Abstract
Background
Besides advances in haemodialysis (HD), mortality rates are still high. The effect of the different types of HD membranes on survival is still a controversial issue. The aim of this COSMOS (Current management Of Secondary hyperparathyroidism: a Multicentre Observational Study) analysis was to survey, in HD patients, the relationship between the use of conventional low- or high-flux membranes and all-cause and cardiovascular mortality.
Methods
COSMOS is a multicentre, open-cohort, 3-year prospective study, designed to evaluate mineral and bone disorders in the European HD population. The present analysis included 5138 HD patients from 20 European countries, 3502 randomly selected at baseline (68.2%), plus 1636 new patients with <1 year on HD (31.8%) recruited to replace patients who died, were transplanted, switched to peritoneal dialysis or lost to follow-up by other reasons. Cox-regression analysis with time-dependent variables, propensity score matching and the use of an instrumental variable (facility-level analysis) were used.
Results
After adjustments using three different multivariate models, patients treated with high-flux membranes showed a lower all-cause and cardiovascular mortality risks {hazard ratio (HR) = 0.76 [95% confidence interval (CI) 0.61–0.96] and HR = 0.61 (95% CI 0.42–0.87), respectively}, that remained significant after matching by propensity score for all-cause mortality (HR = 0.69, 95% CI 0.52–0.93). However, a facility-level analysis showed no association between the case-mix-adjusted facility percentage of patients dialysed with high-flux membranes and all-cause and cardiovascular mortality.
Conclusions
High-flux dialysis was associated with a lower relative risk of all-cause and cardiovascular mortality. However, dialysis facilities using these dialysis membranes to a greater extent did not show better survival.
Keywords: chronic haemodialysis, dialysis, dialysis membranes, mortality, mortality risk
INTRODUCTION
Haemodialysis (HD) is the most applied treatment for end-stage kidney disease (ESKD). On a worldwide scale ∼2 million ESKD patients are on regular HD [1]. Even though life expectancy in the HD population remains substantially shorter than in the general population, a historical trend for improvement in survival in HD patients has been documented in the ERA-EDTA Registry [2]. This favourable trend may depend on various dialysis-related factors such as the dialysis dose [3], the use of convection and/or diffusion technique, the use of different dialysis membranes such as high- or low-flux membranes [4, 5], the chemical composition and microbiological purity of dialysate [6], sodium and volume profiling and the intradialytic volume monitoring [7]. Among them, the permeability of the dialysis membrane has been considered a critical component of extracorporeal dialysis because more permeable membranes (high flux) allow an efficient removal of middle molecules and toxic small solutes, which have been associated with a longer term survival [8, 9].
Despite the better performance of high-flux membranes in the removal of uraemic toxins, randomized clinical trials have not found an overall benefit of high-flux compared with the low-flux membranes [10–12]. A systematic review from the Cochrane Database including 33 studies and 3820 patients concluded that conventional high-flux HD may reduce cardiovascular mortality but not all-cause mortality [13].
In the absence of definitive clinical trials, large observational studies may provide additional circumstantial evidence on whether conventional high-flux HD is superior to conventional low-flux HD. We have therefore used COSMOS (Current management Of Secondary hyperparathyroidism: a Multicentre Observational Study) [14] to assess the association of conventional high- and low-flux membranes with survival in the European dialysis scenario.
MATERIALS AND METHODS
COSMOS is a 3-year, multicentre, open-cohort, prospective study aiming to survey bone and mineral disturbances in adult chronic HD patients >18 years of age, which also collected valuable information on current clinical practices of HD in Europe including the type of dialysis from 227 dialysis centres of 20 European countries (mean 23.9 patients per centre; median 25) with no previous kidney transplant [14]. Patients and facilities were randomly selected. Data collection began in February 2005 and finished in July 2007. The detailed design of this study has been published previously [14–18]. At baseline, demographics, comorbidities, treatments (including the type of dialysis—conventional high flux or conventional low flux) and laboratory values of the previous 6 months (serum parathyroid hormone, phosphate, calcium, albumin and blood haemoglobin) were collected. Every 6 months during the 3-year follow-up, outcomes, management of patients—including treatments, biochemical parameters of the previous 6 months and additional relevant data—were collected. Average values of the previous 6 months were calculated for biochemical parameters. Patients leaving the study by any reason were replaced by new patients (<1 year on HD). The research was conducted according to the principles of the declaration of Helsinki.
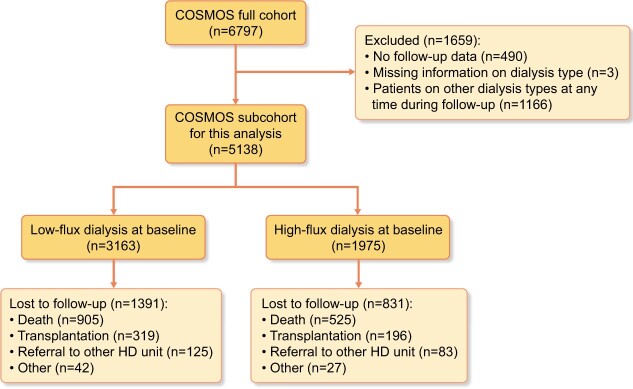
In COSMOS, a total of 6797 patients were recruited, 4500 randomly selected at baseline and 2297 to replace those leaving the study. Patients with no follow-up data, with lacking information on the type of dialysis or dialysed with dialysis techniques other than conventional high and low flux at any time during follow-up (i.e. paired filtration dialysis, haemofiltration, haemodiafiltration, nocturnal daily dialysis and day time daily dialysis), were excluded from the analysis. After exclusions, 5138 patients [3502 (68.2%) randomly selected and 1636 (31.8%) replacements] were available for analysis. More details on the number of patients included/excluded in this study are shown in Figure 1.
FIGURE 1:
Number of patients included and excluded in this study.
The outcomes were all-cause and cardiovascular mortality, and exposure was the type of dialysis membrane (high or low flux) used in conventional HD. The exposure was used as a time-dependent variable (79.3% of patients were always on the same treatment—high flux or low flux—during the whole follow-up period).
Cox’s proportional hazard regression models with time-dependent covariates were used to assess the likely influence of the type of dialysis membrane on survival. Three different multivariate models were used to adjust the relative risk of all-cause and cardiovascular mortality, with a total number of 22 variables in the full model. Model 1: (demographic characteristics and comorbidities) included age, sex, body mass index (BMI), smoking habit, time on HD, aetiology of chronic kidney disease (CKD), diabetes, cardiovascular disease (CVD), parathyroidectomy and calcification (valvular + vascular + calciphylaxis). Model 2: (Model 1 plus management of patients), included the variables of Model 1 plus calcium concentration in the dialysate, hours of HD per week, prescription of erythropoietin-stimulating agents, vitamin D metabolites/analogues (calcitriol, alfacalcidol or paricalcitol), native vitamin D or calcidol, calcimimetics and phosphate-binding agents (PBAs) (calcium-containing PBAs, sevelamer, aluminium-containing PBAs, lanthanum carbonate or other PBAs). Model 3: (Model 2 plus biochemical parameters, full model) included all previous variables plus phosphorus, calcium, parathyroid hormone (PTH), haemoglobin and albumin. The biochemical parameters were categorized as follows: serum phosphorus ≤3.0, 3.0–4.0, 4.0–5.5, 5.5–6.5, >6.5 mg/dL; serum calcium ≤8.5, 8.5–9.0, 9.0–9.5, >9.5 mg/dL; serum PTH ≤50, 50–150, 150–300, 300–500, 500–800, >800 pg/mL; haemoglobin ≤10, 10–11, 11–12, 12–13, >13 g/dL; and serum albumin ≤3.5 and >3.5 g/dL. All variables in Models 2 and 3 as well as BMI in Model 1 were included as time-varying covariates in the multivariate models. In order to take into account potential influences of each centre, all the multivariate models were stratified by centre.
To minimize potential confounding by indication, a propensity score of the likelihood of conventional high-flux HD prescription was calculated at baseline for each patient by using binary logistic regression. This propensity score was used as a covariate for the estimation of the relative risk of mortality of the use of conventional high-flux compared with conventional low-flux dialysis. Additionally, a subcohort of tightly matched exposed and unexposed pairs was selected at baseline. Only pairs of patients with a difference in the propensity score <0.001 were included in this subcohort of patients, as previously described by others [19]. Univariate relative all-cause and cardiovascular mortality risk were calculated in this propensity score-matched subcohort of patients.
A facility-level analysis was also carried out to reduce the effect of unmeasured or unknown confounders [20–22], using the case-mix-adjusted facility percentage of patients on treatment with conventional high-flux HD as instrumental variable. This method is based on a modification of the linear two-stage least squares regression analysis [23]. In the first stage, the case-mix-adjusted percentage of conventional high-flux HD use by facility (instrumental variable) was calculated using a linear regression model with patient conventional high-flux HD treatment (yes/no) as the dependent variable. The independent variables were the facility indicator together with age, sex, time on HD, history of CVD, diabetes and baseline mean serum values of haemoglobin and albumin. The partial F statistic of the coefficient for the effect of instrument (facility) was >10 (F: 35.3), indicating that the instrument is valid [24]. In the second stage, Cox proportional hazard regression analysis was used to estimate relative risk of all-cause and cardiovascular mortality at the patient level, using as exposure the percentage of patients treated with high-flux dialysis calculated in the first stage. Thus, the exposure will be the same for all patients from the same dialysis centre regardless of whether they were treated with high-flux dialysis or not. The relative risk of all-cause and cardiovascular mortality was adjusted using the same multivariate models described above. Additionally, all-cause and cardiovascular mortalities were also assessed in patients from centres with a case-mix-adjusted facility percentage of patients treated with high-flux membranes within the third tertile, using as reference the first tertile [hazard ratio (HR) = 1.0].
In addition, in order to have more specific information related to the reasons to prescribe high- or low-flux dialysis, a multivariate binary logistic regression analysis was performed.
Comparisons between groups were performed with the Student's t-test for continuous variables and the chi-squared test for categorical variables. All statistical analyses were done using R software for statistical computing and graphics version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The main baseline patient characteristics are detailed in Table 1. Overall, the mortality rate was 14.6 deaths per 100 patient-years. At baseline, patients treated with conventional low-flux HD represented 61.6% of the whole cohort, whereas patients treated with conventional high-flux HD constituted the remaining 38.4%. In the latter, we observed more males, younger patients with higher BMI, more smokers, longer vintage and hours of HD per week (Table 1). The propensity score-matched subcohort showed no significant differences in the characteristics of both groups of patients (Table 1). During 3 years of follow-up, 1430 patients died, 515 were transplanted [319 (22.9%) low flux and 196 (23.6%) high flux], 208 referred to other HD units and 69 left the study for other reasons.
Table 1.
Main baseline characteristics of patients included in the study
| Full cohort |
Propensity score matched cohort |
||||||
|---|---|---|---|---|---|---|---|
| All patients (n = 5138) | Low flux (n = 3163) | High flux (n = 1975) | P-value | Low flux (n = 1121) | High flux (n = 1121) | P-value | |
| Males (%) | 3074 (59.8) | 1839 (58.1) | 1235 (62.5) | 0.002 | 676 (60.3) | 679 (60.6) | 0.931 |
| Age [mean (SD)], years | 64.59 (14.38) | 65.26 (14.16) | 63.52 (14.68) | <0.001 | 64.45 (14.00) | 64.42 (14.18) | 0.964 |
| BMI [mean (SD)], kg/m² | 25.28 (5.07) | 25.08 (4.84) | 25.61 (5.41) | <0.001 | 25.23 (4.79) | 25.28 (5.05) | 0.817 |
| Current smokers, % | 702 (13.7) | 402 (12.7) | 300 (15.2) | 0.014 | 163 (14.5) | 165 (14.7) | 0.952 |
| Diabetics, % | 1596 (31.1) | 957 (30.3) | 639 (32.4) | 0.123 | 372 (33.2) | 364 (32.5) | 0.753 |
| CVD history = Yes, % | 3689 (71.9) | 2263 (71.6) | 1426 (72.2) | 0.684 | 810 (72.3) | 817 (72.9) | 0.776 |
| Months on HD [mean (SD)] | 38.20 (49.02) | 34.65 (45.16) | 43.90 (54.17) | <0.001 | 37.44 (46.94) | 37.12 (43.95) | 0.865 |
| Hours of dialysis per week [mean (SD)] | 11.99 (2.13) | 11.84 (2.20) | 12.21 (2.01) | <0.001 | 11.99 (1.91) | 11.95 (1.72) | 0.587 |
| Calcium concentration in dialysate (%) | 0.672 | 0.855 | |||||
| 2.5 mEq/L | 1445 (31.6) | 906 (31.3) | 539 (32.1) | 345 (30.8) | 346 (30.9) | ||
| 3.0 mEq/L | 2221 (48.6) | 1420 (49.1) | 801 (47.7) | 564 (50.3) | 573 (51.1) | ||
| 3.5 mEq/L | 904 (19.8) | 566 (19.6) | 338 (20.1) | 212 (18.9) | 202 (18.0) | ||
| PTH [median (IQR)], pg/mL | 210.00 (109.21, 372.00) | 198.67 (104.00, 351.00) | 229.82 (121.00, 410.50) | <0.001 | 203.00 (106.36, 355.17) | 220.32 (115.00, 379.33) | 0.158 |
| Parathyroidectomy, % | 241 (4.7) | 114 ( 3.6) | 127 ( 6.4) | <0.001 | 42 ( 3.7) | 39 (3.5) | 0.821 |
| Calcium [mean (SD)], mg/dL | 9.06 (0.75) | 9.07 (0.75) | 9.03 (0.75) | 0.080 | 9.07 (0.72) | 9.08 (0.73) | 0.732 |
| Phosphorus [mean (SD)], mg/dL | 5.37 (1.42) | 5.37 (1.43) | 5.38 (1.42) | 0.844 | 5.44 (1.44) | 5.42 (1.44) | 0.760 |
| Albumin [mean (SD)], g/dL | 3.77 (0.48) | 3.76 (0.49) | 3.80 (0.47) | 0.001 | 3.81 (0.50) | 3.80 (0.46) | 0.624 |
| Haemoglobin [mean (SD)], g/dL | 11.38 (1.42) | 11.30 (1.36) | 11.52 (1.51) | <0.001 | 11.46 (1.29) | 11.49 (1.38) | 0.596 |
| Patients treated with PBAs, % | 4354 (84.8) | 2667 (84.4) | 1687 (85.5) | 0.334 | 970 (86.5) | 963 (85.9) | 0.713 |
| Patients treated with VDRAs, % | 2431 (47.4) | 1547 (49.0) | 884 (44.8) | 0.004 | 530 (47.3) | 530 (47.3) | 1.000 |
| Patients treated with calcimimetics, % | 289 (5.7) | 132 (4.2) | 157 (8.0) | <0.001 | 60 (5.4) | 52 (4.6) | 0.497 |
| Patients treated with ESAs, % | 4545 (90.7) | 2785 (90.5) | 1760 (91.1) | 0.532 | 1017 (90.7) | 1024 (91.3) | 0.657 |
VDRAs, vitamin D receptor activators; IQR, interquartile range; ESAs, erythropoietin-stimulating agents.
After multivariate adjustment, patients treated with conventional high-flux HD showed a lower all-cause and cardiovascular mortality relative risk {24% [95% confidence interval (95% CI) 4–39%] and 39% (95% CI 13–58%)}, respectively, in the fully adjusted models (Table 2). Patients treated with conventional high-flux HD showed a lower risk of all-cause mortality in 15 out of the 21 subgroups of patients analysed (Figure 2). After adjustment for propensity score, patients treated with conventional high-flux dialysis also showed a significant lower relative risk of all-cause [0.64 (95% CI 0.51–0.81)] and cardiovascular mortality [0.56 (95% CI 0.39–0.81)] (Table 2). The same association was also found in the propensity score-matched subcohort [0.69 (95% CI 0.52–0.93) and 0.68 (95% CI 0.43–1.08), respectively], although the latter was not statistically significant (Table 2).
Table 2.
Relative all-cause and cardiovascular mortalities in patients prescribed versus not prescribed high-flux HD
| All-cause mortality |
Cardiovascular mortality |
|||||
|---|---|---|---|---|---|---|
| n | HR (95% CI) | P-value | n | HR (95% CI) | P-value | |
| Univariate | 5138 | 0.82 (0.74–0.91) | 0.0002 | 5138 | 0.83 (0.71–0.98) | 0.0270 |
| Model 1 (demographics and comorbidities)a | 5131 | 0.68 (0.56–0.84) | 0.0002 | 5131 | 0.62 (0.45–0.85) | 0.0028 |
| Model 2 (Model 1 + management)a | 4882 | 0.73 (0.59–0.89) | 0.0023 | 5124 | 0.60 (0.43–0.83) | 0.0019 |
| Model 3 (Models 1 + 2 + biochemical parameters)a | 4448 | 0.76 (0.61–0.96) | 0.0218 | 4945 | 0.61 (0.42–0.87) | 0.0063 |
| Adjusted for propensity score (Full cohort)a | 3529 | 0.64 (0.51–0.81) | 0.0002 | 3529 | 0.56 (0.39–0.81) | 0.0021 |
| Propensity score- matched subcohorta | 2242 | 0.69 (0.52–0.93) | 0.0144 | 2242 | 0.68 (0.43–1.08) | 0.1048 |
Stratified by centre.
FIGURE 2:
HRs of unadjusted relative all-cause and cardiovascular mortalities in different subgroups of patients treated with high-flux HD compared with low-flux HD.
In the instrumental variable analysis, the median of the case-mix-adjusted facility percentage of patients treated with conventional high-flux membranes was 25.8%. There were minor differences in patient baseline characteristics among the different tertiles categories for this variable (Table 3). The case-mix-adjusted facility percentage of patients treated with high-flux membranes was not associated with the relative risk of all-cause and cardiovascular mortalities, either when used as a continuous variable (Table 4) or when tertile 3 and 1 were compared (Table 5).
Table 3.
Baseline characteristics of patients by tertiles of case-mix-adjusted facility percentage of high-flux HD prescription
| Tertiles of case-mix-adjusted facility percentage of patients prescribed high flux (median: 25.8%) |
|||
|---|---|---|---|
| ≤1.8% (n = 1779) | 1.8–59.3% (n = 1793) | >59.3% (n = 1523) | |
| Patients prescribed high-flux dialysis, % | 18 (1.0) | 543 (30.3) | 1394 (91.5) |
| Males, % | 1040 (58.5) | 1103 (61.5) | 905 (59.4) |
| Age [mean (SD)], years | 63.88 (14.32) | 65.18 (14.09) | 64.68 (14.80) |
| BMI [mean (SD)], kg/m² | 25.15 (4.90) | 25.33 (5.12) | 25.33 (5.19) |
| Current smokers, % | 224 (12.6) | 272 (15.2) | 203 (13.3) |
| Diabetics, % | 510 (28.7) | 594 (33.1) | 475 (31.2) |
| CVD history = Yes, % | 1284 (72.3) | 1256 (70.1) | 1117 (73.3) |
| HD [mean (SD)], months | 34.55 (43.87) | 37.74 (50.32) | 43.04 (52.82) |
| Hours of dialysis per week [mean (SD)] | 11.96 (2.09) | 11.90 (2.43) | 12.14 (1.77) |
| Calcium concentration in dialysate, % | |||
| 2.5 mEq/L | 514 (32.3) | 544 (31.9) | 367 (29.8) |
| 3.0 mEq/L | 875 (55.1) | 702 (41.2) | 643 (52.1) |
| 3.5 mEq/L | 200 (12.6) | 459 (26.9) | 223 (18.1) |
| PTH [median (IQR)], pg/mL | 215.63 (111.38, 378.00) | 198.00 (106.00, 340.00) | 224.96 (113.62, 413.12) |
| Parathyroidectomy, % | 64 (3.6) | 89 (5.0) | 88 (5.8) |
| Calcium [mean (SD)], mg/dL | 9.02 (0.78) | 9.11 (0.66) | 9.02 (0.79) |
| Phosphorus [mean (SD)], mg/dL | 5.51 (1.51) | 5.28 (1.31) | 5.31 (1.43) |
| Albumin [mean (SD)], g/dL | 3.81 (0.49) | 3.73 (0.47) | 3.80 (0.47) |
| Haemoglobin [mean (SD)] , g/dL | 11.13 (1.36) | 11.53 (1.29) | 11.49 (1.57) |
| Patients treated with PBAs, % | 1557 (87.7) | 1468 (81.9) | 1297 (85.2) |
| Patients treated with VDRAs, % | 890 (50.2) | 872 (48.7) | 656 (43.1) |
| Patients treated with calcimimetics, % | 75 (4.3) | 103 (5.7) | 108 (7.1) |
| Patients treated with ESAs, % | 1571 (90.5) | 1584 (91.2) | 1365 (90.2) |
VDRAs, vitamin D receptor activators; IQR, interquartile range; ESAs, erythropoietin-stimulating agents.
Table 4.
All-cause and cardiovascular mortality per every 10% increase in the case-mix-adjusted facility percentage of high-flux HD prescription
| All-cause mortality |
Cardiovascular mortality |
||||
|---|---|---|---|---|---|
| n | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Univariate | 5095 | 1.00 (0.99–1.02) | 0.6565 | 1.00 (0.98–1.02) | 0.8890 |
| Model 1 (demographics and comorbidities) | 5088 | 1.00 (0.99–1.01) | 0.8596 | 0.99 (0.97–1.01) | 0.4746 |
| Model 2 (Model 1 + management) | 4839 | 1.00 (0.99–1.02) | 0.8127 | 1.00 (0.98–1.02) | 0.8000 |
| Model 3 (Models 1 + 2 + biochemical parameters) | 4427 | 1.01 (1.00–1.03) | 0.1611 | 1.00 (0.98–1.03) | 0.8321 |
Table 5.
All-cause and cardiovascular mortality in patients from centres within the third tertile of case-mix-adjusted facility percentage of patients treated with high-flux membranes (>59.3%) compared with the first tertile (≤1.8%)
| All-cause mortality |
Cardiovascular mortality |
||||
|---|---|---|---|---|---|
| n | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Univariate | 3302 | 0.99 (0.87–1.13) | 0.9020 | 0.94 (0.77–1.14) | 0.5399 |
| Model 1 (demographics and comorbidities) | 3295 | 0.96 (0.84–1.09) | 0.5308 | 0.90 (0.74–1.10) | 0.3175 |
| Model 2 (Model 1 + management) | 3084 | 0.99 (0.86–1.13) | 0.8544 | 0.93 (0.76–1.15) | 0.5283 |
| Model 3 (Models 1 + 2 + biochemical parameters) | 2813 | 1.10 (0.94–1.28) | 0.2250 | 0.99 (0.79–1.25) | 0.9548 |
The binary logistic regression analysis suggests that younger patients and patients with a higher BMI were preferentially prescribed high-flux dialysis (Supplementary data, Table S1).
DISCUSSION
In COSMOS, an observational prospective study representative of the European HD population, the use of high-flux membranes was associated after several analyses (univariate, multivariate adjustments and propensity score—full cohort and matched subcohort) with a lower all-cause and cardiovascular mortality risk. However, the instrumental variable analysis (facility level) showed no association with mortality risk.
High-flux membranes have been found to be more efficient in the removal of middle-size molecules including β2 microglobulin, lowering complications attributed to β2 microglobulin-mediated amyloidosis such as carpal tunnel syndrome, dialysis-associated arthropathy and mortality [25–27]. Due to the better performance of high-flux membranes in the removal of uraemic toxins, it is a subject of discussion whether they might have an impact on hard outcomes such as all-cause and cardiovascular mortality.
Two large randomized clinical trials with different designs, the Haemodialysis Study Group (HEMO) and the Membrane Permeability Outcome Study (MPO), addressed this important topic. The HEMO study, which included patients on dialysis for at least 3 months, showed no significant differences in survival between users of high- and low-flux membranes [10]. However, several secondary analysis of the study showed benefits of high-flux membranes in the risk of death from cardiac causes [28], cerebrovascular accidents [29] and infectious diseases [25, 30]. Other post hoc analyses also showed benefits in the group of long-term dialysis patients (>3.7 years of maintenance dialysis) [31]. In agreement with this post hoc result from the HEMO study, in the present COSMOS analysis, the association between the use of high-flux membranes and mortality risk seemed to be stronger in patients on HD for >5 years (Figure 2), although this association was not found at the facility-level analysis (data not shown).
The MPO trial included incident patients and after a follow-up period of 3–7.5 years, no survival benefit was found with the use of high-flux membranes in the overall population, but a 37% survival benefit was found in patients with serum albumin ≤4 g/dL and a longer follow-up [11], which are consistent with the HEMO and COSMOS results. In addition, a post hoc analysis of MPO showed benefits of high-flux membranes in diabetic patients. A possible influence of low serum albumin was also observed in COSMOS as the use of high-flux membranes was associated with a lower all-cause and cardiovascular mortality risk in patients with a baseline serum albumin <3.5 mg/dL (Figure 2), whereas for serum albumin >3.5 mg/dL, the effect was observed only for all-cause mortality.
Data of the DOPPS study showed that the use of conventional high-flux membranes was not associated with improved outcomes in a population with a mean serum albumin of 3.96 g/dL and a lower percentage of diabetic patients (18.7% in the low-flux group versus 21.3% in the high-flux group) compared with 24.2% of diabetics in the MPO study [32]. A sub-analysis of the Die Deutsche Diabetes Dialyse Studie—a study in which all patients were diabetics—an association between conventional high-flux membranes and survival was observed [33]. On the contrary, in the present COSMOS analysis, the use of high-flux membranes showed benefits in all-cause mortality—not corroborated by the instrumental variable analysis, but independent of important comorbidities such as diabetes, history of CVD and low serum albumin (Figure 2).
Several other observational studies have also shown advantages in the relative risk of mortality with high-flux membranes. A large database (US Renal Data System registry), which included nearly 14 000 HD patients, found a 24% lower relative risk of mortality in patients treated with high-flux dialysis membranes [34]. Similarly, a survival benefit of 38% in the patients on conventional high-flux dialysis was found in a French observational cohort of 650 patients [35]. Other studies showed advantages linked to 24 h-residual urine volume [36], and to greater vitamin B12 clearance [37]; in COSMOS, these specific aspects were not collected.
Finally, a review and meta-analysis published in the Cochrane Database of Systematic Reviews [13] found that the use of conventional high-flux membranes was associated with lower cardiovascular mortality (5 studies, 2612 patients) but not with all-cause mortality (10 studies, 2915 patients). The meta-analysis showed that conventional high-flux membranes were more efficient removing middle molecules (i.e. β2 microglobulin), but the effect on hospitalization, quality of life, carpal tunnel syndrome and amyloid-related arthropathy was not reliably estimated.
In summary, in COSMOS, after multivariate adjustment, propensity score matching and sensitivity analysis, the results of this study are in agreement with several of the above studies, mainly those that are observational. The main limitation of observational studies, including this one, is that confounding cannot be ruled out using these statistical strategies [22, 38]. In this study, confounding by indication may have had a special relevance as a patient’s life expectancy could have influenced the prescription of high-flux dialysis. In fact, a multivariate binary logistic regression analysis (Supplementary data, Table S1) showed that lower age and higher BMI were independently associated with the use of high-flux dialysis.
The instrumental variable method, also used in this study, mimics to some extent a randomized clinical trial and it is used to control unmeasured confounders [22]. In COSMOS, the facility-level analysis (instrumental variable) used as instrument the case-mix-adjusted facility percentage of patients prescribed high-flux. If the use of high-flux dialysis had had an effect on better survival, those facilities using these dialysis membranes in a higher percentage of patients should have had a lower mortality rate. However, in agreement with the two clinical trials published in which high-flux dialysis did not improve survival, no association was found between the case-mix-adjusted facility percentage of patients using high-flux dialysis and all-cause/cardiovascular mortality. The discordance of the facility-level analysis (instrumental variable) with the other methods used could be partly explained by residual confounding due to unmeasured variables such as residual renal function or vascular access type.
The main limitation of the study is that is observational with a ‘bone and mineral’-oriented design and some other variables such as dialysis membrane specifications were not collected in COSMOS, contributing to residual confounding. However, it has a great strength, which is its solid and careful prospective design truly representing the European HD population.
In conclusion, despite univariate, multivariate and propensity score analysis showing that high-flux was associated with a lower all-cause and cardiovascular mortality, a facility-level analysis showed that those facilities using high-flux dialysis in a higher proportion of patients did not show better survival.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the COSMOS participating centres and the group of persons who have collaborated at any stage in COSMOS: José Luis Motellón, Matthew Turner, Julien Chaussy, Bart Molemans, Wal Zani, Dylan Rosser, Bastian Dehmel, Bruno Fouqueray, Brian Bradbury, John Acquavella, Jennifer Hollowell, Dave Carter, Phil Holland, Ana Baños, Caroline Mattin, Cathy Critchlow, Joseph Kim, Charlotte Lewis, Antonia Panayi, Margit Hemetsberger, Stephen Croft, Philippe Jaeger, Prisca Muehlebach, Jane Blackburn, Esther Zumsteg, Andrey Gurevich, Silvia Rodríguez, Angel Pérez, Pau Faner, Irantzu Izco, Susana Traseira, Carmen Castro, Javier Moreno, David Calle and Francesca Pieraccini.
FUNDING
COSMOS is sponsored by the Bone and Mineral Research Unit (Hospital Universitario Central de Asturias), SAFIM (Sociedad Asturiana Fomento Investigaciones Óseas), the European Renal Association–European Dialysis and Transplant Association, the National Program of I + D + I 2008–2011 and Instituto de Salud Carlos III (ISCIII), the ISCIII Retic REDinREN (RD06/0016/1013, RD12/0021/0023 and RD16/0009/0017), the ISCIII (ICI14/00107, PI17/00384 and PI20/00633), Fondo Europeo de Desarrollo Regional (FEDER), Plan Estatal de I + D + I 2013–2016, Plan de Ciencia, Tecnología e Innovación 2013–2017 y 2018–2022 del Principado de Asturias (GRUPIN14-028, IDI-2018-000152), Fundación Renal Íñigo Álvarez de Toledo (FRIAT) and the Spanish Society of Nephrology (Estudio Estratégico de la SEN). Logistics (meetings, secretarial help, printing of materials, development of website for data entry, etc.) have been financially supported by AMGEN Europe and FRIAT. The authors are not aware of any additional relationships, funding or financial holdings that might be perceived as affecting the objectivity of this study. COSMOS participating centres: see Supplementary Appendix.
AUTHORS’ CONTRIBUTIONS
F.L., J.F., M.K., G.L., J.L.G., B.R., A.F., D.P., J.B.C.-A. and J.L.F.-M. were involved in conception and study design; E.S.-Á., M.R.-G., F.L., C.Z., A.M.-M., J.B.C.-A. and J.L.F.-M. were involved analysis design; E.S.-Á., M.R.-G., C.Z., J.B.C.-A. and J.L.F.-M. were involved in statistical analysis; E.S.-Á., M.R.-G., F.L., C.Z., A.M.-M., J.F., A.F., J.B.C.-A. and J.L.F.-M. were involved in interpretation of results; E.S.-Á., M.R.-G., F.L., J.B.C.-A. and J.L.F.-M. contributed to draft writing; E.S.-Á., M.R.-G., F.L., C.Z., A.M.-M., J.F., M.K., G.L., J.L.G., B.R., A.F., D.P., J.B.C.-A. and J.L.F.-M. were involved in manuscript revision; J.B.C.-A. and J.L.F.-M. were involved in acquisition of funding.
CONFLICT OF INTEREST STATEMENT
E.S.-Á. (none), M.R.-G. (none), F.L. (grants and non-financial support from Fresenius Medical Care, personal fees from Baxter and B. Braum), C.Z. (none), A.M.-M. (personal fees from Medtronic, Vifor Pharma, Astellas and AstraZeneca), J.F. (fees from Fresenius Medical Care), M.K. (fees from Fresenius Medical Care), G.L. (none), J.L.G. (none), B.R. (none), A.F. (none), D.P. (none), J.B.C.-A. (grants from Ministerio de Economía y Competitividad/Instituto de Salud Carlos III, grants from Gobierno del Principado de Asturias, grants from Sociedad Española de Nefrología, grants from Fundación Renal Íñigo Álvarez de Toledo, other from European Renal Association–European Dialysis and Transplant Association, grants from Amgen Europe), J.L.F.-M. (grants from Ministerio de Economía y Competitividad/Instituto de Salud Carlos III).
The results presented in this article have not been published previously in whole or part, except in abstract format.
Contributor Information
the COSMOS group:
José Luis Motellón, Matthew Turner, Julien Chaussy, Bart Molemans, Wal Zani, Dylan Rosser, Bastian Dehmel, Bruno Fouqueray, Brian Bradbury, John Acquavella, Jennifer Hollowell, Dave Carter, Phil Holland, Ana Baños, Caroline Mattin, Cathy Critchlow, Joseph Kim, Charlotte Lewis, Antonia Panayi, Margit Hemetsberger, Stephen Croft, Philippe Jaeger, Prisca Muehlebach, Jane Blackburn, Esther Zumsteg, Andrey Gurevich, Silvia Rodríguez, Angel Pérez, Pau Faner, Irantzu Izco, Susana Traseira, Carmen Castro, Javier Moreno, David Calle, and Francesca Pieraccini
REFERENCES
- 1. Robinson BM, Akizawa T, Jager KJ. et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 2016; 388: 294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kramer A, Stel V, Zoccali C. et al. An update on renal replacement therapy in Europe: ERA-EDTA registry data from 1997 to 2006. Nephrol Dial Transplant 2009; 24: 3557–3566 [DOI] [PubMed] [Google Scholar]
- 3. Lertdumrongluk P, Streja E, Rhee CM. et al. Dose of hemodialysis and survival: a marginal structural model analysis. Am J Nephrol 2014; 39: 383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panichi V, Rizza GM, Paoletti S. et al. ; on behalf of the RISCAVID Study Group. Chronic inflammation and mortality in haemodialysis: effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol Dial Transplant 2008; 23: 2337–2343 [DOI] [PubMed] [Google Scholar]
- 5. Locatelli F, Karaboyas A, Pisoni RL. et al. Mortality risk in patients on hemodiafiltration versus hemodialysis: a ‘real-world’ comparison from the DOPPS. Nephrol Dial Transplant 2017; 33: 683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di Iorio B, Di Micco L, Bruzzese D. et al. Ultrapure dialysis water obtained with additional ultrafilter may reduce inflammation in patients on hemodialysis. J Nephrol 2017; 30: 795–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song JH, Park GH, Lee SY. et al. Effect of sodium balance and the combination of ultrafiltration profile during sodium profiling hemodialysis on the maintenance of the quality of dialysis and sodium and fluid balances. J Am Soc Nephrol 2005; 16: 237–246 [DOI] [PubMed] [Google Scholar]
- 8. Vanholder R, Smet RD, Glorieux G. et al. Survival of hemodialysis patients and uremic toxin removal. Artif Organs 2003; 27: 218–223 [DOI] [PubMed] [Google Scholar]
- 9. Massy ZA, Liabeuf S.. Middle-molecule uremic toxins and outcomes in chronic kidney disease. Contrib Nephrol 2017; 191: 8–17 [DOI] [PubMed] [Google Scholar]
- 10. Eknoyan G, Beck GJ, Cheung AK. et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 2002; 347: 2010–2019 [DOI] [PubMed] [Google Scholar]
- 11. Locatelli F, Martin-Malo A, Hannedouche T. et al. Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol 2009; 20: 645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Asci G, Tz H, Ozkahya M. et al. ; EGE Study Group. The impact of membrane permeability and dialysate purity on cardiovascular outcomes. J Am Soc Nephrol 2013; 24: 1014–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palmer SC, Rabindranath KS, Craig JC. et al. High-flux versus low-flux membranes for end-stage kidney disease. Cochrane Database Syst Rev 2012; 2012: CD005016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cannata-Andia JB, Fernandez-Martin JL, Zoccali C. et al. Current management of secondary hyperparathyroidism: a multicenter observational study (COSMOS). J Nephrol 2008; 21: 290–298 [PubMed] [Google Scholar]
- 15. Fernandez-Martin JL, Carrero JJ, Benedik M. et al. COSMOS: the dialysis scenario of CKD-MBD in Europe. Nephrol Dial Transplant 2013; 28: 1922–1935 [DOI] [PubMed] [Google Scholar]
- 16. Cannata-Andia JB, Fernandez-Martin JL, Locatelli F. et al. Use of phosphate-binding agents is associated with a lower risk of mortality. Kidney Int 2013; 84: 998–1008 [DOI] [PubMed] [Google Scholar]
- 17. Fernandez-Martin JL, Martinez-Camblor P, Dionisi MP. et al. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant 2015; 30: 1542–1551 [DOI] [PubMed] [Google Scholar]
- 18. Fernandez-Martin JL, Dusso A, Martinez-Camblor P. et al. ; COSMOS group. Serum phosphate optimal timing and range associated with patients survival in haemodialysis: the COSMOS study. Nephrol Dial Transplant 2019; 34: 673–681 [DOI] [PubMed] [Google Scholar]
- 19. Winkelmayer WC, Liu J, Kestenbaum B.. Comparative effectiveness of calcium-containing phosphate binders in incident U.S. dialysis patients. Clin J Am Soc Nephrol 2011; 6: 175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tentori F, Albert JM, Young EW. et al. The survival advantage for haemodialysis patients taking vitamin D is questioned: findings from the dialysis outcomes and practice patterns study. Nephrol Dial Transplant 2008; 24: 963–972 [DOI] [PubMed] [Google Scholar]
- 21. Lopes AA, Tong L, Thumma J. et al. Phosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): evaluation of possible confounding by nutritional status. Am J Kidney Dis 2012; 60: 90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stel VS, Dekker FW, Zoccali C et al. Instrumental variable analysis. Nephrol Dial Transplant 2013; 28: 1694–1699 [DOI] [PubMed] [Google Scholar]
- 23. Karaboyas A, Zee J, Brunelli SM. et al. Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: results from the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis 2017; 69: 266–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Staiger D, Stock JH.. Instrumental variables regression with weak instruments. Econometrica 1997; 65: 557–586 [Google Scholar]
- 25. Cheung AK, Rocco MV, Yan G. et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol 2006; 17: 546–555 [DOI] [PubMed] [Google Scholar]
- 26. van Ypersele de Strihou C, Jadoul M, Malghem J. et al. Effect of dialysis membrane and patient's age on signs of dialysis-related amyloidosis. The working party on dialysis amyloidosis. Kidney Int 1991; 39: 1012–1019 [DOI] [PubMed] [Google Scholar]
- 27. Kuchle C, Fricke H, Held E. et al. High-flux hemodialysis postpones clinical manifestation of dialysis-related amyloidosis. Am J Nephrol 1996; 16: 484–488 [DOI] [PubMed] [Google Scholar]
- 28. Cheung AK, Sarnak MJ, Yan G.. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int 2004; 65: 2380–2389 [DOI] [PubMed] [Google Scholar]
- 29. Delmez JA, Yan G, Bailey J. et al. Cerebrovascular disease in maintenance hemodialysis patients: results of the HEMO study. Am J Kidney Dis 2006; 47: 131–138 [DOI] [PubMed] [Google Scholar]
- 30. Cheung AK, Greene T, Leypoldt JK. et al. Association between serum 2-microglobulin level and infectious mortality in hemodialysis patients. Clin J Am Soc Nephrol 2008; 3: 69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheung AK, Levin NW, Greene T. et al. Effects of high-flux hemodialysis on clinical outcomes: results of the HEMO study. J Am Soc Nephrol 2003; 14: 3251–3263 [DOI] [PubMed] [Google Scholar]
- 32. Canaud B, Bragg-Gresham JL, Marshall MR. et al. Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int 2006; 69: 2087–2093 [DOI] [PubMed] [Google Scholar]
- 33. Krane V, Krieter DH, Olschewski M. et al. Dialyzer membrane characteristics and outcome of patients with type 2 diabetes on maintenance hemodialysis. Am J Kidney Dis 2007; 49: 267–275 [DOI] [PubMed] [Google Scholar]
- 34. Port FK, Wolfe RA, Hulbert-Shearon TE. et al. Mortality risk by hemodialyzer reuse practice and dialyzer membrane characteristics: results from the USRDS dialysis morbidity and mortality study. Am J Kidney Dis 2001; 37: 276–286 [DOI] [PubMed] [Google Scholar]
- 35. Chauveau P, Nguyen H, Combe C. et al. Dialyzer membrane permeability and survival in hemodialysis patients. Am J Kidney Dis 2005; 45: 565–571 [DOI] [PubMed] [Google Scholar]
- 36. Kim HW, Kim SH, Kim YO. et al. Comparison of the impact of high-flux dialysis on mortality in hemodialysis patients with and without residual renal function. PLoS One 2014; 9: e97184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leypoldt JK, Cheung AK, Carroll CE. et al. Effect of dialysis membranes and middle molecule removal on chronic hemodialysis patient survival. Am J Kidney Dis 1999; 33: 349–355 [DOI] [PubMed] [Google Scholar]
- 38. King G, Nielsen R.. Why propensity scores should not be used for matching. Polit Anal 2019; 27: 435–454 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.