Abstract
Background
Kidney allograft survival in human immunodeficiency virus (HIV)-positive patients is lower than that in the general population. Belatacept increases long-term patient and allograft survival rates when compared with calcineurin inhibitors (CNIs). Its use in HIV-positive recipients remains poorly documented.
Methods
We retrospectively report a French cohort of HIV-positive kidney allograft recipients who were switched from CNI to belatacept, between June 2012 and December 2018. Patient and allograft survival rates, HIV immunovirological and clinical outcomes, acute rejection, opportunistic infections (OIs) and HLA donor-specific antibodies (DSAs) were analysed at 3 and 12 months, and at the end of follow-up (last clinical visit attended after transplantation). Results were compared with HIV-positive recipients group treated with CNI.
Results
Twelve patients were switched to belatacept 10 (2–25) months after transplantation. One year after belatacept therapy, patient and allograft survival rates scored 92% for both, two (17%) HIV virological rebounds occurred due to antiretroviral therapy non-compliance, and CD4+ and CD8+ T-cell counts remained stable over time. Serious adverse events included two (17%) acute steroid-resistant T-cell-mediated rejections and three (25%) OIs. Kidney allograft function significantly increased over the 12 post-switch months (P = 0.009), and DSAs remained stable at 12 months after treatment. The control group showed similar results in terms of patient and kidney allograft survival rates, DSA characteristics and proteinuria
Conclusions
Switch from CNI to belatacept can be considered safe and may increase long-term kidney allograft survival in HIV-positive kidney allograft recipients. These results need to be confirmed in a larger cohort.
Keywords: acute rejection, graft function, graft survival, immunosuppression, kidney transplantation
INTRODUCTION
Discovering highly active antiretroviral (ARV) therapy in the mid-1990s changed human immunodeficiency virus (HIV) infection from a debilitating and life-threatening disease to a chronic manageable condition [1, 2]. Chronic comorbidities, rather than opportunistic infections (OIs), have now become the leading cause of death in people living with HIV (PLHIV) [3]. HIV-associated nephropathy (HIVAN) is the fourth leading cause of end-stage renal disease (ESRD) in African-Americans [4, 5]. Currently, kidney transplantation is a well-recognized therapeutic option in PLHIV with ESRD, provided that their CD4 count is >200/mm3, the HIV viral load is <50 copies/mL and they are on a stable ARV regimen for at least 6 months prior to transplantation [6, 7]. The 3-year survival of patient and of allograft increased significantly, reaching 100 and 96%, respectively, thanks to the wide use of integrase inhibitor-based regimen, where the 1-year acute rejection incidence was 8%, i.e. lowering the risk of rejection by 40% compared with the results of the biggest cohort on HIV kidney allograft recipients treated with protease inhibitors (PIs) [6–8]. HIV infection was well controlled despite induction and maintenance of immunosuppressant (tacrolimus-based regimen) along with transplantation [6–8]. However, allograft survival remained lower than that in HIV-negative recipients [6], essentially because of cardiovascular comorbidity, OI and interactions with ARV regimen. For such, the effect of new immunosuppressive maintenance agents needs to be evaluated in those patients.
Belatacept (CTLA4-Ig), a costimulation blocker, is a new immunosuppressive agent used in kidney transplant recipients as both de novo therapy and conversion therapy from calcineurin inhibitor (CNI). In HIV-negative patients, de novo belatacept has shown efficacy in prolonging patient and allograft survival [9]. Furthermore, belatacept could prevent both CNI metabolic adverse effects and CNI interactions with PI [9]. As a conversion therapy, belatacept seems to be the ideal agent to shift patients from CNI after developing intolerance, marginal kidney function or vascular lesions; numerous conversion trials reported the benefit of belatacept in such settings [10–14]. Conversion to belatacept could particularly be beneficial to HIV patients since allograft loss and cardiovascular morbidity are higher in this specific population [15]. So far, only isolated case reports have demonstrated good outcomes and safety profile of belatacept therapy [16–18].
We performed the first French multicentric retrospective study, which recruited all HIV-positive kidney allograft recipients who were switched from CNI to belatacept. We compared our results with those of a control cohort of HIV-positive recipients maintained on CNI.
MATERIALS AND METHODS
Study design
We conducted a French national retrospective multicentre study including all HIV-positive kidney allograft recipients who were shifted from CNI to belatacept between June 2012 and December 2018. All French transplant programmes were contacted to collect patients. Five centres participated in the study (Henri-Mondor, Necker-Enfants-Malades, Rouen, Clermont-Ferrand and Grenoble). The control group included HIV-positive patients who were engrafted during the same study period, and treated with conventional CNI-based immunosuppressive treatment. Approval from Institutional Review Board was obtained (#00003835).
Study endpoints
Primary endpoints were patient and allograft survival [Modification of Diet in Renal Disease-calculated estimated glomerular filtration rate (eGFR) [19] and proteinuria] and HIV immunovirological status (CD4+ and CD8+ T-cell counts and HIV plasma viral load). Allograft loss was considered if eGFR was ˂6 mL/min/1.73 m2 and/or dialysis was needed.
We also analysed the influence of belatacept treatment on (i) incidence of biopsy-proven acute rejection, as defined by updated Banff classification [20], (ii) evolution of eGFR, (iii) incidence of OI and (iv) donor-specific antibody (DSA) trend.
All endpoints were recorded 3 and 12 months after conversion and at the end of the follow-up (last clinical visit attended after transplantation). Belatacept interruption for any cause was considered as end of follow-up.
HLA-specific antibody screening
HLA-A, HLA-B, Cw, HLA-DR and HLA-DQ genotyping were performed for donors and recipients via low- and high-resolution tests, respectively. All sera obtained before and after transplantation and belatacept switch (M3 and M12) were assessed for the presence of circulating DSA and de novo DSA (dnDSA) using high-resolution Luminex Single Antigen Bead assay technology (One-Lambda, Canoga-Park, CA, USA) on Luminex-platform. Beads showing a normalized mean fluorescence intensity (MFI) >500 were considered positive. For each serum sample, the number of DSA and the highest MFI of all DSAs (MFImax) were calculated.
Causes of switch to belatacept
Known criteria of CNI-to-belatacept switch were (i) chronic allograft dysfunction, (ii) significantly delayed allograft function, (iii) severe histological vascular lesions (cv and ah) or CNI toxicity, and (iv) non-compliance to CNI [10, 12, 13, 20, 21, 23]. New indications like DSA control are currently under evaluation [22].
Immunosuppressive protocol
The induction therapy included thymoglobulin (Genzyme, Cambridge, MA, USA) at 3 mg/kg, the total dose was given >4 days to patients with pre-transplantation panel-reactive antibodies of >85% and/or having DSA before transplantation, otherwise basiliximab (20 mg at transplantation day and 4 days after) was used. The maintenance therapy included CNI, mycophenolate mofetil (MMF) and steroids. MMF could be modified at physician discretion.
Belatacept switch protocol
Conversion protocol was as follows: (i) early-switch (<3 months after transplantation): CNI was stopped at Day 1 and belatacept infusion of 10 mg/kg was administered at Days 1, 5, 14 and 28, Weeks 8 and 12, followed by a 5 mg/kg infusion given every 4–6 weeks, from Week 16 and onwards; (ii) late-switch (>3 months after transplantation): CNI was stepped down to 50% at Day 14 and stopped at Day 28 after the switch, and belatacept infusion of 10 mg/kg was administered at Days 1, 14 and 28, followed by a 5 mg/kg infusion given every 4–6 weeks, from Week 16 and onwards.
Infectious prophylaxis
Participants who had pre-transplantation positive cytomegalovirus (CMV) IgG serology were treated with valganciclovir for 6 months after transplantation.
Participants with latent tuberculosis (LTB; TTS or QuantiFERON positive but showed no signs of active tuberculosis) were treated with isoniazid for 9 months after transplantation.
Pneumocystis jirovecii and Toxoplasma gondii prophylaxes included sulfamethoxazole/trimethoprim (Bactrim) 400/80 mg/day, or pentamidine (pentacarinat) aerosol given for transplant life.
Statistics
Variables were treated as proportions for categorical variables, and median and interquartile range (IQR) for continuous variables. For the continuous variables representing the study endpoints, only their last values were considered in the analysis. Changes in continuous variables from baseline to follow-up were compared using Wilcoxon paired test. Mann–Whitney test was used to compare the differences in continuous variables between groups, and Fisher’s exact or Chi-square test was used to compare the differences of categorical variables between groups. Patient and allograft survival rates were analysed using Kaplan–Meier survival curve.
All reported P-values are two-tailed, with significance set at 0.05. Analyses were done with Prism version 7.0 for Mac.
RESULTS
Twelve HIV-positive kidney allograft recipients had their regimens switched from CNI to belatacept (Belatacept group) (Table 1). Onset of switch was 10 (2–25) months after transplantation with three (25%) early switches. Causes of switch were CNI toxicity (n = 5, 42%), allograft dysfunction (n = 4, 33%) and vascular histological lesions (n = 3, 25%). Median eGFR at the time of switch was 13 (9–19) mL/min/1.73 m2 (Table 2). All patients were on dialysis before transplantation. HIVAN was the leading cause of ESRD (n = 5, 41%). Before transplantation, HIV disease duration was 13 (9–17) years and was under control in all patients. Five (41%) patients were treated for OI prior to transplantation. Almost all patients received kidney allografts from deceased donors (n = 10, 83%) and all received induction therapy (n = 12, 100%), of whom 6 (50%) had thymoglobulin. At transplantation time, all patients were on maintenance immunosuppressive regimen of CNI, MMF and steroids but one (8%), who had imTOR instead of MMF. One (8%) patient was co-infected with Hepatitis C virus (HCV) and three (25%) with Hepatitis B virus (HBV). All HCV-positive patients were treated before kidney transplantation and their Polymerase Chain Reaction (PCR) HCV was negative at the time of transplantation. HBV patients were all treated and checked with negative viral load before and at transplantation.
Table 1.
Patients’ characteristics at the time of kidney transplantation
| Variables | Belatacept | Control | P-value |
|---|---|---|---|
| Recipient, n | 12 | 20 | |
| Age, mean ± SD, years | 50 ± 12 | 51 ± 7 | 0.88 |
| Sex, female, n (%) | 3 (25) | 8 (40) | 0.46 |
| Initial nephropathy | |||
| HIVAN, n (%) | 5 (41) | 11 (55) | 0.13 |
| HTN-diabetes, n (%) | 3 (25) | 5 (25) | – |
| Glomerulopathy, n (%) | 2 (17) | 3 (15) | – |
| Other, n (%) | 2 (17) | 2 (10) | – |
| Dialysis, n (%) | 12 (100) | 20 (100) | 1.00 |
| Haemodialysis, n (%) | 9 (75) | 20 (100) | 0.04 |
| Duration, median (IQR), months | 54 (24–82) | 64 (39–92) | 0.71 |
| HCV+, n (%) | 1 (8) | 2 (10) | 1.00 |
| HBV+, n (%) | 3 (25) | 2 (10) | 0.34 |
| Immunological risk factors | |||
| Transfusions, n (%) | 3 (25) | 6 (30) | 1.00 |
| HLA DSA, n (%) | 6 (50) | 7 (35) | 0.47 |
| HIV disease | |||
| Duration, median (IQR), years | 13 (9–17) | 12 (8–23) | 0.42 |
| CD4 nadir, median (IQR), mm3 | 171 (75–549) | – | – |
| CD4 before transplant, median (IQR), mm3 | 411 (267–565) | 306 (254–445) | 0.09 |
| CD8 before transplant median (IQR), mm3 | 633 (372–709) | 519 (417–846) | 0.40 |
| Donor | |||
| Age, mean ± SD, years | 57 ± 15 | 51 ± 14 | 0.29 |
| Deceased donor, n (%) | 10 (83) | 18 (90) | 0.62 |
| eGFR, median (IQR), mL/min/1.73 m2 | 51 (29–77) | 64 (26–81) | 0.50 |
| Extended-criteria donor, n (%) | 7 (58) | 13 (65) | 0.72 |
| Kidney transplantation | |||
| Cold ischaemia time, mean ± SD, h | 20 ± 5 | 16 ± 5 | 0.07 |
| Induction therapy, n (%) | 12 (100) | 20 (100) | – |
| Thymoglobulin, n (%) | 6 (50) | 10 (50) | 1.00 |
| Maintenance therapy | |||
| CNIs, n (%) | 12 (100) | 20 (100) | – |
| MMF, n (%) | 11 (92) | 18 (90) | 1.00 |
| mTOR inhibitors, n (%) | 1 (8) | 2 (10) | 1.00 |
| Steroids, n (%) | 12 (100) | 20 (100) | – |
HTN, hypertension.
Table 2.
Conversion characteristics and follow-up
| Variables | Belatacept |
|---|---|
| Patients, n | 12 |
| Post-transplantation switch onset, months, median (IQR) | 10 (2–25) |
| Early (<3 months), n (%) | 3 (25) |
| Late (>3 months), n (%) | 9 (75) |
| Cause of switch, n (%) | |
| Vascular | 3 (25) |
| CNI toxicity | 5 (42) |
| Graft dysfunction | 4 (33) |
| eGFR, median (IQR), mL/min/1.73 m2 | 13 (9–19) |
| Proteinuria/creatininuria ratio, median (IQR) | 37 (12–57) |
| Follow-up | |
| 3 months, recipients, n | 11 |
| eGFR, median (IQR), mL/min/1.73 m2 | 18 (14–22) |
| Proteinuria/creatininuria ratio, median (IQR) | 14 (6–35) |
| Acute rejection, n (%) | 1 (8) |
| CD4, median (IQR), mm3 | 213 (90–292) |
| CD8, median (IQR), mm3 | 319 (196–319) |
| HIV reactivation, n (%) | 1 (8) |
| Patient death, n (%) | 0 (0) |
| Kidney allograft loss, n (%) | 1 (8) |
| OI, n (%) | 3 (25) |
| Belatacept stopped, n (%) | 1 (8) |
| 12 months, recipients, n | 9 |
| eGFR, median (IQR), mL/min/1.73 m2 | 18 (15–27) |
| Proteinuria/creatininuria ratio, median (IQR) | 30 (15–63) |
| Acute rejection, n (%) | 1 (9) |
| CD4, median (IQR), mm3 | 318 (150–608) |
| CD8 (mm3), median (IQR) | 567 (299–1036) |
| HIV reactivation, n (%) | 2 (17) |
| Patient death, n (%) | 1 (8) |
| Kidney allograft loss, n (%) | 1 (8) |
| OI, n (%) | 2 (17) |
| Belatacept stopped, n (%) | 3 (25) |
| End of follow-up | |
| eGFR, median (IQR), mL/min/1.73 m2 | 23 (15–27) |
| Proteinuria/creatininuria ratio, median (IQR) | 46 (8–68) |
| Acute rejection, n (%) | 2 (17) |
| CD4, median (IQR), mm3 | 215 (148–380) |
| CD8, median (IQR), mm3 | 483 (282–887) |
| HIV reactivation, n (%) | 2 (17) |
| Patient death, n (%) | 2 (17) |
| Kidney allograft loss, n (%) | 2 (17) |
| OI, n (%) | 3 (25) |
| Belatacept stopped, n (%) | 5 (42) |
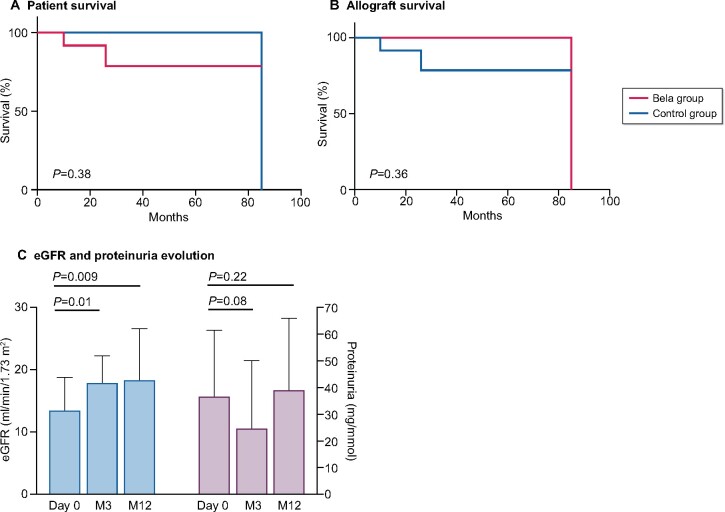
At 3 and 12 months after kidney transplantation, patient survival was, respectively, 100 and 92%, whereas allograft survival was 92%. We compared patient and allograft survival with those of the control group, which included 20 kidney allograft recipients treated with CNI (Table 1). Characteristics of both groups, at the time of transplantation, were similar. No significant difference in patient survival (P = 0.38) and allograft survival (P = 0.36) was detected between the belatacept group and the control group (Figure 1A and B). The two deaths occurred 7 and 8 months after switching to belatacept, at the age of 63 and 72 years old, respectively. Causes of deaths were cardiovascular event for the second and unknown for the first despite the extensive review of the latter’s medical record. The cause of the single allograft loss was refractory acute rejection. In functioning allografts, eGFR increased significantly 12 months after the switch [eGFR = 18 (15–27) mL/min/1.73 m2, P = 0.009, Figure 1C], whereas proteinuria remained similar (P = 0.22; Figure 1C).
FIGURE 1:
Patient and kidney allograft survival after belatacept treatment. (A) Kaplan–Meier estimate of patient survival was similar in HIV-positive kidney allograft recipients converted to belatacept compared with the control group of HIV-positive kidney allograft recipients treated with CNI (P = 0.38). (B) Kaplan–Meier estimate of kidney allograft survival, in HIV-positive kidney allograft recipients converted to belatacept compared with the control group of HIV-positive kidney allograft recipients treated with CNI (P = 0.36). (C) eGFR and proteinuria evolution after belatacept conversion. eGFR increased significantly 3 and 12 months after belatacept conversion (P = 0.01 and P = 0.009, respectively). Proteinuria tended to decrease at 3 months (P = 0.08), which is no longer observed at 12 months (P = 0.22).
After 12 months of belatacept treatment, CD4+ T-cell count was 318 (150–608)/mm3 and HIV viral load rebound was identified in two (17%) patients who admitted non-adherence to ARV therapy for a couple of weeks. However, their viral load decreased as soon as ARV therapy was reinitiated. CD4+ T-cell count significantly decreased at 3 months of belatacept compared with pre-transplantation level (P = 0.02) and remained insignificantly different at 12 months of the therapy (0.29). In contrast, CD8+ T-cell count significantly decreased 3 months after belatacept switch, compared with pre-transplantation (P = 0.02), and significantly increased from 3 to 12 months (P = 0.01), reaching pre-transplantation level (P = 0.47). Results were similar regardless of induction therapy (anti-interleukin-2 receptor or antithymocyte globulin). In the control group, CD4+ and CD8+ T-cell counts remained stable during the 12 months post-transplantation (P = 0.22 and P = 0.18, respectively).
Two patients (17%) suffered T-cell-mediated rejection (TCMR) at 2 and 24 months of conversion. Both were early switch, i.e. ˂3 months after transplantation. Despite high doses of steroids, both TCMR episodes led to rapid allograft loss and arrest of belatacept. None had DSA and they were not treated with thymoglobulin. The control group had a similar incidence of acute rejection in four (20%) patients (P = 1.00).
Distribution of DSA at 12 months after belatacept switch was similar to the baseline value (Table 3). DnDSA was observed at 12 months of belatacept in one patient (8%), who did not present acute rejection. In the control group, the distribution of DSA at 12 months after transplantation was similar to that of pre-transplantation.
Table 3.
HLA DSAs and kidney allograft function evolution in belatacept and control groups
| Treatment |
Belatacept |
Control |
||||
|---|---|---|---|---|---|---|
| Variables | Before belatacept | 12 months after belatacept | P-value | Before transplantation | 12 months after transplantation | P-value |
| Immunological variables | ||||||
| Recipient, n | 12 | 9 | 20 | 19 | – | |
| HLA DSA Class 1 | ||||||
| n (%) | 2 (17) | 4 (44) | 0.12 | 8 (40) | 4 (21) | 0.30 |
| MFI max, median (IQR) | 2878 (2006–3750) | 1884 (1059–2661) | 1.00 | 832 (520–1324) | 1921 (870–4175) | – |
| MFI sum, median (IQR) | 3875 (3750–4000) | 2137 (1592–2661) | 1.00 | 832 (520–1805) | 2211 (1015–4175) | – |
| HLA DSA Class 2 | ||||||
| n (%) | 6 (50) | 4 (44) | 1.00 | 5 (25) | 4 (21) | 1.00 |
| MFI max, median (IQR) | 2205 (1309–10 349) | 2835 (2168–4060) | 1.00 | 849 (620–1378) | 2617 (223–4574) | 0.25 |
| MFI sum, median (IQR) | 3038 (1440–11 291) | 4971 (2501–5195) | 1.00 | 1315 (620–1969) | 2773 (223–5816) | 0.25 |
| Kidney allograft function (conversion) | ||||||
| Recipient, n | 12 | 9 | ||||
| eGFR, median (IQR), mL/min/1.73 m2 | 13 (9–19) | 18 (15–27) | 0.009 | – | – | – |
| Proteinuria/creatininuria ratio, median (IQR) | 37 (12–57) | 30 (15–63) | 0.22 | – | – | – |
Three patients (25%) developed OIs in the 12 after-switch months: one had disseminated tuberculosis with macrophage activation syndrome that incurred belatacept disruption 3 months after conversion and 12 months after transplantation, one CMV colitis developed 57 months after conversion and 60 months after transplantation, and one had chronic norovirus diarrhoea with good outcome after reducing immunosuppressant doses. Two of the three patients experienced OI before transplantation. The one with disseminated tuberculosis was treated for LTB for 9 months before transplantation. At the time of OI, the three patients had CD4+ T-cell counts of 92, 419 and 152/mm3, respectively. Another two patients (17%) presented CMV viraemia without developing CMV disease at 1 and 27 months after the switch. Two patients (17%) presented BK viraemia at 1 and 25 months after the switch. In the control group, the incidences of OI [n = 1 (5%); P = 0.28], CMV viraemia [n = 4 (33%); P = 1.00] and BK viraemia [n = 2 (10%); P = 0.62] were not significantly different when compared with their counterparts.
Belatacept was interrupted in five (42%) patients, 5 (3–19) months after conversion, because of acute rejection (n = 2), personal convenience (n = 2) and severe tuberculosis with macrophage activation syndrome (n = 1).
DISCUSSION
We present here the first series of HIV-positive kidney allograft recipients whose medication was shifted from CNI to belatacept. We first demonstrated the safety of belatacept conversion in HIV-positive recipients with satisfactory patient survival and significant increase of allograft function after the switch. In addition, we did not observe major adverse effects over the 1 year of treatment. HIV infection remained under control without viral load rebound despite non-adherence to ARV therapy in two cases. CD4+ and CD8+ T-cell counts remained stable for 12 months after conversion. When compared with the control group, our results suggest that belatacept is a safe alternative to CNI in terms of acute rejection, survival and HIV disease control.
In our work, patient and allograft survival rates seemed to be lower than those reported in the most recent US HIV-positive kidney allograft recipients’ cohort, which described 100% patient and allograft survival rates at 1 year, and 100 and 96%, respectively, at 3 years [7]. Our recipients were in the same age class. However, almost all of our donors were deceased donors, versus 60% in the US cohort, they were significantly older and 50% were extended-criteria donors [7]. Our data are at least equivalent to reported patient and allograft survival rates after CNI–belatacept switch [14, 22]. The control cohort, comprising HIV-positive kidney allograft recipients treated with CNI, showed higher insignificant patient survival and similar allograft survival. It is worth mentioning that our control group was small and these results should be taken with caution and verified in larger studies. We did not have enough HIV-positive kidney allograft recipients treated with CNI to fairly match each belatacept switch to one or two controls. In HIV-negative patients, belatacept is now commonly used as a switch therapy since it has proven its superiority to CNI in maintaining allograft function in recipients with chronic vascular lesions regardless of the timing after transplantation [14, 15]. Allograft survival reported within 6 months after the switch was 85% [14], and no allograft survival benefit has been identified after the switch [12].
According to the literature, eGFR rose over time in patients switched to belatacept [11, 12, 14, 15, 22]. In our study, eGFR significantly increased over the 12 months after conversion and accrued until the end of the follow-up. In addition, proteinuria tended to be lower compared with baseline. Whether CNI type (cyclosporine or tacrolimus) can modify these results is a probability that merits exploration.
Belatacept safety was considered satisfactory in our cohort with ˂20% of acute rejection and OI, which was similar to our control group findings. Acute rejection occurred in two patients of the early switch group (<3 months), of whom one was close to switch. We observed a higher incidence of acute rejection (9%) compared with HIV-negative patients [22]. In the latter, acute rejection episodes occurred significantly more often in patients converted to belatacept within the first three post-transplantation months [22]. Given this fact and the higher rates of acute rejection in HIV-positive recipients within the first post-transplantation year (up to 40%) [4, 6], we recommend early switch to belatacept in HIV-positive kidney recipients with low immunological risk profile (no or low DSA titre) [14, 18]. Nonetheless, avoiding belatacept conversion in the early post-transplantation period or associating it with low doses of CNI during the first three post-transplantation months could be a reasonable approach to prevent acute rejection occurring after CNI-belatacept switch [24]. The probability that belatacept could decrease the elevated risk of acute rejection in patients requiring PI should be explored as interaction between CNI and PI has largely been suspected, especially after reporting low acute rejection incidence in patients treated with integrase inhibitors [8].
Half of our recipients had DSA at the time of belatacept switch, albeit we did not observe any significant changes 12 months after the switch, even with ˂10% of dnDSA. Our findings confirmed the low risk of dnDSA developing after belatacept treatment irrespective of whether DSAs were present before the switch or not [23, 25]. However, as previously reported, we did not observe a significant decrease of DSA after belatacept treatment compared with the control group [10].
Considering OI, no lymphoproliferative disorder was observed and ˂10% of patients contracted severe OI requiring arrest of belatacept regimen. Although this information is encouraging, longer follow-up is necessary as we recently showed that OIs occur later in the new immunosuppressive era [26]. More recently, significant incidence of OI has been reported in HIV-negative kidney allograft recipients switched to belatacept, particularly pneumocystis pneumonia and CMV disease [27]. In HIV-positive recipients, pneumocystis pneumonia is easily prevented; however, CMV disease risk should be carefully analysed in a larger cohort with longer follow-up period to establish specific CMV monitoring or preventive strategy in this particular population. To limit the risk of OI, screening for CD4+ T-cell count before the switch should be recommended.
Almost 20% of patients showed HIV viral load rebound because of voluntary cessation of ARV therapy and non-adherence to it. There was no evidence of belatacept involvement in both HIV rebounds. Belatacept is simple to use in practice and does not interact with any ARV treatment since its metabolism involves neither Cytochrome 450 (CYP450) nor Uridine diphospho (UDP)-glucuronosyltransferase. CD4+ T-cell count has been reported as an independent risk factor for mortality in HIV-positive kidney allograft recipients, linked to antithymocyte globulin induction [6]. In our study, CD4+ and CD8+ T-cell counts remained stable for 3 months after belatacept treatment initiation regardless of the induction therapy. However, CD4+ T-cell count evolution before and after conversion needs further investigation.
Belatacept was interrupted in almost half of the patients, and for medical reasons in only a quarter of our cohort. The disruption rate is comparable to former studies on HIV-negative patients [28]. Before switching, patient’s residency needs to be carefully considered as belatacept can only be administered in developed countries and a large part of our HIV-positive patients live between Africa and Europe. Furthermore, the time interval between two infusions is up to 6 weeks.
It would have been interesting to have the details of CNI treatment, but the small size of our cohort did not allow us to draw conclusions.
In conclusion, despite the small sample size and the non-matched control group, we provide herein the first retrospective cohort of HIV-positive kidney allograft recipients who underwent conversion from CNI to belatacept. One year after the switch, patient and allograft survival rates were satisfactory, eGFR increased significantly and proteinuria remained stable. Incidence of acute rejection and OI was acceptable. Early switch should probably be avoided or associated with low doses of CNI to prevent acute rejection, especially in high immunological risk recipients. Infectious screening should be more frequent after the switch, especially in case of pre-transplantation OI. Interestingly, HIV disease remained under control after belatacept treatment and CD4+ and CD8+ T-cell counts remained stable for 12 months after the switch. Our results need to be confirmed in a larger cohort to precisely define the place of belatacept in the therapeutic arsenal for HIV-positive kidney allograft recipients, especially those requiring PI, and to further analyse its benefits in terms of long-term survival.
AUTHORS’ CONTRIBUTIONS
M.M. and K.E.S. designed the study and analysed data. K.E.S., M.M. and P.G. wrote the article. G.M., A.S., D.B., C.G., P.M., P.R., A.Moktefi, A.I., C.C., J.-D.L., D.K., A.Morel, D.M., P.A., P.G. and M.M. participated in research design and data collecting.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1. Abraham AG, Althoff KN, Jing Y. et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis 2015; 60: 941–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krentz HB, Kliewer G, Gill MJ.. Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Med 2005; 6: 99–106 [DOI] [PubMed] [Google Scholar]
- 3. Rosenthal E, Roussillon C, Salmon-Céron D. et al. ; the Mortalité 2010 and GERMIVIC study groups. Liver-related deaths in HIV-infected patients between 1995 and 2010 in France: the Mortavic 2010 study in collaboration with the Agence Nationale de Recherche sur le SIDA (ANRS) EN 20 Mortalité 2010 survey. HIV Med 2015; 16: 230–239 [DOI] [PubMed] [Google Scholar]
- 4. Sawinski D. Kidney transplantation for HIV-positive patients. Transplant Rev (Orlando) 2017; 31: 42–46 [DOI] [PubMed] [Google Scholar]
- 5. Herman ES, Klotman PE.. HIV-associated nephropathy: epidemiology, pathogenesis, and treatment. Semin Nephrol 2003; 23: 200–208 [DOI] [PubMed] [Google Scholar]
- 6. Stock PG, Barin B, Murphy B. et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med 2010; 363: 2004–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malat GE, Boyle SM, Jindal RM. et al. Kidney transplantation in HIV-positive patients: a single-center, 16-year experience. Am J Kidney Dis 2019; 73: 112–118 [DOI] [PubMed] [Google Scholar]
- 8. Matignon M, Lelièvre JD, Lahiani A. et al. ; the ANRS 153 TREVE study group. Low incidence of acute rejection within 6 months of kidney transplantation in HIV-infected recipients treated with raltegravir: the Agence Nationale de Recherche sur le Sida et les Hépatites Virales (ANRS) 153 TREVE trial. HIV Med 2019; 20: 202–213 [DOI] [PubMed] [Google Scholar]
- 9. Vincenti F, Rostaing L, Grinyo J. et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 2016; 374: 333–343 [DOI] [PubMed] [Google Scholar]
- 10. Rostaing L, Massari P, Garcia VD. et al. Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: a randomized phase II study. Clin J Am Soc Nephrol 2011; 6: 430–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grinyó JM, Del Carmen Rial M, Alberu J. et al. Safety and efficacy outcomes 3 years after switching to belatacept from a calcineurin inhibitor in kidney transplant recipients: results from a phase 2 randomized trial. Am J Kidney Dis 2017; 69: 587–594 [DOI] [PubMed] [Google Scholar]
- 12. Brakemeier S, Kannenkeril D, Dürr M. et al. Experience with belatacept rescue therapy in kidney transplant recipients. Transpl Int 2016; 29: 1184–1195 [DOI] [PubMed] [Google Scholar]
- 13. Le Meur Y, Aulagnon F, Bertrand D. et al. Effect of an early switch to belatacept among calcineurin inhibitor-intolerant graft recipients of kidneys from extended-criteria donors. Am J Transplant 2016; 16: 2181–2186 [DOI] [PubMed] [Google Scholar]
- 14. Bertrand D, Cheddani L, Etienne I. et al. Belatacept rescue therapy in kidney transplant recipients with vascular lesions: a case control study. Am J Transplant 2017; 17: 2937–2944 [DOI] [PubMed] [Google Scholar]
- 15. Choi AI, Li Y, Deeks SG. et al. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation 2010; 121: 651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen EA, Mulligan D, Kulkarni S. et al. De novo belatacept in a human immunodeficiency virus-positive kidney transplant recipient. Am J Transplant 2016; 16: 2753–2757 [DOI] [PubMed] [Google Scholar]
- 17. Nair V, Liriano-Ward L, Kent R. et al. Early conversion to belatacept after renal transplantation. Clin Transplant 2017; 31: e12951. [DOI] [PubMed] [Google Scholar]
- 18. Ebcioglu Z, Liu C, Shapiro R. et al. Belatacept conversion in an HIV-positive kidney transplant recipient with prolonged delayed graft function. Am J Transplant 2016; 16: 3278–3281 [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Coresh J, Greene T. et al. Expressing the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007; 53: 766–772 [DOI] [PubMed] [Google Scholar]
- 20. Loupy A, Haas M, Solez K. et al. The Banff 2015 kidney meeting report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant 2017; 17: 28–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Darres A, Ulloa C, Brakemeier S. et al. Conversion to belatacept in maintenance kidney transplant patients: a retrospective multicenter European study. Transplantation 2018; 102: 1545–1552 [DOI] [PubMed] [Google Scholar]
- 22. Ulloa CE, Anglicheau D, Snanoudj R. et al. Conversion from calcineurin inhibitors to belatacept in HLA-sensitized kidney transplant recipients with low-level donor-specific antibodies. Transplantation 2019; 103: 2150–2156 [DOI] [PubMed] [Google Scholar]
- 23. Leibler C, Matignon M, Moktefi A. et al. Belatacept in renal transplant recipient with mild immunologic risk factor: a pilot prospective study (BELACOR). Am J Transplant 2019; 19: 894–906 [DOI] [PubMed] [Google Scholar]
- 24. Adams AB, Goldstein J, Garrett C. et al. Belatacept combined with transient calcineurin inhibitor therapy prevents rejection and promotes improved long-term renal allograft function. Am J Transplant 2017; 17: 2922–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vincenti F, Larsen CP, Alberu J. et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant 2012; 12: 210–217 [DOI] [PubMed] [Google Scholar]
- 26. Attias P, Melica G, Boutboul D. et al. Epidemiology, risk factors, and outcomes of opportunistic infections after kidney allograft transplantation in the era of modern immunosuppression: a monocentric cohort study. J Clin Med 2019; 8: 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bertrand D, Chavarot N, Gatault P. et al. Opportunistic infections after conversion to belatacept in kidney transplantation. Nephrol Dial Transplant 2020; 35: 336–345 [DOI] [PubMed] [Google Scholar]
- 28. Vincenti F, Blancho G, Durrbach A. et al. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol 2010; 21: 1587–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]