Abstract
OBJECTIVE
To explore associations between reductions in diabetes distress (DD) and improvements in glycemic outcomes among adults with type 1 diabetes (T1D) in the context of a DD randomized clinical trial.
RESEARCH DESIGN AND METHODS
Adults with T1D (N = 301) participated in a two-arm trial aimed at reducing DD (DD-focused OnTrack group vs. education-oriented KnowIt group). Mean age was 45.1 years; mean baseline HbA1c was 8.8% (73 mmol/mol). Individuals were assessed at baseline and 9 months later on DD, self-care, HbA1c, and frequency of hypoglycemia. Structural equation models evaluated hypothesized pathways among changes in DD, self-care, and glycemic outcomes in the total sample and by intervention group.
RESULTS
Reductions in DD were significantly and independently associated with better self-care, including fewer missed insulin boluses, more frequent insulin adjustment, improved problem-solving skills, more blood glucose monitoring, and greater adoption of continuous glucose monitoring (all P < 0.05). In turn, better self-care was linked with better glycemic outcomes, including fewer episodes of hypoglycemia and improved HbA1c over time. Fit indices indicated good fit of the model to the data (confirmatory fit index = 0.94, root mean square error of approximation = 0.05), with stronger and more meaningful associations for OnTrack than for KnowIt.
CONCLUSIONS
In the context of an intervention to reduce DD for adults with T1D, results indicate that reductions in DD do not affect glycemic outcomes directly but through improvements in self-care behavior. Findings support the importance of integrating disease management with DD interventions to maximize improvements in glycemic outcomes.
Introduction
Diabetes distress (DD) refers to the personal, often hidden side of diabetes: It reflects the unique emotional burdens and strains that individuals with diabetes often experience as they manage a demanding chronic disease over time (1,2). Cross-sectional studies have demonstrated significant positive associations between elevated DD and glycemic outcomes (3–6) as well as similar linkages between DD and core aspects of diabetes self-care. These include missed insulin boluses (3), unhealthy diet, and low physical activity (6). These studies, alongside sparse longitudinal results that span both type 1 diabetes (T1D) and type 2 diabetes, indicate consistent, but modest covariations between DD and HbA1c such that increases or decreases in DD co-occur with similar increases or decreases in HbA1c over time (5,7–9). While literature examining linkages between DD and hypoglycemic episodes is sparse, results similarly support cross-sectional associations between elevated DD and frequency of hypoglycemia (10) as well as decreases in both DD and frequency of hypoglycemia following glucose monitoring interventions (11,12), suggesting that changes in hypoglycemia and DD may also covary.
The consistency of these significant associations across studies raises an important question with implications for clinical care: How and through which behavioral mechanisms is DD linked with glycemic outcomes? Answers to this question have import when designing and delivering interventions. For example, should interventions focus on reducing DD with the idea that once DD is reduced, improvements in glycemic outcomes will follow? And if so, which self-care or educational constructs are key to attenuating this relationship? Furthermore, identifying important moderating factors in these processes would clarify for whom these interventions are most and least effective.
Theories of emotion regulation (ER) provide a useful conceptual platform from which to explore the potential impact of DD on diabetes management. This approach suggests that as DD increases over time, emotions become more intense and less well modulated, which leads to a narrowing of critical attention, an inability to consider realistic behavioral options, and a reduced ability to choose alternative diabetes management approaches (13). For example, the Broaden-and-Build and Dynamic Models of Affect (13,14) indicate that increases in DD, with its altered ER, may act as a “brake” on the application of existing diabetes knowledge and the utilization of more effective behavioral strategies to improve glycemic management. This brake can in turn lead to an exacerbation of negative outcomes and a further increase in DD in a cyclic fashion. Similarly, high DD may limit the potential uptake of diabetes education and willingness to trying new technologies, making individuals less responsive to a variety of educational interventions. Releasing the DD brake and remodulating ER through targeted DD intervention could end the negative cycle. This hypothesis was supported in our previous cross-sectional study in adults with T1D such that multiple indicators of poor ER were significantly associated with higher DD, and, subsequently, higher DD was significantly associated with skipped insulin boluses and higher HbA1c (3).
Building on these initial cross-sectional studies, we now seek to understand further the critical linkages among DD, self-care, and glycemic outcomes longitudinally, providing additional evidence for understanding their associations when examined during a dynamic period of time. Given the conceptual linkages between targeted management distress and the behavioral and glycemic outcomes under study, the high prevalence of this source of DD in clinical populations (2,8), and the high correlation between management and total distress (2), we focus the current study on the impact of management distress on glycemic outcomes. As part of a two-arm randomized controlled trial in adults with T1D (called Reducing Distress and Enhancing Effective Management for T1D Adults [T1-REDEEM]), we previously reported dramatic decreases in DD and statistically significant decreases in HbA1c over the 9 months following participation in each of the two active intervention programs: OnTrack, a DD-focused, affective-based intervention, and KnowIt, an education/management-based intervention (12). Because T1-REDEEM was designed to reduce DD and improve glycemic outcomes, it provides a unique opportunity to investigate further the mechanisms and directionality through which one set of variables affects the others, thus enhancing our knowledge of their potential causative impact with major implications for developing strategies of intervention (8).
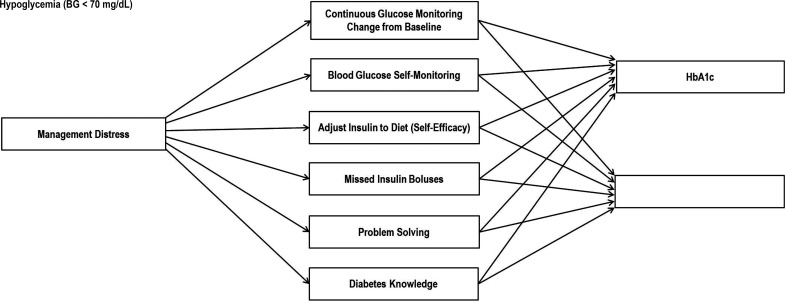
Given the impact of emotional dysregulation during periods of stress, the primary aim of the current study was to examine the longitudinal association between change in DD and change in glycemic outcomes. We hypothesized that decreases in DD, as a function of intervention, would be associated with improvements in both diabetes knowledge and diabetes self-care, which, in turn, would be associated with improved glycemic outcomes (Fig. 1). That is, reductions in DD would affect changes in glycemic outcomes indirectly through changes in diabetes self-care. We hypothesized that these effects would operate differently in the two study arms. Because of the affective focus of OnTrack, the path between DD and glycemic outcomes in this study arm would be stronger and more consistent than for the KnowIt study arm, thus assessing the relative impact of an affective-focused intervention compared with a solely knowledge-based intervention. Finally, to more fully investigate other potential paths of influence and further explicate the hypothesized model (Fig. 1), we explored an alternative model: that changes in self-care drive changes in glycemic outcomes either directly or through DD.
Figure 1.
Measurement model. BG, blood glucose.
Research Design and Methods
Participants
Adults with T1D were recruited from diabetes clinics, registries, support groups, and diabetes organizations in the western U.S. (California, Oregon, Arizona) and Ontario, Canada, to ensure a diverse sample. Inclusion criteria were as follows: ≥19 years of age; diagnosis of T1D for at least 12 months; ability to read, write, and speak English; mean item score of ≥2 on the Type 1 Diabetes Distress Scale, indicating elevated DD (2); HbA1c ≥7.5% (within the past 3 months); no severe complications (e.g., end-stage renal disease); and absence of psychosis or dementia.
Procedures
A description of the full study protocol and the intervention programs has been previously published (15). In brief, individuals who met inclusion criteria were identified through either a community-based opt-in procedure in which interested individuals contacted the research team or a clinic-based opt-out procedure through letters from each clinic informing them that they would receive a telephone call from a project representative if they did not opt out by calling or returning an enclosed postcard. All participants were screened for eligibility by telephone and, if interested, e-mailed a personal link to a Health Insurance Portability and Accountability Act–protected online survey and informed consent form. Participants also provided permission for their health care provider to release their most recent HbA1c results (within 3 months). If a timely HbA1c result was not available, a prepaid laboratory slip was mailed to the participant for HbA1c collection at a local facility. Following survey completion, participants were randomized to either the KnowIt or the OnTrack group, using a computer-generated, random number protocol (1:1 ratio). Both interventions required the same participant time commitment: attendance at a 1-day group workshop with a trained group leader and participation in four 1-h group online video meetings over the following 3 months. KnowIt, led by a certified diabetes educator, included a diabetes update of key factors regarding the causes and management of T1D and focused on strategies to address specific management problems. OnTrack, led by a psychologist with diabetes experience, focused on ways to deal with the emotional side of diabetes, with specific techniques drawn from programs of empowerment-based communication (16,17) and motivational interviewing (18,19), and included several ER elements (e.g., keeping feelings in perspective to reduce overreactions, separating feelings from appraisals of self-worth). The online survey was repeated 9 months after the initial survey, including permission for the release of the most recent HbA1c result. Participants were sent electronic gift cards ($25 for the initial survey and $45 for the 9-month survey). The study received approval from the University of California, San Francisco, committee on human research. Data were collected in 2014–2017 and analyzed in 2017–2020.
Measures
All measures were collected at baseline and 9-month follow-up to document change over time. Demographic measures included age, sex, education level, and age at diagnosis. Diabetes-specific measures included number of diabetes complications (from a list of 14), pump usage, and use of continuous glucose monitoring (CGM).
Diabetes management distress was measured by a four-item subscale of the Type 1 Diabetes Distress Scale (2) (α = 0.76), which assesses worries and burdens around the daily diabetes management regimen. Management distress is among the most commonly reported sources of DD and is most directly linked to management and glycemic outcomes, the focus of the current study (2,6,8,20). Items are rated on a six-point scale from “not a problem” to “a very serious problem.” Mean item scores of ≥2 are considered clinically meaningful (moderate distress 2.0–2.9, high distress ≥3).
Six measures of diabetes self-care were included. 1) Missed insulin boluses over the past week was assessed by one item: “How many times did you typically miss or skip a bolus that you probably should have taken during a typical day over the past week?” 2) Frequency of blood glucose checks per day was assessed by one item: “How many times did you typically check your blood sugar during a typical day over the past week?” 3) Diabetes knowledge was measured by a 27-item assessment derived from the Revised Brief Diabetes Knowledge Test (21), with expanded items to include content presented in the KnowIt intervention. 4) Adjusting insulin to diet (self-efficacy) was assessed by one item: “Adjust your insulin correctly when you eat more or less than usual.” Respondents rated their confidence on a five-point scale, with lower ratings indicating less confidence. 5) Problem solving was assessed by the nine-item Effective Problem-Solving subscale (α = 0.86) of the Health Problem Solving Scale (22). Respondents rated items on a five-point scale, with lower ratings indicating less problem-solving skill. A total summed score was calculated. 6) Change in CGM during the 9-month period was assessed with a binary variable based on CGM status at baseline and follow-up. Glycemic outcomes were assessed by HbA1c obtained from clinic records or laboratory tests within 3 months of survey completion and self-reported number of hypoglycemic episodes (<70 mg/dL) in the past 7 days.
Data Analysis
Sample size and power estimates are based on a two-sided α = 0.05 and Student t tests on change from baseline to 3 and 9 months. Conservatively estimating a 20% attrition rate, a sample of 145 per group allows for detection of small to moderate DD effect sizes (d = 0.35–0.40 SD unit differences) and mean changes in HbA1c of ≥0.48% (21–25). Structural equation modeling was used to examine relationships among DD, self-care, and glycemic outcomes as outlined in Fig. 1. Models were estimated using Mplus version 6.1 software (26). Mplus uses an expectation maximization algorithm to handle missing data, allowing for inclusion of all participants’ data in analyses. Analyses were specified to estimate regression parameters, covariances, means, and variances according to hypothesized relationships. The self-care measures were regressed on DD and were specified to covary with each other. Glycemic outcomes were regressed on the self-care measures and were specified to covary with each other. In the model, 9-month follow-up P values for all variables were regressed on baseline values to adjust for initial levels. To test for the effects of treatment group, multiple-group (by treatment condition) structural equation modeling was used (27). These analyses tested for significant differences by treatment group in regression parameters, covariances, means, and variances, using the “model test” command in Mplus. Parameter estimates that did not significantly differ were constrained to be equal across groups; estimates that did significantly differ were allowed to be freely estimated. Patient characteristics (i.e., age, sex) that had previously been associated with DD and glycemic outcomes (2,7,8,12) were explored in the models.
Results
The sample included 301 adults with T1D (149 participants in KnowIt and 152 participants in OnTrack). Losses after randomization (17 from KnowIt, 27 from OnTrack) (12) were due to lack of time or interest or moving outside the area. Attrition at 9-month follow-up was minimal (12% total, 9.4% KnowIt, 16.4% OnTrack) and did not differ by study arm. Those who dropped out were younger (40.6 vs. 45.7 years of age) and had significantly higher baseline management DD scores (3.5 vs. 3.1) and HbA1c (9.2% vs. 8.7%) and more complications (3.0 vs. 2.7) compared with those who completed the study. Mean (SD) age was 45.1 (15.0) years, 69.1% were female, and mean (SD) baseline HbA1c was 8.80% (1.12%) (73 [15.5] mmol/mol). Participant characteristics by intervention group are reported in Table 1. Participants randomized to KnowIt were slightly older than OnTrack participants, and OnTrack participants scored higher at baseline on diabetes knowledge and reported more missed insulin boluses than KnowIt participants (Table 1).
Table 1.
Participant characteristics by treatment group (N = 301)
| Variable | KnowIt (n = 149) | OnTrack (n = 152) | Difference, P value |
|---|---|---|---|
| Age (years) | 47.32 (14.53) | 42.82 (15.14) | 0.009 |
| Education (years) | 15.65 (3.60) | 15.24 (3.63) | 0.32 |
| Number of children | 1.10 (1.30) | 0.93 (1.04) | 0.20 |
| Age at diagnosis (years) | 21.20 (14.36) | 19.46 (13.68) | 0.10 |
| Years with diabetes | 26.12 (13.97) | 23.17 (13.26) | 0.06 |
| Number of complications | 2.84 (2.56) | 2.65 (2.47) | 0.51 |
| Female, % | 70.5 | 67.8 | 0.61 |
| White, % | 82.6 | 77.6 | 0.29 |
| With partner, % | 61.7 | 67.5 | 0.29 |
| With insulin pump, % | 63.8 | 67.8 | 0.46 |
| With CGM, % | 37.6 | 38.8 | 0.83 |
| Baseline DD management | 3.29 (1.10) | 3.46 (1.15) | 0.20 |
| Baseline self-efficacy | 2.87 (0.71) | 2.90 (0.73) | 0.67 |
| Baseline problem solving | 26.95 (5.62) | 27.36 (6.27) | 0.56 |
| Baseline missed insulin boluses | 1.12 (1.32) | 1.66 (1.99) | 0.006 |
| Baseline times checked glucose per day | 4.70 (2.55) | 4.92 (2.51) | 0.45 |
| Baseline diabetes knowledge (% correct) | 70.44 (13.84) | 74.79 (11.48) | 0.01 |
| Baseline HbA1c % | 8.77 (1.13) | 8.83 (1.11) | 0.65 |
| Baseline glucose <70 mg/dL | 2.60 (1.99) | 2.51 (2.03) | 0.08 |
Data are mean (SD) unless otherwise indicated. P values were derived from independent samples t tests for continuous variables and χ2 tests for categorical variables.
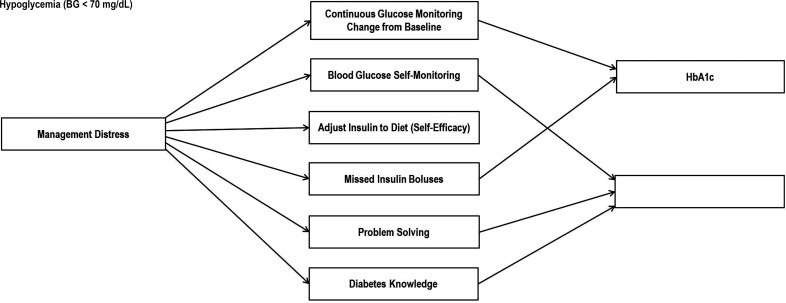
The final structural equation modeling path for the total sample is illustrated in Fig. 2, and the model estimates are presented in Table 2. The structural equation model fit indices indicated good fit of the model to the data (χ2 [df = 197] = 259, P = 0.002, comparative fit index = 0.94, Tucker Lewis index = 0.94, root mean square error of approximation = 0.046). In the final model, reductions in DD were significantly associated with improved diabetes self-care, which was, in turn, significantly linked with better glycemic outcomes. Patient characteristics (i.e., age, sex) were included in initial models; however, their effects were minimal and nonsignificant and, therefore, not retained in the final model.
Figure 2.
Significant structural equation modeling pathways linking change in management distress with change in glycemic outcomes in the final model. Linkages shown are significant pathways (P < 0.05) (see Table 2) in the final model. All pathways denoted here reached statistical significance for the OnTrack and KnowIt groups, with the exception of pathways from problem solving and diabetes knowledge to hypoglycemia episodes for the KnowIt group (P > 0.05). Further variations in strength of associations by intervention group are presented in Table 2. BG, blood glucose.
Table 2.
Regression effects and correlations in the final model
| KnowIt | OnTrack | |
|---|---|---|
| Management distress | ||
| Change in glucose monitoring | −0.14* | −0.14* |
| Adjust insulin to diet (self-efficacy) | −0.14** | −0.14** |
| Problem solving | −0.24*** | −0.24*** |
| Missed insulin boluses | +0.20*** | +0.22*** |
| Glucose self-monitoring | −0.15** | −0.15** |
| Diabetes knowledge | 0.10* | +0.10* |
| Change in glucose monitoring | ||
| HbA1c | −0.12*** | −0.12*** |
| Hypoglycemia (blood glucose <70 mg/dL) | −0.004 | −0.004 |
| Adjust insulin to diet (self-efficacy) | ||
| HbA1c | −0.01 | −0.01 |
| Hypoglycemia (blood glucose <70 mg/dL) | +0.003 | +0.003 |
| Problem solving | ||
| HbA1c | −0.04 | −0.04 |
| Hypoglycemia (blood glucose <70 mg/dL) | −0.08 | +0.16* |
| Missed insulin boluses | ||
| HbA1c | +0.08* | +0.10* |
| Hypoglycemia (blood glucose <70 mg/dL) | −0.03 | −0.03 |
| Glucose self-monitoring | ||
| HbA1c | −0.03 | −0.03 |
| Hypoglycemia (blood glucose <70 mg/dL) | +0.12* | +0.12* |
| Diabetes knowledge | ||
| HbA1c | −0.05 | −0.05 |
| Hypoglycemia (blood glucose <70 mg/dL) | +0.06 | −0.20** |
| Change in glucose monitoring with | ||
| Adjust insulin to diet (self-efficacy) | −0.02 | −0.02 |
| Problem solving | −0.04 | −0.04 |
| Missed insulin boluses | −0.09* | −0.09* |
| Glucose self-monitoring | +0.08 | +0.08 |
| Diabetes knowledge | −0.06 | −0.06 |
| Adjust insulin to diet (self-efficacy) with | ||
| Problem solving | +0.23*** | +0.23*** |
| Missed insulin boluses | −0.03 | −0.03 |
| Glucose self-monitoring | −0.02 | −0.02 |
| Diabetes knowledge | +0.08 | +0.08 |
| Problem-solving with | ||
| Missed insulin boluses | −0.14 | −0.14 |
| Glucose self-monitoring | +0.16 | +0.16 |
| Diabetes knowledge | −0.002 | −0.002 |
| Missed insulin boluses with | ||
| Glucose self-monitoring | −0.11 | −0.11 |
| Diabetes knowledge | −0.24 | −0.24 |
| Glucose self-monitoring with diabetes knowledge | +0.08 | +0.08 |
| HbA1c with hypoglycemia (blood glucose <70 mg/dL) | −0.22** | +0.06 |
Effects in boldface type denote a significant difference between the two groups (equality constraint relaxed).
P < 0.05,
P < 0.01,
P < 0.001.
Three aspects of the findings regarding hypothesis one are noteworthy. First, in no case was a reduction in DD directly linked to an improvement in glycemic outcome. When considering each of the self-care indicators in the model, the effect of DD on glycemic outcomes operated only indirectly through changes in self-care behavior. Second, reductions in management distress were significantly and independently linked with changes in each of the six self-care variables: fewer missed insulin boluses (B = 0.20 KnowIt and 0.22 OnTrack; P < 0.001), increased problem-solving skills (B = –0.24; P < 0.001), increased blood glucose monitoring (B = –0.15; P < 0.01), improved perceived ability to make adjustments to insulin regimen in relation to diet (B = –0.14; P < 0.01), and increased likelihood of CGM initiation (B = –0.14; P < 0.05). Change in management distress, however, was linked with diabetes knowledge in an unexpected direction, with decreases in distress associated with decreases in diabetes knowledge (B = 0.10; P < 0.05). Third, five of the six self-care variables were significantly and independently linked to at least one of the two glycemic outcome measures, with diet-adjusted insulin the single variable remaining unrelated. Reductions in missed boluses (B = 0.08 KnowIt and 0.10 OnTrack; P < 0.05) and CGM initiation (B = –0.12; P < 0.001) were each significantly and independently linked to reductions in HbA1c, and decreases in frequency of blood glucose checks (B = 0.12; P < 0.05) were linked with reductions in number of hypoglycemic episodes.
Tests of invariance in the model allow for understanding the significant differences found in these processes between the two intervention groups (Table 2). Diabetes knowledge (B = –0.20; P < 0.01) and self-reported problem solving (B = 0.16; P < 0.01) were linked with less frequent hypoglycemic episodes over the 9-month period for the OnTrack group only. Furthermore, the linkage between skipped boluses and HbA1c (B = 0.10 and B = 0.08; P < 0.05) as well as the association between DD and skipped boluses and HbA1c (B = 0.22 and B = 0.20; P < 0.001) were significant for both groups but relatively stronger for OnTrack. Thus, where intervention group differences emerged, a more robust set of linkages were noted for the DD-focused (OnTrack) versus the education/management (KnowIt) intervention.
In supplementary analyses, we tested an alternative model in which the hypothesized order of influence between DD and self-care behavior was reversed. That is, the model tested whether changes in self-care as a result of intervention led to subsequent changes in DD and, in turn, whether changes in DD led to changes in glycemic outcomes. While the model fit indices were acceptable statistically (χ2 [df = 194] = 273; P < 0.001; comparative fit index = 0.93; Tucker Lewis index = 0.92; root mean square error of approximation = 0.052), the model itself did not prove meaningful. None of the DD or self-care variables in this alternative model were significantly associated with changes in glycemic outcomes over time. Thus, the model presented in Fig. 1 provides a more useful and parsimonious explanation of the proposed underlying mechanisms of change.
Conclusions
Among adults with T1D participating in T1-REDEEM, we explored the pathways linking changes in DD to changes in diabetes self-care and, subsequently, to improvements in glycemic outcomes. The results identify multiple, potentially causative mechanisms through which decreases in DD may lead to improvements in glycemic outcomes. Of primary importance, reductions in DD do not affect changes in glycemic outcomes directly; instead, their significant effect operates exclusively through changes in diabetes self-care behavior. This suggests that reductions in DD through intervention display proximal effects on self-care and that improvements in self-care are required to achieve more distal effects on glycemic outcomes. In contrast, results of our alternative, reverse model were not meaningful; that is, we find no support that changes in self-care behavior drive improvements in glycemic outcomes through reductions in DD. These results support further the ER premise that DD may act as a brake on the effectiveness of educational or self-care interventions to improve glycemic outcomes and that releasing the brake through the inclusion of DD-targeted interventions enhances the impact of both to drive improvements in glycemic outcomes. Consideration of participant demographics did not impact the model results, suggesting that these associations apply across the T1D population.
Reductions in DD display significant and independent effects on multiple aspects of diabetes self-care behavior among adults with T1D, demonstrating its pervasive negative influence on overall, day-to-day diabetes management. For example, we find that decreases in DD are significantly and independently linked with changes over time in all six self-care behaviors examined. Thus, DD affects not just isolated or specific individual aspects of self-care (7,12) but, instead, has a pervasive influence on diabetes management in general, highlighting its global importance and clinical impact.
Despite the unified influence of reductions in DD on improvements in all self-care variables examined, the impact of changes in self-care on glycemic outcomes are more specific: Improvements in some self-care behaviors targeted only specific glycemic outcomes. For example, decreases in missed insulin boluses and initiation of CGM were each only linked with reductions in HbA1c over 9 months, not with a reduction in hypoglycemic episodes. This result supports and extends previous work pointing to how reductions in missed insulin boluses and adoption of CGM technology serve as critical explanatory pathways from DD to HbA1c (3); that is, reduced DD leads to both fewer missed insulin boluses and greater adoption of CGM technology, which, in turn, leads to reduced HbA1c. In contrast, other self-care indicators appear to be more targeted toward reducing episodes of hypoglycemia. For example, improved diabetes knowledge was associated only with decreases in self-reported hypoglycemia frequency, not with reductions in HbA1c. Thus, although reductions in DD seem to have a general effect on multiple aspects of self-care, improvements in self-care appear to affect specific and targeted glycemic outcomes.
One unexpected finding occurred: Improvements in self-reported glucose self-monitoring and self-reported problem solving as a result of intervention were associated with increased, rather than decreased, self-reported frequency of hypoglycemic episodes. One explanation is that participants who increased the frequency of glucose testing and who improved their problem-solving skills as a result of intervention may have become more aware of and more likely to report episodes of hypoglycemia. Extending follow-up to ascertain whether with increased awareness a subsequent reduction in actual episodes occurs over time will be helpful.
Substantive differences occurred in the model tests for the two intervention arms (OnTrack vs. KnowIt). While many of the model pathways were similar for both groups, there were notable differences in the strength, direction, and meaningfulness of individual pathways. In general, the strength of associations seen in the pathways and the substance of the model were stronger for the DD-focused OnTrack intervention than for the management-focused KnowIt intervention. For example, stronger linkages occurred in OnTrack than KnowIt between the paths linking decreases in DD, reduced missed insulin boluses, and reductions in HbA1c. The greater efficiency and meaningfulness of OnTrack, a DD emotion-focused intervention, than KnowIt, an education/management intervention, further underscores the importance of addressing DD directly rather than assuming that education or management assistance alone will most efficiently address glycemic outcomes in the context of high DD. Doing so provides added value and when delivered either simultaneously or sequentially may enhance the overall effectiveness of education and self-care programs to maximize glycemic outcomes (12,28).
This pattern of results is in agreement with reviews that have cited the positive impact of DD interventions delivered in a group setting on a variety of diabetes-related outcomes (23,24,29) and has implications for addressing DD in routine clinical care. Our previous work related to OnTrack and KnowIt points to the relatively modest costs of training and implementing these interventions in clinic settings (30), with $250 per participant and cost per unit change in DD of $364 for KnowIt and $335 for OnTrack. However, as Skinner et al. (29) pointed out, there will always be a shortage of trained mental health professionals relative to the need. Thus, a critical next step will be to expand programs of care that address DD by leveraging existing diabetes health teams to deliver evidence-based interventions, such as OnTrack, directly. Most likely, this will require that these programs be adapted to fit the skill sets of existing clinic staff, include sufficient training and follow-up, and be structured to mesh with clinic protocols and patient flow seamlessly.
This study has several strengths. It included a diverse sample with both elevated DD and HbA1c from several geographical and clinical settings, followed a randomized controlled design, led to low attrition, and yielded significant decreases in DD and HbA1c. Several limitations, however, should be considered when interpreting these results. First, although the current study included multiple self-care and glycemic outcome measures, there are undoubtedly additional aspects of diabetes management (e.g., social support, time in range) as well as other contextual constructs (e.g., lifestyle behaviors such as physical activity or substance use, additional health or mental health diagnoses, medication use that could impact glycemic management) that should be addressed. It also will be important to document the specific impact of DD on self-care and glycemic outcomes for specific groups of adults with T1D and to explore sources of DD other than management distress that may be linked to these outcomes. Second, to enable the inclusion of a diverse sample recruited from multiple settings, most study measures were self-reported. Confirmation of the findings using more “objective” measures (e.g., CGM, insulin pens) would be beneficial. Finally, the study design was limited to two time points spanning 9 months. While providing a critical glimpse into the processes and mechanisms of change, the adoption of more micro longitudinal studies (e.g., daily reporting) and extended longitudinal studies with more assessment points will enable the modeling of trajectories or slopes of change over time among DD, self-care, and glycemic outcomes.
In conclusion, in the context of a randomized controlled trial to reduce DD for adults with T1D, results are in alignment with ER theory to support the brake hypothesis: that DD acts as a brake on efforts to improve self-care behaviors in ways that enhance glycemic outcomes. Through intervention, the effects of reduced DD on improved glycemic outcomes operate only indirectly through improvements in self-care behaviors. Thus, DD reductions have positive effects on proximal self-care behavior, which, in turn, impacts more distal changes in glycemic outcomes. Among adults with T1D, results indicate the importance of directing interventions to reduce DD through DD-targeted interventions as a starting point in improving both self-care and glycemic outcomes.
Article Information
Acknowledgments. The authors thank the following site collaborators for their assistance: Andrew Almanns (Oregon Health & Science University) Marina Basina (Stanford University), Ian Blumer (University of Toronto), Charles Chioe (University of California, San Diego), Sara Kim (University of California, San Francisco), Ann Peters (University of Southern California), Karen Weissmann (California Pacific Medical Center), Umesh Masharani (University of California, San Francisco), and Patricia Wu (Kaiser Permanente). They also acknowledge the contributions of project associates Vicky Bowyer, Britnee Ochabski, Hannah Martin, and Meredith Craven (University of California, San Francisco).
Funding: Funding for the research was provided by National Institute of Diabetes and Digestive and Kidney Diseases award R01-DK-094863.
The funder was not involved in the study design, data collection or analysis, or manuscripts.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.H. conceived the idea and analysis plan. D.H. and L.F. had the primary responsibility of writing the manuscript. L.S. carried out the analyses. L.F., supported by D.H., oversaw the overall conduct of the study. D.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
Clinical trial reg. no. NCT02175732, clinicaltrials.gov.
References
- 1. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care 2007;30:542–548 [DOI] [PubMed] [Google Scholar]
- 2. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications 2015;29:572–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fisher L, Hessler D, Polonsky W, et al. Emotion regulation contributes to the development of diabetes distress among adults with type 1 diabetes. Patient Educ Couns 2018;101:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Bastelaar KM, Pouwer F, Geelhoed-Duijvestijn PH, et al. Diabetes-specific emotional distress mediates the association between depressive symptoms and glycaemic control in type 1 and type 2 diabetes. Diabet Med 2010;27:798–803 [DOI] [PubMed] [Google Scholar]
- 5. Strandberg RB, Graue M, Wentzel-Larsen T, Peyrot M, Rokne B. Relationships of diabetes-specific emotional distress, depression, anxiety, and overall well-being with HbA1c in adult persons with type 1 diabetes. J Psychosom Res 2014;77:174–179 [DOI] [PubMed] [Google Scholar]
- 6. Joensen LE, Tapager I, Willaing I. Diabetes distress in type 1 diabetes--a new measurement fit for purpose. Diabet Med 2013;30:1132–1139 [DOI] [PubMed] [Google Scholar]
- 7. Hessler DM, Fisher L, Polonsky WH, et al. Diabetes distress is linked with worsening diabetes management over time in adults with type 1 diabetes. Diabet Med 2017;34:1228–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hessler D, Fisher L, Glasgow RE, et al. Reductions in regimen distress are associated with improved management and glycemic control over time. Diabetes Care 2014;37:617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care 2012;35:2472–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al Sayah F, Yeung RO, Johnson JA. Association of depressive symptoms and diabetes distress with severe hypoglycemia in adults with type 2 diabetes. Can J Diabetes 2019;43:316–321 [DOI] [PubMed] [Google Scholar]
- 11. Al Hayek AA, Robert AA, Al Dawish MA. Effectiveness of the FreeStyle Libre flash glucose monitoring system on diabetes distress among individuals with type 1 diabetes: a prospective study. Diabetes Ther 2020;11:927–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polonsky WH, Hessler D, Ruedy KJ; DIAMOND Study Group . The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 2017;40:736–741 [DOI] [PubMed] [Google Scholar]
- 13. Fredrickson BL, Branigan C. Positive emotions broaden the scope of attention and thought-action repertoires. Cogn Emotion 2005;19:313–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reich J, Zautra A, Davis M. Dimensions of affect relationships: Models and their integrative implications. Rev Gen Psychol 2003;7:66–83 [Google Scholar]
- 15. Fisher L, Hessler D, Polonsky WH, et al. T1-REDEEM: a randomized controlled trial to reduce diabetes distress among adults with type 1 diabetes. Diabetes Care 2018;41:1862–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Funnell MM, Tang TS, Anderson RM. From DSME to DSMS: developing empowerment-based diabetes self-management support. Diabetes Spectr 2007;20:221–226 [Google Scholar]
- 17. Madmoli M. A systematic review study on the results of empowerment-based interventions in diabetic patients. Int Res Med and Health Sci 2019;2:1–7 [Google Scholar]
- 18. Rollnick S, Allison J. Motivational interviewing. In The Essential Handbook of Treatment and Prevention of Alcohol Problems. Heather N, Stockwell T, Eds. Chichester, U.K., Wiley, 2004, pp. 105–116 [Google Scholar]
- 19. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. New York, Guilford Press, 2012 [Google Scholar]
- 20. Fisher L, Hessler D, Polonsky W, Strycker L, Masharani U, Peters A. Diabetes distress in adults with type 1 diabetes: prevalence, incidence and change over time. J Diabetes Complications 2016;30:1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fitzgerald JT, Funnell MM, Anderson RM, Nwankwo R, Stansfield RB, Piatt GA. Validation of the Revised Brief Diabetes Knowledge Test (DKT2). Diabetes Educ 2016;42:178–187 [DOI] [PubMed] [Google Scholar]
- 22. Hill-Briggs F, Yeh HC, Gary TL, Batts-Turner M, D’Zurilla T, Brancati FL. Diabetes problem-solving scale development in an adult, African American sample. Diabetes Educ 2007;33:291–299 [DOI] [PubMed] [Google Scholar]
- 23. Sturt J, Dennick K, Hessler D, Hunter BM, Oliver J, Fisher L. Effective interventions for reducing diabetes distress: systematic review and meta-analysis. Int Diabetes Nurs 2015;12:40–55 [Google Scholar]
- 24. Schmidt CB, van Loon BJP, Vergouwen ACM, Snoek FJ, Honig A. Systematic review and meta-analysis of psychological interventions in people with diabetes and elevated diabetes-distress. Diabet Med 2018;35:1157–1172 [DOI] [PubMed] [Google Scholar]
- 25. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care 2013;36:2551–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muthén LK, Muthén BO. Mplus user’s guide (6th ed.). Los Angeles, Muthén & Muthén, 2011 [Google Scholar]
- 27. Duncan TE, Duncan SC, Strycker LA. An Introduction to Latent Variable Growth Curve Modeling: Concepts, Issues, and Applications. 2nd ed. Mahwah, NJ, Lawrence Erlbaum, 2006, pp. 81–92 [Google Scholar]
- 28. Fisher L, Polonsky WH, Hessler D. Addressing diabetes distress in clinical care: a practical guide. Diabet Med 2019;36:803–812 [DOI] [PubMed] [Google Scholar]
- 29. Skinner TC, Joensen L, Parkin T. Twenty-five years of diabetes distress research. Diabet Med 2020;37:393–400 [DOI] [PubMed] [Google Scholar]
- 30. Shumway M, Fisher L, Hessler D, Bowyer V, Polonsky WH, Masharani U. Economic costs of implementing group interventions to reduce diabetes distress in adults with type 1 diabetes mellitus in the T1-REDEEM trial. J Diabetes Complications 2019;33:107416. [DOI] [PMC free article] [PubMed] [Google Scholar]