Abstract
OBJECTIVE
Rates of diagnosis of prediabetes and uptake of the National Diabetes Prevention Program (NDPP) are low. We evaluated a proactive three-level strategy to identify individuals with prediabetes in a population with employer-sponsored health insurance.
RESEARCH DESIGN AND METHODS
We studied 64,131 insured employees, dependents, and retirees ≥18 years of age without diagnosed diabetes, 19,397 (30%) of whom were estimated to have prediabetes. Individuals with prediabetes were identified by 1) searching claims diagnoses and previously performed HbA1c test results, 2) risk stratifying people 40–64 years of age without diabetes, prediabetes, or documented normal HbA1c to identify individuals at higher risk and encourage them to be tested, and 3) using a media campaign to encourage employees not otherwise targeted to self-screen and, if at higher risk, to be tested.
RESULTS
Using claims and laboratory data, 11% of the population was identified as having prediabetes. Of those 40–64 years of age, 25% were identified as being at higher risk, and 27% of them were tested or diagnosed within 1 year. Of employees exposed to the media campaign, 14% were tested or diagnosed within 1 year. Individuals with prediabetes were older, heavier, and more likely to have hypertension and dyslipidemia. Testing and diagnosis were associated with receiving medical care and provider outreach. A total of 8,129 individuals, or 42% of those with prediabetes, were identified.
CONCLUSIONS
Analysis of existing health insurance data facilitated the identification of individuals with prediabetes. Better identification of people with prediabetes is a first step in increasing uptake of the NDPP.
Introduction
In 2018, 88 million U.S. adults aged ≥18 years had prediabetes (1). Unfortunately, despite compelling evidence of the efficacy (2) and cost-effectiveness (3,4) of a targeted lifestyle intervention for diabetes prevention as studied in the Diabetes Prevention Program and the nationwide rollout of the National Diabetes Prevention Program (NDPP) (5), uptake of the NDPP has been low (6). Although offered through >400 venues and paid for by many commercial and state employee health plans, state Medicaid programs, and Medicare, <36,000 individuals enrolled in the NDPP during its first 4 years of implementation (6). This suggests that by 2015, only 1 in 10,000 adults with prediabetes had availed themselves to the NDPP each year. There are a number of possible explanations for this low uptake (7). First, prediabetes is often undiagnosed, and, even when diagnosed, information about the diagnosis may not be communicated to patients. In 2018, only 15% of U.S. adults with prediabetes reported being told by a health professional that they had prediabetes (1). Second, there is often a lack of proactive outreach to people with prediabetes to engage them in targeted prevention interventions. Most organizations that offer the DPP and payers who cover it have relied on passive self-referral. Third, both the length and intensity of the NDPP may deter some individuals from participating. The 1-year NDPP requires at least 16 sessions during the first 6 months and monthly sessions thereafter. Finally, the pay-for-performance reimbursement model used for the NDPP may deter NDPP suppliers from enrolling candidates whom they feel might not adhere to the program (8).
Beginning in August 2015, the University of Michigan (U-M) decided to offer its insured employees, dependents, and retirees with prediabetes their choice of four Centers for Disease Control and Prevention–recognized 1-year NDPPs at no out-of-pocket cost. We used this natural experiment to evaluate three population-based strategies to increase awareness of and screening for prediabetes and promote enrollment in the NDPP.
In this study, we describe the characteristics of the U-M population with previously diagnosed prediabetes, the population identified as being at high risk for prediabetes, and the population of U-M employees exposed to the media campaign who were not otherwise identified as having or being at high risk for prediabetes. We then describe the rates and outcomes of screening and the rates of diagnosis of prediabetes according to the target population and the identification strategy used. The results highlight the yield of these complimentary strategies to identify and to target screening to identify individuals with prediabetes.
Research Design and Methods
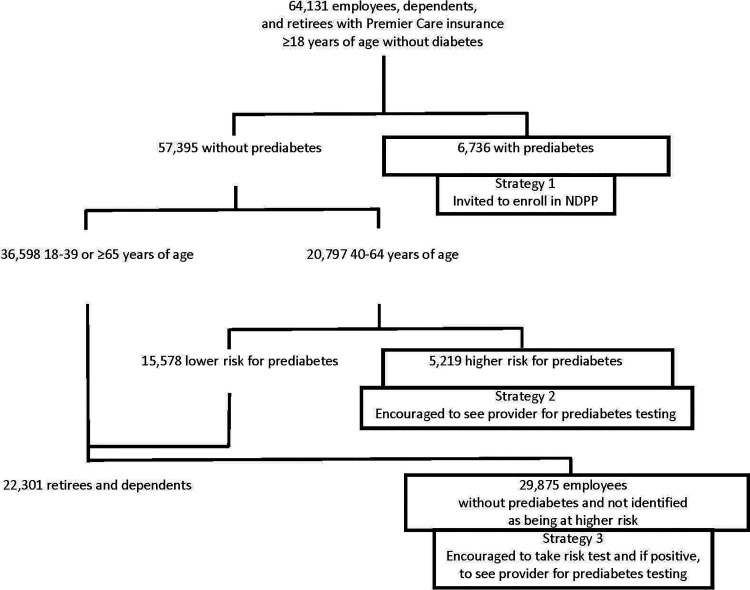
U-M is a public research university in Ann Arbor, MI, with smaller regional campuses in Flint and Dearborn, MI. Approximately 85,000 university employees, dependents, and retirees are insured by Premier Care, the U-M’s self-funded commercial health insurance program. Blue Care Network (BCN) is the claims manager for Premier Care. Recognizing the proven effectiveness and cost-effectiveness of the DPP (2–4), the U-M Benefits Office decided to offer the NDPP at no out-of-pocket cost to overweight and obese employees, dependents, and retirees ≥18 years of age with prediabetes who had Premier Care. Recognizing the historically low uptake of the NDPP, the Benefits Office chose to test three proactive identification strategies in a 3-year pilot study and to facilitate our rigorous evaluation of those strategies (Fig. 1).
Figure 1.
CONSORT diagram.
Strategy 1
Strategy 1 involved the health plan identifying individuals without diagnosed diabetes but with a claims diagnosis consistent with prediabetes or a previous HbA1c level of 5.7–6.4% (39–46 mmol/mol). Beginning in August 2015, BCN used enrollment, claims, pharmacy, and laboratory data to identify Premier Care members ≥18 years of age with no evidence of diabetes who had at least one claim for impaired fasting glucose (ICD-9 790.21 or ICD-10 R7301), impaired glucose tolerance (ICD-9 790.22 or ICD-10 R7302), or other abnormal glucose (ICD-9 790.29 or ICD-10 R73, R730, R7309, and R739) or an HbA1c level between 5.7% and 6.4% in the past 3 years. These criteria were then applied approximately every 6 months, and invitations to enroll in the NDPP were mailed to newly identified individuals meeting these criteria and to individuals identified to the health plan as having prediabetes by their primary care physicians (PCPs). Letters were mailed to a total of 6,736 individuals: 2,539 in August 2015, 948 in November 2015, 1,145 in October 2016, 561 in January 2017, 698 in July 2017, 557 in January 2018, and 239 in July 2018. Invitation letters were also mailed to 49 individuals who were not identified as having prediabetes by BCN but who were identified as having prediabetes by their PCPs. These individuals were likely diagnosed as having prediabetes based upon glucose criteria without a claims diagnosis code or a qualifying HbA1c level.
Strategy 2
Strategy 2 involved the health plan applying a validated risk stratification algorithm to claims data for individuals 40–64 years of age without diabetes, prediabetes, or documented normal glucose regulation (HbA1c <5.7%) to identify higher-risk individuals and encourage them to see their PCPs to be tested for prediabetes. Previously, we developed and validated an algorithm that used health plan enrollment and demographic, claims, pharmacy, and laboratory data (but not HbA1c or fasting glucose levels) to identify nonpregnant adults 40–64 years of age with impaired fasting glucose (defined as fasting glucose 110–125 mg/dL) or previously undiagnosed diabetes (7). Four models were developed that used demographic information and progressively more comprehensive health plan data related to diabetes risk factors (9). Approximately every 6 months, BCN applied these models to Premier Care data. Individuals in the highest three deciles of risk using the most comprehensive of the four models for which they had data were sent letters by BCN, notifying them of their increased risk and encouraging them to follow up with their PCPs for diagnostic testing. A total of 5,219 individuals 40–64 years of age received strategy 2 letters including 1,010 in July 2016, 1,371 in January 2017, 629 in July 2017, 1,450 in January 2018, and 759 in July 2018. Each strategy 2 letter was followed in ˜60 days by a single reminder letter. A few provider groups elected to perform additional outreach to their patients at high risk for prediabetes by sending electronic medical record portal messages or letters or by contacting their patients by telephone. The impact of this provider group outreach was assessed.
Strategy 3
Strategy 3 involved the U-M Benefits Office implementing a digital and print media campaign and sending an e-mail to all employees encouraging them to screen themselves for prediabetes using an online questionnaire and, if positive, to obtain follow-up diagnostic tests from their PCPs. In January 2018, the U-M Benefits Office launched a media campaign to its employees describing the DPP benefit. The campaign used print and electronic announcements, articles, advertisements, and social media. In addition, 29,875 employees, but not dependents or retirees, received e-mails encouraging them to self-screen for prediabetes with a simple, online five-item questionnaire (10) and, if positive, to see their PCPs to be tested for prediabetes. We assessed the number of times per week the link to the online screening questionnaire was accessed. We also assessed the numbers of employees who had not previously been targeted by strategy 1 or strategy 2 and who had HbA1c tests performed within 1 year of the date of the e-mail.
Data Analysis
Data for these analyses were obtained from BCN. Claims diagnoses were based on the presence of at least one ICD-9 or ICD-10 code in specific categories in the 3 years before the initial contact letter. Residential address zip codes were merged with data from the U.S. Census FactFinder Tool (11) to describe zip code–specific median household income, percent unemployment, and percent participation in the Supplemental Nutrition Assistance Program (or food stamps) based on 5-year averages derived from the American Community Survey. To evaluate the impact of the strategies individually and in aggregate, we described the characteristics of those with and without prediabetes, those who were identified as being at high risk and those who were at lower risk, and the characteristics of those who were screened and not screened. Differences between groups were assessed using t tests for continuous variables and χ2 tests for categorical variables. The study was reviewed and approved by the U-M Institutional Review Board, and all analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
The study population included 64,131 U-M employees, dependents, and retirees ≥18 years of age without diabetes who were enrolled in the University’s self-funded health insurance plan (Fig. 1). Table 1 shows the characteristics of the population. Mean age (± SD) was 39 ± 14 years. Fifty-five percent of the population were women, and 84% were White. Approximately half of the population had seen a PCP and specialist in the past year. Mean BMI was 28.3 ± 6.7 kg/m2.
Table 1.
Baseline characteristics of U-M employees, dependents, and retirees ≥18 years of age with Premier Care insurance, without diabetes stratified by prediabetes status
| Total | No prediabetes | Prediabetes | P value | |
|---|---|---|---|---|
| N (%) | 64,131 | 57,395 (89) | 6,736 (11) | — |
| Age (years) | 39 ± 14 | 38 ± 13 | 50 ± 12 | <0.0001 |
| Sex | <0.0001 | |||
| Women | 35,244 (55) | 31,338 (55) | 3,906 (58) | |
| Men | 28,887 (45) | 26,057 (45) | 2,830 (42) | |
| Race | <0.0001 | |||
| Asian | 3,382 (8) | 2,851 (8) | 531(10) | |
| Black | 2,688 (7) | 2,229 (6) | 459 (8) | |
| White | 34,575 (84) | 30,227 (84) | 4,348 (80) | |
| Other | 651 (2) | 579 (2) | 72 (1) | |
| At least one PCP visit in past year | 34,574 (54) | 28,654 (50) | 5,920 (88) | <0.0001 |
| At least one specialist visit in past year | 29,687 (46) | 25,046 (44) | 4,641 (69) | <0.0001 |
| BMI (kg/m2) | 28.3 ± 6.7 | 27.6 ± 6.3 | 32.4 ± 7.3 | <0.0001 |
| Blood pressure (mmHg) | ||||
| Systolic | 120 ± 15 | 119 ± 15 | 125 ± 15 | <0.0001 |
| Diastolic | 72 ± 10 | 72 ± 10 | 75 ± 10 | <0.0001 |
| Cholesterol (mg/dL) | ||||
| Total cholesterol | 194 ± 38 | 194 ± 38 | 194 ± 39 | 0.7565 |
| HDL | 55 ± 16 | 56 ± 17 | 52 ± 15 | <0.0001 |
| Women | 61 ± 16 | 63 ± 17 | 57 ± 15 | <0.0001 |
| Men | 48 ± 13 | 49 ± 13 | 46 ± 12 | <0.0001 |
| Triglycerides | 129 ± 88 | 122 ± 87 | 147 ± 91 | <0.0001 |
| LDL | 113 ± 32 | 113 ± 32 | 113 ± 33 | 0.8387 |
| HbA1c (%) | 5.7 ± 0.6, N = 9,134 (14) | 5.3 ± 0.5, N = 4,504 (8) | 5.8 ± 0.5, N = 4,630 (69) | <0.0001 |
| Claims diagnosis | ||||
| Overweight/obesity | 8,598 (13) | 6,223 (11) | 2,375 (35) | <0.0001 |
| Hypertension | 8,108 (13) | 5,539 (10) | 2,569 (38) | <0.0001 |
| Any antihypertensive medication | 8,168 (13) | 5,761 (10) | 2,407 (35) | <0.0001 |
| Dyslipidemia | 6,651 (10) | 4,062 (7) | 2,389 (35) | <0.0001 |
| Any lipid-lowering medication | 3,619 (6) | 2,232 (4) | 1,387 (20) | <0.0001 |
| Smoking | 1,737 (3) | 1,271 (2) | 466 (7) | <0.0001 |
| Women | 850 (2) | 624 (2) | 226 (6) | <0.0001 |
| Men | 887 (3) | 647 (2) | 240 (8) | <0.0001 |
| Cardiovascular disease | 1,917 (3) | 1,219 (2) | 698 (10) | <0.0001 |
| Geocoded indicators | ||||
| Median neighborhood income ($) | 70,228 | 70,290 | 69,704 | 0.0123 |
| Percent unemployment | 35.3 ± 5.0 | 35.4 ± 5.0 | 35.0 ± 4.7 | <0.0001 |
| Percent receiving food stamps | 7.9 ± 6.0 | 7.7 ± 5.9 | 8.7 ± 6.6 | <0.0001 |
Data are n (%) or mean ± SD.
At baseline, 11% of employees, dependents, and retirees ≥18 years of age without diabetes had a claims diagnosis for prediabetes or an HbA1c of 5.7–6.4% recorded in the previous 3 years. Table 1 shows the characteristics of these 6,736 individuals. Compared with those without prediabetes, those with prediabetes were significantly older, more likely to be women, and less likely to be White. Individuals with prediabetes were significantly more likely to visit a PCP or specialist in the past year. They had significantly higher BMI, systolic and diastolic blood pressures, and triglyceride levels and had lower HDL-cholesterol levels. Individuals with prediabetes were significantly more likely to have had HbA1c tests performed, and HbA1c levels were significantly higher. Individuals with prediabetes were more likely to have claims diagnoses of overweight or obesity, hypertension, dyslipidemia, smoking, and cardiovascular disease. They were also more likely to have filled prescriptions for blood pressure and lipid-lowering medications. Geocoding indicated that those with prediabetes were more likely to live in neighborhoods with lower median incomes and in neighborhoods with a lower percentage of unemployment but a higher percentage of residents receiving food stamps.
Table 2 shows the baseline characteristics of the 20,797 U-M employees, dependents, and retirees 40–64 years of age without known diabetes, prediabetes, or normal HbA1c levels stratified according to their calculated risk of having prediabetes or undiagnosed diabetes. The 5,219 (25%) subjects identified as being at higher risk were stratified according to whether or not they were tested for prediabetes with an HbA1c test or diagnosed with prediabetes or diabetes within 1 year after their initial strategy 2 high-risk notification. Compared with the 15,578 individuals 40 to 64 years of age at lower risk, those at higher risk were older, more likely to be men, more likely to be White, and more likely to have made at least one PCP visit and one specialist visit in the past year. They were more likely to have higher BMI and blood pressure levels and to have less favorable HDL and triglyceride profiles. They were also more likely to have claims diagnoses for cardiovascular risk factors and cardiovascular disease and more likely to be treated for hypertension and dyslipidemia. They did not differ from lower-risk individuals with respect to neighborhood characteristics.
Table 2.
Baseline characteristics of U-M employees, dependents, and retirees 40–64 years of age with Premier Care insurance, without known diabetes, prediabetes, or normal HbA1c stratified by risk for prediabetes, testing, and diagnosis
| Aged 40–64 years | Lower risk for prediabetes | Higher risk for prediabetes | P value | Any HbA1c or prediabetes or diabetes diagnosis | No HbA1c or prediabetes or diabetes diagnosis | P value | |
|---|---|---|---|---|---|---|---|
| N (%) | 20,797 (36) | 15,578 (75) | 5,219 (25) | — | 1,416 (27) | 3,803 (73) | — |
| Age (years) | 51 ± 7 | 50 ± 7 | 56 ± 6 | <0.0001 | 56 ± 6 | 56 ± 6 | 0.6209 |
| Sex | <0.0001 | <0.0001 | |||||
| Women | 11,679 (56) | 10,667 (68) | 1,012 (19) | 369 (26) | 643 (17) | ||
| Men | 9118 (44) | 4,911 (32) | 4,207 (81) | 1047 (74) | 3,160 (83) | ||
| Race | <0.0001 | 0.0278 | |||||
| Asian | 1,322 (8) | 976 (8) | 346 (8) | 97 (8) | 249 (7) | ||
| Black | 817 (5) | 741 (7) | 76 (2) | 31 (3) | 45 (1) | ||
| White | 14,402 (86) | 10,334 (85) | 4,068 (89) | 1,076 (88) | 2,992 (90) | ||
| Other | 212 (1) | 154 (1) | 58 (1) | 19 (2) | 39 (1) | ||
| At least one PCP visit in past year | 13,677 (66) | 9,860 (63) | 3,817 (73) | <0.0001 | 1,079 (76) | 2,738 (72) | 0.0023 |
| At least one specialist visit in past year | 11,062 (53) | 8,102 (52) | 2,960 (57) | <0.0001 | 868 (61) | 2,092 (55) | <0.0001 |
| BMI (kg/m2) | 28.7 ± 6.3 | 27.3 ± 5.7 | 32.6 ± 6.3 | <0.0001 | 33.4 ± 6.7 | 32.2 ± 6.0 | <0.0001 |
| Blood pressure (mmHg) | |||||||
| Systolic | 122 ± 16 | 119 ± 14 | 132 ± 15 | <0.0001 | 132 ± 15 | 132 ± 15 | 0.9500 |
| Diastolic | 74 ± 10 | 72 ± 10 | 79 ± 10 | <0.0001 | 78 ± 10 | 79 ± 10 | 0.0071 |
| Cholesterol (mg/dL) | |||||||
| Total cholesterol | 201 ± 37 | 202 ± 36 | 197 ± 39 | <0.0001 | 195 ± 39 | 197 ± 37 | 0.1325 |
| HDL | 57 ± 17 | 61 ± 17 | 47 ± 12 | <0.0001 | 48 ± 14 | 47 ± 11 | 0.3526 |
| Women | 64 ± 17 | 65 ± 17 | 53 ± 13 | <0.0001 | 54 ± 15 | 52 ± 12 | 0.2497 |
| Men | 49 ± 13 | 52 ± 14 | 46 ± 11 | <0.0001 | 45 ± 12 | 46 ± 11 | 0.5739 |
| Triglycerides | 129 ± 91 | 115 ± 73 | 161 ± 117 | <0.0001 | 164 ± 134 | 159 ± 110 | 0.4770 |
| LDL | 118 ± 32 | 118 ±31 | 118 ± 33 | 0.9484 | 114 ± 33 | 119 ± 33 | 0.0016 |
| Claims diagnosis of | |||||||
| Overweight/obesity | 3,538 (17) | 1,788 (11) | 1,750 (34) | <0.0001 | 581 (41) | 1,169 (31) | <0.0001 |
| Hypertension | 4,139 (20) | 1,818 (12) | 2,321 (44) | <0.0001 | 701 (50) | 1,620 (43) | <0.0001 |
| Any antihypertensive medication | 3,945 (19) | 1,915 (12) | 2,030 (39) | <0.0001 | 631 (45) | 1,299 (37) | <0.0001 |
| Dyslipidemia | 3,126 (15) | 1,624 (10) | 1,502 (29) | <0.001 | 464 (33) | 1,038 (27) | 0.0001 |
| Any lipid-lowering medication | 1,791 (9) | 724 (5) | 1,067 (20) | <0.0001 | 346 (24) | 721 (19) | <0.0001 |
| Smoking | 681 (3) | 427 (3) | 254 (5) | <0.0002 | 73 (5) | 181 (5) | 0.5544 |
| Women | 343 (3) | 290 (3) | 53 (5) | <0.0001 | 23 (6) | 30 (5) | 0.2813 |
| Men | 338 (4) | 137 (3) | 201 (5) | <0.0001 | 50 (5) | 151 (5) | 0.9969 |
| Cardiovascular disease | 843 (4) | 421 (3) | 422 (8) | <0.0001 | 138 (10) | 284 (7) | 0.0073 |
| Geocoded indicators | |||||||
| Median neighborhood income ($) | 72,239 | 72,232 | 72,263 | 0.9168 | 71,764 | 72,448 | 0.2194 |
| Percent unemployment | 34.9 ± 4.7 | 34.8 ± 4.8 | 34.9 ± 4.6 | 0.4613 | 34.9 ± 4.7 | 34.9 ± 4.6 | 0.7631 |
| Percent receiving food stamps | 7.7 ± 5.7 | 7.7 ± 5.8 | 7.7 ± 5.6 | 0.6638 | 7.9 ± 5.6 | 7.6 ± 5.6 | 0.1522 |
Data are N (%) or mean ± SD.
A total of 1,416 out of the 5,219 individuals identified as being at higher risk for prediabetes at baseline (27%) had HbA1c tests or claims diagnoses for prediabetes or diabetes within 1 year after their initial strategy 2 notification (Table 2). Of them, 582 were diagnosed with prediabetes (41% of those tested). Compared with those who were not tested or diagnosed, those who were tested or diagnosed were more likely to be women and less likely to be White. They were more likely to have made PCP and specialist visits. They had significantly higher BMI but lower diastolic blood pressure and LDL-cholesterol levels. Based on claims diagnoses, they were more likely to be overweight or obese, have hypertension and dyslipidemia, and have cardiovascular disease. They were more likely to be taking medications for blood pressure and lipid management and were not more likely to be smokers than those who were not tested or diagnosed. They did not differ from those who were not tested or diagnosed with respect to neighborhood characteristics. Two percent of provider groups performed additional outreach (portal messages, letters, or telephone calls) to their high-risk patients, encouraging them to be tested for prediabetes. Within provider groups that performed additional outreach, individuals who received outreach were more likely to be tested or diagnosed with prediabetes than those who did not receive individual outreach (42% vs. 30%; P = 0.002).
Table 3 shows the characteristics of the 29,875 U-M employees who did not have diabetes or prediabetes and were not identified as being at higher risk for prediabetes by strategy 2, but who received e-mails encouraging them to self-screen with a risk questionnaire. In 1 month, the screening questionnaire was opened 6,300 times. If employees had a positive screening test result, they were encouraged to see their PCPs for diagnostic testing. We further stratified the population of employees into those who had HbA1c tests or were diagnosed with prediabetes or diabetes within 1 year after the e-mail and those who were not. A total of 4,056 of the target population of 29,875 (14%) had HbA1c tests or diagnoses of prediabetes or diabetes (Table 3). Of them, 813 were diagnosed with prediabetes (20% of those tested). Those who were tested or diagnosed were significantly older, more likely to be women, and less likely to be White than those who were not tested or diagnosed. They were more likely to have made at least one PCP and specialist visit. They had significantly higher BMI and blood pressure levels and less favorable lipid profiles. Based on claims data, they were more likely to be overweight or obese and to have hypertension, dyslipidemia, or cardiovascular disease and more likely to be treated with blood pressure and lipid-lowering medications. Men who were tested or diagnosed were slightly more likely to be smokers. Those who were tested or diagnosed lived in neighborhoods with lower percentages of unemployment but higher percentages of residents receiving food stamps.
Table 3.
Baseline characteristics of U-M employees with Premier Care insurance, without diabetes, prediabetes, or at high risk for prediabetes who were encouraged to self-screen, stratified by those who were tested or diagnosed or not tested or diagnosed within 1 year
| Total | Any HbA1c or diagnosis of prediabetes or diabetes | No HbA1c or diagnosis of prediabetes or diabetes | P value | |
|---|---|---|---|---|
| N (%) | 29,875 | 4,056 (14) | 25,819 (86) | — |
| Age (years) | 38 ± 12 | 44 ± 12 | 37 ± 11 | <0.0001 |
| Sex | <0.0001 | |||
| Women | 18,993 (64) | 2,897 (71) | 16,096 (62) | |
| Men | 10,882 (36) | 1,159 (29) | 9,723 (38) | |
| Race | 0.0072 | |||
| Asian | 1,272 (8) | 244 (9) | 1,028 (8) | |
| Black | 1,172 (7) | 228 (8) | 944 (7) | |
| White | 13,798 (84) | 2,307 (82) | 11,491 (84) | |
| Other | 211 (1) | 28 (1) | 183 (1) | |
| At least 1 PCP visit in past year | 15,211 (51) | 2,455 (61) | 12,756 (49) | <0.001 |
| At least 1 specialist visit in past year | 13,967 (47) | 2,105 (52) | 11,862 (45) | <0.0001 |
| BMI (kg/m2) | 27.1 ± 6.1 | 29.4 ± 7.0 | 26.7 ± 5.8 | <0.0001 |
| Blood pressure (mmHg) | ||||
| Systolic | 118 ± 14 | 120 ± 15 | 117 ± 14 | <0.0001 |
| Diastolic | 72 ± 10 | 72 ± 10 | 71 ± 10 | <0.0001 |
| Cholesterol (mg/dL) | ||||
| Total cholesterol | 194 ± 37 | 196 ± 37 | 193 ± 37 | 0.0062 |
| HDL | 59 ± 17 | 58 ± 17 | 60 ± 17 | 0.0007 |
| Women | 63 ± 17 | 62 ± 17 | 64 ± 16 | 0.0004 |
| Men | 50 ± 13 | 48 ± 13 | 51 ± 13 | 0.0003 |
| Triglycerides | 112 ± 75 | 126 ± 90 | 109 ± 71 | <0.0001 |
| LDL | 112 ± 32 | 114 ± 32 | 112 ± 31 | 0.0148 |
| Claims diagnosis of | ||||
| Overweight/obesity | 2,974 (10) | 811 (20) | 2,163 (8) | <0.0001 |
| Hypertension | 2,171 (7) | 571 (14) | 1,600 (6) | <0.0001 |
| Any antihypertensive medication | 2,598 (9) | 568 (14) | 2,030 (8) | <0.0001 |
| Dyslipidemia | 1,669 (6) | 401 (10) | 1,268 (5) | <0.0001 |
| Any lipid-lowering medication | 737 (2) | 205 (5) | 532 (2) | <0.0001 |
| Smoking | 520 (2) | 83 (2) | 437 (2) | 0.1092 |
| Women | 379 (2) | 58 (2) | 321 (2) | 0.9780 |
| Men | 141 (1) | 25 (2) | 116 (1) | 0.0061 |
| Cardiovascular disease | 503 (2) | 108 (3) | 395 (2) | <0.0001 |
| Geocoded Indicators | ||||
| Median neighborhood income ($) | 69,709 | 69,605 | 69,726 | 0.6887 |
| Percent unemployment | 35.5 ± 5.1 | 35.0 ± 4.8 | 35.6 ± 5.1 | <0.0001 |
| Percent receiving food stamps | 7.7 ± 5.9 | 8.5 ± 6.5 | 7.5 ± 5.8 | <0.0001 |
Data are N (%) or mean ± SD.
Conclusions
We found that in a large, predominantly White, employed, and insured population of adults ≥18 years of age who were overweight or obese but generally had well-controlled blood pressure and lipid levels, 11% of adults without diabetes had claims diagnoses or met HbA1c criteria for prediabetes based on data available through their health plan. No additional screening or biochemical testing was required to identify these individuals. Although substantially lower than the 30% prevalence of prediabetes estimated by applying age-specific National Health and Nutrition Examination Survey prediabetes prevalence rates to the U-M population, this prevalence was twice as high as the 5% prevalence of diagnosed prediabetes reported by U.S. adults (1). This suggests that even without additional screening or biochemical testing, more than one-third of the U-M population with prediabetes (6,736 individuals or ∼35%) could be readily identified.
Unfortunately, many individuals who have claims for prediabetes or HbA1c levels diagnostic of prediabetes may not be aware of the diagnosis. Only ∼15% of American adults with screen-detected prediabetes report ever having been told by a health care provider about their high-risk status (1). We found that only 49% of individuals with prediabetes identified with strategy 1 who subsequently responded to mailed surveys answered that they had prediabetes. Twenty-nine percent responded that they did not have prediabetes, and 22% responded that they were not sure if they had prediabetes (data not shown) (7,12). This may reflect in part providers’ perception of the term “prediabetes” and their views on its meaning and impact (13). It also suggests that the diagnosis may not be communicated to patients in ways that are understandable or actionable and highlights the need for better risk communication between providers and patients. Health care providers’ awareness of the NDPP program also appears to be limited. In prior studies, fewer than one-quarter of PCPs reported ever having made a referral to the NDPP (14,15).
In addition to using health plan data to identify people with diagnosed prediabetes who may or may not be aware of the diagnosis, we showed that it is feasible to use available health plan data to risk stratify individuals 40–64 years of age without diabetes, prediabetes, or normal HbA1c levels to identify those at higher risk and to target them for definitive diagnostic testing. After the health plan applied risk stratification algorithms to its claims data, we sent targeted mailings to individuals in the highest quartile of risk encouraging them to be tested. A total of 27% of those who received these targeted letters were subsequently tested within 1 year, and, of those tested, 41% were diagnosed with prediabetes. This suggests that available health plan data can be used to proactively and efficiently identify individuals at higher risk for undiagnosed prediabetes or diabetes and to target them for testing. Unfortunately, this strategy identified only 582 individuals or 3.0% of all individuals estimated to have prediabetes in the population.
We also assessed the impact of a mass media campaign and e-mail that encouraged employees without diabetes, prediabetes, or known high risk for prediabetes to self-screen for prediabetes with a questionnaire and to follow-up with their PCPs for definitive diagnostic testing. A total of 14% of employees had follow-up HbA1c testing or were diagnosed with prediabetes or diabetes within 1 year of the e-mail. Of those tested, only 20% were diagnosed with prediabetes. The yield of this approach to identify people with prediabetes was approximately one-half that of the targeted approach and was consistent with the results of programs that use mass media campaigns alone to encourage screening for prediabetes (16–18). Despite its lower yield, this strategy identified 813 individuals or 4.2% of all individuals estimated to have prediabetes in the population.
Essentially all of those identified with prediabetes displayed the risk factors highlighted by the American Diabetes Association (10). They were significantly older, more likely to be women, and more likely to be non-White than those without prediabetes. They were also significantly more likely to have made outpatient visits to PCPs and specialists in the past year and to have higher BMI and blood pressure levels and less favorable lipid profiles. In addition, they were more likely to have claims diagnoses for overweight or obesity, hypertension, dyslipidemia, smoking, and cardiovascular disease. They also lived in neighborhoods with lower median incomes and higher percentages of residents receiving food stamps.
Taken together, these results suggest that a sequential, opportunistic approach to case finding, conducted using an insured population’s existing health insurance records, can identify as many as 42% of all individuals with prediabetes in the population. The vast majority (83%) of individuals with prediabetes who were identified were identified using existing health plan claims data or previously measured HbA1c levels. No further testing was required. An additional 7% were identified by encouraging targeted testing, and 10% were identified using population screening. Since the proportion of positive tests was twofold higher when targeted screening was performed compared with population screening, it is reasonable to consider expanding the use of targeted screening of high-risk individuals by including a larger proportion of those at higher risk and by encouraging provider groups to perform additional outreach. The fact that men were less likely to have diagnosed prediabetes and less likely to respond to targeted or general outreach suggests that additional efforts will be needed to identify and engage men with prediabetes (19–21).
Although more recent reports indicate that enrollment in the NDPP has increased substantially since 2015 (22,23), additional interventions will be needed to increase provider awareness of prediabetes and facilitate communication of risk to patients with prediabetes. The American Medical Association and the YMCA of the U.S. have developed tools and tested the feasibility of establishing clinical–community linkages to facilitate the systematic identification of patients with prediabetes and their referral to NDPPs at YMCAs (8). Such population-level strategies will be required to expand the reach and uptake of targeted NDPPs to address the global epidemic of type 2 diabetes.
Article Information
Acknowledgments. The authors thank Marsha Manning, Manager, Medical Benefits and Strategy at U-M; Ashley Weigl, Associate Director, MHealthy; and Marc D. Keshishian, and Dawn Beaird, Blue Cross Blue Shield of Michigan, for their contributions to this project.
Funding. This work was supported by National Institutes of Health, National Institute of Diabetes, Digestive, and Kidney Diseases grant R01 DK109995.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. W.H.H. researched the data, wrote the manuscript, and reviewed and edited the manuscript. K.J. and T.H. contributed to the discussion and reviewed and edited the manuscript. L.N.M. researched the data, contributed to the discussion, and reviewed and edited the manuscript. W.H.H. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This work was presented at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
References
- 1. Centers for Disease Control and Prevention . Prevention of Diabetes. Accessed 3 March 2021. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- 2. Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herman WH, Hoerger TJ, Brandle M, et al.; Diabetes Prevention Program Research Group . The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diabetes Prevention Program Research Group . The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS [published correction appears in Diabetes Care 2013;36:4173–4175]. Diabetes Care 2012;35:723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the U.S.: the National Diabetes Prevention Program. Am J Prev Med 2013;44(Suppl. 4):S346–S351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ely EK, Gruss SM, Luman ET, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care 2017;40:1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ali MK, McKeever Bullard K, Imperatore G, et al. Reach and use of diabetes prevention services in the United States, 2016-2017. JAMA Netw Open 2019;2:e193160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ritchie ND, Baucom KJW, Sauder KA. Current perspectives on the impact of the National Diabetes Prevention Program: building on successes and overcoming challenges. Diabetes Metab Syndr Obes 2020;13:2949–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McEwen LN, Adams SR, Schmittdiel JA, Ferrara A, Selby JV, Herman WH. Screening for impaired fasting glucose and diabetes using available health plan data. J Diabetes Complications 2013;27:580–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S14–S31 [DOI] [PubMed] [Google Scholar]
- 11. U.S. Census Bureau . Data.census.gov. Accessed 6 February 2020. Available from https://data.census.gov/cedsci/
- 12. Hafez D, Nelson DB, Martin EG, Cohen AJ, Northway R, Kullgren JT. Understanding type 2 diabetes mellitus screening practices among primary care physicians: a qualitative chart-stimulated recall study. BMC Fam Pract 2017;18:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas JJ, Moring JC, Baker S, et al. Do words matter? Health care providers’ use of the term prediabetes. Health Risk Soc 2017;19:301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ackermann RT, O’Brien MJ. Evidence and challenges for translation and population impact of the Diabetes Prevention Program. Curr Diab Rep 2020;20:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nhim K, Khan T, Gruss SM, et al. Primary care providers’ prediabetes screening, testing, and referral behaviors. Am J Prev Med 2018;55:e39–e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zigmont VA, Shoben AB, Kaye GL, et al. An evaluation of reach for a work site implementation of the National Diabetes Prevention Program focusing on diet and exercise. Am J Health Promot 2018;32:1417–1424 [DOI] [PubMed] [Google Scholar]
- 17. Chambers EC, Gonzalez JS, Marquez ME, Parsons A, Rehm CD. The reach of an urban hospital system-based Diabetes Prevention Program: patient engagement and weight loss characteristics. Diabetes Educ 2019;45:616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahern AL, Aveyard P, Boyland EJ, Halford JC; WRAP trial team . Inequalities in the uptake of weight management interventions in a pragmatic trial: an observational study in primary care. Br J Gen Pract 2016;66:e258–e263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsai SA, Lv N, Xiao L, Ma J. Gender differences in weight-related attitudes and behaviors among overweight and obese adults in the United States. Am J Men Health 2016;10:389–398 [DOI] [PubMed] [Google Scholar]
- 20. Rose SA, Poynter PS, Anderson JW, Noar SM, Conigliaro J. Physician weight loss advice and patient weight loss behavior change: a literature review and meta-analysis of survey data. Int J Obes 2013;37:118–128 [DOI] [PubMed] [Google Scholar]
- 21. Venkataramani M, Pollack CE, Yeh HC, Maruthur NM. Prevalence and correlates of Diabetes Prevention Program referral and participation. Am J Prev Med 2019;56:452–457 [DOI] [PubMed] [Google Scholar]
- 22. Gruss SM, Nhim K, Gregg E, Bell M, Luman E, Albright A. Public health approaches to type 2 diabetes prevention: the US National Diabetes Prevention Program and beyond [published correction appears in Curr Diab Rep 2020;20:36]. Curr Diab Rep 2019;19:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burd C, Gruss S, Albright A, Zina A, Schumacher P, Alley D. Translating knowledge into action to prevent type 2 diabetes: Medicare expansion of the National Diabetes Prevention Program lifestyle intervention. Milbank Q 2020;98:172–196 [DOI] [PMC free article] [PubMed] [Google Scholar]