol 2007; 18: 886–895 [DOI] [PubMed] [Google Scholar]
- 18. Hutchison CA, Bradwell AR, Cook M. et al. Treatment of acute renal failure secondary to multiple myeloma with chemotherapy and extended high cut-off hemodialysis. Clin J Am Soc Nephrol 2009; 4: 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bridoux F, Carron PL, Pegourie B. et al. Effect of high-cutoff hemodialysis vs. conventional hemodialysis on hemodialysis independence among patients with Myeloma cast nephropathy: a randomized clinical trial. J Am Med Assoc 2017; 318: 2099–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hutchison CA, Cockwell P, Moroz V. et al. High cutoff versus high-flux haemodialysis for myeloma cast nephropathy in patients receiving bortezomib-based chemotherapy (EuLITE): a phase 2 randomised controlled trial. Lancet Haematol 2019; 6: e217–e228 [DOI] [PubMed] [Google Scholar]
- 21. Durie BGM, Harousseau JL, Miguel JS. et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–1473 [DOI] [PubMed] [Google Scholar]
- 22. Wells GO, Peterson D, Welch JV. et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (1 September 2019,. date last accessed)
- 23. Peters NO, Laurain E, Cridlig J. et al. Impact of free light chain hemodialysis in myeloma cast nephropathy: a case-control study. Hemodial Int 2011; 15: 538–545 [DOI] [PubMed] [Google Scholar]
- 24. Curti A, Schwarz A, Trachsler J. et al. Therapeutic efficacy and cost effectiveness of high cut-off dialyzers compared to conventional dialysis in patients with cast nephropathy. PLoS One 2016; 11: e0159942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gerth HU, Pohlen M, Görlich D. et al. Impact of high-cut-off dialysis on renal recovery in dialysis-dependent multiple myeloma patients: results from a case-control study. PLoS One 2016; 11: e0154993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dimopoulos MA, Delimpasi S, Katodritou E. et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol 2014; 25: 195–200 [DOI] [PubMed] [Google Scholar]
- 27. Hutchison CA, Harding S, Mead G. et al. Serum free-light chain removal by high cutoff hemodialysis: optimizing removal and supportive care. Artif Organs 2008; 32: 910–917 [DOI] [PubMed] [Google Scholar]
- 28. Alhaj Moustafa M, Rajkumar SV, Dispenzieri A. et al. Utility of serum free light chain measurements in multiple myeloma patients not achieving complete response to therapy. Leukemia 2015; 29: 2033–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dejoie T, Corre J, Caillon H. et al. Serum free light chains, not urine specimens, should be used to evaluate response in light-chain multiple myeloma. Blood 2016; 128: 2941–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Veas Silva JLG, Guitarte CB, Valladares PM. et al. Prognostic value of serum free light chains measurements in multiple myeloma patients. PLoS One 2016; 11: e0166841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hutchison CA, Cockwell P, Stringer S. et al. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol 2011; 22: 1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yadav P, Sathick IJ, Leung N. et al. Serum free light chain level at diagnosis in myeloma cast nephropathy—a multicenter study. Blood Cancer J 2020; 10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yadav P, Cockwell P, Cook M. et al. Serum free light chain levels and renal function at diagnosis in patients with multiple myeloma. BMC Nephrol 2018; 19: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Drayson M, Begum G, Basu S. et al. Effects of paraprotein heavy and light chain types and free light chain load on survival in myeloma: an analysis of patients receiving conventional-dose chemotherapy in Medical Research Council UK multiple myeloma trials. Blood 2006; 108: 2013–2019 [DOI] [PubMed] [Google Scholar]
- 35. Rajkumar SV, Dimopoulos MA, Palumbo A. et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15: e538–e548 [DOI] [PubMed] [Google Scholar]
- 36. Ludwig H, Adam Z, Hajek R. et al. Light chain-induced acute renal failure can be reversed by bortezomib-doxorubicin-dexamethasone in multiple myeloma: results of a phase II study. J Clin Oncol 2010; 28: 4635–4641 [DOI] [PubMed] [Google Scholar]
- 37. Dimopoulos MA, Roussou M, Gkotzamanidou M. et al. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia 2013; 27: 423–429 [DOI] [PubMed] [Google Scholar]
- 38. Haase M, Bellomo R, Baldwin I. et al. Hemodialysis membrane with a high-molecular-weight cutoff and cytokine levels in sepsis complicated by acute renal failure: a phase 1 randomized trial. Am J Kidney Dis 2007; 50: 296–304 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.