Abstract
Objective:
This study aims to explore and determine the effectiveness of current pharmacologic agents for the prevention of noise-induced hearing loss (NIHL) via a systematic review.
Databases Reviewed:
The PubMed, Scopus, ClinicalTrials.gov, and Cochrane Library databases were searched from inception through February 6, 2020.
Methods:
Full-text, English language articles detailing prospective randomized and non-randomized clinical trials with pharmacological interventions administered to prevent NIHL were included in accordance with PRISMA guidelines. The detailed search terms are included in the Appendix.
Results:
Eleven articles were included in this review with 701 patients receiving a pharmacologic prevention for various noise exposures. Various regimens included administration of alpha-lipoic acid (ALA), ambient oxygen, beta-carotene, carbogen, ebselen, Mg-aspartate, N-acetylcysteine (NAC), and vitamins C, E, and B12. A number of studies demonstrated statistically significant amelioration of NIHL with pharmacologic intervention. Two studies demonstrated significantly better hearing outcomes for pharmacological prophylaxis with carbogen or ebselen as compared to placebo for the 4 kHz frequency, where the noise-notch is most likely to be encountered. Given the considerable heterogeneity in agents and methodologies, however, it was not possible to conduct a meta-analysis.
Conclusions:
While several heterogenous articles demonstrated promising results for Mg-aspartate, carbogen, vitamin B12, and ALA, the clinical significance of these pharmaceuticals remains unclear. Initial data from this study alongside future clinical trials might potentially contribute to the generation of clinical practice guidelines to prevent NIHL.
Level of Evidence:
2
Keywords: noise-induced hearing loss, prevention, pharmacologic, NIHL, systematic review
Introduction
Noise exposure is an extremely common cause of sensorineural hearing loss that can lead to auditory, physiological, and psychosocial deterioration. It is estimated that approximately 5% of the world’s population suffers from noise-induced hearing loss (NIHL), and it is the most prevalent occupational-related disease in the United States (1). Globally, a staggering 1.1 billion adolescents and young adults are at risk for noise-related hearing loss (2).
The severity, duration, and types of noise exposure determine the degree of hearing loss. Both genetic and environmental factors play a role in the susceptibility of individuals to NIHL. Typically, NIHL will first affect hearing in the 4000 to 6000 Hz range, leading to a noise-notch configuration on an audiogram (3). Individuals with longer durations of noise exposure often demonstrate patterns of more profound hearing loss above and below these frequencies. At the microscopic level, noise injury leads to cochlear hair cell loss and a progressive degeneration of acoustic afferent nerve fibers, resulting in permanent hearing loss.
Currently, the management and treatment of NIHL is poorly understood. Previous literature regarding treatment regimens exhibited variable and inconsistent results (4,5). With this as a background, prevention of noise-induced hearing loss, rather than its treatment, is a major public health initiative and is the focus of this study. Prevention of NIHL with consistent use of earplugs and other hearing protection devices has been evaluated extensively, but user-reported rates are variable (6,7). Therefore, it is not always possible to prevent noise exposure itself nor reasonable to expect the consistent use of hearing protection devices.
Preliminary data in animal models have provided evidence supporting various classes of pharmacological agents including anti-inflammatories, antioxidants, minerals, calcium antagonists, vitamins, hemodilution agents, and others to prevent NIHL (8). More recently, prospective clinical trials conducted in humans have demonstrated promise in using pharmacological agents to prevent NIHL. The primary purpose of this study is to perform a systematic review to identify, critically appraise, and evaluate the outcomes of various pharmacological agents that have been investigated for the prevention of NIHL.
Materials and Methods
Literature Search
Since the nature of this article was a systematic review, it was deemed to be exempt from local institutional review board (IRB) approval. The search methodology of this study was designed a priori and completed with the assistance of a senior medical librarian (EAB) in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines and checklist (9). The PubMed (U.S. National Library of Medicine, National Institute of Health), Scopus (Elsevier), ClinicalTrials.gov (U.S. National Library of Medicine, National Institute of Health), and Cochrane Library (Wiley) databases were queried for clinical trials with terms such as: “noise-induced hearing loss”, and “prevention” or “protection”, and the detailed search methodology including utilized medial subject heading (MeSH) terms is included in the Appendix. These databases were searched from inception through February 6, 2020. Additional articles were found via hand-searching of the reference lists of included articles and cited articles, when appropriate. The EndNote software (Version 9.3, Clarivate Analytics, Philadelphia, PA) was utilized to review references for inclusion in this study.
Articles were screened for relevance independently by two authors (AG and SK) for full-text review. There was generally a strong degree of inter-reviewer agreement for the inclusion of an article among the two primary review authors, and a third author (SAN) was available if necessary to reach consensus. The inclusion criteria for this review (Table 1) were determined by using the PICO (Population, Intervention, Comparison, and Outcome) framework for the following: a study population consisting of subjects that did not demonstrate prior evidence of NIHL, an intervention detailing a pharmacologic regimen prior to a noise exposure, comparisons of placebo/control versus interventions (if possible), and hearing outcome assessments by audiometric evaluation. Studies were excluded if the subjects were non-human, if the manuscript was not written in the English language, available only in print, if prior evidence of hearing loss was demonstrated, if a treatment was provided after noise exposure, or if the pharmacological regimen was not detailed. Case reports, book chapters, conference proceedings, and review articles were also excluded.
Table 1.
Inclusion criteria for selection of articles.
| Inclusion Criteria |
|---|
| Population: No evidence of prior noise-induced hearing loss in subjects |
| Intervention: Pharmacologic agent was provided to subjects prior to noise-exposure(s) |
| Comparison: Pharmacologic intervention versus placebo intervention (if possible) |
| Outcome: Audiometric efficacy of provided pharmacologic regimen |
Quality Assessment and Critical Appraisal
The Cochrane Risk of Bias assessment tool was used to independently appraise the methodological quality of the included trials in our systematic review (10). Additionally, articles were independently assigned a hierarchical evidence level using the Oxford Center for Evidence-Based Medicine (OCEBM) Levels of Evidence Table (11). If there was disagreement regarding the assignment of bias or level of evidence, a third author (SAN) was consulted in order to reach consensus. Risk of bias items included the following: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The risk of bias for each aspect is graded as “low,” “unclear,” or “high.”
Data Extraction
The following variables were extracted by two authors (AG and SK) from full-text articles, when possible, for qualitative analyses: authors of the study, country of origin, sample sizes of patients, demographic characteristics including mean age and range, types and durations of noise exposures utilized or encountered, pharmacological agents utilized in the prevention strategy, regimen and dosing, primary endpoint of hearing assessment, and mean corresponding threshold shifts in decibels (dB) with standard deviations (SD) and statistical p values. Data were not extracted from graphical representations that did not subsequently present exact numerical values. Multiple attempts were made to contact corresponding authors of the included articles. Data collection and analysis were performed up to February 6, 2020.
Statistical Methods
Given the heterogeneity and lack of adequate data in the outcome metrics, it was not possible to perform a meta-analysis or statistical tests of comparison.
Results
The previously outlined search strategy yielded a total of 962 articles (Figure 1). After initial de-duplication, 637 articles remained for screening. After title and abstract screening, 565 articles were excluded. The remaining 72 articles underwent full-text review and 11 articles were included for qualitative review (12–22).
Figure 1.
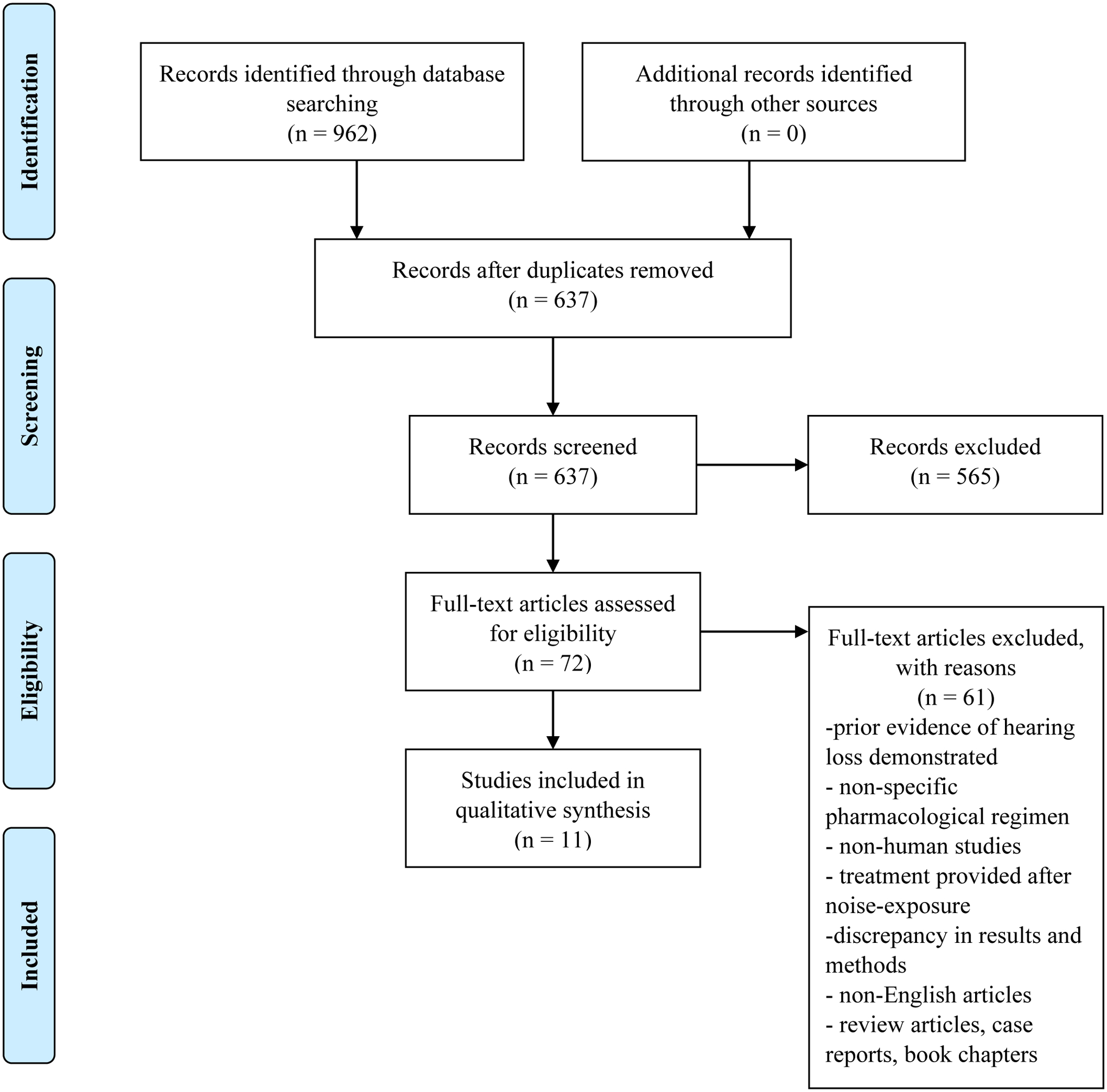
Flowchart of literature review process using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Characteristics of Included Studies
The majority of included articles were randomized, placebo-controlled, clinical trials. Tables 2 and 3 outline the baseline characteristics, methodologies, and results of the included studies. The earliest year of publication for included studies was 1980, and the mean year of publication was 2002. Studies were conducted in many different countries, including the United States, Germany, India, Israel, and Italy. Across all studies, 701 patients received a prophylactic pharmacologic agent. The majority of all patients were male (89%) and tended to be young to middle-aged adults in both civilian and military populations for studies with available gender data.
Table 2.
Baseline characteristics of included studies.
| Study | Country | Study Design | Pharmacologic Agent | Sample Size | Mean Age (range) | Male/Female (%) | OCEBM Evidence Level |
|---|---|---|---|---|---|---|---|
| Attias et al. 1994(12) | Israel & Germany | RCT | 1) 167 Mg-Asp 2) placebo |
1) 125 2) 130 |
(17.5–18.5) | 100/0 | 2 |
| Attias et al. 2004(13) | Israel & Germany | RCT | 1) 122 mg Mg-Asp 2) placebo |
1) 20 2) 20 |
21 (16–37) | 100/0 | 2 |
| Chaturvedi et al. 1984(14) | India | NRCT | 1) carbogen 2) ambient O2 |
1) 12 2) 12 |
(22–28) | 100/0 | 4 |
| Joachims et al. 1992(15) | Israel | RCT | 1) 6.7 mmol Mg-Asp 2) placebo |
1) 125 2) 130 |
18 | 100/0 | 2 |
| Kil et al. 2017(16) | United States | RCT | 1) 200 mg ebselen 2) 400 mg ebselen 3) 600 mg ebselen 4) placebo |
1) 19 2) 20 3) 18 4) 20 |
1) 20.8 (19–25) 2) 20.6 (19–23) 3) 20 (18–21) 4) 21.4 (18–26) |
1) 59/41 2) 55/45 3) 57/43 4) 35/65 |
2 |
| Kopke et al. 2015(17) | United States | RCT | 1) 2700 mg NAC 2) placebo |
1) 277 2) 289 |
1) 19.4 2) 19.8 (18–35) |
100/0 | 2 |
| Kramer et al. 2006(18) | United States | RCT | 1) 900 mg NAC 2) placebo |
1) 15 2) 16 |
22 (19–29) | 45/55 | 2 |
| Le Prell et al. 2016(19) | United States | RCT | 1) beta-carotene, vitamins C & E, Mg 2) placebo |
1) 35 1) 35 |
21.6 (18–28) | 46/54 | 2 |
| Quaranta et al. 2004(20) | Italy | RCT | 1) vitamin B12 regimen 2) placebo |
1) 10 2) 10 |
(20–30) | −/− | 2 |
| Quaranta et al. 2012(21) | Italy | RCT | 1) 600 mg ALA one-time 2) 600 mg ALA regimen 3) control |
1) 10 2) 10 3) 10 |
23.9 (20–30) | 50/50 | 2 |
| Witter et al. 1980(22) | United States | NRCT | 1) carbogen 2) compressed air |
1) 5 2) 5 |
(25–35) | 40/60 | 4 |
Abbreviations: ALA = alpha-lipoic acid, Asp = Aspartate, NAC = N-acetylcysteine, NRCT = non-randomized clinical trial, OCEBM = Oxford Center for Evidence-Based Medicine, RCT = randomized clinical trial
Table 3.
Reported outcomes of included studies.
| Study | Type of Exposure (average/range dB) | Exposure Duration | Post-Exposure Measurement | Range of Frequencies (kHz) | Audiometric Outcome | Significant Result (p < 0.05) |
|---|---|---|---|---|---|---|
| Attias et al. 1994(12) | M-16 rifle (164) | 8 weeks | 7–10 days | 2 to 8 | Greater incidence of post-exposure PTS among placebo versus Mg group | Yes (p < 0.05) |
| Attias et al. 2004(13) | monaural white noise (90) | 10 min | immediate | 1 to 8 | Greater incidence of post-exposure TTS among placebo versus Mg group | Yes (p = 0.001) |
| Chaturvedi et al. 1984(14) | white noise (100) | 20 min daily for 3 days | 2 min | 0.25 to 8 | Lower TTS was observed after carbogen inhalation versus atmospheric oxygen | Yes (p < 0.05) |
| Joachims et al. 1992(15) | firearms (164) | 2 months | 7 days | 3 to 8 | Greater incidence of post-exposure PTS among placebo versus Mg group | Yes (p < 0.05) |
| Kil et al. 2017(16) | music recordings (100) | 4 hours | 15 min | 0.25 to 8 | 68% reduction of TTS at 4 kHz in 400 mg ebselen prophylaxis compared to placebo | Yes (p = 0.0025) |
| Kopke et al. 2015(17) | impulse, steady-state noise, simulated explosions, M-16 rifle (98 to 112) | 16 days | 10 days | 2 to 20 | No reduction in STS for placebo versus NAC intervention group | No |
| Kramer et al. 2006(18) | live nightclub music (98.1) | 2 hours | immediate | 2 to 8 | No reduction in TTS for placebo versus NAC intervention group | No |
| Le Prell et al. 2016(19) | music recordings (95) | 4 hours | 15 min | 0.25 to 8 | No reduction in TTS at any tested frequency in placebo versus intervention group | No |
| Quaranta et al. 2004(20) | 3 kHz narrowband noise (112) | 10 min | 2 min | 0.25 to 8 | Reduction in TTS at 3 kHz in B12 group versus placebo group | Yes (p < 0.001) |
| Quaranta et al. 2012(21) | 3 kHz pure tone sound (90) | 10 min | 2 min | 3 to 6 | Reduction in TTS at 6 kHz in ALA regimen group versus control group | Yes (p = 0.023) |
| Witter et al. 1980(22) | 1 kHz pure tone sound (100) | 10 min | immediate | 0.5 to 4 | Some protective value of carbogen inhalation compared to placebo | Not analyzed |
Abbreviations: ALA = alpha-lipoic acid, PTS = permanent threshold shift, STS = significant threshold shift (author-defined), TTS = temporary threshold shift
In regard to methodological evaluation of the included articles, types of noise exposures were variable and included recorded music, live music, white noise, and firearms. The mean level of noise exposure ranged from 90 to 164 dB, and the duration of noise exposure varied from 10 minutes to 2 months. Primary endpoints of measurements ranged from immediately after exposure to up to 10 days after the final noise exposure. Frequencies evaluated ranged from 0.5 kHz to 20 kHz. There were insufficient individual frequency data to conduct further statistical comparisons among the included studies via a meta-analysis of continuous measures.
Pharmacologic Interventions and Hearing Outcomes
Various pharmacologic agents were studied, including alpha-lipoic acid (ALA) (antioxidant and free radical scavenger), ambient oxygen, beta-carotene, carbogen (95% oxygen, 5% carbon dioxide), ebselen (novel glutathione peroxidase 1 (GPx1) mimic), Mg-aspartate, N-acetylcysteine (NAC), and vitamins C, E, and B12. Routes of administration were mostly either oral or inhalation, with the exception of intramuscular vitamin B12. The pharmacologic agents were generally well-tolerated, with a small minority of studies reporting complications of headache, drowsiness, otalgia, tinnitus, increased urination, and gastrointestinal symptoms such as indigestion, vomiting, diarrhea and others. There were no life-threatening or severe adverse events.
Seven articles demonstrated statistically significant results for their audiometric hearing outcomes as detailed in Table 2 (12–16,20,21). These outcomes included overall differences in the incidences of permanent threshold shifts (PTS) and temporary threshold shifts (TTS) among placebo/control groups versus intervention groups at various ranges of tested frequencies. Interventions that produced statistically significant results included administrations of Mg-aspartate, carbogen, vitamin B12, and ALA. The dosing, routes of administration, schedule of administration, and possible adverse events (Lexicomp Online, Wolters Kluwer Health, Riverwoods, IL) for these pharmaceutical agents that produced statistically significant results are highlighted in Table 4. Pharmacological agents that did not produce significant results included administrations of NAC and a combination regimen of beta-carotene, vitamins C &E, and Mg. Additionally, one study by Witter et al.(22) using carbogen at a rate of 10 L/min for 30 minutes demonstrated a faster recovery of threshold shift for the experimental group as compared to the control group, but no formal statistical testing was conducted.
Table 4.
Pharmaceutical agents with demonstrated efficacy.
| Study | Pharmacologic Agent | Dosing | Route of Administration | Administration Schedule | Possible Adverse Events |
|---|---|---|---|---|---|
| Attias et al. 1994(12) | Mg-Aspartate | 167 mg | oral | once daily for 8 weeks | abdominal cramps, diarrhea, flatulence |
| Attias et al. 2004(13) | Mg-Aspartate | 122 mg | oral | once daily for 10 days | abdominal cramps, diarrhea, flatulence |
| Chaturvedi et al. 1984(14) | carbogen | 95% O2, 5% CO2 | inhalation | 20 min for 3 days | not defined |
| Joachims et al. 1992(15) | Mg-Aspartate | 4g Mg granulate verum | oral | daily for 2 months | abdominal cramps, diarrhea, flatulence |
| Kil et al. 2017(16) | ebselen | 200 mg 400 mg 600 mg |
oral | twice daily for 4 days | not defined |
| Quaranta et al. 2004(20) | vitamin B12 | 7 doses 1 mg 1 dose 5 mg |
intramuscular | once daily for total of 8 doses | headache, infection, asthenia, paresthesia, glossitis, rhinitis, cardiac failure, thrombosis, pruritis, skin rash, hypokalemia, diarrhea, polycythemia, anaphylactic shock, pulmonary edema, swelling |
| Quaranta et al. 2012(21) | alpha-lipoic acid | 600 mg one-time 600 mg regimen |
oral | one-time once daily for 10 days |
hypersensitivity |
Data for possible adverse events were obtained from the Lexicomp Online Drug Database (Wolters Kluwer Health, Riverwoods, IL)
For data specifically available at the 4 kHz frequency where the noise-notch is most frequently encountered, only 2 studies demonstrated significantly better hearing outcomes for pharmacological prophylaxis as compared to placebo (14,16). In Chaturvedi et al.(14), subjects receiving carbogen inhalation had a significantly lower (p < 0.001) mean threshold shift at the 4 kHz frequency 2 minutes after a 20-minute 100 dB white noise exposure when compared to atmospheric air inhalation (10.04 ± 2.29 dB vs. 22.50 ± 3.67 dB). However, Quaranta et al.(21) also measured the threshold shift 2 minutes after a 10-minute 90 dB 3 kHz pure tone exposure at 4 kHz but found non-significant (p = 0.521) differences when comparing two different ALA regimens versus a control group (11.7 ± 4.2 dB for single-dose ALA, 10.9 ± 6.5 dB for 10-day ALA, and 10.4 ± 7.9 dB for control group). When the threshold shift was measured at 15-minutes post-exposure to 100 dB music for 4 hours in a study by Kil et al.(16) there was a significant difference (p = 0.0025) in the mean threshold shift at 4 kHz after a course of 400 mg of ebselen (1.32 ± 4.07 dB) versus placebo (4.07 ± 4.02 dB) in 20 patients.
Levels of Evidence and Risk of Bias Assessment
The mean OCEBM level of evidence for the articles included in the review was 2.4 and ranged from 2 to 4 (on a scale of 1 to 4, with 1 being the highest level of evidence). In regard to the bias assessment of these studies (Figure 2), there were generally low risks of performance and detection biases. Additionally, there were mostly unclear risks for random sequence generation, allocation concealment, selective reporting, and other biases with inadequate descriptions of randomization methods and reporting of data for inclusion in meta-analyses or lack of pre-specified primary outcomes.
Figure 2.
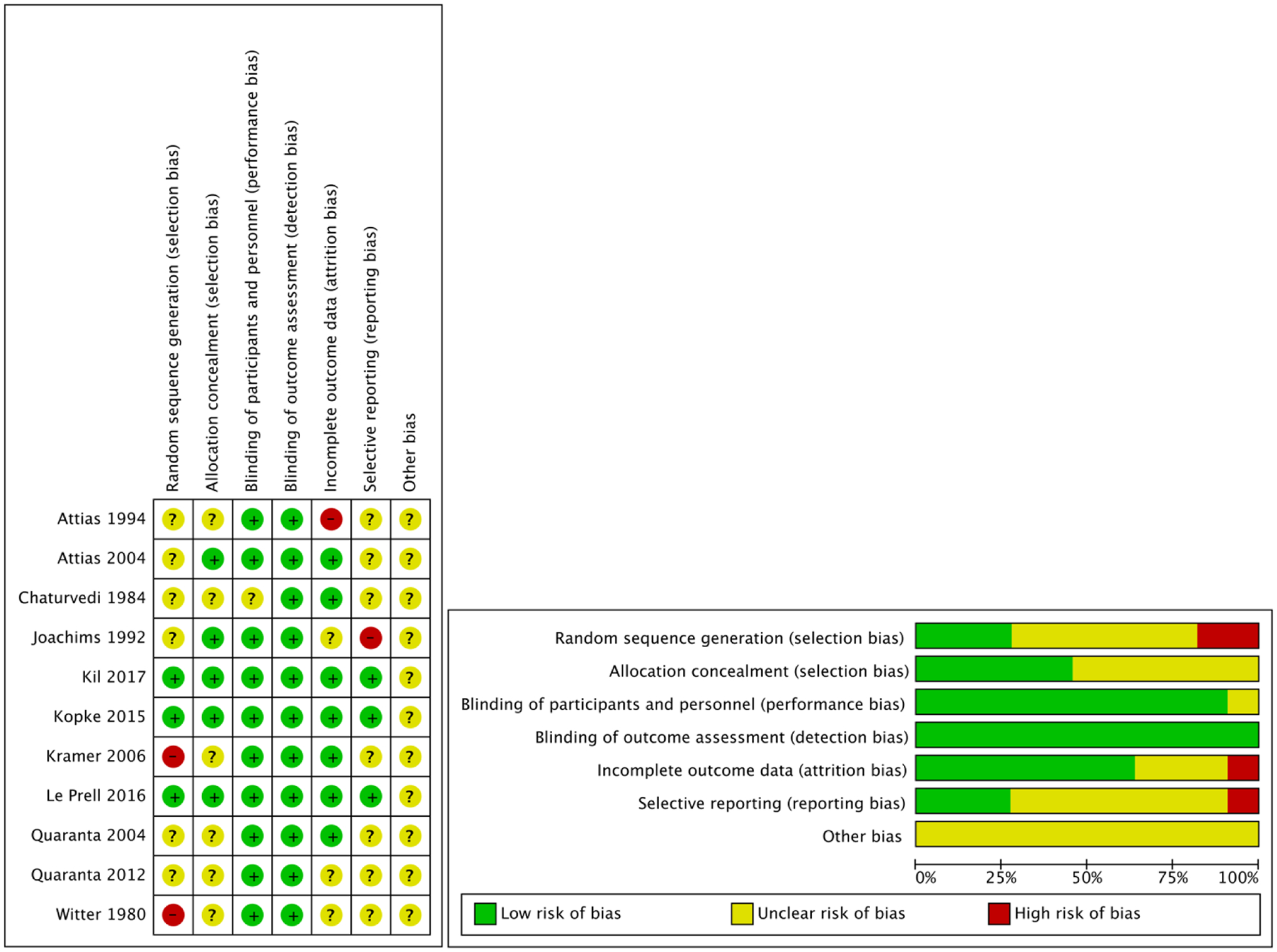
Risk of bias appraisal for included studies.
Plus sign (+) indicates low risk of bias, question mark (?) indicates unclear risk of bias, minus sign (−) indicates high risk of bias.
Discussion
To the best of our knowledge, this review represents one of the first attempts to systematically evaluate the outcomes of various pharmacologic agents that have been investigated for the prevention of NIHL. Limited literature was available for qualitative review prior to more recent randomized clinical trials. Although a previous Cochrane review addressed strategies to prevent NIHL, the focus of the review was limited to non-pharmaceutical interventions for occupational noise exposure (23). There was very low-quality evidence to support the adherence of using hearing protection devices (HPDs) in conjunction with hearing loss prevention programs in that review. Moreover, recent literature demonstrated that only 8% of participants in a survey of 6,357 American adults reported consistent HPD use for loud entertainment or sporting events (7). There is a fundamental gap in the prevention strategies for NIHL, as the use of HPDs is not sufficient in all circumstances. For example, in military combat it might not always be feasible to have adequate team communication and use HPDs consistently. Pharmacologic agents can possibly address this gap and are emerging as feasible solutions to address this prevalent and preventable condition which afflicts both civilian and military personnel.
While it is known that there are currently no known Food and Drug Administration (FDA) approved agents with an indication for NIHL prevention, prior preclinical literature has focused upon agents such as antioxidants, neurotrophins, calcineurin inhibitors, diuretics, glucocorticoids, growth factors, iron chelators, c-Jun-N-terminase kinase (JNK) inhibitors, magnesium, N-methyl-D-aspartate (NMDA) antagonists, nitric oxide synthase (NOS) inhibitors, and others (24). It is theorized that NIHL is caused by irreversible damage to the outer cochlear hair cells due to the generation of free radical species with subsequent loss of cochlear amplification (25). Therefore, the mechanism of action of most of the included agents in our review targeted either free radical scavenging, modulation of oxygen delivery, vascular endothelial repair, and stabilization of the cochlear hair cells. This current systematic review identified clinical trials for several well-tolerated agents and known supplements such as ALA, ambient oxygen, beta-carotene, carbogen, ebselen, Mg-aspartate, NAC, vitamin C, vitamin E, and vitamin B12. Interestingly, both articles that utilized NAC did not demonstrate statistically significant results in their primary hearing outcomes when compared to placebo agents (17,18). However, to the heterogenous nature of the reported data, we were unable to conclude if one particular agent had a statistically superior effect in preventing NIHL.
The majority of the articles included in our study were randomized clinical trials but had varying methodologies and reporting of results, which contributed to the considerable heterogeneity of the studies included. Interestingly, the majority of studies reported statistically significant results of their primary outcomes, but these outcomes were heterogenous in nature with regard to clinical relevance. The non-randomized study by Chaturvedi et al.(14) demonstrated statistically significant (p < 0.05) differences in their outcome of using carbogen as compared to ambient air inhalation at 2 minutes post-exposure across all tested frequencies. The resulting magnitude of this change also appeared to be clinically relevant (10.04 ± 2.29 dB for the carbogen group vs. 22.50 ± 3.67 dB for the control). However, these results were short-lived without any significant differences in hearing outcomes for the carbogen inhalation group after 60 minutes. It is currently unknown if any long-term benefit or harm exists for pharmacologic agents for the prevention of hearing loss. Additionally, the dosing and administration of the different agents were variable. For example, Kil et al.(16) demonstrated a significant (p = 0.0004) mean difference of 75% with use of 400 mg of ebselen at 4 kHz and at an average of 3, 4, and 6 kHz frequencies. However, it is important to note that this difference in the ebselen treatment versus placebo group was only 2.4 dB. The clinical efficacy of this treatment regimen should be interpreted with caution. Additionally, statistically significant results were not reproducible with administration of either 200 mg or 600 mg dosages, indicating variability in dosing and reproducibility of results. It was also difficult to ascertain which exposure type, duration, or noise level were predictive of statistically significant results across all included studies. Many authors also did not define or had varying definitions of what was considered to be a significant threshold shift, as this ranged from changes of 5 dB up to 25 dB. Inherently, is it not possible to compare the effect of hearing recovery among different individuals that were exposed to various types, intensities, and durations of noise-exposures after varying regimens and doses of different pharmaceutical agents. Furthermore, there were insufficient individual frequency data to pool data for quantitative analyses.
Results from our study suggest that there were promising statistically significant effects for using pharmacologic agents such as Mg-aspartate, carbogen, vitamin B12, and ALA in the prevention of NIHL. However, these results should be interpreted with caution, as there were variable study designs, pharmacologic agents, and reporting of outcome data, suggesting that research concerning the effects of various pharmacologic agents is at an early stage. Moreover, the clinical relevance and applicability of our findings are yet to be elucidated, as even fewer studies demonstrated clinically relevant differences in their audiometric outcomes. While clinical practice guidelines exist for sudden sensorineural hearing loss suggesting that clinicians have the option to offer steroids or hyperbaric oxygen as initial therapy as a treatment(26), there is no current recommendation for pharmaceutical prophylaxis in the context of NIHL. While our study provides initial data for some heterogenous clinical trials, clinicians should cautiously interpret the risk-benefit ratio for administration of an agent that is not FDA-approved for the particular indication of NIHL.
Limitations of this Review
The studies selected for this systematic review had considerable heterogeneity, varying methodologies, and were conducted across many different locations and years. In addition, publication bias exists in the quantity and quality of available literature for a systematic review, such as the exclusion of articles published only in print or those not available in the English language. There were limited data to compare the pharmacologic agents, and therefore it was not possible to determine if one pharmacologic regimen was statistically superior. Additionally, there exists considerable heterogeneity in the measurement of pure tone audiometry and available data for individual frequencies, limiting the ability to directly compare all available frequencies. Future clinical trials might consider protocols with a more standardized format for the reporting of hearing outcome data with long-term follow up in order to allow for reproducibility and direct comparisons (27).
Conclusions
Various experimental pharmacologic regimens were evaluated for the prevention of NIHL, and while there were statistically significant effects for the administration of Mg-aspartate, carbogen, vitamin B12, and ALA, the overall clinical value of pharmacological prevention remains unclear. There were a limited number of heterogenous studies in this systematic review. Future prospective, double-blinded, randomized, placebo-controlled clinical trials with standardized reporting of audiometric data are necessary to evaluate the clinical efficacy of pharmacological prevention for NIHL.
Supplementary Material
Appendix. Detailed search methodology of selected databases for initial identification of studies for the pharmacological prevention of noise-induced hearing loss.
Acknowledgments
This work was supported by the “Interdisciplinary Research Training in Otolaryngology and Communication Sciences” NIH T32 DC014435 Grant
Footnotes
No disclosures, financial or otherwise
No conflicts of interest
References
- 1.Basner M, Babisch W, Davis A et al. Auditory and non-auditory effects of noise on health. Lancet 2014;383:1325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy WJ, Eichwald J, Meinke DK et al. CDC Grand Rounds: Promoting Hearing Health Across the Lifespan. MMWR Morb Mortal Wkly Rep 2018;67:243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson DI, Nelson RY, Concha-Barrientos M et al. The global burden of occupational noise-induced hearing loss. Am J Ind Med 2005;48:446–58. [DOI] [PubMed] [Google Scholar]
- 4.Sha SH, Schacht J. Emerging therapeutic interventions against noise-induced hearing loss. Expert Opin Investig Drugs 2017;26:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakat MS, Kilic K, Bercin S. Pharmacological agents used for treatment and prevention in noise-induced hearing loss. Eur Arch Otorhinolaryngol 2016;273:4089–101. [DOI] [PubMed] [Google Scholar]
- 6.Kraaijenga VJC, van Munster J, van Zanten GA. Association of Behavior With Noise-Induced Hearing Loss Among Attendees of an Outdoor Music Festival: A Secondary Analysis of a Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg 2018;144:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichwald J, Scinicariello F, Telfer JL et al. Use of Personal Hearing Protection Devices at Loud Athletic or Entertainment Events Among Adults - United States, 2018. MMWR Morb Mortal Wkly Rep 2018;67:1151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le TN, Straatman LV, Lea J et al. Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J Otolaryngol Head Neck Surg 2017;46:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JP, Altman DG, Gotzsche PC et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durieux N, Vandenput S, Pasleau F. [OCEBM levels of evidence system]. Rev Med Liege 2013;68:644–9. [PubMed] [Google Scholar]
- 12.Attias J, Weisz G, Almog S et al. Oral magnesium intake reduces permanent hearing loss induced by noise exposure. Am J Otolaryngol 1994;15:26–32. [DOI] [PubMed] [Google Scholar]
- 13.Attias J, Sapir S, Bresloff I et al. Reduction in noise-induced temporary threshold shift in humans following oral magnesium intake. Clin Otolaryngol Allied Sci 2004;29:635–41. [DOI] [PubMed] [Google Scholar]
- 14.Chaturvedi RC, Sharma RK, Lakhera SC et al. Role of carbogen in protection against noise induced hearing loss in man. Indian J Med Res 1984;80:583–9. [PubMed] [Google Scholar]
- 15.Joachims ZZ. Oral magnesium supplementation as prophylaxis for noise-induced hearing loss: results of a double blind field study. Schriftenreihe des Vereins fur Wasser-, Boden- und Lufthygiene 1993;88:503–16. [PubMed] [Google Scholar]
- 16.Kil J, Lobarinas E, Spankovich C et al. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017;390:969–79. [DOI] [PubMed] [Google Scholar]
- 17.Kopke R, Slade MD, Jackson R et al. Efficacy and safety of N-acetylcysteine in prevention of noise induced hearing loss: a randomized clinical trial. Hear Res 2015;323:40–50. [DOI] [PubMed] [Google Scholar]
- 18.Kramer S, Dreisbach L, Lockwood J et al. Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music. J Am Acad Audiol 2006;17:265–78. [DOI] [PubMed] [Google Scholar]
- 19.Le Prell CG, Fulbright A, Spankovich C et al. Dietary supplement comprised of beta-carotene, vitamin C, vitamin E, and magnesium: failure to prevent music-induced temporary threshold shift. Audiol Neurotol Extra 2016;6:20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quaranta A, Scaringi A, Bartoli R et al. The effects of ‘supra-physiological’ vitamin B12 administration on temporary threshold shift. Int J Audiol 2004;43:162–5. [DOI] [PubMed] [Google Scholar]
- 21.Quaranta N, Dicorato A, Matera V et al. The effect of alpha-lipoic acid on temporary threshold shift in humans: a preliminary study. Acta Otorhinolaryngol Ital 2012;32:380–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Witter HL, Deka RC, Lipscomb DM et al. Effects of prestimulatory carbogen inhalation on noise-induced temporary threshold shifts in humans and chinchilla. Am J Otol 1980;1:227–32. [PubMed] [Google Scholar]
- 23.Tikka C, Verbeek JH, Kateman E et al. Interventions to prevent occupational noise-induced hearing loss. Cochrane Database Syst Rev 2017;7:CD006396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch ED, Kil J. Compounds for the prevention and treatment of noise-induced hearing loss. Drug Discov Today 2005;10:1291–8. [DOI] [PubMed] [Google Scholar]
- 25.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 2009;29:14077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrasekhar SS, Tsai Do BS, Schwartz SR et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngol Head Neck Surg 2019;161:S1–S45. [DOI] [PubMed] [Google Scholar]
- 27.Gurgel RK, Jackler RK, Dobie RA et al. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg 2012;147:803–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix. Detailed search methodology of selected databases for initial identification of studies for the pharmacological prevention of noise-induced hearing loss.


