Abstract
OBJECTIVE
We examined whether the presence of microvascular complications was associated with increased subsequent risk of cardiovascular disease (CVD) among participants with type 1 diabetes in the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study followed for >35 years.
RESEARCH DESIGN AND METHODS
Standardized longitudinal data collection included: 1) stereoscopic seven-field retinal fundus photography centrally graded for retinopathy stage and clinically significant macular edema; 2) urinary albumin excretion rate (AER) and estimated glomerular filtration rate (eGFR); 3) cardiovascular autonomic neuropathy (CAN) reflex testing; and 4) adjudicated CVD events, including death from CVD, nonfatal myocardial infarction, stroke, subclinical myocardial infarction on electrocardiogram, confirmed angina, or coronary artery revascularization. Cox proportional hazards models assessed the association of microvascular complications with subsequent risk of CVD.
RESULTS
A total of 239 participants developed CVD, including 120 participants who suffered major adverse cardiovascular events (MACE) defined as nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. The presence of microvascular disease (diabetic retinopathy, kidney disease, or CAN) was associated with increased risk of subsequent CVD and MACE (hazard ratios 1.86 to 3.18 and 2.09 to 3.63, respectively), associations that remained significant after adjusting for age and HbA1c. After adjustment for traditional CVD risk factors, however, only sustained AER ≥30 mg/24 h occurring alone and/or with eGFR <60 mL/min/1.73 m2 and the presence of both retinal and kidney disease remained associated with CVD.
CONCLUSIONS
Advanced microvascular disease, especially moderate to severe albuminuria or eGFR <60 mL/min/1.73 m2, conveyed an increased risk of subsequent cardiovascular disease in the DCCT/EDIC cohort.
Introduction
Early detection of cardiovascular disease (CVD), the leading cause of death in patients with type 1 diabetes (T1D), provides opportunities for early interventions (1–5). Identifying additional clinical features of individuals with diabetes with the highest risk for CVD can prospectively guide the need and timing for additional testing. Given that the systemic microcirculation is exposed to hyperglycemia and non-diabetes-related CVD risk factors, the development of diabetic microvascular disease in the retinal, renal, and neurovascular beds may herald the onset of CVD in patients with T1D, as well as provide additional information toward early CVD detection and risk stratification.
Several studies have investigated whether assessment of the microcirculation in organ systems affected early in the course of diabetes might identify individuals at higher risk for impending macrovascular events (6–9). These studies demonstrated associations among retinopathy, kidney disease, and neuropathy with CVD. Yet, the strength of the associations varied due to differences in methodologies used to assess microvascular disease and to measure other risk factors. Association results also differed due, in part, to the extent of microvascular disease present in the cohort studied, as well as the cohort sample sizes and durations of follow-up.
Using the extensive longitudinal data collection of CVD risk factors, diabetes-related complications, and CVD events in the large and well-characterized cohort of participants with T1D in the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study over the past 35 years, we examined the impact of presence of microvascular disease on the risk of subsequent CVD in the DCCT/EDIC cohort. Specifically, we evaluated retinopathy, kidney disease, and neuropathy for their association with CVD above and beyond common pathogenic CVD risk factors.
Research Design and Methods
Subjects
The methods of the DCCT/EDIC studies have been previously described (10). Briefly, the DCCT randomly assigned 1,441 participants, aged 13–39 years, to receive either intensive treatment (n = 711, with the goal of reducing blood glucose levels as close to the nondiabetic range as safely possible) or conventional treatment (n = 730, aimed at reducing symptoms of hypo- and hyperglycemia but with no target glucose levels). The study consisted of two cohorts. The primary prevention cohort included participants with diabetes duration between 1 and 5 years, albumin excretion rate (AER) <40 mg/24 h, and no retinopathy as determined by fundus photography at baseline. The secondary intervention cohort included participants with diabetes duration between 1 and 15 years, AER ≤200 mg/24 h, and at least one microaneurysm in either eye, but no more than moderate nonproliferative diabetic retinopathy. Of note, no participants had a history of CVD or major adverse cardiovascular events (MACE) prior to enrollment in DCCT.
At the end of DCCT (1993), all participants were instructed in intensive therapy and referred to their primary health care providers for subsequent diabetes care. The participants have continued to be followed in the EDIC study, which started in 1994 and enrolled 98% of the surviving DCCT cohort. The current analyses include data from the combined DCCT/EDIC follow-up period for all 1,441 participants up to EDIC study year 24 (around 2017, >30 years from the start of the DCCT).
Cardiovascular Outcomes
Time to any CVD and the time to any MACE were assessed. CVD was defined as a composite time to the first of any of the following: death from CVD, nonfatal myocardial infarction (MI), stroke, subclinical MI on electrocardiogram, confirmed angina, or the need for coronary artery revascularization. MACE was defined as a composite of time to the first of any of the following: nonfatal MI, nonfatal stroke, or death from CVD. All cardiovascular events were adjudicated by physician review of medical records blinded to glycemic control parameters (11).
Microvascular Complications
Retinopathy was assessed using standardized stereoscopic seven-field fundus photography every 6 months in DCCT and roughly every 4 years in EDIC, with all active participants receiving an examination during EDIC study years 4 and 10. Retinal photos were centrally graded using the Early Treatment Diabetic Retinopathy Study scale, which, along with the presence of pan-retinal photocoagulation, was used to determine the presence of proliferative diabetic retinopathy (PDR). Additionally, clinically significant macular edema (CSME) was determined by fundus photography or the presence of focal photocoagulation or self-reported anti–vascular endothelial growth factor treatment. During this time, ocular coherence tomography to evaluate macular edema was not available.
Kidney disease was assessed in both DCCT and EDIC using urine specimen collection and assay measurement. Serum creatinine, measured annually, was used to determine estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration equation. Sustained eGFR <60 mL/min/1.73 m2 was used as a microvascular outcome. Urinary AER was measured annually in DCCT and every 2 years in EDIC using a 4-h urine collection. Sustained moderate albuminuria (AER ≥30 mg/24 h) and severe albuminuria (AER ≥300 mg/24 h) were additional microvascular outcomes used in these analyses (12).
Cardiovascular autonomic neuropathy (CAN) was determined using standardized cardiovascular reflex testing that assessed the R-R response to paced breathing (R-R variation), the Valsalva maneuver, and postural changes in blood pressure. These tests were administered biennially during DCCT and at DCCT closeout and EDIC years 13/14 and 15/16. CAN was defined as an R-R variation <15, an R-R variation 15–19.9 in combination with a Valsalva ratio ≤1.5, or a decrease of >10 mmHg in diastolic blood pressure (13).
Covariates
HbA1c was assayed using high-performance liquid chromatography quarterly during DCCT and annually during EDIC. Lipids, sitting blood pressures, and heart rates were measured using standardized methods during the annual study visit. Smoking was self-reported annually, and the use of ACE inhibitors and angiotensin-2 receptor blockers were self-reported during EDIC. The family history of MI was collected at the DCCT baseline visit (14).
The covariates were captured either as the current (most recent) value (e.g., current triglyceride levels) or as the updated mean from baseline (such as the mean updated HbA1c value). The updated mean is the weighted average of prior values using weights proportional to the time interval between the measurements (i.e., one-fourth for quarterly visits during DCCT and one for annual visits during EDIC).
Statistical Analysis
Baseline clinical characteristics of the DCCT/EDIC participants are presented separately by CVD and MACE status over the follow-up period using means (SDs) for quantitative variables and percentages for discrete variables. The number of participants with CVD or MACE are reported both overall and separately by microvascular complication status. Only microvascular complications that occurred prior to the occurrence of a CVD event or MACE were considered. Kaplan-Meier curves described the cumulative incidence of CVD and MACE.
Cox proportional hazards models assessed the associations between microvascular complications and the subsequent risk of CVD and MACE with follow-up censored at either the last study visit or the time of CVD or MACE. Each microvascular complication was included as a time-varying covariate defined as 0 prior to the onset and 1 after the onset of that complication. Separate models assessed the association between CVD and MACE with one microvascular complication at a time. Given the more frequent data collection of both the retinal and kidney outcomes compared with CAN, a combined outcome for retinal disease, including PDR and/or CSME, and for kidney disease, including sustained moderate albuminuria (sustained AER ≥30 mg/24 h) and/or eGFR<60 mL/min/1.73 m2, was also investigated. In addition, we investigated the association between the risk of CVD and MACE and the combined presence of retinal or kidney disease, a quantitative variable defined as 0, no prior complications; 1, prior (PDR and/or CSME) or (sustained moderate albuminuria (sustained AER ≥30 mg/24 h) and/or eGFR<60 mL/min/1.73 m2) but not both; and 2, prior (PDR and/or CSME) and (sustained moderate albuminuria (sustained AER ≥30 mg/24 h) and/or eGFR<60 mL/min/1.73 m2).
Models were unadjusted, minimally adjusted for baseline age and mean updated HbA1c, or fully adjusted for the covariates determined previously in separate models for CVD and for MACE (11). Baseline age and baseline duration of T1D were fixed covariates, while mean updated HbA1c, sitting blood pressure, heart rate, LDL, triglycerides, and use of ACEs and angiotensin-2 receptor blockers were time-varying covariates. While we have previously reported that baseline family history of MI was a nominally significant correlate for any CVD in a prior analysis and current smoking status for MACE (11), neither was significant in these updated analyses with additional follow-up data and were not used in the models in this study. Additionally, the fully adjusted models include adjustment for ACE inhibitors, the only medication that was significant in the multivariable CVD risk models developed previously, and, similarly, sex was not retained (significant) in the multivariable models for CVD or MACE, a finding that was previously discussed (11). To investigate the risk factors (such as systolic blood pressure) that explained the differences in the observed associations between microvascular complications and the risk of CVD in the minimally adjusted versus the fully adjusted models, we added one covariate at a time (e.g., systolic blood pressure) to the minimally adjusted models. P values <0.05 were considered nominally significant.
Results
Participant Characteristics
At study baseline, DCCT/EDIC participants had a mean age of 27 years (SD 7 years), diabetes duration 70 months (SD 50 months), and HbA1c 8.9% (SD 1.6%), and 53% were men. Table 1 summarizes the baseline characteristics of study participants by whether or not they developed CVD or experienced MACE during DCCT and EDIC follow-up. At DCCT baseline, participants who subsequently experienced CVD events during DCCT or EDIC were older, had a longer duration of T1D, and were more likely to have a family history of MI and to be smokers. They also had higher systolic blood pressure, heart rate, LDL, triglycerides, and HbA1c, as well as lower eGFR values, than participants who did not experience a CVD event. Similar differences in baseline characteristics were evident for those participants experiencing MACE, with the exceptions that females had lower risk then males, and that a higher heart rate and a family history of MI did not reach statistical significance.
Table 1.
Baseline characteristics of the DCCT/EDIC participants by status of CVD or MACE over the combined DCCT/EDIC follow-up
| Any CVD* | MACE** | ||||||
|---|---|---|---|---|---|---|---|
| Baseline characteristics | Overall (n = 1,441) | No CVD (n = 1,202) | With CVD (n = 239) | P value^ | No MACE (n = 1,321) | With MACE (n = 120) | P value^ |
| Age (years) | 26.8 (7.1) | 26.2 (7.1) | 29.9 (6.3) | <0.0001 | 26.5 (7.1) | 30.3 (6.1) | <0.0001 |
| Sex (% female) | 47.2 | 47.8 | 43.9 | 0.1875 | 47.9 | 39.2 | 0.0419 |
| T1D duration (months) | 69.8 (49.7) | 67.9 (49.0) | 79.4 (51.9) | 0.0048 | 68.6 (49.1) | 82.7 (54.3) | 0.0103 |
| Family history of MI (%) | 48.9 | 46.8 | 59.0 | 0.0011 | 48.4 | 53.3 | 0.8743 |
| Smoking (%) | 18.5 | 16.6 | 28.0 | 0.0028 | 17.1 | 33.3 | 0.0003 |
| Systolic blood pressure (mmHg) | 114.5 (11.4) | 114.2 (11.3) | 116.2 (11.4) | 0.0105 | 114.3 (11.3) | 116.7 (11.3) | 0.0322 |
| Pulse (bpm) | 76.1 (11.1) | 75.8 (11.1) | 77.6 (11.1) | 0.0356 | 75.9 (11.1) | 77.9 (11.2) | 0.0904 |
| LDL (mg/dL) | 109.7 (29.1) | 107.9 (28.6) | 118.8 (30.0) | <0.0001 | 109.0 (28.9) | 117.5 (30.2) | 0.0008 |
| Triglycerides (mg/dL) | 81.3 (47.5) | 79.9 (46.7) | 88.6 (50.7) | 0.0069 | 80.1 (46.9) | 94.8 (51.6) | 0.0008 |
| HbA1c (%) | 8.9 (1.6) | 8.9 (1.6) | 9.1 (1.6) | 0.0116 | 8.9 (1.6) | 9.2 (1.7) | 0.0116 |
| HbA1c (mmol/mol) | 74 (17.5) | 74 (17.5) | 76 (17.5) | 0.0116 | 74 (17.5) | 77 (18.6) | 0.0116 |
| AER (mg/24 h) | 15.9 (18.8) | 15.7 (18.7) | 17.0 (19.0) | 0.4179 | 15.8 (18.5) | 17.8 (21.7) | 0.3618 |
| eGFR (mL/min/1.73 m2) | 126.1 (14.2) | 126.6 (14.3) | 123.9 (13.5) | 0.0040 | 126.4 (14.2) | 122.7 (14.0) | 0.0031 |
bpm, beats per minute.
Any CVD: composite time to first of any of the following: death from CVD, nonfatal MI, stroke, subclinical MI on electrocardiogram, confirmed angina, or the need for coronary artery revascularization.
MACE: composite of time to first of any of the following: nonfatal MI, nonfatal stroke, or death from CVD.
P values from unadjusted Cox proportional hazards models. Boldface type indicates significance at P < 0.05.
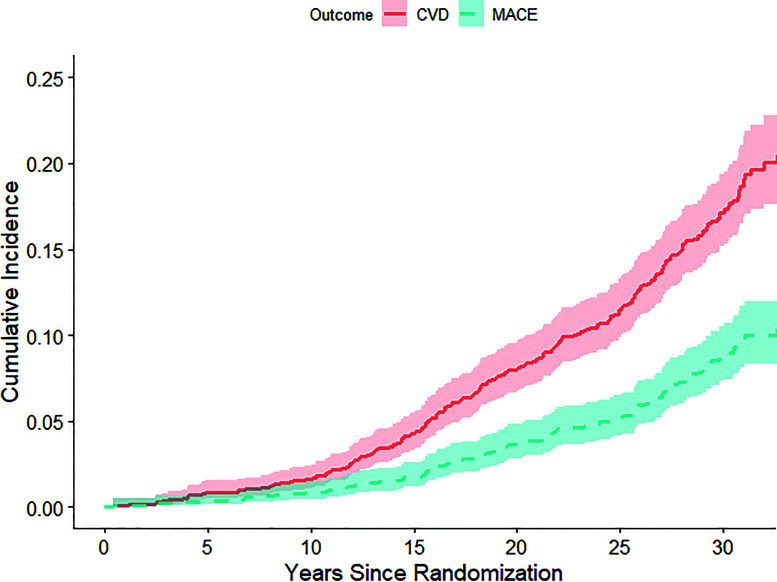
Cumulative Incidence of CVD/MACE in the DCCT/EDIC Cohort
Figure 1 presents the cumulative incidence for CVD/MACE. There were 239 individuals with CVD (6.1 cases/1,000 patient-years), of which 120 individuals had MACE (3.0 cases/1,000 patient-years). The rates of any CVD and MACE were higher in the presence of microvascular complications (Table 2). Notably, participants with prior PDR and/or CSME (n = 525) had an average of 12.8 CVD events/1,000 patient-years, compared with an average of 4.4 CVD events/1,000 patient-years among those without prior PDR or CSME. Likewise, participants with prior PDR and/or CSME had an average of 7.0 MACE/1,000 patient-years, compared with an average of 1.9 MACE/1,000 patient-years among participants without prior CSME or PDR.
Figure 1.
Cumulative incidence functions (with 95% CIs) for CVD and MACE.
Table 2.
Numbers and rates of CVD events and MACE both overall and separately by preceding microvascular event status
| Any CVD, n (rate*) | MACE, n (rate*) | |
|---|---|---|
| Overall | 239 (6.1) | 120 (3.0) |
| Microvascular outcome | ||
| PDR (n = 360) | ||
| Yes | 69 (14.1) | 39 (7.4) |
| No | 170 (5.0) | 81 (2.3) |
| CSME (n = 413) | ||
| Yes | 76 (13.0) | 45 (7.2) |
| No | 163 (4.9) | 75 (2.2) |
| Three-step progression (n = 972) | ||
| Yes | 150 (8.2) | 82 (4.3) |
| No | 89 (4.3) | 38 (1.8) |
| PDR and/or CSME (n = 525) | ||
| Yes | 99 (12.8) | 58 (7.0) |
| No | 140 (4.4) | 62 (1.9) |
| AER ≥300 (n = 172) | ||
| Yes | 39 (16.1) | 27 (10.3) |
| No | 200 (5.4) | 93 (2.5) |
| Sustained AER ≥30 (n = 457) | ||
| Yes | 103 (12.4) | 57 (6.4) |
| No | 136 (4.4) | 63 (2.0) |
| eGFR <60 (n = 147) | ||
| Yes | 31 (28.4) | 20 (15.9) |
| No | 208 (5.5) | 100 (2.6) |
| Sustained AER ≥30 mg/24 h and/or eGFR <60 mL/min/1.73 m2 (n = 498) | ||
| Yes | 110 (12.8) | 60 (6.5) |
| No | 129 (4.2) | 60 (1.9) |
| CAN (n = 589) | ||
| Yes | 99 (12.1) | 57 (6.5) |
| No | 140 (4.5) | 63 (2.0) |
| Presence of retinal and kidney disease+ | ||
| 0 | 94 (3.5) | 41 (1.5) |
| 1 | 81 (9.1) | 40 (4.3) |
| 2 | 64 (17.1) | 39 (9.6) |
Per 1,000 patient-years. +Presence of retinal and kidney disease is a quantitative variable defined as 0, no prior complications; 1, prior (PDR and/or CSME) or (severe albuminuria (AER ≥300 mg/24 h) and/or sustained eGFR <60 mL/min/1.73 m2) but not both; and 2, prior (PDR and/or CSME) and (severe albuminuria (AER ≥300 mg/24 h) and/or sustained eGFR <60 mL/min/1.73 m2).
Associations of Microvascular Complications With CVD and MACE
Table 3 reports the associations between microvascular complications and any CVD (Table 3A) and MACE (Table 3B). In simple unadjusted analyses, all of the microvascular indices except three-step progression for retinopathy were highly significantly associated with subsequent risk of CVD and MACE with hazard ratios (HRs) ranging from 1.86 to 3.18 and from 2.09 to 3.63, respectively. The strongest associations were between eGFR <60 mL/min/1.73 m2 with CVD and MACE. After adjustment for age and the updated mean HbA1c, all HRs were reduced for both any CVD and MACE, with the strongest associations between eGFR <60 mL/min/1.73 m2 and any CVD (HR 2.12) and MACE (HR 2.06), which represented over a doubling of the risk among those with eGFR <60 mL/min/1.73 m2. In the fully adjusted models, which account for traditional CVD risk factors, moderate albuminuria (HR 1.47 [95% CI 1.09, 1.97]; P = 0.0119), sustained AER ≥30 mg/24 h and/or eGFR <60 mL/min/1.73 m2 (HR 1.54 [1.15, 2.07]; P = 0.0038), and the combined presence of retinal and kidney disease (HR 1.28 [1.05, 1.56]; P = 0.0127) remained significantly associated with CVD.
Table 3.
Associations (HRs, 95% CIs, and P values) of prior microvascular complications with subsequent risk of CVD events (A) and MACE (B)
| Unadjusted models | Minimally adjusted models* | Fully adjusted models^ | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| A. CVD | ||||||
| PDR and/or CSME | 1.98 (1.51, 2.58) | <0.0001 | 1.47 (1.11, 1.94) | 0.0069 | 1.13 (0.84, 1.53) | 0.4033 |
| PDR | 1.91 (1.44, 2.55) | <0.0001 | 1.44 (1.06, 1.95) | 0.0187 | 1.09 (0.79, 1.51) | 0.5852 |
| CSME | 1.86 (1.40, 2.46) | <0.0001 | 1.33 (0.99, 1.77) | 0.0562 | 1.03 (0.77, 1.40) | 0.8244 |
| Three-step progression | 1.18 (0.90, 1.55) | 0.2391 | 0.89 (0.66, 1.19) | 0.4329 | 0.76 (0.56, 1.03) | 0.0816 |
| Sustained AER ≥30 mg/24 h and/or eGFR <60 mL/min/1.73 m2 | 2.36 (1.82, 3.05) | <0.0001 | 1.99 (1.51, 2.61) | <0.0001 | 1.54 (1.15, 2.07) | 0.0038 |
| AER ≥300 mg/24 h | 2.13 (1.51, 3.01) | <0.0001 | 1.67 (1.16, 2.41) | 0.0059 | 1.15 (0.78, 1.70) | 0.4884 |
| Sustained AER ≥30 mg/24 h | 2.22 (1.71, 2.87) | <0.0001 | 1.91 (1.45, 2.52) | <0.0001 | 1.47 (1.09, 1.97) | 0.0119 |
| eGFR <60 mL/min/1.73 m2 | 3.18 (2.15, 4.69) | <0.0001 | 2.12 (1.43, 3.15) | 0.0002 | 1.48 (0.97, 2.23) | 0.0658 |
| CAN | 1.91 (1.45, 2.51) | <0.0001 | 1.36 (1.03, 1.80) | 0.0313 | 1.11 (0.83, 1.49) | 0.4767 |
| Presence of retinal and kidney disease+ | 1.81 (1.54, 2.12) | <0.0001 | 1.55 (1.30, 1.85) | <0.0001 | 1.28 (1.05, 1.56) | 0.0127 |
| B. MACE | ||||||
| PDR and/or CSME | 2.45 (1.69, 3.56) | <0.0001 | 1.61 (1.09, 2.40) | 0.0178 | 1.29 (0.85, 1.95) | 0.2342 |
| PDR | 2.09 (1.41, 3.11) | 0.0002 | 1.34 (0.88, 2.04) | 0.1735 | 1.00 (0.64, 1.56) | 0.9877 |
| CSME | 2.22 (1.52, 3.25) | <0.0001 | 1.39 (0.93, 2.07) | 0.1087 | 1.16 (0.77, 1.75) | 0.4695 |
| Three-step progression | 1.49 (0.99, 2.23) | 0.0541 | 1.00 (0.65, 1.54) | 0.9866 | 0.88 (0.56, 1.36) | 0.5561 |
| Sustained AER ≥30 mg/24 h and/or eGFR <60 mL/min/1.73 m2 | 2.59 (1.80, 3.72) | <0.0001 | 1.93 (1.31, 2.85) | 0.0009 | 1.43 (0.94, 2.18) | 0.0913 |
| AER ≥300 mg/24 h | 2.99 (1.94, 4.61) | <0.0001 | 2.12 (1.33, 3.38) | 0.0015 | 1.56 (0.94, 2.59) | 0.0878 |
| Sustained AER ≥30 mg/24 h | 2.51 (1.74, 3.61) | <0.0001 | 1.93 (1.30, 2.85) | 0.0010 | 1.41 (0.92, 2.14) | 0.1134 |
| eGFR <60 | 3.63 (2.20, 5.97) | <0.0001 | 2.06 (1.23, 3.44) | 0.0058 | 1.54 (0.90, 2.65) | 0.1182 |
| CAN | 2.23 (1.52, 3.26) | <0.0001 | 1.49 (1.01, 2.20) | 0.0471 | 1.14 (0.76, 1.73) | 0.5195 |
| Presence of retinal and kidney disease+ | 2.03 (1.62, 2.54) | <0.0001 | 1.59 (1.24, 2.04) | 0.0003 | 1.31 (0.99, 1.73) | 0.0572 |
Associations with P values <0.05 are reported in boldface.
Adjusted for baseline age and mean updated HbA1c.
CVD: adjusted for baseline age, mean updated HbA1c, mean updated systolic blood pressure, current triglycerides, mean updated pulse, baseline duration, use of ACE inhibitors, family history of MI, and mean updated LDL; MACE: adjusted for baseline age, mean updated HbA1c, mean updated pulse, current triglycerides, mean updated systolic BP, current smoking status, baseline duration, use of ACE inhibitors, and current LDL. See ref. 11. +Presence of retinal and kidney disease is a quantitative variable defined as 0, no prior complications; 1, prior (PDR and/or CSME) or (severe albuminuria (AER ≥300 mg/24 h) and/or sustained eGFR <60 mL/min/1.73 m2) but not both; and 2, prior (PDR and/or CSME) and (severe albuminuria (AER ≥300 mg/24 h) and/or sustained eGFR <60 mL/min/1.73 m2).
Supplementary Table 1 reports results for multivariable models that included CAN; PDR and/or CSME; and sustained AER ≥30 mg/24 h and/or eGFR <60 mL/min/1.73 m2 in the same model. Without further adjustment, PDR and/or CSME (CVD: HR 1.47 [1.10, 1.95], P = 0.0087; MACE: HR 1.77 [1.19, 2.65], P = 0.0049), sustained AER ≥30 mg/24 h and/or eGFR <60 mL/min/1.73 m2 (CVD: HR 1.94 [1.47, 2.56]; P ≤ 0.0001; MACE: HR 1.95 [1.32, 2.88], P = 0.0008), and CAN (CVD: HR 1.54 [1.16, 2.04], P = 0.0030; MACE: HR 1.69 [1.14, 2.53], P = 0.0089) were all significantly associated with higher risk of CVD and MACE. After adjusting for baseline age and mean updated HbA1c, only sustained AER ≥30 mg/24 h and/or eGFR <60 mL/min/1.73 m2 remained significantly associated with CVD (HR 1.84 [1.39, 2.45]; P ≤ 0.0001) and MACE (HR 1.72 [1.15, 2.59]; P = 0.0087). In fully adjusted multivariate models, only sustained AER ≥30 mg/24 h and/or eGFR <60 mL/min/1.73 m2 remained significantly associated with CVD (HR 1.52 [1.13, 2.05]; P = 0.0058).
Supplementary Table 2 reports risk factors from the fully adjusted CVD models, including mean updated heart rate, systolic blood pressure, baseline diabetes duration, triglycerides, and current LDL, each added one at a time to the minimally adjusted models. Adjustment for mean updated systolic blood pressure had the strongest effect. More specifically, after adjustment for mean updated systolic blood pressure (in addition to age and mean updated HbA1c), the associations with eGFR <60 mL/min/1.73 m2, sustained AER ≥30 mg/24 h, and the combined outcome of sustained AER ≥30 mg/24 h and/or eGFR <60 mL/min/1.73 m2, along with the combined presence of retinal and kidney disease, remained significant. Qualitatively similar results were observed for MACE (Supplementary Table 3) and after further adjustment for other risk factors in addition to the mean updated systolic blood pressure (Supplementary Table 4 for CVD and Supplementary Table 5 for MACE).
Conclusions
The results of this study demonstrate the association of the development of microvascular complications with an increased risk of subsequent CVD and MACE. Notably, the rates of CVD and MACE were higher among those individuals with advanced diabetic retinopathy, kidney disease, and CAN than those without microvascular disease. However, the strongest associations observed were between moderate to severe albuminuria or eGFR <60 mL/min/1.73 m2 and CVD/MACE, which represented over a doubling of the risk compared with those without kidney disease. While the independent associations of microvascular disease with CVD or MACE are similar in magnitude in the models fully adjusted for other known CVD risk factors, the associations with MACE do not reach statistical significance, likely due to the smaller number of MACE (120 MACE vs. 239 CVD events).
Our findings are similar to other studies that reported the presence of PDR and/or kidney disease was associated with an ∼1.5–2.0-fold increase in the risk of subsequent CVD/MACE (6,9). In our fully adjusted models, PDR alone was not associated with an increased risk of CVD, which is unlike the findings in the FinnDiane study (9). This may be in part due to: 1) differences in the cohort characteristics at baseline, with the FinnDiane participants being older and with longer duration of T1D compared with the DCCT/EDIC participants; 2) differences in the classification of severe diabetic retinopathy, in which the FinnDiane study included only individuals with a history of photocoagulation; and 3) use of microvascular disease as a fixed covariate in the FinnDiane study in contrast to a time-varying covariate in our analyses. Although diabetic kidney disease is known to be associated with age, mean updated HbA1c, systolic blood pressure, and diabetes duration, kidney disease still has a significant association with the risk of CVD even after adjustment for these factors. Our results also indicate that even early moderate albuminuria manifested by sustained AER ≥30 mg/24 h can impart an increased risk for future CVD.
We have previously reported that CAN at DCCT closeout was associated with a higher risk of CVD events during EDIC, although the effect was diminished after adjusting for glucose control over time as documented by the updated mean HbA1c throughout DCCT/EDIC (15). The prior analyses investigated the association between presence of CAN at the end of DCCT (as a time-fixed exposure) and the risk of CVD events during EDIC. In contrast, the current analyses used CAN as a time-varying exposure using both the biennial DCCT evaluations and the EDIC years 13/14 and 15/16 evaluations. In these updated analyses, CAN was associated with higher risk of both CVD and MACE after adjustment for age and HbA1c, but the associations did not remain significant in the fully adjusted models. Of note, when considering CAN in relation to other microvascular diseases, there are complex interplays between CAN and kidney disease in patients with diabetes due to shared pathophysiologic mechanisms underlying both conditions, such as sympathetic overactivity leading to constriction of renal preglomerular vessels, proteinuria, and further deterioration in kidney function that will subsequently further impact CAN (16).
As for the retinal microvasculature, the pathophysiology of diabetic retinopathy includes progressive endothelial cell dysfunction and alterations in perfusion of the retina (17,18). Our data suggest that progression of diabetic retinopathy by three or more steps prior to the development of PDR or CSME is not associated with a significant increased risk for CVD and MACE. However, in contrast to our findings for kidney disease, systolic blood pressure and baseline diabetes duration largely explained the increased CVD/MACE risk associated with the presence of PDR, as individual adjustments for these covariates reduced the HR to a nonsignificant level. As others have proposed, this suggests a potential difference in the underlying mechanisms and susceptibility between the glomerular vessels and retinal microvasculature, a balance of protective and pathogenic factors (6), that may, ultimately, lead to new therapies that prevent the development of microvascular disease and perhaps benefit, as well, the microvessels involved in CVD and MACE.
The strength of our study is the extensive longitudinal clinical phenotyping of participants with T1D over an extended period of time. Diabetes complications and proposed risk factors were continuously monitored with sensitive and specific standardized measures during the study and cardiovascular events adjudicated carefully over time. The integrity and completeness of the data collection along with the presence and severity of microvascular and macrovascular disease that has developed over prolonged periods of time provided the power to carefully detect associations between these complications as well as other intermediary risk factors.
Our study has certain limitations. Retinopathy and CAN were not assessed as routinely as kidney outcomes, and this may have reduced the power to detect their associations with CVD. Additionally, the DCCT/EDIC cohort was initially selected to participate in a randomized controlled clinical trial; as such, self-motivation, social support, educational level, and socioeconomic status of this largely Caucasian cohort may differ from the general population with T1D. However, most of the outcomes (220 out of the 239 CVD events and all 120 MACE) have occurred over the ∼23 years of the EDIC observational phase when the participants received standard diabetes care.
In conclusion, the development of moderate to severe albuminuria, eGFR <60 mL/min/1.73 m2, PDR, CSME, and CAN are strongly associated with increased risk of subsequent CVD and MACE, above and beyond the risk explained by age and historic glycemic control. Therapies to preserve kidney function, in addition to the usually recommended intensive management of glycemia and hypertension, could reduce the risk of CVD over time in patients with T1D.
Article Information
Funding. The DCCT/EDIC has been supported by cooperative agreement grants (1982–1993, 2012–2017, and 2017–2022) and contracts (1982–2012) with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (current grants U01 DK094176 and U01 DK094157), and through support from the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993–2007), and Clinical Translational Science Center Program (2006–present), Bethesda, MD. Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly and Company (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), LifeScan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi (Bridgewater, NJ).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.G.-K., J.M.L., and I.B. designed the study. R.G.-K. wrote the initial draft of the manuscript. X.G. conducted all analyses under the supervision of I.B. The other authors contributed to the specification of the analyses and critically reviewed and edited the manuscript. I.B. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. nos. NCT00360893 and NCT00360815, clinicaltrials.gov.
A complete list of the investigators and members of the DCCT/EDIC Research Group can be found in N Engl J Med 2017;376:1507-1516.
This article contains supplementary material online at https://doi.org/10.2337/figshare.14356943.
References
- 1. Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Writing Group for the DCCT/EDIC Research Group; Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, Backlund JY, Lachin JM. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463–1469 [DOI] [PubMed] [Google Scholar]
- 4. Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care 2006;29:2528–2538 [DOI] [PubMed] [Google Scholar]
- 5. Livingstone SJ, Looker HC, Hothersall EJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med 2012;9:e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordin D, Harjutsalo V, Tinsley L, et al. Differential association of microvascular attributions with cardiovascular disease in patients with long duration of type 1 diabetes. Diabetes Care 2018;41:815–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carbonell M, Castelblanco E, Valldeperas X, et al. Diabetic retinopathy is associated with the presence and burden of subclinical carotid atherosclerosis in type 1 diabetes. Cardiovasc Diabetol 2018;17:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garofolo M, Gualdani E, Giannarelli R, et al. Microvascular complications burden (nephropathy, retinopathy and peripheral polyneuropathy) affects risk of major vascular events and all-cause mortality in type 1 diabetes: a 10-year follow-up study. Cardiovasc Diabetol 2019;18:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pongrac Barlovic D, Harjutsalo V, Gordin D, et al.; FinnDiane Study Group . The association of severe diabetic retinopathy with cardiovascular outcomes in long-standing type 1 diabetes: A longitudinal follow-up. Diabetes Care 2018;41:2487–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 11. Diabetes Control and Complications Trial (DCCT)-Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Risk factors for cardiovascular disease in type 1 diabetes. Diabetes 2016;65:1370–137926895792 [Google Scholar]
- 12. Perkins BA, Bebu I, de Boer IH, et al.; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Risk factors for kidney disease in type 1 diabetes. Diabetes Care 2019;42:883–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pop-Busui R, Low PA, Waberski BH, et al.; DCCT/EDIC Research Group . Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009;119:2886–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The DCCT/EDIC Research Group . Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pop-Busui R, Braffett BH, Zinman B, et al.; DCCT/EDIC Research Group . Cardiovascular autonomic neuropathy and cardiovascular outcomes in the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes Care 2017;40:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ang L, Dillon B, Mizokami-Stout K, Pop-Busui R. Cardiovascular autonomic neuropathy: a silent killer with long reach. Auton Neurosci 2020;225:102646. [DOI] [PubMed] [Google Scholar]
- 17. Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR. Diabetic retinopathy: breaking the barrier. Pathophysiology 2017;24:229–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrett EJ, Liu Z, Khamaisi M, et al. Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab 2017;102:4343–4410 [DOI] [PMC free article] [PubMed] [Google Scholar]