Abstract
Background
Sodium zirconium cyclosilicate (SZC; formerly ZS-9) is a potassium (K+) binder for treatment of hyperkalemia in adults. SZC binds K+ in exchange for sodium (Na+) or hydrogen (H+) in the gastrointestinal tract, conveying potential for systemic absorption of Na+.
Methods
This single-center Phase 1 study evaluated the effects of SZC on Na+ and K+ excretion in healthy, normokalemic adults. During an initial run-in period (Days 1–2), participants started a high K+/low Na+ diet. After baseline (Days 3–4), SCZ 5 or 10 g once daily (QD) was administered (Days 5–8). The primary endpoint was mean change in urinary Na+ excretion from baseline (Days 3–4) to the treatment period (Days 7–8).
Results
Of 32 enrolled participants, 30 entered and completed the study; the first 15 received 5 g and the next 15 received 10 g. Nonsignificant changes from baseline in urinary Na+ excretion were observed with SZC 5 g (mean ± SD −0.93 ± 25.85 mmol/24 h) and 10 g (−5.47 ± 13.90 mmol/24 h). Statistically significant decreases from baseline in urinary K+ excretion (mean ± SD −21.17 ± 21.26 mmol/24 h; P = 0.0017) and serum K+ concentration (−0.25 ± 0.24 mmol/L; P = 0.0014) were observed with the 10-g dose. There were few adverse events and no clinically meaningful changes in vital signs or laboratory safety measures.
Conclusions
Treatment with SZC 5 or 10 g QD reduced serum K+ concentration and urinary K+ excretion, with no significant effect on urinary Na+ excretion, and was well tolerated.
Keywords: excretion, hyperkalemia, Phase 1 study, potassium, sodium, sodium zirconium cyclosilicate
INTRODUCTION
Hyperkalemia or elevated serum potassium (K+) concentrations may occur because of a disruption in K+ homeostasis triggered by a combination of factors, including excessive dietary K+ intake, insufficient K+ excretion, use of certain medications or abnormal distribution of intracellular and extracellular K+ [1, 2]. Hyperkalemia is frequent among patients with chronic kidney disease, heart failure, Type 2 diabetes and hypertension [2, 3]. In addition to the increased potential for life-threatening cardiac arrhythmias in patients with severe hyperkalemia (6.0 mmol/L) [4], large retrospective cohort studies have demonstrated an increased risk of mortality even with mild hyperkalemia in various patient populations [5–7].
K+-binding agents that entrap K+ ions in the gastrointestinal (GI) tract and increase K+ elimination from the body have shown promise for treating hyperkalemia. K+ binders, including sodium or calcium polystyrene sulfonate, patiromer and sodium zirconium cyclosilicate (SZC; formerly ZS-9), form bound K+ complexes that move through the GI tract and are excreted in the feces [8]. Notably, sodium polystyrene sulfonate (SPS) and patiromer are nonspecific and may also bind other electrolytes, including magnesium and/or calcium. SPS and patiromer may also take several hours to have an effect because their primary site of action is in the colon, where K+ concentrations are highest [8, 9]. SPS use is also limited by the potential for increased sodium (Na+) load and serious GI adverse events (AEs), such as ischemic colitis, colonic necrosis and intestinal perforation [8].
SZC (chemical formula Na∼1.5H∼0.5ZrSi3O9·2–3H2O) is an orally administered, inorganic, nonabsorbed, selective K+ binder that is currently approved for the treatment of hyperkalemia in adults in the USA and European Union [10, 11]. The zirconium cyclosilicate ring of SZC exchanges the initially bound hydrogen (H+) or Na+ ions for K+ and ammonium () in a 1:1 ratio through a specific interaction driven both by cation charge and the size of the nonhydrated cation [12]. SZC has the potential to bind K+ through the entire GI tract [12]. One key clinical concern of SZC treatment is how much Na+ is released during K+ exchange and systemically absorbed or excreted in the urine.
This Phase 1 study evaluated the effects of SZC 5 or 10 g once daily (QD) administration >4 days on the excretion of Na+, K+ and other electrolytes in healthy adults. To maximize detection of Na+ following release from SZC and to ensure safe concentration of K+ during SZC administration, study participants were given a standardized diet with more K+ and less Na+ than the typical US diet [13].
MATERIALS AND METHODS
Study design
The study was conducted at Riverside Clinical Research (Edgewater, FL, USA). The study protocol was approved by the institutional review board (Liberty IRB, now Advarra) at the study investigational center, and the study was performed in accordance with regulations governing clinical trials, including the declaration of Helsinki. All participants provided written informed consent prior to study entry.
This Phase 1, single-center, nonblinded, inpatient study in healthy adults assigned participants to receive either SZC 5 g QD (first 15 participants enrolled) or 10 g QD (next 15 participants enrolled; Figure 1).
FIGURE 1:
Study design.
aParticipants began a standardized diet of 920 mg or 40 mmol (±10%) Na+ and 5005 mg or 128 mmol (±10%) K+ per day (continued until study Day 9). bSZC was administered to two dose groups (5 or 10 g QD). cParticipants discharged from clinic at study Day 9. dDefined as nothing by mouth except water for ≥8 h prior to collection of any sample.
Enrolled participants entered the clinical facility on the morning of study Day 1 after fasting for ≥8 h and remained at the clinic until study Day 9. During a 2-day run-in period (study Days 1–2), participants started a standardized diet that was continued through study Day 8. After a 2-day baseline period (study Days 3–4), participants received SZC 5 or 10 g QD for 4 days (study Days 5–8) and were discharged from the clinic on study Day 9 (Figure 1).
Study participants
The study included healthy adults (aged ≥18 years) with a history of daily defecation and the ability to undergo repeated blood sample collection or venous catheterization. Women of childbearing potential were required to have a negative pregnancy test within 1 day before starting the standardized diet, and sexually active women of childbearing potential were required to use two forms of medically acceptable contraception, including at least one barrier method.
Major exclusion criteria included history of constipation or erratic bowel transit time (>48 h) or history of any irregular bowel movements, including unexplained diarrhea; any condition which, according to the investigator, placed the individual at undue risk or potentially jeopardized the quality of generated data; and use of a drug or device within the last 30 days that had not received regulatory approval at the time of study entry. Individuals were also excluded for the use of concomitant medications that might have potentially confounded the study results, including dietary supplements and vitamins, without approval of the medical monitor. Women who were pregnant, lactating or planning on becoming pregnant, as well as individuals with known hypersensitivity or previous anaphylaxis to SZC or any of its components, were not permitted to participate in the study.
Study endpoints
The primary endpoint was mean change in urinary Na+ excretion from baseline (study Days 3–4; 48-h collection) to the SZC treatment period (study Days 7–8; 48-h collection). Secondary endpoints included mean change in cumulative urinary K+ excretion and serum K+ concentration (central laboratory) from baseline to the SZC treatment period (study Days 7–8). Safety assessments, including vital signs (blood pressure, body temperature, heart rate), body weight, standard hematologic laboratory values (hemoglobin, hematocrit, blood cell counts), spontaneously reported AEs and laboratory safety, were also recorded. Additional endpoints included mean change in fecal Na+ and K+ excretion, urinary pH and urinary creatinine excretion from baseline to the SZC treatment period (study Days 7–8).
Standardized diet
During the 9-day study period, study participants were required to consume a standardized high K+/low Na+ diet containing 920 mg or 40 mmol (±10%) Na+ and 5005 mg or 128 mmol (±10%) K+ per day from study Day 1 until study Day 8. The standardized diet was developed by a registered dietitian and designed to provide 300 mg or 13.1 mmol (±15%) Na+ and 1200 mg or 30.7 mmol (± 15%) K+ per meal across breakfast, lunch and dinner, with additional Na+ and K+ from between-meal snacks and beverages. Each 5 g dose of SZC contains ∼400 mg (17.4 mmol) of Na+ [11]. According to National Health and Nutrition Examination Survey 2017–18 data, US adults (aged ≥20 years) typically consume an average of 3531 mg or 154 mmol of Na+ and 2618 mg or 67 mmol of K+ per day [13]. Therefore, compared with a typical US diet, the standardized diet had very low Na+ content to maximize detection of Na+ release from SZC and any changes in urinary and fecal Na+ excretion and high K+ content to minimize the risk of hypokalemia.
All meals were prepared and provided to the participants at the study site, where the weight of food and volume of beverages provided and of any items not consumed were recorded. The total daily intake of Na+ and K+ was calculated for each participant and recorded in the electronic case report form.
Clinical laboratory evaluations
Blood samples were collected under fasting conditions on study Day 1 of the 2-day run-in period, after which the standardized diet was started. During the subsequent 2-day baseline period (study Days 3–4), urinary and fecal Na+ and K+ excretion was measured by collecting two 24-h samples. During the treatment period, urinary and fecal excretion of electrolytes was assessed by collecting two 24-h samples on study Days 7 and 8. Urinary and fecal excretion of Na+ and K+ and urinary pH and creatinine excretion were measured from each 24-h collection period (study Days 3, 4, 7 and 8). Fasting serum K+ was also measured each day by central laboratory and the point-of-care device i-STAT (Abbott Point of Care, Inc., Princeton, NJ, USA).
Statistical analysis
All calculations were performed using SAS statistical software, version 9.1 or later, and StatXact, version 10 or later. Because the study was powered only for within-treatment comparisons, comparison of the effect between the two dose groups could not be addressed. A sample size of 15 participants per dose group (30 participants total) was determined to be sufficient for assessing mean change from baseline for each of the urinary Na+ and K+ excretion endpoints. There were no missing data. The effect size was calculated as the change divided by the standard deviation (SD) of the change. The study had 80% power to detect a 0.675 effect size (5% one-sided Type I error); an effect size of >0.446 would rule out the null hypothesis in favor of the alternative hypothesis.
Unless otherwise specified, descriptive statistics were used to summarize data for each dose group. Efficacy analyses included all participants who had received SZC per the study protocol and had Na+ and K+ excretion measurements made at baseline and during the treatment period. Daily results were averaged by dose group within the study period to derive individual baseline and mean treatment period data values. The null hypothesis was based on the premise that mean change from baseline is 0. This was tested using a one-sample, one-sided, paired t-test for each endpoint under study and performed separately for each dose group. A one-sided P of ≤0.05 was considered statistically significant. A one-sided 95% confidence interval (CI) on the change from baseline was constructed to provide the upper bound of the expected change. The alternative hypothesis was that the change from baseline is not 0. The safety population consisted of all study participants who received at least one dose of SZC. Missing AE or laboratory data were not imputed for the safety analysis.
RESULTS
Study participants
Of the 33 individuals screened, 32 met the study criteria and were enrolled in the study between 28 January 2015 and 4 March 2015 (Figure 2). Two individuals withdrew consent before receiving the study drug. In total, 30 healthy adults participated in the study; the first 15 enrolled participants received SZC 5 g QD and the next 15 received SZC 10 g QD. All 30 participants completed the study and were included in the efficacy and safety analyses.
FIGURE 2:
Study participant disposition. The broken line indicates sequential SZC administration in the two dose groups (the SZC 5 g group was evaluated first, followed by the SZC 10 g group).
The demographic and baseline characteristics of the study participants were generally similar between the two dose groups (Table 1). Most participants were male (76.7%) and White (83.3%) and had a mean age of 33 years. The proportion of male participants was greater in the 5 g dose group (93.3%) than that in the 10 g dose group (60.0%). Concomitant medications were received by four participants (26.7%) in the 5 g dose group (all received paracetamol) and by three participants (20.0%) in the 10-g dose group (all received paracetamol and ketorolac tromethamine; one participant received ibuprofen, one received levonorgestrel and one received medroxyprogesterone acetate).
Table 1.
Participant baseline demographics and characteristics
| SZC 5 g QD (n = 15) | SZC 10 g QD (n = 15) | |
|---|---|---|
| Age, years | 33.6 ± 11.9 | 32.2 ± 12.9 |
| Male, n (%) | 14 (93.3) | 9 (60.0) |
| Race, n (%) | ||
| White | 11 (73.3) | 14 (93.3) |
| Black or African American | 4 (26.7) | 1 (6.7) |
| Hispanic ethnicity,an (%) | 3 (20.0) | 2 (13.3) |
| Body weight, kg | 81.2 ± 20.0 | 82.4 ± 13.2 |
| Height, cm | 175.7 ± 6.2 | 173.2 ± 8.9 |
| BMI, kg/m2 | 26.2 ± 5.8 | 27.4 ± 3.6 |
| Blood pressure, mmHg | ||
| Systolic | 125.3 ± 11.6 | 127.7 ± 10.2 |
| Diastolic | 79.3 ± 7.8 | 81.3 ± 4.7 |
| Hemoglobin, g/dL | 14.8 ± 0.9 | 14.3 ± 1.4 |
| Hematocrit, % | 45.9 ± 2.7 | 44.6 ± 3.7 |
| Serum K+, mmol/L | 4.5 ± 0.3 | 4.6 ± 0.3 |
| Serum Na+, mmol/L | 136.9 ± 1.8 | 137.2 ± 1.4 |
| Serum creatinine, mmol/L | 0.092 ± 0.017 | 0.083 ± 0.015 |
| Urinary pH | 6.45 ± 0.44 | 6.50 ± 0.31 |
Data are mean ± SD unless stated otherwise.
Individuals of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin.
BMI, body mass index.
Primary endpoint
For the primary endpoint, mean ± SD urinary Na+ excretion over study Days 7–8 numerically decreased from baseline (study Days 3–4) following treatment with SZC 5 g QD [−0.93 ± 25.85 mmol/24 h (95% CI −15.25, 13.38 mmol/24 h); P > 0.05] and 10 g QD [−5.47 ± 13.90 mmol/24 h (95% CI −13.17, 2.23 mmol/24 h); P > 0.05; Figure 3A]. This corresponded to a decrease in urinary Na+ excretion of ∼21 mg (0.9 mmol) and ∼126 mg (5.5 mmol) with SZC 5 and 10 g, respectively.
FIGURE 3:
Mean change from baseline (Days 3–4) to treatment period (Days 7–8) in (A) urinary sodium excretion, (B) urinary potassium excretion, (C) fecal sodium excretion and (D) fecal potassium excretion with SZC 5 or 10 g QD. Error bars are the SD. Circles and squares show values for individual participants in the SZC 5 g and SZC 10 g groups, respectively. *P < 0.01 versus baseline. **P < 0.05 versus baseline.
Secondary endpoints
Mean ± SD numerical decreases from baseline in urinary K+ excretion were observed over study Days 7–8 for SZC 5 g QD [−9.67 ± 18.33 mmol/24 h (95% CI −19.82, 0.48 mmol/24 h); P = 0.0604] and were statistically significant for 10 g QD [−21.17 ± 21.26 mmol/24 h (95% CI −32.94, −9.39 mmol/24 h); P = 0.0017; Figure 3B]. This corresponded to a decrease in urinary K+ excretion of ∼379 mg (9.7 mmol) and ∼829 mg (21.2 mmol) with SZC 5 and 10 g, respectively.
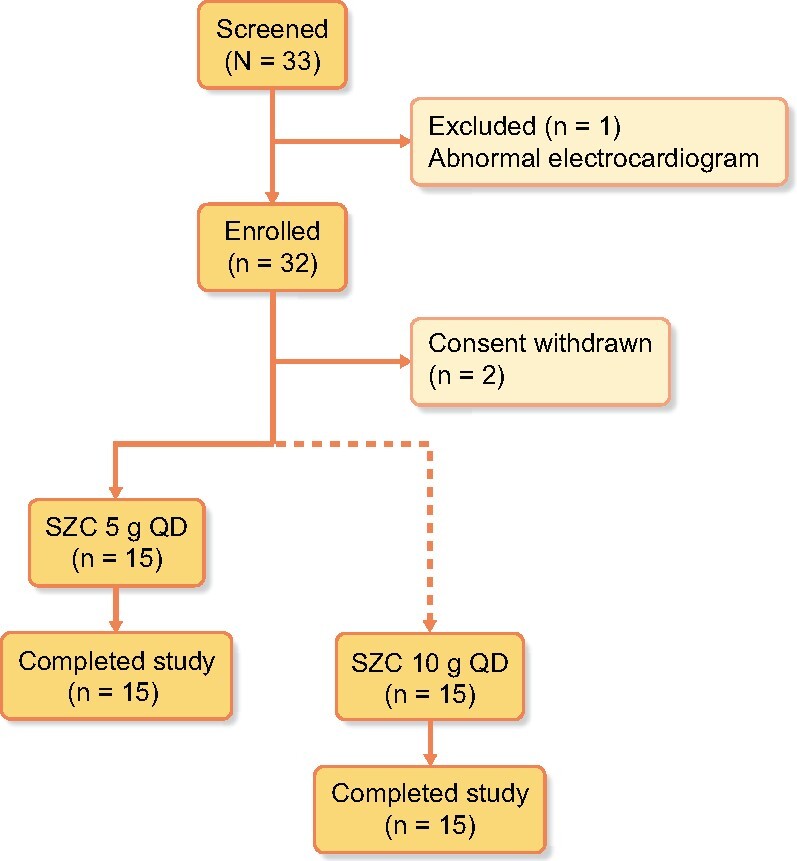
Both the 5 and 10 g SZC doses reduced mean serum K+ concentration over the treatment period (4.46 and 4.33 mmol/L, respectively, averaged over study Days 7–8) compared with baseline (4.52 and 4.57 mmol/L, respectively); however, the change was statistically significant for only the 10 g dose group (mean change −0.25 ± 0.24 mmol/L, P = 0.0014; Figure 4).
FIGURE 4:
Mean change from baseline (Days 3–4) to treatment period (Days 7–8) in serum potassium concentration with SZC 5 or 10 g QD. Error bars are the SD. Circles and squares show values for individual participants in the SZC 5 g and SZC 10 g groups, respectively. *P < 0.01 versus baseline.
Additional endpoints
Mean ± SD fecal Na+ excretion was numerically decreased from baseline with SZC 5 g QD [−0.01 ± 1.60 mmol (95% CI −0.90, 0.88 mmol)] but was numerically increased by 1.48 ± 3.73 mmol (95% CI −0.58, 3.55 mmol) with 10 g QD; however, the observed changes were not statistically significant (Figure 3C). Mean ± SD fecal Na+ excretion showed high variability for each SZC dose tested, whether taken on study Days 3–4 or study Days 7–8 (Figure 5A).
FIGURE 5:
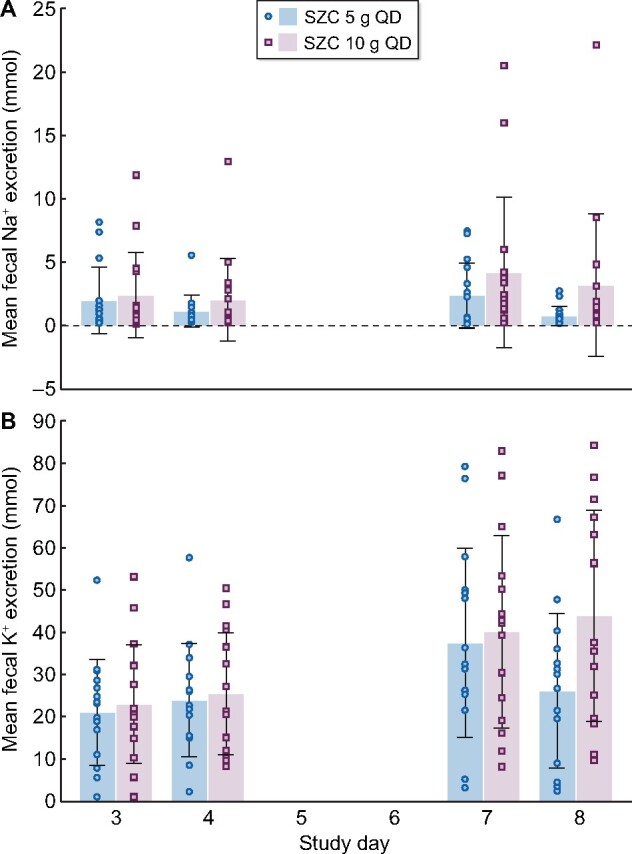
Mean daily fecal excretion of (A) sodium and (B) potassium on study Days 3 and 4 (baseline) and study Days 7 and 8 (treatment period). Error bars are the SD. Circles and squares show values for individual participants in the SZC 5 g and SZC 10 g groups, respectively.
Fecal K+ excretion was significantly increased from baseline in both the 5 g dose group [mean ± SD change 9.31 ± 14.97 mmol (95% CI 1.02, 17.60 mmol); P = 0.0304] and 10 g dose group [17.79 ± 18.31 mmol (95% CI 7.65, 27.93 mmol); P = 0.0021; Figure 3D]. Similar to Na+ excretion, there was high variability in mean ± SD K+ excretion between study Days 3 and 4 and between study Days 7 and 8 (Figure 5B).
Mean ± SD serum Na+ concentration showed no significant change from baseline with SZC 5 g QD on study Day 9 (−0.10 ± 2.25 mmol/L) but showed a significant increase with 10 g QD (1.20 ± 1.42 mmol/L; Supplementary data, Table S1). A significant increase in mean ± SD urinary pH from baseline was observed with SZC 5 g but not with SZC 10 g, and mean ± SD urinary creatinine excretion showed no significant change from baseline with SZC 5 and 10 g (Supplementary data, Table S2 and Figure S1).
Safety
No clinically meaningful changes in blood pressure, body weight, hemoglobin or hematocrit were observed during the study period (Table 2). However, statistically significant reductions from baseline in diastolic blood pressure and body weight were observed with both SZC doses. Similarly, there were no clinically meaningful changes from baseline in other chemistry evaluations following treatment with SZC (Supplementary data, Table S1). During the run-in and baseline periods, eight AEs were reported by six participants, including headache (five events), toothache (one event) and gingival abscess (one event) with gingival pain (one event). One treatment-emergent AE of mild headache was reported in the SZC 5 g dose group; this was not considered study drug related. There were no deaths, serious AEs or study drug discontinuations due to AEs.
Table 2.
Mean change from baseline in vital signs and hematologic evaluations following QD treatment with SZC 5 or 10 g for 4 days
| SZC 5 g QD (n = 15) | SZC 10 g QD (n = 15) | |
|---|---|---|
| Systolic blood pressure, mmHg | ||
| Baseline | 125.30 ± 11.60 | 127.70 ± 10.17 |
| Study Day 9 | 122.30 ± 8.66 | 121.10 ± 10.32 |
| Change to study Day 9 (95% CI) | −3.1 ± 13.31 (−10.40, 4.30) | −6.5 ± 13.83 (−14.20, 1.10) |
| Diastolic blood pressure, mmHg | ||
| Baseline | 79.30 ± 7.78 | 81.30 ± 4.65 |
| Study Day 9 | 73.10 ± 5.72 | 76.70 ± 6.20 |
| Change to study Day 9 (95% CI) | −6.1 ± 7.03 (−10.00, −2.20) | −4.6 ± 7.73 (−8.90, −0.30) |
| Body weight, kg | ||
| Baseline | 81.17 ± 20.01 | 82.37 ± 13.19 |
| Study Day 9 | 78.55 ± 19.31 | 79.97 ± 12.15 |
| Change to study Day 9 (95% CI) | −2.61 ± 1.85 (−3.64, −1.59) | −2.39 ± 1.39 (−3.16, −1.62) |
| Hemoglobin, g/dL | ||
| Baseline | 14.76 ± 0.95 | 14.33 ± 1.37 |
| Study Day 9 | 15.05 ± 1.02 | 14.50 ± 1.12 |
| Change to study Day 9 (95% CI) | 0.29 ± 0.72 (−0.11, 0.68) | 0.17 ± 0.79 (−0.27, 0.60) |
| Hematocrit, % | ||
| Baseline | 45.87 ± 2.67 | 44.55 ± 3.70 |
| Study Day 9 | 46.45 ± 3.12 | 45.58 ± 3.68 |
| Change to study Day 9 (95% CI) | 0.58 ± 2.45 (−0.78, 1.94) | 1.03 ± 2.94 (−0.60, 2.66) |
Data are mean ± SD unless stated otherwise.
DISCUSSION
This Phase 1 single-center study in healthy adults showed that while serum and urinary K+ excretion were significantly reduced, urinary Na+ excretion was not significantly altered during administration of SZC (5 or 10 g QD) over 4 days. K+ binders are emerging as a potential tool for managing hyperkalemia over time. Previous randomized controlled studies of SZC in patients with hyperkalemia have demonstrated significant reductions in serum K+ within 48 h of SZC administration at dosages of 2.5–10 g three times daily [14–16] and sustained normokalemia (serum K+ 3.5–4.9 mmol/L) during maintenance treatment for 12 or 28 days at dosages of 5, 10 or 15 g QD [15, 16]. Nevertheless, a key clinical concern regarding SZC treatment is what happens to the Na+ counterion.
The mechanism of action for SZC predicts that consumption of food containing K+ would selectively release H+ and Na+ from SZC in exchange for K+ or , with K+ or being retained as part of the SZC complex and removed from the body in the feces [12]. An initial consideration in conducting this Phase 1 study of SZC in healthy individuals was that Na+ release from SZC could increase fluid retention, while K+ absorption and removal could result in hypokalemia. However, this study showed that neither dose of SZC tested had any notable effect on urinary Na+ excretion. Furthermore, fecal Na+ excretion did not change with either dose, and there were no clinically meaningful changes in body weight, blood pressure, hemoglobin or hematocrit, which may be expected with increased fluid retention [17]. These results are consistent with results of the DIALIZE trial, which observed no fluid retention or worsening hypertension in patients on hemodialysis who were treated with SZC [18].
While it is tempting to conclude from this study that SZC does not release Na+ for absorption and therefore excretion, these results should be interpreted with caution because the study conditions may have influenced outcomes. A previous study of urinary Na+ excretion under conditions of Na+ depletion allowed 9 days for equilibration of Na+ concentration [19], a longer time than the 2-day period included in this study. Thus, it is possible that participants in this study were still adapting to the low Na+ diet when SZC administration was started. If study participants had not fully adapted to the low Na+ diet, ongoing loss of Na+ and water may explain the ∼2.5 kg reduction in body weight that was observed between baseline to study Day 9. The lack of any notable increase in urinary or fecal Na+ excretion might suggest that reduction in dietary Na+ (from an average of 154 mmol/day in a typical US diet [13] to 40 mmol/day with the standardized diet) masked any increase in Na+ provided by either 5 or 10 g of SZC. Each 5 g dose of SZC contains 400 mg of Na+ [11], thus the maximum Na+ amount in each dose is 17.4 mmol, and it is unlikely that all Na+ in SZC is exchanged.
Mean serum K+ concentration and urinary K+ excretion each showed significant reductions with SZC in this study, while fecal K+ excretion was significantly increased from baseline, consistent with the K+-binding effects of SZC within the GI tract [12]. Importantly, no hypokalemia was observed with the significant reductions in serum K+ concentration. In Phases 2 and 3 clinical studies of SZC administration in patients with hyperkalemia in which there was no standardized diet, no increase in urinary Na+ excretion (measured by spot test) or any clinically significant changes in serum Na+, blood pressure or body weight were seen [14–16].
Similar to the observed decrease in urinary K+ excretion with both SZC doses in this study, a previous study evaluating the effects of patiromer on urinary ion excretion in healthy adults (n = 32) showed dose-dependent decreases in urinary K+ excretion with patiromer over 7 days of treatment that were significantly greater than placebo (P < 0.01) [20]. However, unlike the results of this study, patiromer was also associated with significant dose-dependent decreases in urinary Na+ excretion (P < 0.01), consistent with patiromer binding Na+ in the GI tract as part of its nonspecific, cation-exchange-driven (calcium counterion) mechanism of action [20].
In contrast to SZC, SPS is associated with excess delivery of Na+ ions. The recommended daily dose of SPS is 15 g taken up to four times daily, and each 15-g dose of SPS contains 1500 mg or 65.25 mmol of Na+ [21]. Therefore, SPS is associated with a Na+ intake of up to 246 mmol/day. Although no studies have investigated Na+ excretion with SPS in humans, a study of K+-binding agents in mice demonstrated significant increases in Na+ excretion with SPS compared with controls (P < 0.0001) [22].
The incidence of AEs following 4-day treatment with SZC was low, with only one patient from the 5 g dose group experiencing headache, which was mild in severity and not considered to be study drug related. In these healthy participants, no hypokalemia, GI AEs, edema or blood pressure increase was reported. Furthermore, there were no reported serious AEs, AEs leading to study drug discontinuation or deaths.
This study has several potential limitations. A conservative approach was adopted, whereby therapeutic doses of SZC were used to investigate the K+ exchange process during consumption of a high K+/low Na+ diet. While the low Na+ consumption helped to maximize detection of Na+ release from SZC, it does not represent the physiologic environment, and changes in regulatory hormones may have affected Na+ absorption. Similarly, the high K+ consumption may have reduced the risk of hypokalemia in the healthy participants with normal K+ values but also does not reflect normal physiology. The variability of fecal electrolyte amounts between individual study days may also preclude meaningful conclusions being drawn regarding fecal Na+ and K+ excretion. It should also be noted that this study was not powered to compare the effects between doses of SZC. Most importantly, the participants did not have hyperkalemia or comorbidities that are often associated with hyperkalemia, such as chronic kidney disease or hypertension, and were not taking the medications commonly administered to patients with these conditions that may affect electrolyte metabolism. Thus, additional study of Na+ metabolism and excretion with SZC in patients with hyperkalemia on their usual diet and medications is warranted.
CONCLUSIONS
This study showed that SZC 5 or 10 g QD administration in healthy adults on a high K+/low Na+ diet did not significantly influence urinary or fecal Na+ excretion and was well tolerated. Consistent with its mechanism of action, SZC administered to healthy individuals resulted in significant reductions in serum K+ concentration and urinary K+ excretion, and significantly increased fecal K+ excretion.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
Sarah Greig, PhD (Auckland, New Zealand), and Elizabeth Strickland, PhD (Philadelphia, PA, USA), of inScience Communications, Springer Healthcare, provided medical writing support funded by ZS Pharma, Inc., a subsidiary of AstraZeneca. Mary Beth DeYoung of AstraZeneca and Carl Felton of Paragon, Prime Global Medical Group (Knutsford, UK), provided critical review of the manuscript.
FUNDING
This study was supported by ZS Pharma, Inc., a member of the AstraZeneca family of companies.
AUTHORS’ CONTRIBUTIONS
M.N. and D.W.B. provided scientific interpretation of the results and critical review of the manuscript. B.S. assisted with the conception and design of the study, review of the protocol, analysis and scientific interpretation of the results, and drafting and critical review of the manuscript. All authors approved the final version of the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
M.N. and D.W.B. are employees and shareholders of AstraZeneca. B.S. was an employee of ZS Pharma Inc. at the time of the study.
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
REFERENCES
- 1. Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol 2014; 10: 653–662 [DOI] [PubMed] [Google Scholar]
- 2. Linder KE, Krawczynski MA, Laskey D.. Sodium zirconium cyclosilicate (ZS-9): a novel agent for the treatment of hyperkalemia. Pharmacotherapy 2016; 36: 923–933 [DOI] [PubMed] [Google Scholar]
- 3. Betts KA, Woolley JM, Mu F. et al. The prevalence of hyperkalemia in the United States. Curr Med Res Opin 2018; 34: 971–978 [DOI] [PubMed] [Google Scholar]
- 4. An JN, Lee JP, Jeon HJ. et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care 2012; 16: R225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collins AJ, Pitt B, Reaven N. et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol 2017; 46: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Einhorn LM, Zhan M, Hsu VD. et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169: 1156–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goyal A, Spertus JA, Gosch K. et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA 2012; 307: 157–164 [DOI] [PubMed] [Google Scholar]
- 8. Chaitman M, Dixit D, Bridgeman MB.. Potassium-binding agents for the clinical management of hyperkalemia. P T 2016; 41: 43–50 [PMC free article] [PubMed] [Google Scholar]
- 9. Meaney CJ, Beccari MV, Yang Y. et al. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37: 401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AstraZeneca. Lokelma summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004029/WC500246774.pdf (8 March 2019, date last accessed)
- 11.AstraZeneca. Lokelma™ prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207078s000lbl.pdf (8 March 2019, date last accessed)
- 12. Stavros F, Yang A, Leon A. et al. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One 2014; 9: e114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Health and Nutrition Examination Survey. What we eat in America. http://www.ars.usda.gov/nea/bhnrc/fsrg (8 March 2019, date last accessed)
- 14. Ash SR, Singh B, Lavin PT. et al. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int 2015; 88: 404–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kosiborod M, Rasmussen HS, Lavin P. et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA 2014; 312: 2223–2233 [DOI] [PubMed] [Google Scholar]
- 16. Packham DK, Rasmussen HS, Lavin PT. et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015; 372: 222–231 [DOI] [PubMed] [Google Scholar]
- 17. Ellison DH. Treatment of disorders of sodium balance in chronic kidney disease. Adv Chronic Kidney Dis 2017; 24: 332–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fishbane S, Ford M, Fukagawa M. et al. A phase 3b, randomized, double-blind, placebo-controlled study of sodium zirconium cyclosilicate for reducing the incidence of predialysis hyperkalemia. J Am Soc Nephrol 2019; 30: 1723–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flier JS, Underhill LH, Laragh JH.. Atrial natriuretic hormone, the renin-aldosterone axis, and blood pressure-electrolyte homeostasis. N Engl J Med 1985; 313: 1330–1340 [DOI] [PubMed] [Google Scholar]
- 20. Bushinsky DA, Spiegel DM, Gross C. et al. Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol 2016; 11: 1769–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Concordia Pharmaceutical Inc. Kayexalate prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/011287s026lbl.pdf (8 March 2019, date last accessed)
- 22. Davidson JP, King AJ, Kumaraswamy P. et al. Evaluation of the pharmacodynamic effects of the potassium binder RDX7675 in mice. J Cardiovasc Pharmacol Ther 2018; 23: 244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure