Abstract
OBJECTIVE
Advances in diabetes technology have transformed the treatment paradigm for type 1 diabetes, yet the burden of disease is significant. We report on a pivotal safety study of the first tubeless, on-body automated insulin delivery system with customizable glycemic targets.
RESEARCH DESIGN AND METHODS
This single-arm, multicenter, prospective study enrolled 112 children (age 6–13.9 years) and 129 adults (age 14–70 years). A 2-week standard therapy phase (usual insulin regimen) was followed by 3 months of automated insulin delivery. Primary safety outcomes were incidence of severe hypoglycemia and diabetic ketoacidosis. Primary effectiveness outcomes were change in HbA1c and percent time in sensor glucose range 70–180 mg/dL (“time in range”).
RESULTS
A total of 235 participants (98% of enrolled, including 111 children and 124 adults) completed the study. HbA1c was significantly reduced in children by 0.71% (7.8 mmol/mol) (mean ± SD: 7.67 ± 0.95% to 6.99 ± 0.63% [60 ± 10.4 mmol/mol to 53 ± 6.9 mmol/mol], P < 0.0001) and in adults by 0.38% (4.2 mmol/mol) (7.16 ± 0.86% to 6.78 ± 0.68% [55 ± 9.4 mmol/mol to 51 ± 7.4 mmol/mol], P < 0.0001). Time in range was improved from standard therapy by 15.6 ± 11.5% or 3.7 h/day in children and 9.3 ± 11.8% or 2.2 h/day in adults (both P < 0.0001). This was accomplished with a reduction in time in hypoglycemia <70 mg/dL among adults (median [interquartile range]: 2.00% [0.63, 4.06] to 1.09% [0.46, 1.75], P < 0.0001), while this parameter remained the same in children. There were three severe hypoglycemia events not attributable to automated insulin delivery malfunction and one diabetic ketoacidosis event from an infusion site failure.
CONCLUSIONS
This tubeless automated insulin delivery system was safe and allowed participants to significantly improve HbA1c levels and time in target glucose range with a very low occurrence of hypoglycemia.
Introduction
Diabetes care has been transformed by rapid innovation in glucose sensor and insulin pump technology; however, glycemic outcomes continue to be suboptimal across all populations, and the burden of disease for type 1 diabetes is immense (1–5). Automated insulin delivery systems, which combine a subcutaneous insulin infusion pump, glucose sensor, and automated dosing algorithm, have improved glycemic control while reducing hypoglycemia exposure (6–8). Currently, four systems are commercially available in the U.S. and Europe (6,9–12). Each system differs through the combination of specific devices, the system configuration and settings, and the proprietary dosing algorithm. The first automated insulin delivery systems to become available had limited options for customizing glucose targets and interoperability of devices, and so, this has become an area of focus for enhancing the capabilities of more recent systems.
All systems available to date have utilized tethered pumps, which deliver insulin through a length of tubing (46–110 cm) that connects the pump to the infusion site cannula and, therefore, the pump must be located somewhere on the person to accommodate the tubing (e.g., belt, pocket) (13). The algorithm is hosted either on the pump itself, in which case the tethered device must be accessed frequently for user interactions, or on a smartphone that controls the pump, in which case the smartphone must remain within communication range of the pump. In either case, the tubing typically needs to be disconnected for certain activities, such as swimming, exercise, and bathing, during which time no insulin is delivered (12).
The Omnipod 5 Automated Insulin Delivery System (Insulet Corporation, Acton, MA) is a new system that at the time of writing is under review by the U.S. Food and Drug Administration. In addition to a novel algorithm with customizable glucose targets, this system has a unique configuration that utilizes a tubeless insulin pump (Pod), which is a small (3.9 × 5.2 × 1.45 cm) adhesive patch pump worn on the body. The cannula is automatically deployed directly under the Pod, creating an infusion site without external tubing. The Pod is waterproof (IP28) and worn continuously for up to 72 h. All user interactions are conducted wirelessly through a mobile app on a smartphone. The algorithm itself is located on the Pod, and the glucose sensor communicates directly with the Pod through Bluetooth wireless technology. Therefore, the system can continuously provide automated insulin delivery through the wearable on-body components alone (Pod and sensor) without the smartphone needing to be nearby.
We present the results of the first 3-month outpatient pivotal trial of this automated insulin delivery system that uses a wearable tubeless insulin pump with an embedded algorithm that receives communication directly from a glucose sensor through Bluetooth wireless technology (14). The system has been designed to minimize hypoglycemia while maximizing time in glycemic target range, ensuring safety and effectiveness across a broad range of people with diabetes from children to seniors (15,16). Preliminary versions of the algorithm were evaluated in several clinical studies (15–18), and an early trial of the commercial configuration demonstrated positive results (14).
Research Design and Methods
Study Conduct and Oversight
A single-arm, multicenter, prospective clinical study was conducted at 17 sites in the U.S. The protocol was approved by a central institutional review board and relevant local review boards. Informed consent was obtained from participants aged ≥18 years. For participants <18 years, assent and consent were obtained from participants and their parents or guardians, respectively, according to state requirements. The U.S. Food and Drug Administration approved an investigational device exemption. An independent data and safety monitoring board as well as a medical monitor provided trial oversight.
Study Design and Participants
Eligible participants were aged 6–70 years, diagnosed with type 1 diabetes for at least 6 months, had a point-of-care screening HbA1c <10.0% (86 mmol/mol), and did not have a history of severe hypoglycemia or diabetic ketoacidosis in the past 6 months (complete eligibility criteria are listed in Supplementary Table 1). Participants were enrolled into two cohorts: children (age 6–13.9 years) and adolescents/adults (age 14–70 years, subsequently referred to as adults). Both cohorts followed the same protocol and used an identical automated insulin delivery system.
After screening, participants completed a 2-week standard therapy phase to collect glucose sensor data on their usual diabetes care regimen to be used as a comparator to automated insulin delivery. Participants wore a glucose sensor in blinded mode during the standard therapy phase if a glucose sensor was not part of their usual regimen. Participants were then trained on the investigational system, which consisted of a tubeless insulin pump (Pod) with an embedded automated insulin delivery algorithm (Omnipod 5), an interoperable glucose sensor (Dexcom G6; Dexcom, San Diego, CA), and a mobile app (Omnipod 5 app) on a locked-down Android phone.
Participants controlled the system through the mobile app for daily interactions, including delivering meal and correction boluses and setting target glucose profiles. The mobile app was also used for initial setup and to start automated mode. Both the Pod and the continuous glucose monitor (CGM) were worn on the body, and the CGM communicated directly with the algorithm on the Pod. Therefore, the smartphone containing the app did not need to be present for automated insulin delivery to continue once initiated since the algorithm was embedded directly onto the on-body Pod.
During automated mode, each Pod delivers insulin microboluses every 5 min on the basis of current and projected glycemic values to bring the glucose level toward the selected target. The main determinant of automated insulin delivery is the user-selected glucose target, which can range from 110 to 150 mg/dL in 10 mg/dL increments and can be programmed to vary by time of day. The insulin delivery is continuously adjusted to meet this target and is based on an adaptive basal rate determined from the total daily insulin delivered, which is tracked and updated with each subsequent Pod change. The basal rates entered by the user at first-time setup are only relevant for the first Pod use in automated mode or when the system is in manual insulin delivery mode.
Study participants were educated on additional system options, such as the HypoProtect feature that could be used to temporarily set a higher glucose target of 150 mg/dL with additional restrictions on insulin delivery if anticipating lower insulin needs, such as during exercise. Participants were encouraged to use the bolus calculator within the mobile app to deliver meal and correction boluses through the Pod. These boluses were calculated on the basis of user-defined insulin-to-carbohydrate ratios, correction factors, and glucose targets. The bolus calculator adjusted the suggested insulin bolus on the basis of insulin on board as well as the CGM trend arrow in both automated and manual mode. Further details on system function have been previously published (14).
The system was used for 3 months, with nine follow-up study visits occurring in person or by phone (Supplementary Table 2). During each visit, participants were asked about adverse events, medication use, and device issues, and the study team reviewed the system data history and recommended changes to pump settings as needed. Although study teams had access to real-time monitoring of system data, they were not instructed to conduct surveillance outside of study visits, and there were no automated alerts to investigators related to glycemia. Reportable adverse events included adverse device events, adverse events associated with study procedures or hyperglycemia associated with ketosis (>1 mmol/L), or serious adverse events such as severe hypoglycemia, defined as requiring assistance because of altered consciousness, and diabetic ketoacidosis (19).
HbA1c was measured at baseline and the final study visit using laboratory measures, which could differ from point-of-care measures used for eligibility screening. Additional optional measurements were added at the start and end of the study pause (discussed below). HbA1c (Tosoh Bioscience) was measured by a central laboratory (University of Minnesota Advanced Research and Diagnostic Laboratory).
Use of automated insulin delivery was paused study-wide from 28 February 2020 to 4 June 2020 because of a software anomaly with the potential to impact insulin delivery as a result of erroneous system inputs in certain uncommon circumstances. The study was paused as a precaution, and there were no adverse events associated with the anomaly. The median (interquartile range) number of days completed before and after the pause were 46 (36, 56) and 49 (38, 59) for children and 43 (30, 52) and 49 (41, 63) for adults, respectively. During the study pause, participants could continue study system use without activation of automated insulin delivery features where the device functioned as a stand-alone insulin pump (77% of participants), or they could use another insulin regimen (23%). Once the software update was implemented, participants resumed automated delivery, which occurred remotely for most participants because of the COVID-19 pandemic.
Outcomes
The primary safety outcomes were incidence rates of severe hypoglycemia and diabetic ketoacidosis. The primary effectiveness outcomes were change in HbA1c and percentage of time in glucose target range 70–180 mg/dL (“time in range”) measured by the glucose sensor during the 3-month automated insulin delivery phase compared with the 2-week standard therapy phase. Secondary outcomes included percent time <70 mg/dL and >180 mg/dL, percent time in additional glycemic ranges (<54 mg/dL, ≥250 mg/dL, ≥300 mg/dL), additional glycemic measures (mean, SD, and coefficient of variation of sensor glucose), and additional clinical measures (total daily dose of insulin, total daily basal or bolus insulin delivery, BMI). Measures related to system use were also assessed (percentage of study time spent in automated mode, number and type of device deficiencies observed).
Statistical Methods
This study was designed to provide 90% power and a one-sided significance level of 2.5% or 1.25% for the two primary effectiveness outcomes. The sample size estimation assumed a mean difference and SD of the difference in HbA1c of 0.5% (5.5 mmol/mol) and 0.8% (8.7 mmol/mol) and time in range of 10% and 15%. Although 35 and 31 participants were required for the two effectiveness outcomes, respectively, enrollment of up to 240 participants was planned for a robust safety evaluation.
Analyses were performed on a modified intention-to-treat data set of participants who entered the automated insulin delivery phase. Any sensor or insulin delivery data collected during the study pause were not included in the analyses of outcomes, and any adverse events or device deficiencies during the pause were listed separately. Sensor data were primarily obtained from the investigational app through cloud-based transmission, with data from Dexcom Clarity as a secondary source. No imputations for missing data were planned or performed.
Analyses were conducted separately for children and adults. Results are presented as means with SDs or medians with interquartile ranges as appropriate. The primary safety outcome was considered a success if the incidence rates of severe hypoglycemia and diabetic ketoacidosis were considered acceptable compared with published rates (3,4). Effectiveness outcomes were evaluated using paired t tests, or using Wilcoxon signed rank tests if there were <10 participants in a group or if Shapiro-Wilk tests of normality were significant (P < 0.05). All P values were considered significant at a two-sided level of 0.05. Analysis was performed using SAS 9.4 statistical software.
Results
Participant Characteristics
Between 19 December 2019 and 28 February 2020, 241 participants were enrolled, and 240 entered the automated insulin delivery phase and were included in the modified intention-to-treat data set. Baseline characteristics are included in Table 1. Ninety-eight percent of enrolled participants completed the main study (Supplementary Fig. 1). Of these, 224 (95%) elected to participate in an ongoing extension phase.
Table 1.
Characteristics at baseline of the study participants in the modified intention-to-treat data set
| Characteristic | Children (age 6–13.9 years) | Adults (age 14–70 years) |
|---|---|---|
| n | 112 | 128 |
| Age (years) | 10.3 ± 2.2 (6.0, 14.0#) | 36.9 ± 13.9 (14.5, 69.8) |
| Duration of diabetes (years) | 4.7 ± 2.6 (0.6, 11.6) | 17.9 ± 11.6 (1.0, 51.0) |
| BMI (kg/m2) | 18.6 ± 3.2 (13.7, 32.4) | 26.6 ± 4.7 (18.9, 41.4) |
| Female sex, n (%) | 60 (53.6) | 78 (60.9) |
| Race/ethnicity, n (%)‡ | ||
| White | 104 (92.9) | 117 (91.4) |
| Hispanic or Latino | 8 (7.1) | 6 (4.7) |
| Not Hispanic or Latino | 96 (85.7) | 111 (86.7) |
| Black or African American, White | 3 (2.7) | — |
| Black or African American | 2 (1.8) | 5 (3.9) |
| Hispanic or Latino | — | 1 (0.8) |
| Not Hispanic or Latino | 2 (1.8) | 4 (3.1) |
| Asian | — | 2 (1.6) |
| Asian, White | 2 (1.8) | — |
| Asian, Native Hawaiian or Pacific Islander, White | 1 (0.9) | — |
| American Indian or Alaska Native, White | — | 1 (0.8) |
| American Indian or Alaska Native | — | 3 (2.3) |
| Hispanic or Latino | — | 3 (2.3) |
| Not Hispanic or Latino | — | 0 (0.0) |
| HbA1c (%)§ | 7.67 ± 0.95 (5.80, 10.30) | 7.16 ± 0.86 (5.20, 9.80) |
| HbA1c (mmol/mol)§ | 60 ± 10.4 (40, 89) | 55 ± 9.4 (33, 84) |
| Daily insulin dose (units/kg)ǁ | 0.85 ± 0.24 (0.25, 1.47) | 0.61 ± 0.22 (0.16, 1.31) |
| Previous¶ or current CGM use, n (%) | 108 (96.4) | 126 (98.4) |
| Previous¶ or current pump use, n (%) | 100 (89.3) | 115 (89.8) |
| Use of multiple daily injections as standard therapy method, n (%) | 13 (11.6) | 20 (15.6) |
Data are mean ± SD (minimum, maximum) unless otherwise indicated.
Age was determined at the date of informed consent. The birth date of one participant fell immediately after the informed consent date, resulting in inclusion in the children cohort despite the age being 14.0 years after rounding.
Race and ethnicity were reported by the participants and are displayed exactly as reported. As shown, several participants chose more than one racial category. Ethnicity delineation is shown for racial categories where at least one person identified as Hispanic or Latino.
Participant eligibility for the study was determined using a point-of-care HbA1c measurement performed at screening, which in some cases differed from the laboratory assessment displayed here and used for analysis.
Baseline total daily insulin dose was determined from data collected during the standard therapy phase.
Previous use is defined as having used the device for any duration in the past.
Effectiveness Outcomes
HbA1c was reduced in children by 0.71% (7.8 mmol/mol) (7.67 ± 0.95% to 6.99 ± 0.63% [60 ± 10.4 to 53 ± 6.9 mmol/mol], P < 0.0001) and in adults by 0.38% (4.2 mmol/mol) (7.16 ± 0.86% to 6.78 ± 0.68% [55 ± 9.4 to 51 ± 7.4 mmol/mol], P < 0.0001) (Table 2). Improvement was seen in both age-groups regardless of their baseline glycemic control (20) (Fig. 1).
Table 2.
Primary and secondary effectiveness outcomes
| Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|
| Children (age 6–13.9 years, n = 112) | Adults (age 14–70 years, n = 128) | |||||||
| Parameter | Baseline§ or standard therapy phase | Follow-up§ or automated insulin delivery phase | Change | P valueǁ | Baseline§ or standard therapy phase | Follow-up§ or automated insulin delivery phase | Change | P valueǁ |
| Overall (24 h) | ||||||||
| Primary effectiveness outcome, HbA1c† % | 7.67 ± 0.95, 7.50 (7.00, 8.30) | 6.99 ± 0.63, 6.90 (6.50, 7.40) | –0.71 ± 0.63, –0.65 (–1.10, –0.30) | <0.0001 | 7.16 ± 0.86, 7.10 (6.60, 7.60) | 6.78 ± 0.68, 6.70 (6.40, 7.15) | –0.38 ± 0.54, –0.30 (–0.70, –0.00) | <0.0001 |
| mmol/mol | 60 ± 10.4, 58 (53, 67) | 53 ± 6.9, 52 (48, 57) | –7.8 ± 6.9, –7.1 (–12, 3.3) | — | 55 ± 9.4, 54 (49, 60) | 51 ± 7.4, 50 (46, 55) | –4.2 ± 5.9, –3.3 (–7.7, 0.0) | — |
| Primary effectiveness outcome, time 70–180 mg/dL, % | 52.5 ± 15.6, 52.8 (39.3, 63.2) | 68.0 ± 8.1, 68.2 (63.3, 73.5) | 15.6 ± 11.5, 15.0 (7.8, 24.6) | <0.0001 | 64.7 ± 16.6, 67.1 (51.3, 77.6) | 73.9 ± 11.0, 75.8 (68.0, 81.1) | 9.3 ± 11.8, 7.9 (0.9, 15.5) | <0.0001 |
| Mean sensor glucose, mg/dL | 183 ± 32, 181 (163, 205) | 160 ± 15, 159 (150, 171) | –23 ± 23, –21 (–37, –7) | <0.0001 | 161 ± 28, 156 (138, 178) | 154 ± 17, 151 (143, 163) | –8 ± 20, –6 (–16, 7) | 0.0002 |
| SD of sensor glucose, mg/dL | 68 ± 13, 68 (59, 77) | 60 ± 10, 59 (52, 65) | –9 ± 8, –8 (–14, –3) | <0.0001 | 57 ± 14, 55 (46, 67) | 49 ± 11, 48 (42, 55) | –8 ± 9, –7 (–13, –1) | <0.0001 |
| CV of sensor glucose, %‡ | 37.5 ± 5.1, 37.1 (33.8, 41.0) | 37.0 ± 3.9, 37.6 (34.3, 39.8) | –0.4 ± 4.2, –0.6 (–3.5, 2.5) | 0.2893 | 35.2 ± 5.7, 34.6 (31.2, 37.9) | 31.7 ± 4.7, 32.1 (28.5, 34.6) | –3.5 ± 4.3, –3.7 (–6.0, –0.3) | <0.0001 |
| Time in glucose range, % | ||||||||
| <54 mg/dL | 0.41 ± 0.83, 0.10 (0.00, 0.41) | 0.32 ± 0.33, 0.23 (0.08, 0.42) | –0.08 ± 0.66, 0.04 (–0.12, 0.20) | 0.1132 | 0.62 ± 1.24, 0.22 (0.00, 0.77) | 0.23 ± 0.27, 0.17 (0.06, 0.28) | –0.39 ± 1.16, –0.08 (–0.46, 0.04) | <0.0001 |
| <70 mg/dL | 2.21 ± 2.66, 1.38 (0.42, 2.67) | 1.78 ± 1.37, 1.48 (0.65, 2.23) | –0.43 ± 2.01, 0.06 (–0.66, 0.68) | 0.8153 | 2.89 ± 3.07, 2.00 (0.63, 4.06) | 1.32 ± 1.10, 1.09 (0.46, 1.75) | –1.57 ± 2.55, –0.89 (–2.23, –0.01) | <0.0001 |
| >180 mg/dL | 45.3 ± 16.7, 44.8 (35.2, 58.9) | 30.2 ± 8.7, 29.7 (24.1, 35.5) | –15.1 ± 12.2, –14.3 (–24.5, –6.8) | <0.0001 | 32.4 ± 17.3, 28.9 (17.6, 46.6) | 24.7 ± 11.2, 22.8 (17.0, 31.4) | –7.7 ± 12.1, –6.2 (–13.9, 1.6) | <0.0001 |
| ≥250 mg/dL | 19.1 ± 13.1, 15.9 (9.7, 26.3) | 9.6 ± 5.4, 8.9 (5.7, 12.4) | –9.4 ± 9.8, –6.1 (–14.4, –2.1) | <0.0001 | 10.1 ± 10.5, 6.6 (2.3, 15.4) | 5.8 ± 5.5, 3.9 (2.2, 7.7) | –4.3 ± 7.7, –2.3 (–6.9, 0.3) | <0.0001 |
| ≥300 mg/dL | 8.5 ± 8.9, 5.8 (2.5, 11.5) | 3.5 ± 2.9, 2.7 (1.4, 4.8) | –5.1 ± 7.1, –2.6 (–7.5, –0.5) | <0.0001 | 3.7 ± 5.5, 1.5 (0.2, 5.0) | 1.7 ± 2.5, 0.8 (0.3, 2.2) | –2.0 ± 4.3, –0.4 (–2.8, 0.1) | <0.0001 |
| Overnight (00:00–06:00 h) | ||||||||
| Primary effectiveness outcome, time 70–180 mg/dL, % | 55.3 ± 19.0, 55.8 (41.2, 70.6) | 78.1 ± 10.8, 79.4 (70.9, 85.9) | 22.9 ± 14.8, 23.5 (12.8, 33.8) | <0.0001 | 64.3 ± 19.5, 66.7 (50.1, 79.5) | 78.1 ± 13.9, 79.9 (69.4, 89.6) | 13.8 ± 14.0, 12.3 (4.5, 20.7) | <0.0001 |
| Mean sensor glucose, mg/dL | 177 ± 35, 173 (154, 200) | 149 ± 17, 147 (136, 158) | –29 ± 27, –27 (–48, –9) | <0.0001 | 160 ± 34, 158 (134, 178) | 149 ± 21, 147 (134, 161) | –11 ± 24, –7 (–23, 4) | <0.0001 |
| SD of sensor glucose, mg/dL | 61 ± 15, 61 (50, 73) | 48 ± 12, 46 (39, 56) | –13 ± 13, –13 (–22, –5) | <0.0001 | 56 ± 17, 55 (43, 67) | 44 ± 13, 42 (34, 52) | –12 ± 12, –12 (–18, –5) | <0.0001 |
| CV of sensor glucose, %‡ | 34.6 ± 7.1, 34.2 (29.0, 38.7) | 31.9 ± 5.6, 31.5 (28.2, 35.6) | –2.8 ± 7.5, –2.5 (–7.5, 1.9) | 0.0002 | 35.0 ± 7.9, 34.4 (29.8, 39.2) | 28.9 ± 5.8, 29.1 (24.8, 32.8) | –6.2 ± 7.0, –6.1 (–9.7, –1.4) | <0.0001 |
| Time in glucose range, % | ||||||||
| <54 mg/dL | 0.57 ± 1.65, 0.00 (0.00, 0.30) | 0.22 ± 0.31, 0.09 (0.02, 0.32) | –0.35 ± 1.52, 0.02 (–0.18, 0.13) | 0.9451 | 0.95 ± 1.86, 0.00 (0.00, 1.06) | 0.24 ± 0.38, 0.09 (0.02, 0.30) | –0.70 ± 1.74, 0.00 (–0.85, 0.09) | 0.0002 |
| <70 mg/dL | 2.51 ± 4.21, 0.78 (0.00, 2.84) | 1.17 ± 1.19, 0.78 (0.37, 1.49) | –1.34 ± 3.81, 0.01 (–1.56, 0.55) | 0.0456 | 3.64 ± 4.49, 2.07 (0.50, 5.54) | 1.17 ± 1.27, 0.82 (0.31, 1.62) | –2.46 ± 4.03, –0.86 (–3.76, 0.14) | <0.0001 |
| >180 mg/dL | 42.2 ± 20.0, 40.1 (27.1, 57.9) | 20.7 ± 10.8, 18.6 (12.7, 26.6) | –21.5 ± 16.0, –21.8 (–33.7, –12.1) | <0.0001 | 32.1 ± 20.2, 29.3 (15.6, 44.5) | 20.7 ± 14.1, 18.8 (8.7, 29.8) | –11.3 ± 14.4, –9.1 (–18.9, –2.2) | <0.0001 |
| ≥250 mg/dL | 16.3 ± 15.0, 11.7 (5.1, 26.4) | 5.4 ± 5.1, 3.5 (1.9, 7.3) | –10.9 ± 12.0, –7.2 (–18.2, –2.3) | <0.0001 | 10.6 ± 12.7, 6.6 (1.5, 15.0) | 4.8 ± 7.0, 2.5 (0.9, 6.7) | –5.7 ± 8.9, –3.2 (–9.0, 0.0) | <0.0001 |
| ≥300 mg/dL | 6.7 ± 9.1, 3.1 (0.5, 10.2) | 1.8 ± 2.5, 0.9 (0.3, 2.6) | –4.8 ± 7.5, –1.7 (–7.3, 0.0) | <0.0001 | 4.2 ± 8.0, 0.7 (0.0, 4.9) | 1.5 ± 3.1, 0.5 (0.0, 1.7) | –2.7 ± 6.4, 0.0 (–2.7, 0.2) | <0.0001 |
Data are mean ± SD, median (interquartile range). To convert the values for glucose to mmol/L, multiply by 0.05551. CV, coefficient of variation.
Baseline HbA1c values were available for 112 children and 128 adults. Final HbA1c values were available for 108 children and 124 adults who completed the study. The change and P values were calculated only for those who had values available at both baseline and end of study (see Supplementary Table 7 for HbA1c outcomes at additional time points).
CV of sensor glucose is SD divided by the mean.
Baseline and follow-up data were used for the primary effectiveness outcome of HbA1c; the remaining outcomes are described for the standard therapy phase and the automated insulin delivery phase.
P value determined using unadjusted two-sided paired t tests, unless otherwise specified as follows: two-sided Wilcoxon signed rank tests were used for HbA1c for adults, mean sensor glucose (except for children overnight), SD for adults overnight, CV for adults overnight, and percentage of time in glucose ranges 70–180 mg/dL for adults, <54 mg/dL, <70 mg/dL, >180 mg/dL for adults, ≥250 mg/dL, and ≥300 mg/dL.
Figure 1.
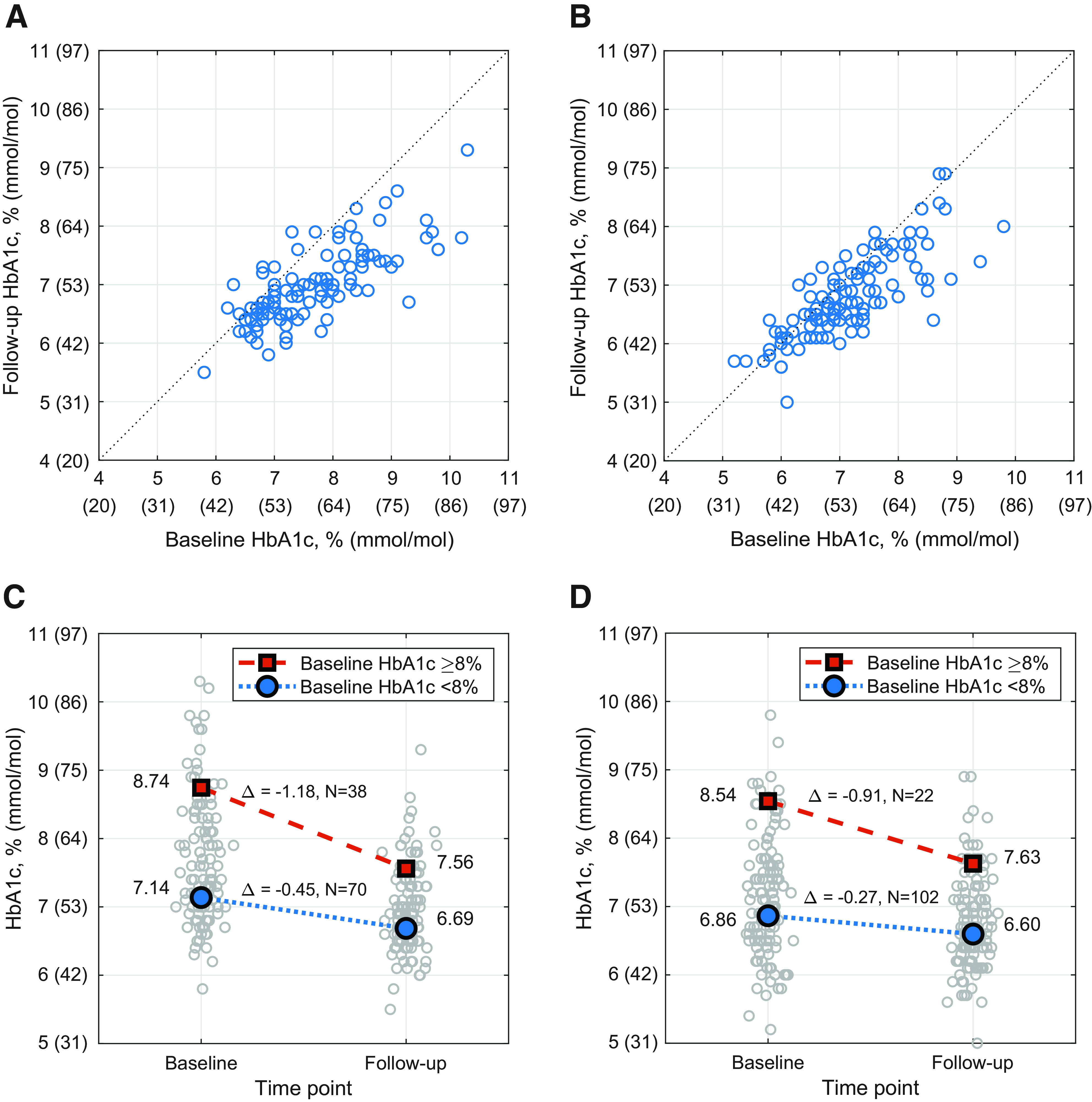
Changes in HbA1c. Individual participant HbA1c results are shown before (baseline) and after (follow-up) the 3-month automated insulin delivery phase for all participants with measurements available at both time points. HbA1c at follow-up plotted vs. HbA1c at baseline for children age 6–13.9 years (n = 108) (A) and adults age 14–70 years (n = 124) (B), with each circle representing a single participant. Mean HbA1c (%) values at baseline and follow-up when stratified into two groups by baseline HbA1c <8% (blue circle) and ≥8% (red square) for children (n = 108) (C) and adults (n = 124) (D), with the distribution of individual participant results at each time point shown as gray circles. Mean HbA1c (mmol/mol) values for children (C) with baseline HbA1c <64 mmol/mol (blue circle) and ≥64 mmol/mol (red square) are 55 and 72 mmol/mol at baseline and 50 and 59 mmol/mol at follow-up (change −4.9 and −12.9 mmol/mol), respectively. HbA1c values for adults (D) with baseline HbA1c <64 mmol/mol (blue circle) and ≥64 mmol/mol (red square) are 51 and 70 mmol/mol at baseline and 49 and 60 mmol/mol at follow-up (change −3.0 and −9.9 mmol/mol), respectively. In the analysis of change in HbA1c stratified by baseline HbA1c, the change was significant for each combination of age-group and baseline HbA1c category (all P < 0.0001 by paired t test). The cutoff of HbA1c <8.0% (<64 mmol/mol) was selected as a measure of adequate HbA1c control set by the Comprehensive Diabetes Care Healthcare Effectiveness Data and Information Set (20).
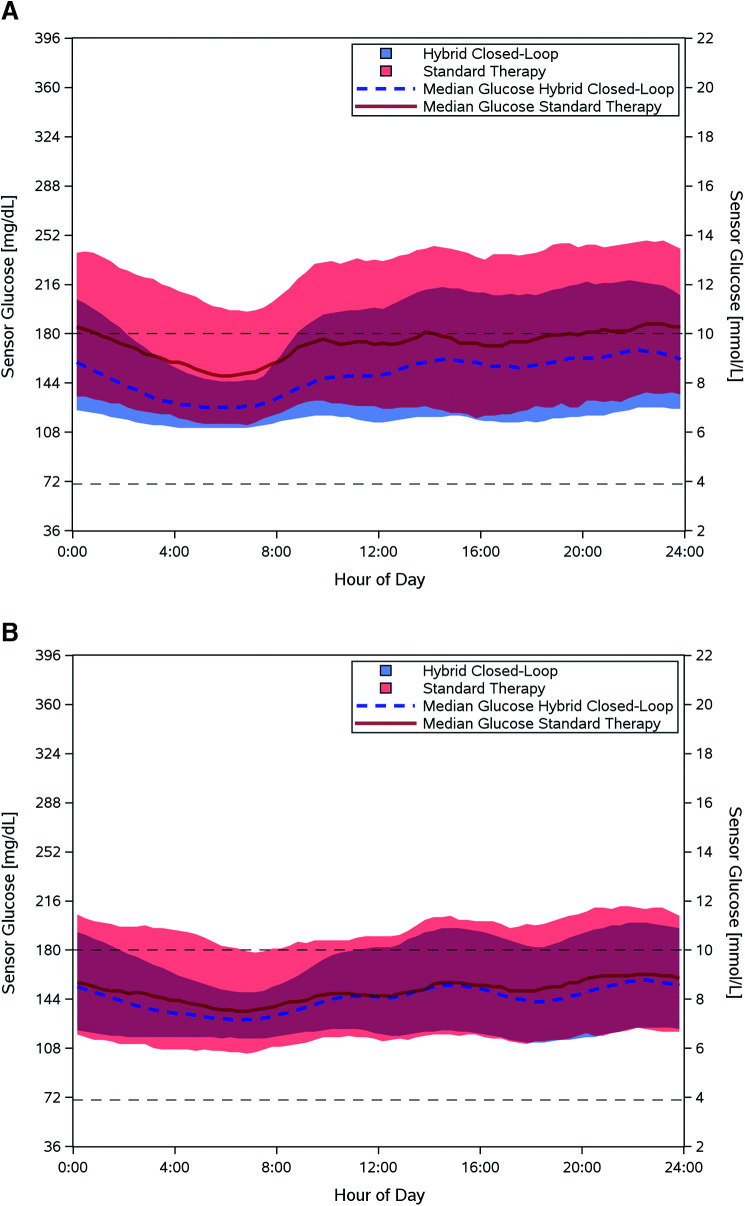
Time in range increased by 15.6% in children (52.5 ± 15.6% to 68.0 ± 8.1%, P < 0.0001) and 9.3% in adults (64.7 ± 16.6% to 73.9 ± 11.0%, P < 0.0001) (Table 2 and Supplementary Table 3). Sensor glucose profile by time of day is presented in Fig. 2. Improvement in time in range was achieved rapidly, with adults achieving 73.5% over days 1–3 after system initiation and children achieving 62.6% in days 1–3 and 68.0% in days 4–6. Time in range remained stable thereafter. The number of participants who met the established clinical targets (1,2) for glycemic measures is included in Supplementary Table 4.
Figure 2.
Sensor glucose measurements. Median sensor glucose measurements are shown for children (age 6–13.9 years, n = 112) (A) and adults (age 14–70 years, n = 128) (B) during the automated insulin delivery phase (blue dashed line) and the standard therapy phase (red line), with blue and red shaded areas indicating the interquartile range for each phase. The target range (70–180 mg/dL) is indicated by black dashed lines. Measurements represent a 24-h period from midnight to midnight.
Secondary outcomes demonstrated a reduction in hypoglycemia for adults and a reduction in hyperglycemia in both age-groups. Time <70 mg/dL for adults declined by a median of 0.89% (interquartile range 2.00%, 1.09%; P < 0.0001). Adults also had a median reduction of 0.08% in time spent <54 mg/dL (P < 0.0001). Time <54 mg/dL and <70 mg/dL remained unchanged for children. Time >180 mg/dL declined by a mean of 15.1% (from 45.3% to 30.2%, P < 0.0001) for children and by 7.7% (from 32.4% to 24.7%, P < 0.0001) for adults. Mean sensor glucose was also reduced in both groups (Table 2). Subgroup analysis by baseline demographic characteristics, including device-naive participants, demonstrated glycemic improvement similar to the overall study cohort (Supplementary Table 5).
Most participants selected the glucose target of 110 mg/dL for automated insulin delivery (61% of cumulative person-days for children and 80% for adults). Patterns in glycemic outcomes at the 110 mg/dL target for both groups were similar to those overall (Supplementary Table 6). With the 110 mg/dL target, time in range was 68.4 ± 9.1% for children and 75.6 ± 9.9% for adults. Glycemic outcomes when using the other targets are listed in Supplementary Tables 8 and 9.
BMI increased slightly in children (18.6 ± 3.2 to 19.2 ± 3.6 kg/m2, P < 0.0001 by paired t test), although the BMI z-score was the same at baseline and follow-up (0.4 ± 0.8), indicating that the increase in BMI was related to normal growth. There was no change in BMI in adults. Total daily insulin requirements increased in children (0.85 ± 0.24 to 0.92 ± 0.25 units/kg, P < 0.0001) and decreased slightly in adults (0.61 ± 0.22 to 0.59 ± 0.21 units/kg, P = 0.02) (Supplementary Table 10).
Safety Outcomes
The observed incidence rates of severe hypoglycemia and diabetic ketoacidosis during the automated insulin delivery phase were 4.8 and 1.2 events per 100 person-years, respectively (Supplementary Table 11). These are lower than the respective rates of 25.2 severe hypoglycemia events and 10.8 diabetic ketoacidosis events per 100 person-years reported in the U.S. T1D Exchange Registry (3,4). There were three severe hypoglycemia events: two in adults following user-initiated boluses, with appropriate insulin suspension by the system, and one in a child following delayed eating after a preprandial bolus. There was one case of diabetic ketoacidosis in a child because of a suspected infusion site failure. Additional details on all adverse events recorded during the automated insulin delivery phase in each age-group and overall are available in Supplementary Table 11.
System Use
Children spent median 96.4% (interquartile range: 93.8 to 97.9%) and adults spent median 96.7% (interquartile range: 93.4 to 98.0%) of total study time in automated mode (mean±SD: 95.2 ± 4.0% and 94.8 ± 6.0% of time in children and adults, respectively). There was approximately 1 device deficiency per person-month of system use: 608 related to the Pod, 83 to the app/handheld device, 20 to the glucose sensor, and 1 each to the glucose and ketone meters.
Conclusions
This multicenter trial of adults and children with type 1 diabetes using a tubeless automated insulin delivery system for 3 months demonstrated the safety of the system in this population. We also observed significant and clinically important improvements in glycemic outcomes with system use compared with participants’ standard therapy. Adults and children achieved a mean HbA1c of 6.78% (51 mmol/mol) and 6.99% (53 mmol/mol), respectively. Adults attained a time in range of 73.9% (an improvement of 2.2 h/day from standard therapy) or 75.6% when using the 110 mg/dL target, with a reduction in hypoglycemia (<70 mg/dL) exposure by 13 min/day. Children attained a time in range of 68.0%, which increased their time in range by 3.7 h/day from standard therapy without an increase in hypoglycemia. In both age-groups, the glycemic improvements were particularly prominent overnight and were driven by a clinically meaningful reduction in hyperglycemia. While using the system, 82% of children and 69% of adults met established clinical targets for time in range (children >60%, adults >70%) (2), and 53% of children and 66% of adults had an HbA1c <7% (<53 mmol/mol) (1). Importantly, gains in glycemic control were not associated with a concomitant rise in hypoglycemia. Median percent time <70 mg/dL was 1.48% and 1.09% for children and adults, respectively, which is well below the recommendation of <4% (2). In fact, 93% of children and 95% of adults achieved this target. The safety outcomes of severe hypoglycemia and diabetic ketoacidosis incidence were below observed rates in type 1 diabetes (3–5) and were not attributable to automated insulin delivery malfunction.
These results are consistent with published findings of similar systems. The pivotal trials for two systems commercially available in the U.S. reported improved HbA1c and time in range in both adults and children after 3–6 months of use (6,7,10,21). One system resulted in an HbA1c of 6.9% (change from baseline of −0.5%) in adults and 7.5% (−0.4%) in children after 3 months, with improved time in range, achieving 72.2% (+5.5%) and 65% (+8.8%) for adults and children, respectively (10,21). Another system resulted in an HbA1c of 7.06% (−0.34%) in adults and 7.0% (−0.6%) in children after 6 and 4 months, respectively, and an improved time in range, achieving 71% (+10%) and 67% (+14%) (6,7). The results of the current study show notably low rates of hypoglycemia, with the majority of participants reaching glycemic targets for their age. While differences in study design limit direct outcome comparisons, the results presented here indicate that the present system compares favorably with current commercially available devices.
An unexpected challenge to this study was the 3-month pause that was initiated once a software anomaly was detected. System use evaluation time was no longer continuous and thus, presented a complication for the planned analysis. For some participants, the final HbA1c was preceded by as few as 28 days of continuous system use, while HbA1c is known to reflect glycemic changes over longer periods of time (22); thus, our results may provide an underestimation of effectiveness. Nevertheless, a stable time in range was achieved quickly after system initiation, and outcomes that were based on sensor data are not expected to have been affected by the pause.
During this first outpatient evaluation of the system, there was about one device deficiency recorded per person-month of use, with the majority of these relating to the Pod. Pod deficiencies were generally related to hazard alarms occurring before the end of the expected 72-h duration of use. These alarms indicate to the user that the Pod must be replaced. Many of these alerts are standard for insulin pump systems and arise from fault detection, such as detection of an occlusion, low battery voltage, or interference in electrical circuit integrity. Pod change is routine, and the rate of Pod device deficiencies equated to approximately an additional 2.5 Pod changes throughout the duration of the study. This would have a negligible effect on the study results. Information from the device deficiencies observed in this study was used to make improvements to the system. Thus, there is expected to be a much lower rate of error occurrences in future uses of the system.
Strengths of this study include the wide range of participant ages (6–70 years) and baseline HbA1c levels (5.2–10.3% [33–89 mmol/mol]). In addition, the trial enrolled both pump and multiple daily injection users with no requirement for prestudy sensor use, with successful implementation across diverse pediatric and adult centers. Participant engagement and endorsement of the system was evident, with 95% choosing to enroll in the extension phase of the trial. Connectivity of the on-body devices was excellent, allowing use of automated insulin delivery for median 96.4% and 96.7% of the possible time for children and adults, respectively. Furthermore, the study used a remote data monitoring system that allowed for cloud-based transmission of data, obviating the need for manual uploads initiated by the user. This critical component of care to allow for seamless data review may help to alleviate the burden sometimes generated by diabetes technologies. While in this study a mobile app on a provided locked-down Android phone was used to interact with the system, it is planned for this app to be available for download and use on compatible personal smartphones when the system becomes commercially available.
Limitations of this study include the single-arm design without a control group that did not account for effects related to participation in a study. This important design limitation could result in overestimation of effectiveness outcomes because it does not account for potential improvements through study-related interactions alone. The standard therapy comparator phase was also of shorter duration than the treatment phase. This limitation is mitigated in part by using glycemic measures that have been validated to reflect underlying glycemia across shorter durations (10–14 days of sensor use) (2,23). Participants had relatively well-controlled baseline glycemic metrics, with many already using insulin pumps and glucose sensors, which may limit generalizability. However, the fact that there were improvements in this generally well-controlled group is encouraging.
In conclusion, this tubeless, on-body automated insulin delivery system with customizable targets was safe, achieving significant improvements in HbA1c and glycemic measures with a low rate of hypoglycemia in a heterogeneous participant group with varied age, baseline glycemia, and prior device use. This system allowed the majority of participants to achieve current glycemic targets set by the American Diabetes Association and international consensus guidelines (1,2).
Article Information
Acknowledgments. The authors extend sincere thanks to the participants in this study and their families. The authors also thank Jodi Bernstein, of Jodi Bernstein Medical Writing (Toronto, Ontario, Canada), who received payment from Insulet Corporation for assisting with revisions and formatting of the manuscript. The authors are grateful to the medical monitor, Dr. Roy Beck of the Jaeb Center for Health Research, and the data and safety monitoring board for time spent reviewing the data and providing feedback throughout the study. The authors also thank the Insulet clinical team, including Nikia Trinward, Rachel McElligott, Tanya Meletlides, Anny Fonseca, Michaela Sorrell, Leslie Barrett, Brenda Ferris, and Alex Nguyen, for contributions to the conduct of the study, and the Omnipod 5 research and development teams, including Yibin Zheng, Connor Gullifer, Kyle Grover, John Hardy, Steve Cardinali, and Sam Carl, for contributions to the development and technical support of the study device. The authors thank the dedicated staff at each clinical site who made this study possible.
Funding and Duality of Interest. This study was funded by Insulet Corporation. S.A.B. reports grants from Insulet and nonfinancial support from Dexcom during the conduct of the study and grants and nonfinancial support from Tandem Diabetes Care, nonfinancial support from Roche Diagnostics, grants from Tolerion, and grants and nonfinancial support from Dexcom outside the submitted work. G.P.F. reports grants and personal fees from Insulet during the conduct of the study and grants and personal fees from Medtronic, grants and personal fees from Dexcom, grants from Abbott, grants and personal fees from Tandem, grants and personal fees from Eli Lilly, and grants and personal fees from Beta Bionics outside the submitted work. B.W.B. reports grant support to his employer Atlanta Diabetes Associates. J.E.P. reports grant support and product support provided to his institution from Insulet, grant support provided to his institution and consulting fees and speaker fees from Tandem Diabetes Care, grant support provided to his institution and advisory board fees from Medtronic, grant support provided to his institution and consulting fees from Eli Lilly, and product support provided to his institution from Dexcom. C.J.L. reports research support from Dexcom and Abbott Diabetes, which have been paid to her institution, and has received an honorarium for serving on an advisory board for Dexcom. A.B.C. reports grants from Insulet during the conduct of the study and grants from Dexcom, grants and other from Medtronic, grants from Abbott Diabetes, grants and other from Sanofi, grants and other from Eli Lilly, and other from Bigfoot Biomedical outside the submitted work. D.W.H. reports grants from Insulet during the conduct of the study and grants from Medtronic and Boehringer Ingelheim. I.B.H. reports research support from Medtronic Diabetes, Insulet, and Beta Bionics and personal fees from Abbott Diabetes Care and Bigfoot Biomedical. A.L.C. reports grants from Insulet during the conduct of the study and grants from Dexcom, grants and other from Medtronic, grants from Abbott Diabetes, grants and other from Sanofi, grants and other from Eli Lilly, and other from Bigfoot Biomedical outside the submitted work. R.B.M. reports research support, consulting, or service on a scientific advisory board for Abbott Diabetes Care, Ascensia, Bigfoot Biomedical, Dexcom, Hygieia, Eli Lilly, Medtronic, Novo Nordisk, Onduo, Roche, Sanofi, and United Healthcare and grants from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and Helmsley Charitable Trust. R.M.B.’s employer, the nonprofit HealthPartners Institute, contracts for his services, and no personal income goes to him. J.L.S. reports research support from Insulet during the conduct of the study and research support from Medtronic and NIDDK. She has served on advisory boards for Bigfoot Biomedical, Cecelia Health, Insulet, and Medtronic Diabetes. Consulting work has been done for Cecelia Health, Eli Lilly, Lexicon, Insulet, Medtronic, and Sanofi. S.N.M. reports grants from Insulet outside the submitted work. L.M.L. reports grants from Insulet during the conduct of the study and personal fees from Novo Nordisk, Eli Lilly, Sanofi, Roche, Johnson & Johnson, Dexcom, Insulet, Boehringer Ingelheim, Convatec, Medtronic, Laxmi, Insulogic, and Lifescan outside the submitted work. V.N.S. reports grants from Dexcom, Insulet, Eli Lilly, Novo Nordisk, Mylan, vTv Therapeutics, EyeNuk, NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases and NIDDK), and Abbott; grants and other from Sanofi; and other from Medscape outside the submitted work. A.B. reports grant support to his employer Iowa Diabetes Research. R.S.W. reports grants from Insulet during the conduct of the study and grants from Eli Lilly, Medtronic, Tolerion, Diasome Pharmaceuticals, Boehringer Ingelheim, and Kowa outside the submitted work. S.A.M. reports personal fees from Insulet during the conduct of the study. D.J.D. reports grants from Insulet during the conduct of the study and personal fees from Dexcom and Insulet outside the submitted work. T.C.J. reports grant support from Insulet to his employer East Coast Institute for research pertaining to and outside the submitted work. G.A. reports research support from AstraZeneca, Dexcom, Eli Lilly, Insulet, and Novo Nordisk and has served as a consultant for Dexcom and Insulet. B.A.B. reports grants and personal fees from Insulet during the conduct of the study and grants and personal fees from Medtronic, grants and nonfinancial support from Tandem, nonfinancial support from Dexcom outside the submitted work, and grants and personal fees from Convatec. In addition, B.A.B. has a patent (no. 61197230) issued. T.T.L. is a full-time employee of and owns stock in Insulet. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.A.B., G.P.F., B.W.B., J.E.P., C.J.L., A.B.C., D.W.H., A.L.C., R.M.B., J.L.S., S.N.M., L.M.L., A.B., R.S.W., S.A.M., D.J.D., T.C.J., B.A.B., and T.T.L. acquired data for the study. S.A.B., G.P.F., B.W.B., J.E.P., C.J.L., A.B.C., I.B.H., A.L.C., R.M.B., J.L.S., L.M.L., V.N.S., R.S.W., D.J.D., G.A., B.A.B., and T.T.L. interpreted the data for this study. S.A.B., G.P.F., B.W.B., J.E.P., A.B.C., A.L.C., V.N.S., D.J.D., B.A.B., and T.T.L. analyzed the data for the study. S.A.B., G.P.F., I.B.H., and T.T.L. wrote the manuscript. S.A.B., G.P.F., and T.T.L. designed the study. S.A.B., B.W.B., J.E.P., C.J.L., A.B.C., D.W.H., A.L.C., R.M.B., J.L.S., S.N.M., L.M.L., V.N.S., A.B., R.S.W., S.A.M., D.J.D., T.C.J., G.A., B.A.B., and T.T.L. critically revised the work for important intellectual content. G.P.F., B.W.B., and T.T.L. created the concept of the study. All authors agreed to be accountable for the work presented in this study. T.T.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at ENDO 2021, 20–23 March 2021, and the 14th International Conference on Advanced Technologies & Treatments for Diabetes, 2–5 June 2021.
Footnotes
S.A.B. and G.P.F. contributed equally to this article.
A complete list of the Omnipod 5 Research Group can be found in the supplementary material online.
Clinical trial reg. no. NCT04196140, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.14423636.
Contributor Information
Collaborators: Sue A. Brown, Mary Voelmle, Emma Emory, Gregory P. Forlenza, R. Paul Wadwa, Robert Slover, Erin Cobry, Laurel H. Messer, Cari Berget, Susan McCoy, Viral N. Shah, Halis K. Akturk, Nicole Schneider, Hal Joseph, Prakriti Joshee, Christie Beatson, Bruce W. Bode, Brooke Narron, Tricia Lopez, Jordan E. Pinsker, Mei Mei Church, Kristin Castorino, Molly Piper, Jimena Perez, Carol J. Levy, David W. Lam, Camilla Levister, Grenye O’Malley, Selassie Ogyaadu, Dushyanthy Arasaratnam, Mitchell Plesser, Emily Nosova, Suzan Bzdick, David W. Hansen, Sheri L. Stone, Ruth S. Weinstock, Irl B. Hirsch, Subbulaxmi Trikudanathan, Nancy Sanborn, Dori Khakpour, Anders L. Carlson, Amy B. Criego, Richard M. Bergenstal, Thomas Martens, Aimee Grieme, Jamie Hyatt, Alina Punel, Diane Whipple, Jennifer L. Sherr, Michelle Van Name, Michelle Brei, Melinda Zgorski, Amy Steffen, Lori Carria, Sanjeev N. Mehta, Lori M. Laffel, Lindsay Roethke, Margaret Fisher, Rebecca Ortiz La Banca, Lisa Volkening, Louise Ambler-Osborn, Christine Turcotte, Emily F. Freiner, Anuj Bhargava, Lisa Borg, Sarah A. MacLeish, Jamie R. Wood, Beth A. Kaminski, Terri L. Casey, Wendy Campbell, Daniel J. DeSalvo, Siripoom McKay, Mary Kylie DeLaO, Carolina Villegas, Thomas C. Jones, Barry Russel Johns, Ashwini Gore, Leslie Harvill, Kayla Merritt, Jennifer Stanfield, Jennifer Sheldon, Lisa Hichkad, Erica Burnett, Alicia Castelot, Lindsay Bounds, Kaitlyn Preston, Rebecca Goldfaden, Grazia Aleppo, Jelena Kravarusic, Anupam Bansal, Bruce A. Buckingham, Laya Ekhlaspour, Ryan Kingman, Kaisa Kivilaid, Krista Kleve, Trang T. Ly, Bonnie Dumais, Todd Vienneau, Lauren M. Huyett, Joon Bok Lee, Jason O’Connor, and Eric Benjamin
References
- 1. American Diabetes Association . 6. Glycemic Targets: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43(Suppl. 1):S66–S76 [DOI] [PubMed] [Google Scholar]
- 2. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 5. Pettus JH, Zhou FL, Shepherd L, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care 2019;42:2220–2227 [DOI] [PubMed] [Google Scholar]
- 6. Brown SA, Kovatchev BP, Raghinaru D, et al.; iDCL Trial Research Group . Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Breton MD, Kanapka LG, Beck RW, et al. iDCL Trial Research Group . A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020;383:836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512 [DOI] [PubMed] [Google Scholar]
- 9. Tauschmann M, Thabit H, Bally L, et al.; APCam11 Consortium . Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018;392:1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 11. Leelarathna L, Choudhary P, Wilmot EG, et al. Hybrid closed-loop therapy: where are we in 2021? Diabetes Obes Metab 2021;23:655–660 [DOI] [PubMed] [Google Scholar]
- 12. Boughton CK, Hovorka R. New closed-loop insulin systems. Diabetologia 2021;64:1007–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berget C, Messer LH, Forlenza GP. A clinical overview of insulin pump therapy for the management of diabetes: past, present, and future of intensive therapy. Diabetes Spectr 2019;32:194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forlenza GP, Buckingham BA, Brown SA, et al. First outpatient evaluation of a tubeless automated insulin delivery system with customizable glucose targets in children and adults with type 1 diabetes. Diabetes Technol Ther 18 January 2021 [Epub ahead of print]. DOI: 10.1089/dia.2020.0546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buckingham BA, Forlenza GP, Pinsker JE, et al. Safety and feasibility of the Omnipod hybrid closed-loop system in adult, adolescent, and pediatric patients with type 1 diabetes using a personalized model predictive control algorithm. Diabetes Technol Ther 2018;20:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forlenza GP, Buckingham BA, Christiansen MP, et al. Performance of Omnipod personalized model predictive control algorithm with moderate intensity exercise in adults with type 1 diabetes. Diabetes Technol Ther 2019;21:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sherr JL, Buckingham BA, Forlenza GP, et al. Safety and performance of the Omnipod hybrid closed-loop system in adults, adolescents, and children with type 1 diabetes over 5 days under free-living conditions. Diabetes Technol Ther 2020;22:174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buckingham BA, Christiansen MP, Forlenza GP, et al. Performance of the Omnipod personalized model predictive control algorithm with meal bolus challenges in adults with type 1 diabetes. Diabetes Technol Ther 2018;20:585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 20. National Committee on Quality Assurance . Comprehensive Diabetes Care (CDC), 2020. Accessed. Available from https://www.ncqa.org/hedis/measures/comprehensive-diabetes-care
- 21. Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. Safety evaluation of the MiniMed 670G system in children 7-13 years of age with type 1 diabetes. Diabetes Technol Ther 2019;21:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rohlfing CL, Wiedmeyer H-M, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care 2002;25:275–278 [DOI] [PubMed] [Google Scholar]
- 23. Riddlesworth TD, Beck RW, Gal RL, et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther 2018;20:314–316 [DOI] [PubMed] [Google Scholar]