Abstract
OBJECTIVE
It is controversial whether adults who are obese but “metabolically healthy” have cardiovascular disease (CVD) risk comparable with that of normal-weight adults. High-sensitivity cardiac troponin T (hs-cTnT), a biomarker of myocardial damage, is useful in characterizing subclinical CVD. We categorized obesity phenotypes and studied their associations with subclinical and clinical CVD and CVD subtypes, including heart failure (HF).
RESEARCH DESIGN AND METHODS
We conducted cross-sectional and prospective analyses of 9,477 adults in the Atherosclerosis Risk in Communities (ARIC) study. We used the Adult Treatment Panel III criteria and BMI to define obesity phenotypes as follows: metabolically healthy normal weight, metabolically healthy overweight, metabolically healthy obese, metabolically unhealthy normal weight, metabolically unhealthy overweight, and metabolically unhealthy obese.
RESULTS
At baseline (1990–1992), mean age was 56 years, 56% were female, 23% were Black, and 25% had detectable hs-cTnT (≥6 ng/L). Over a median of 17 years of follow-up, there were 2,603 clinical CVD events. Those with the metabolically healthy obese (hazard ratio [HR] 1.38, 95% CI 1.15–1.67), metabolically unhealthy normal weight (HR 1.51, 95% CI 1.30–1.76), metabolically unhealthy overweight (HR 1.60, 95% CI 1.41–1.82), and metabolically unhealthy obese (HR 2.14, 95% CI 1.88–2.44) phenotypes had higher CVD risks in comparison with metabolically healthy normal weight. Detectable hs-cTnT (≥6 ng/L) was associated with higher CVD risk, even among metabolically healthy normal-weight adults. Metabolically healthy obese adults had higher HF risk (HR 1.65, 95% CI 1.30–2.09) in comparison with metabolically healthy normal weight.
CONCLUSIONS
The metabolically healthy obese phenotype was associated with excess burden of clinical CVD, primarily driven by an excess risk of HF. hs-cTnT was useful in stratifying CVD risk across all obesity phenotypes, even among obese individuals who appear otherwise metabolically healthy.
Introduction
Obesity affects 40% of U.S. adults (1). However, obesity is a heterogeneous condition, and it is unclear why some individuals develop cardiovascular disease (CVD) and others do not. Obesity has been subtyped according to specific metabolic abnormalities to better define cardiometabolic health (2,3). Almost one-third of obese adults in the U.S. are considered “metabolically healthy” based on typical criteria.
There is debate about whether the metabolically healthy obese phenotype is a benign condition. The full spectrum of CVD risk among those with the metabolically healthy obese phenotype is unclear. Prior studies have demonstrated a higher risk of CVD in metabolically healthy obese in comparison with metabolically healthy normal-weight individuals (3–11). However, these studies were limited to mostly White adults, and a few have examined CVD subtypes, such as coronary heart disease (CHD) or heart failure (HF). Furthermore, the definition of metabolic health has not included novel CVD biomarkers, which can improve the characterization of subclinical CVD risk. The metabolically healthy overweight phenotype has also received less attention in the literature than the metabolically healthy obese.
High-sensitivity cardiac troponin T (hs-cTnT) is a biomarker of subclinical cardiac damage and is a potent risk factor for the development of CVD and mortality in the general population (12–16). Prior studies have shown that small elevations in hs-cTnT predict incident HF, left ventricular hypertrophy, CHD, CVD, and all-cause mortality in the general population (12,14,17). However, the association of hs-cTnT in the setting of different obesity phenotypes with overall CVD risk and CVD subtypes has not been examined. We sought to 1) examine the cross-sectional associations between obesity phenotypes (metabolically healthy normal weight, metabolically healthy overweight, metabolically healthy obese, metabolically unhealthy normal weight, metabolically unhealthy overweight, and metabolically unhealthy obese) and subclinical myocardial damage among Black and White men and women and 2) investigate the independent and combined associations of obesity phenotypes and subclinical myocardial damage with incident CVD and CVD subtypes including CHD and HF.
Research Design and Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) study is a prospective cohort of 15,792 adults aged 45–64 years at baseline from four U.S. communities (Forsyth County, NC; Jackson, MS; suburban Minneapolis, MN; and Washington County, MD). Visit 2 (1990–1992) was the baseline for this study, as it constituted the first ARIC visit with hs-cTnT measurements available. Of the participants who attended visit 2, there were 13,956 Black or White participants who were free of prevalent CVDs or stroke. We excluded participants who were missing data on hs-cTnT (n = 932) or covariates (n = 747), were nonfasting (n = 370), or had BMI <18.5 kg/m2 (n = 112); who self-reported cancer diagnosis (n = 700), lung disease (n = 436), or diagnosed diabetes (n = 1,182); and African Americans in the Minnesota and Washington County cohorts (because of small numbers)—leaving 9,477 adults for this study. All protocols were approved by institutional review boards at the study sites, and all participants provided written informed consent.
Definition of Obesity Phenotypes
We evaluated six mutually exclusive obesity phenotypes based on metabolic status and BMI categories: metabolically healthy normal weight, metabolically healthy overweight, metabolically healthy obese, metabolically unhealthy normal weight, metabolically unhealthy overweight, and metabolically unhealthy obese. BMI was calculated as weight in kilograms divided by the square of height in meters and classified as normal weight (<25 kg/m2), overweight (25 to <30 kg/m2), or obese (≥30 kg/m2) (18). We used the National Cholesterol Education Program–Adult Treatment Panel III criteria (19) to define metabolic status. We classified participants as “metabolically healthy” or “metabolically unhealthy.” A person was “metabolically unhealthy” with two or more of the following: triglycerides level ≥150 mg/dL or treated for dyslipidemia; HDL cholesterol <40 mg/dL in men and <50 mg/dL in women; systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥85 mmHg, or use of antihypertensive drugs; diabetes diagnosis; fasting glucose ≥100 mg/dL; or medications for diabetes. Although waist and hip circumference were not used to define the obesity phenotypes due to the strong association with BMI, we evaluated the association between these anthropometric indices and hs-cTnT.
Outcomes
The primary outcome was a composite incident CVD, which included adjudicated fatal or nonfatal CHD, coronary revascularization, silent and unrecognized myocardial infarction, fatal or nonfatal ischemic stroke or hemorrhagic stroke confirmed by imaging, or HF hospitalization or death from HF (adjudicated after 2005), with follow-up through 2017 as previously described (20,21). We also examined CHD and HF separately as secondary outcomes.
hs-cTnT
hs-cTnT was measured in stored serum samples collected from participants during visit 2 at the University of Minnesota in 2012–2013 with a sandwich immunoassay method with a Roche Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, IN) (13). Intra-assay coefficients of variation were 2.1% at a mean hs-cTnT concentration of 26 ng/L and 1.0% at 1,990 ng/L. Interassay coefficients of variation were 6.0% at a mean hs-cTnT concentration of 25 ng/L and 3.7% at 1,940 ng/L (22). We categorized hs-cTnT as “undetectable” (<6 ng/L), detectable (≥6 ng/L and <14 ng/L), or elevated (≥14 ng/L) (23). We used a cutoff of 6 ng/L for consistency with prior ARIC studies (13,24) and the manufacturer’s definition of reliable detection of hs-cTnT.
Statistical Analyses
We used multinomial logistic regression to examine the cross-sectional associations of obesity phenotypes with hs-cTnT categories (hs-cTnT<6 ng/L (reference), 6–13 ng/L, and ≥14 ng/L). Model 1 was adjusted for age, sex, and race-center. Model 2 was adjusted for model 1 variables plus health behaviors: smoking status and physical activity. Model 3 included all variables in model 2 plus potential mediators: hs-CRP, estimated glomerular filtration rate (eGFR), and N-terminal pro–B-type natriuretic peptide (NT-proBNP). We used Poisson regression to estimate the incidence rates of CVD in each of the obesity phenotypes. We used Cox regression analyses to examine the association between obesity phenotypes with or without elevated hs-cTnT and incident CVD, CHD, and HF. For examination of the combined associations of obesity phenotypes and detectable hs-cTnT with incident CVD, interaction terms were created for the two exposures, and statistical interaction was tested on the multiplicative scale. We performed sensitivity analyses and limited follow-up of CVD, CHD, and HF events to 5 years to limit the effect of transitions in obesity phenotypes. All statistical analyses were performed with Stata, version 16.0 (StataCorp, College Station, TX).
Results
Study Population Characteristics
There were 9,477 participants followed for a median of 27 years (through 2016). The mean age was 56 years, 56% were female, and 23% were Black (Table 1). The metabolically healthy obese phenotype (7%) was the least common, while the metabolically unhealthy overweight (23%) and obese (19%) phenotypes were the most common. Female and Black participants were more likely to have the metabolically healthy obese phenotype than male and White participants. Compared with those who were metabolically healthy normal weight or metabolically healthy overweight, those who were metabolically healthy obese had higher systolic blood pressure, diastolic blood pressure, and BMI and were more likely to be diagnosed with hypertension. The presence of detectable hs-cTnT (≥6 ng/L) was common among individuals who were overweight (21%) or obese (22%) but otherwise considered metabolically healthy according to traditional risk factors. Furthermore, greater waist circumference and hip circumference were associated with higher hs-cTnT levels in men and women (Supplementary Table 5).
Table 1.
Baseline characteristics of the ARIC study population without CVD at visit 2 (1990–1992), by obesity phenotypes N = 9,477
| Characteristics | Metabolically healthy | Metabolically unhealthy | ||||
|---|---|---|---|---|---|---|
| Normal weight (N = 1,943, 21%) | Overweight (N = 1,726, 18%) | Obese (N = 696, 7%) | Normal weight (N = 1,073, 11%) | Overweight (N = 2,212, 23%) | Obese (N = 1,827, 19%) | |
| Age, years | 55.8 (5.6) | 55.8 (5.6) | 55.3 (5.3) | 57.9 (5.7) | 57.3 (5.6) | 56.5 (5.6) |
| Female | 67.2 | 53.1 | 71.7 | 55.5 | 41.7 | 58.7 |
| African American | 14.2 | 21.9 | 42.8 | 16.3 | 21.0 | 31.0 |
| ≥College education | 54.9 | 52.3 | 46.4 | 45.8 | 49.4 | 42.5 |
| Ideal physical activity* | 43.8 | 42.9 | 32.5 | 39.8 | 41.3 | 30.7 |
| Current smoker | 23.8 | 18.0 | 11.6 | 31.7 | 21.9 | 16.2 |
| Systolic blood pressure, mmHg | 111.9 (15.7) | 114.8 (14.5) | 120.1 (16.6) | 124.0 (19.1) | 124.3 (18.2) | 128.7 (18.7) |
| Diastolic blood pressure, mmHg | 68.2 (9.1) | 70.4 (9.0) | 72.8 (9.0) | 72.8 (10.9) | 74.0 (10.2) | 75.9 (10.3) |
| Hypertension | 10.9 | 11.9 | 21.7 | 41.0 | 46.0 | 58.2 |
| Antihypertension medicationv | 61.6 | 61.7 | 72.9 | 71.4 | 76.9 | 78.6 |
| BMI, kg/m2 | 22.6 (1.6) | 27.2 (1.4) | 33.8 (3.8) | 23.1 (1.5) | 27.5 (1.4) | 34.5 (4.6) |
| Waist circumference, cm | 83.4 (8.0) | 95.5 (7.7) | 109.0 (10.9) | 87.0 (7.5) | 98.2 (7.0) | 112.9 (11.4) |
| Hip circumference, cm | 97.2 (4.7) | 104.6 (4.8) | 117.1 (9.1) | 96.9 (4.7) | 103.9.5 (4.7) | 117.2 (10.5) |
| Total cholesterol, mg/dL | 202.6 (34.5) | 207.0 (36.6) | 206.9 (36.2) | 213.1 (42.9) | 214.6 (39.1) | 212.0 (40.2) |
| Statin use | 0.7 | 0.5 | 0.3 | 4.3 | 3.8 | 2.6 |
| HDL-C, mg/dL | 60.9 (16.5) | 55.2 (14.8) | 56.2 (13.5) | 47.2 (16.8) | 42.7 (13.6) | 42.9 (13.3) |
| LDL-C, mg/dL | 123.6 (33.2) | 132.3 (35.1) | 130.8 (34.9) | 137.7 (40.7) | 139.9 (35.5) | 137.7 (36.0) |
| Triglycerides, mg/dL | 90.2 (38.6) | 97.4 (36.2) | 98.9 (33.9) | 141.6 (73.1) | 161.6 (82.4) | 1,594 (93.1) |
| Fasting blood glucose, mg/dL | 95.9 (8.0) | 98.3 (8.8) | 99.2 (9.2) | 104.4 (9.9) | 107.2 (10.2) | 109.2 (10.8) |
| eGFR, mL/min/1.73 m2 | 97.7 (13.1) | 97.8 (13.8) | 100.3 (15.4) | 95.3 (14.7) | 94.2 (15.1) | 96.7 (16.0) |
| NT-proBNP, ng/L | 85.3 (35.3–102.3) | 65.5 (25.9–81.2) | 68.2 (24.5–86.6) | 128.9 (30.7–108.1) | 75.4 (23.2–83.2) | 86.7 (24.7–87.4) |
| hs-CRP, mg/dL | 2.5 (0.6–2.5) | 3.3 (0.9–3.3) | 5.1 (1.7–6.3) | 3.5 (0.9–3.5) | 4.0 (1.2–4.3) | 5.9 (2.1–7.2) |
| Detectable hs-cTnT (≥6 ng/L) | 17.3 | 21.0 | 21.7 | 26.6 | 29.0 | 31.9 |
Data are mean (SD) or median (Q1–Q3) for continuous variables and % for categorical variables. HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
American Heart Association definition: 150+ min/week moderate, 75+ min/week vigorous, or 150+ min/week moderate or vigorous physical activity.
Cross-sectional Associations of Obesity Phenotypes and hs-cTnT
Those with metabolically healthy obese (prevalence ratio [PR] 1.31, 95% CI 1.03–1.67), metabolically unhealthy normal weight (PR 1.23, 95% CI 1.00–1.50), metabolically unhealthy overweight (PR 1.28, 95% CI 1.08–1.51), and metabolically unhealthy obese (PR 1.78, 95% CI 1.49–2.11) phenotypes were more likely to have hs-cTnT levels in the 6 to <14 ng/L range than those with the metabolically healthy normal weight phenotype after adjustment for other risk factors (Table 2 [model 3]). The metabolically healthy obese (PR 2.29, 95% CI 1.23–4.32), metabolically unhealthy overweight (PR 1.79, 95% CI 1.12–2.86), and metabolically unhealthy obese (PR 3.16, 95% 1.78–5.61) phenotypes had higher odds of elevated hs-cTnT levels (≥14 ng/L) than the metabolically healthy normal weight (Table 2 [model 3]).
Table 2.
Cross-sectional association,* relative risk ratio (95% CI), of obesity phenotypes with hs-cTnT, N = 9,477
| N (%) | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| hs-cTnT <6 ng/L (N = 7,118) | 1 (Ref) | 1 (Ref) | 1 (Ref) | |
| hs-cTnT 6 to < 14 ng/L (N = 2,068) | ||||
| Metabolically healthy | ||||
| Normal | 311 (15) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Overweight | 331 (16) | 1.03 (0.86–1.24) | 1.01 (0.84–1.21) | 1.07 (0.89–1.29) |
| Obese | 133 (7) | 1.30 (1.02–1.64) | 1.24 (0.97–1.57) | 1.31 (1.03–1.67) |
| Metabolically unhealthy | ||||
| Normal weight | 249 (12) | 1.24 (1.02–1.52) | 1.27 (1.04–1.56) | 1.23 (1.00–1.51) |
| Overweight | 564 (27) | 1.26 (1.07–1.49) | 1.26 (1.06–1.48) | 1.28 (1.08–1.51) |
| Obese | 480 (23) | 1.74 (1.47–2.06) | 1.71 (1.44–2.03) | 1.78 (1.49–2.11) |
| hs-cTnT ≥14 ng/L (N = 291) | ||||
| Normal | 26 (9) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Metabolically healthy | ||||
| Overweight | 31 (11) | 1.10 (0.64–1.87) | 1.11 (0.65–1.90) | 1.22 (0.71–2.11) |
| Obese | 18 (6) | 2.05 (1.10–3.84) | 2.06 (1.10–3.86) | 2.29 (1.23–4.32) |
| Metabolically unhealthy | ||||
| Normal weight | 36 (12) | 2.00 (1.18–3.37) | 1.99 (1.18–3.37) | 1.40 (0.80–2.44) |
| Overweight | 77 (27) | 1.90 (1.20–3.01) | 1.92 (1.21–3.04) | 1.79 (1.12–2.86) |
| Obese | 103 (35) | 4.40 (2.81–6.88) | 4.40 (2.81–6.90) | 4.16 (2.63–6.59) |
Boldface type indicates P < 0.05. Ref, reference.
Multinomial logistic regression model with hs-cTnT <6 ng/L as the base outcome. Model 1: adjustment for age, sex, and race-center. Model 2: model 1 adjustments plus smoking status and physical activity. Model 3: Model 2 adjustments plus hs-CRP, eGFR, and NT-proBNP.
Prospective Associations of Obesity Phenotypes and Incident CVD
The median time to event for participants who developed CVD (N = 2,603) was 17 years. The incidence rates of CVD for the metabolically healthy obese phenotype were intermediate between the metabolically healthy normal weight and metabolically unhealthy obese phenotypes (Table 3). After adjustment for age, sex, race-center, smoking status, physical activity, hs-CRP, eGFR, and NT-proBNP, those with the metabolically healthy obese phenotype were more likely to develop CVD than the metabolically healthy normal weight (hazard ratio [HR] 1.38, 95% CI 1.15–1.67). The metabolically unhealthy obese group had an almost twofold higher risk of CVD than the metabolically healthy normal-weight group (HR 2.14, 95% CI 1.88–2.44). The metabolically healthy obese group had a higher risk of HF (HR 1.65, 95% CI 1.30–2.09) than the metabolically healthy normal-weight group, but the association was not significant for CHD (HR 1.34, 95% CI 0.98–1.82) (Supplementary Tables 1 and 2).
Table 3.
IRs and HRs for the association of obesity phenotypes and incident cardiovascular disease, N = 9,477
| Metabolic status | Obesity status | Events/n | IR/1,000 person-years (95% CI) | Model 1, HR (95% CI) | Model 2, HR (95% CI) |
|---|---|---|---|---|---|
| Metabolically healthy | Normal weight | 358/1,942 | 8.5 (7.6–9.4) | 1(Ref) | 1 (Ref) |
| Overweight | 364/1,725 | 9.8 (8.8–10.8) | 1.05 (0.91–1.22) | 1.08 (0.93–1.25) | |
| Obese | 165/696 | 10.9 (9.4–12.7) | 1.28 (1.06–1.54) | 1.38 (1.15–1.67) | |
| Metabolically unhealthy | Normal weight | 331/1,073 | 15.8 (14.2–17.6) | 1.67 (1.43–1.94) | 1.51 (1.30–1.76) |
| Overweight | 740/2,212 | 16.6 (15.5–17.89) | 1.64 (1.45–1.87) | 1.60 (1.41–1.82) | |
| Obese | 645/1,825 | 18.4 (17.0–19.8) | 2.12 (1.86–2.41) | 2.14 (1.88–2.44) |
Boldface type indicates P < 0.05. Model 1: adjustment for age, sex, and race-center. Model 2: model 1 adjustments plus smoking status, physical activity, hs-CRP, eGFR, and NT-proBNP. IR, incidence rate; Ref, reference.
Prospective Associations of Cross Categories of Obesity Phenotypes and hs-cTnT With Incident CVD
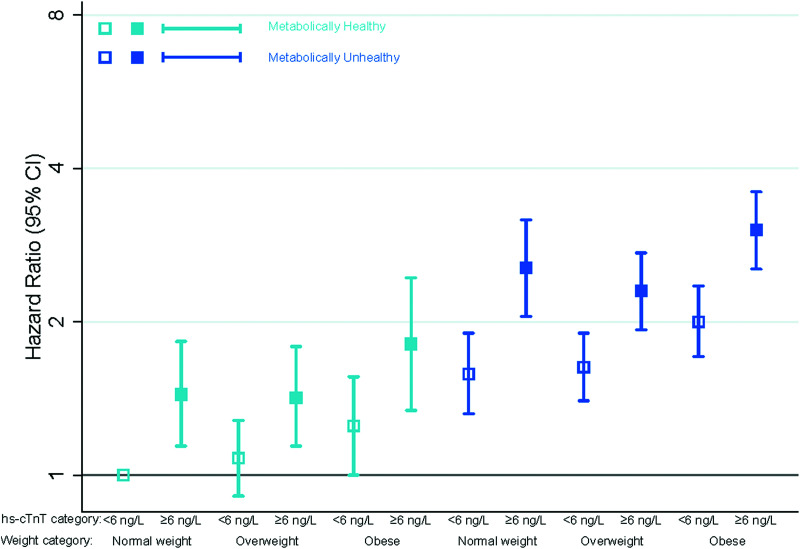
Adults with detectable hs-cTnT had a significantly higher risk of CVD than those with undetectable hs-cTnT, across all obesity phenotypes (Fig. 1 and Table 4). Detectable hs-cTnT tended to be more strongly associated with HF in comparison with CHD (Supplementary Tables 3 and 4). Individuals who were obese and had detectable hs-cTnT but were classified as being metabolically healthy had a significantly elevated risk of both CHD (HR 1.75, 95% CI 1.07–2.88) and HF (HR 2.48, 95% CI 1.71–3.60) compared with normal-weight individuals without detectable hs-cTnT (Supplementary Fig. 1 and 2).
Figure 1.
Adjusted HRs (95% CIs) for the association of obesity phenotypes and incident CVD by hs-cTnT levels.
Table 4.
IRs and adjusted HRs for incident CVD according to cross categories of obesity phenotypes and hs-cTnT levels, N = 9,477
| Metabolic status† | Obesity status | hs-cTnT level, ng/L | Events/n | IR/1,000 person-years (95% CI) | Adjusted,* HR (95% CI) |
|---|---|---|---|---|---|
| Metabolically healthy | Normal weight | <6 ng/L | 265/1,606 | 7.4 (6.6–8.4) | 1 (Reference) |
| ≥6 ng/L | 93/336 | 14.2 (11.6–17.4) | 1.48 (1.17–1.88) | ||
| Overweight | <6 ng/L | 253/1,364 | 8.4 (7.5–9.5) | 1.09 (0.92–1.30) | |
| ≥6 ng/L | 111/361 | 15.2 (12.6–18.3) | 1.53 (1.22–1.92) | ||
| Obese | <6 ng/L | 113/545 | 9.4 (7.79–11.3) | 1.37 (1.10–1.71) | |
| ≥6 ng/L | 52/151 | 17.1 (13.0–22.4) | 1.95 (1.44–2.64) | ||
| Metabolically unhealthy | Normal weight | <6 ng/L | 207/788 | 12.9 (11.2–14.8) | 1.49 (1.24–1.79) |
| ≥6 ng/L | 124/285 | 25.7 (21.4–30.5) | 2.23 (1.78–2.80) | ||
| Overweight | <6 ng/L | 454/1,571 | 13.9 (12.7–15.2) | 1.56 (1.33–1.81) | |
| ≥6 ng/L | 286/641 | 24.4 (21.7–27.4) | 2.38 (2.00–2.83) | ||
| Obese | <6 ng/L | 376/1,244 | 14.8 (13.4–16.4) | 2.04 (1.74–2.40) | |
| ≥6 ng/L | 269/581 | 27.5 (24.4–31.0) | 3.06 (2.57–3.65) |
Boldface type indicates P < 0.05. IR, incidence rate.
Adjustment for age, sex, race-center, smoking status, physical activity, hs-CRP, eGFR, and NT-proBNP.
Metabolic healthy, fewer than two Adult Treatment Panel III criteria; metabolically unhealthy, two or more Adult Treatment Panel III criteria.
Conclusions
In a community-based sample of middle-aged adults, who were free of CVD or diabetes at baseline, the presence of subclinical myocardial damage was common in adults with the metabolically healthy obese phenotype. These individuals also had an excess risk of clinical CVD, intermediate between that of the metabolically healthy normal-weight and metabolically unhealthy obese groups. hs-cTnT was associated with CVD risk regardless of obesity or metabolic health status. This study demonstrated that hs-cTnT, a potent marker of CVD risk, distinguishes cardiometabolic health among adults classified as metabolically healthy based on traditional risk factors.
The question of whether obesity is associated with CVD has been studied extensively. However, exceptions to the “more fat, worse metabolic health” premise have been described as the metabolically healthy obese phenotype. Several epidemiological studies have presented evidence in support of cardioprotection among adults who are metabolically healthy but obese in comparison with the metabolically unhealthy. However, existing studies have defined metabolic health as the absence of traditional CVD risk factors and have not considered hs-cTnT, a potent biomarker of morbidity and mortality, which can improve characterization of CVD risk across the full range of obese phenotypes.
Beyond BMI, the International Diabetes Federation consensus group (25) suggests measurement of additional parameters to improve the characterization of the metabolic syndrome, including proinflammatory state, dysglycemia, vascular regulation, and prothrombotic state among others in the “Platinum standard” definition. There is growing evidence that clinical CVD is preceded by decades of subclinical disease. Echouffo-Tcheugui et al. (26) demonstrated that assessing coronary artery calcification provided additional information about the risk of hypertension and diabetes among those who were otherwise classified as metabolically healthy obese in the Framingham Heart Study.
In our cross-sectional analyses, we demonstrated that middle-aged adults without clinical CVD who had the metabolically unhealthy overweight or obese phenotypes were more likely to have elevated hs-cTnT levels than metabolically healthy normal-weight individuals. In a general population, hs-cTnT may reflect subclinical myocardial insults and microvascular damage to the heart (14,27). Assessment of hs-cTnT among overweight or obese people without clinical CVD could lead to early detection and diagnosis and prompt treatment of subclinical CVD through intensive lifestyle modification.
We found that the CVD risk among those with the metabolically healthy obese phenotype was intermediate between that of the metabolically healthy normal weight and the metabolically unhealthy obese phenotypes. We also observed that a single measurement of hs-cTnT among the metabolically healthy overweight and obese groups was informative in identifying those individuals at higher risk for overall CVD, CHD, and HF. Since those with the metabolically healthy overweight phenotype with detectable hs-cTnT levels had almost twofold higher risk of CVD, CHD, or HF in comparison with those with the metabolically healthy normal weight phenotype, they should also be targeted for intensive lifestyle modification to stave off CVD.
The associations with CVD in our study were largely driven by the robust associations with HF. Our findings are consistent with those of prior studies demonstrating strong, independent associations of metabolically healthy obesity with future HF risk and less robust associations with CHD (28–30). Our work builds on these prior studies and further demonstrates that detectable hs-cTnT is associated with HF and CHD risk and provides useful prognostic information, even among those otherwise classified as being “metabolically healthy.” Our findings suggest that hs-cTnT is useful in identifying adults at high risk for future clinical CVD, even among people who appear metabolically healthy in other respects.
This study had several strengths. First, this investigation included a large, community-based prospective cohort study of >11,000 middle-aged White and Black participants with active surveillance and adjudicated cardiovascular outcomes over 25 years of follow-up. We had standardized and rigorous measurements of traditional cardiovascular risk factors and hs-cTnT. However, there were some limitations to this study. First, we did not include data on impaired glucose tolerance, an additional marker of metabolic dysfunction. Second, information on diet was not assessed at visit 2 in the ARIC study. Third, our cutoff point of 6 ng/L for hs-cTnT was consistent with that of prior ARIC studies (13,24) but may not be generalizable to other assays. Fourth, we excluded participants missing hs-cTnT at visit 2 (9% of the population). Those who were missing hs-cTnT were more likely to be older, to be male, to have diabetes, to have dyslipidemia, and to smoke than those who were not missing hs-cTnT. In the ARIC study, this missingness was primarily related to the availability of stored samples for the measurement of hs-cTnT. Finally, as with any observational study, we cannot eliminate the possibility of residual confounding.
Conclusions
The metabolically healthy obese phenotype was uncommon (7%) in this middle-aged adult population but more common in younger adults, women, and Black individuals. In comparison with individuals with the metabolically healthy normal weight phenotype, those with the metabolically healthy obese phenotype had higher overall CVD risk and evidence of subclinical myocardial damage. The associations were more robust for HF than CHD. Detectable hs-cTnT levels (≥6 ng/L) were associated with higher overall CVD risk in all obesity phenotype groups, suggesting that hs-cTnT is a useful biomarker for stratifying CVD risk. Routine screening of CVD risk with hs-cTnT among the obese, regardless of current metabolic health, may provide an opportunity to institute intensive lifestyle changes targeting weight loss and pharmacological therapy to prevent subsequent CVD.
Article Information
Acknowledgements. The authors thank the staff and participants of the ARIC study for their important contributions.
Funding. The ARIC study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), Department of Health and Human Services (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Support was received from the National Center for Advancing Translational Sciences (5KL2TR001077-05 to Y.C.-M.), National Institute of Diabetes and Digestive and Kidney Diseases (F30 DK120160 to O.T. and R01 DK089174 to E.S.), and National Heart, Lung, and Blood Institute (R01 HL134320 to C.M.B. and E.S. and K24 HL152440 to E.S.) of the NIH. Reagents for the hs-cTnT assay were donated by Roche Diagnostics.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Duality of Interest. V.N. is supported by a VA MERIT Award and has a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers in prediction of HF. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.C.-M. and D.W. conducted the analyses. Y.C.-M. and E.S. designed the study and drafted the manuscript. Y.C.-M., M.L., O.T., J.B.E.-T., C.E.N., V.N., D.W., C.B., and E.S. provided data interpretation and meaningful contributions to the revision of the manuscript. Y.C.-M and E.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14342321.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief 2017;208:1–8 [PubMed] [Google Scholar]
- 2. Bluher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 2010;21:38–43 [DOI] [PubMed] [Google Scholar]
- 3. Stefan N, Haring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol 2013;1:152–162 [DOI] [PubMed] [Google Scholar]
- 4. Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann Intern Med 2013;159:758–769 [DOI] [PubMed] [Google Scholar]
- 5. Fan J, Song Y, Chen Y, Hui R, Zhang W. Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Int J Cardiol 2013;168:4761–4768 [DOI] [PubMed] [Google Scholar]
- 6. Eckel N, Meidtner K, Kalle-Uhlmann T, Stefan N, Schulze MB. Metabolically healthy obesity and cardiovascular events: a systematic review and meta-analysis. Eur J Prev Cardiol 2016;23:956–966 [DOI] [PubMed] [Google Scholar]
- 7. Zheng R, Zhou D, Zhu Y. The long-term prognosis of cardiovascular disease and all-cause mortality for metabolically healthy obesity: a systematic review and meta-analysis. J Epidemiol Community Health 2016;70:1024–1031 [DOI] [PubMed] [Google Scholar]
- 8. Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol 2018;6:714–724 [DOI] [PubMed] [Google Scholar]
- 9. Mongraw-Chaffin M, Foster MC, Anderson CAM, et al. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol 2018;71:1857–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med 2008;168:1617–1624 [DOI] [PubMed] [Google Scholar]
- 11. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56–e528 [DOI] [PubMed] [Google Scholar]
- 12. Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities study. Circulation 2011;123:1367–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McEvoy JW, Chen Y, Nambi V, et al. High-sensitivity cardiac troponin T and risk of hypertension. Circulation 2015;132:825–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ndumele CE, Coresh J, Lazo M, et al. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail 2014;2:600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ndumele CE, Cobb L, Lazo M, et al. Weight history and subclinical myocardial damage. Clin Chem 2018;64:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Lemos JA, Grundy SM. Low levels of circulating troponin as an intermediate phenotype in the pathway to heart failure. J Am Coll Cardiol 2012;59:490–492 [DOI] [PubMed] [Google Scholar]
- 17. deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010;304:2494–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995;854:1–452 [PubMed] [Google Scholar]
- 19. ATP III Guidelines At-A-Glance Quick Desk Reference, 2001 . National Institutes of Health, U.S. Department of Health and Human Services. Accessed 04 May 2020. Available from https://www.nhlbi.nih.gov/files/docs/guidelines/atglance.pdf
- 20. White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years’ experience. J Clin Epidemiol 1996;49:223–233 [DOI] [PubMed] [Google Scholar]
- 21. Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999;30:736–743 [DOI] [PubMed] [Google Scholar]
- 22. McEvoy JW, Chen Y, Ndumele CE, et al. Six-year change in high-sensitivity cardiac troponin T and risk of subsequent coronary heart disease, heart failure, and death. JAMA Cardiol 2016;1:519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010;56:254–261 [DOI] [PubMed] [Google Scholar]
- 24. Whelton SP, McEvoy JW, Lazo M, Coresh J, Ballantyne CM, Selvin E. High-sensitivity cardiac troponin T (hs-cTnT) as a predictor of incident diabetes in the Atherosclerosis Risk in Communities study. Diabetes Care 2017;40:261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alberti KGMM, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group . The metabolic syndrome--a new worldwide definition. Lancet 2005;366:1059–1062 [DOI] [PubMed] [Google Scholar]
- 26. Echouffo-Tcheugui JB, Short MI, Xanthakis V, et al. Natural history of obesity subphenotypes: dynamic changes over two decades and prognosis in the Framingham Heart Study. J Clin Endocrinol Metab 2019;104:738–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011;58:1332–1339 [DOI] [PubMed] [Google Scholar]
- 28. Caleyachetty R, Thomas GN, Toulis KA, et al. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol 2017;70:1429–1437 [DOI] [PubMed] [Google Scholar]
- 29. Morkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trondelag Health Study), Norway. J Am Coll Cardiol 2014;63:1071–1078 [DOI] [PubMed] [Google Scholar]
- 30. Ndumele CE, Matsushita K, Lazo M, et al. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc 2016;5:e003921. [DOI] [PMC free article] [PubMed] [Google Scholar]