Abstract
OBJECTIVE
To evaluate the efficacy of aluminum-formulated intralymphatic glutamic acid decarboxylase (GAD-alum) therapy combined with vitamin D supplementation in preserving endogenous insulin secretion in all patients with type 1 diabetes (T1D) or in a genetically prespecified subgroup.
RESEARCH DESIGN AND METHODS
In a multicenter, randomized, placebo-controlled, double-blind trial, 109 patients aged 12–24 years (mean ± SD 16.4 ± 4.1) with a diabetes duration of 7–193 days (88.8 ± 51.4), elevated serum GAD65 autoantibodies, and a fasting serum C-peptide >0.12 nmol/L were recruited. Participants were randomized to receive either three intralymphatic injections (1 month apart) with 4 μg GAD-alum and oral vitamin D (2,000 IE daily for 120 days) or placebo. The primary outcome was the change in stimulated serum C-peptide (mean area under the curve [AUC] after a mixed-meal tolerance test) between baseline and 15 months.
RESULTS
Primary end point was not met in the full analysis set (treatment effect ratio 1.091 [CI 0.845–1.408]; P = 0.5009). However, GAD-alum–treated patients carrying HLA DR3-DQ2 (n = 29; defined as DRB1*03, DQB1*02:01) showed greater preservation of C-peptide AUC (treatment effect ratio 1.557 [CI 1.126–2.153]; P = 0.0078) after 15 months compared with individuals receiving placebo with the same genotype (n = 17). Several secondary end points showed supporting trends, and a positive effect was seen in partial remission (insulin dose–adjusted HbA1c ≤9; P = 0.0310). Minor transient injection site reactions were reported.
CONCLUSION
Intralymphatic administration of GAD-alum is a simple, well-tolerated treatment that together with vitamin D supplementation seems to preserve C-peptide in patients with recent-onset T1D carrying HLA DR3-DQ2. This constitutes a disease-modifying treatment for T1D with a precision medicine approach.
Introduction
Many individuals with type 1 diabetes (T1D) have a long life, with reasonably good quality of life, but even with modern treatment, the disease still causes serious morbidity (1) and increased mortality (2,3). Even a limited residual β-cell function facilitates glycemic control, decreases the risk of both acute and late complications, and reduces mortality (4). The most efficient immune therapy evaluated to date for preservation of β-cell function is treatment with anti-CD3 monoclonal antibodies (teplizumab) (5,6); tumor necrosis factor-α inhibitors (7), ATG (8), alefacept (9), and rituximab (10) have also displayed efficacy, which is encouraging, but sometimes these therapies have adverse events, risks, and heavy treatment burdens, and therefore, additional studies are needed.
An alternative approach is treatment with autoantigens to modulate the immune system. Most autoantigen immunotherapies have failed to meet their therapeutic end points or have shown inconclusive results (11–14). One reason for this may be the heterogeneity of T1D (15). Disease endotype should be considered in the design and evaluation of clinical trials (16). The appearance of GAD65 autoantibodies (GADA) is linked to the HLA DR3-DQ2 haplotype, and the emergence of insulin autoantibodies is linked to DR4-DQ8 (15,16). Importantly, a meta-analysis of data from clinical trials evaluating subcutaneous GAD-alum immunotherapy (12–14) indicated that patients carrying the HLA DR3-DQ2 haplotype showed a significant and positive dose-dependent preservation of β-cell function following GAD-alum treatment (17).
The immunological impact of antigen can be improved by direct administration into lymph nodes. This strategy has been successful in allergy immunotherapy (18) and was tested in T1D in a pilot trial with GAD-alum injected into an inguinal lymph node, in combination with oral vitamin D, with encouraging results (19).
Therefore, in this randomized, double-blind, placebo-controlled phase IIb trial, we aimed to determine the efficacy of three intralymphatic injections of 4 μg GAD-alum into inguinal lymph nodes in preserving β-cell function in individuals with recent-onset T1D. Based on the results from the meta-analysis suggesting efficacy of GAD-alum in individuals carrying the HLA DR3-DQ2 (defined as DRB1*03, DQB1*02:01) haplotype (17), the analysis of efficacy end points in the subpopulation carrying this genotype was introduced as an amendment to the original clinical study protocol ahead of treatment unblinding.
Research Design and Methods
Study Conduct
The coordinating investigator (J.L.) designed the study based on a previous pilot trial (DIAGNODE-1; EudraCT2014-001417-79) (19) together with the sponsor, Diamyd Medical (Stockholm, Sweden). The first and last authors vouch for the data, analysis, and fidelity of the report to the study protocol.
The study was approved by the relevant regulatory authorities and research ethics boards of the participating sites and countries. All patients and their caregivers provided written informed consent. The study was conducted in accordance with the clinical study protocol and the statistical analysis plan.
Study Design and Patients
This study was a two-arm, multicenter, randomized, double-blind, placebo-controlled trial performed at 18 diabetes clinics in the Czech Republic, the Netherlands, Spain, and Sweden. Patients were recruited by the clinicians, and screening was performed between 7 December 2017 and 16 April 2019. The study intended to enroll 106 patients aged between ≥12 and <25 years at screening with a diagnosis of T1D within the previous 6 months. Major inclusion criteria were elevated serum GADA and fasting serum C-peptide >0.12 nmol/L (>0.36 ng/mL). Major exclusion criteria were prior or current treatment with immunosuppressant therapy, continuous treatment with an anti-inflammatory drug, treatment with any oral or injected antidiabetic medication (other than insulin), and concomitant treatment with vitamin D.
Randomization and Masking
Patients were randomized at a 1:1 ratio in blocks of four by an interactive web response system using a computer-generated randomization list. Randomization was stratified by level of serum GADA and by country. The randomization list was kept strictly confidential until database lock at 15 months. The identity of the treatments (GAD-alum and vitamin D) was masked using matching placebo with identical packaging, labeling, appearance, and schedule of administration. Matching placebo used for GAD-alum was aluminum only.
Study Treatments and Procedures
Patients were randomized in a 1:1 ratio to receive one of the following:
Three intralymphatic injections with 4 μg Diamyd on days 30, 60, and 90 and 2,000 IE oral vitamin D daily for 4 months (from day 1 through 120) if the vitamin D serum level was <100 nmol/L (40 ng/mL) at screening.
Three intralymphatic injections of placebo for Diamyd on days 30, 60, and 90 and oral placebo for vitamin D daily for 4 months (from day 1 through 120) if the vitamin D serum level was <100 nmol/L (40 ng/mL) at screening.
Each patient kit contained three vials, which were labeled with the same treatment number.
Follow-up visits were performed after 180 and 450 days. An extra follow-up visit after 720 days will be performed in a subset of patients. Patients, investigators, and study personnel will remain blinded to the treatment assignments until the follow-up visits are completed.
Medical examination was performed at all visits including baseline (day 1) and months 1, 2, 3, 6, and 15. Mixed-meal tolerance tests (MMTT) (20) were performed at baseline (day 1) and months 6 and 15. Investigator assessment of injection site reactions was collected after each injection, and adverse events were collected throughout the study. Patients documented insulin dose information for 4 days before each visit. Patients wore blinded FreeStyle Libre Pro continuous glucose monitoring devices during 2 weeks postvisit for visit 1 (screening), month 6, and month 15. Injection site reactions after each injection were reported by patients in a diary, and any severe episodes of hypoglycemia were collected at each study visit postbaseline.
HLA genotyping (month-1 visit) as well as clinical chemistry, hematology, urine, GADA, HbA1c, and C-peptide analyses were performed centrally by Synlab Pharma Institute (Munich, Germany), using its standardized techniques. GADA levels were assessed by means of ELISA, with results measured in IU/mL. C-peptide quantification was performed with the use of dual-sided chemiluminescence immunoassay, using two antibodies (Siemens C-peptide assay no. 03649928), with calibration standards based on the World Health Organization National Institute for Biological Standards and Control International Reference Reagent standards (product no. 84/510). HLA typing (HLA-DR-B1 and HLA-DQ-B1) was performed using a sequence-specific oligonucleotides kit (One Lambda).
For cytokine quantification, peripheral blood mononuclear cells (PBMC) were cultured for 7 days in the presence of 5 μg/mL recombinant human GAD65 (Diamyd Medical) or in medium (AIM-V) alone at 37°C in 5% carbon dioxide, as previously described (21). Interleukin-10 (IL-10) and IL-13 were measured in cell culture supernatants using the Bio-Plex Pro Cytokine Panel (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Data were collected using the Luminex 200 (Luminex xMAP Corporation, Austin, TX). The antigen-induced cytokine secretion level was calculated by subtracting the spontaneous secretion (i.e., secretion from PBMC cultured in medium alone) from that following stimulation with GAD65.
To quantify PBMC proliferation, PBMC were resuspended in AIM-V medium and incubated in triplicate in round-bottom 96-well plates with 5 μg/mL recombinant human GAD65 (Diamyd Medical) or in AIM-V medium alone. After 3 days, cells were pulsed for 18 h with 0.2 μCi of [3H] thymidine per well (PerkinElmer) and thereafter harvested. Proliferation was recorded using a cell counter and expressed as stimulation index, calculated as the median of triplicates in presence of stimulus divided by the median of triplicates with medium alone.
Outcomes
The primary outcome was the change in stimulated serum C-peptide (mean area under the curve [AUC] over the 2-h period after an MMTT between the baseline visit and the 15-month visit).
The key secondary outcomes were changes between baseline and 15 months in insulin dose–adjusted HbA1c (IDAA1c) (22), HbA1c, and daily exogenous insulin dose. Other secondary end points were:
Change in glycemic variability/fluctuations (evaluated from data from continuous glucose monitoring FreeStyle LibrePro and flash glucose monitoring) over a 14-day period between screening and 15 months.
Proportions of patients at 15 months with IDAA1c ≥9, a stimulated maximum C-peptide >0.2 nmol/L (0.6 ng/mL), and a stimulated 90-min C-peptide >0.2 nmol/L (0.6 ng/mL).
Number of self-reported episodes of severe hypoglycemia (defined as needing help from others and/or seizures and/or unconsciousness) between baseline and 15 months.
Number of patients with at least 1 severe hypoglycemic event between baseline and 15 months
Change in maximum C-peptide during MMTT between baseline and 15 months
Change in fasting C-peptide between baseline and 15 months
A number of secondary end points were evaluated to assess safety, such as occurrences of adverse events, reactions at the injection site, physical and neurological assessments, and clinical chemistry and hematology measurements.
Analyses of primary and key secondary end points were preplanned in the statistical analysis plan and clinical trial protocol for the subgroup of patients carrying the HLA DR3-DQ2 haplotype. This subgroup analysis was not part of the initial protocol but rather was prespecified ahead of treatment unblinding in the statistical analysis plan and in the clinical study protocol through a protocol amendment. The scientific rationale for this subgroup is based on the results of a meta-analysis (17) of previous GAD-alum studies showing efficacy mainly in patients carrying HLA DR3-DQ2.
Statistical Analyses
Sample size was calculated such that the study would have 90% power to detect a 50% difference in the primary end point (mean C-peptide AUCmean 0–120 min) during an MMTT at 15 months between the actively treated group and the placebo group at a two-sided significance level of 5%, including allowance for a 10% dropout rate. This was based on a t test using ln(x + 1) normalizing transformation of the primary end point and assumed mean and SD estimates, on the transformed scale, of 0.134 and 0.20, respectively (23).
Analyses of efficacy end points included all randomized patients who received at least one injection and had at least one postbaseline assessment of any efficacy end point.
For the primary efficacy and key secondary end points, between-group comparisons of mean changes from baseline were analyzed using a restricted maximum likelihood–based repeated measures approach (mixed-model repeated measures). The model for analysis included fixed categorical effects of treatment, randomization strata (GAD65 antibody level), visit and treatment-by-visit interactions, and the continuous fixed covariate of log-transformed baseline C-peptide AUCmean 0–120 min during an MMTT. The treatment effect within the impact of the HLA haplotype subgroups on the primary efficacy analysis was analyzed by adding a class variable (HLA haplotype as DR3-DQ2 vs. not DR3-DQ2) and the interaction term between this variable and the treatment variable (i.e., treatment ∗ Visit ∗ HLA) in the mixed-model repeated measures analysis. An unstructured (full sample analyses) and compound symmetry (HLA subgroup analyses) (co)variance structure was used to model the within-patient errors. Because the model accounts for missing data, no imputations were made.
Data and Resource Availability
The data sets are available from the corresponding author on reasonable request.
Results
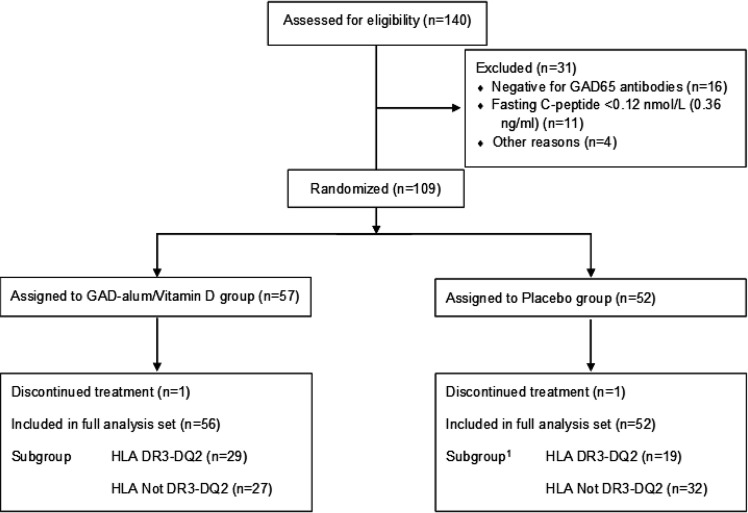
Patients (n = 140) were screened between 7 December 2017 and 16 April 2019, of whom 109 were randomized to active treatment (n = 57) or placebo (n = 52). The primary analysis was performed in randomized patients who received at least one injection and had been evaluated for at least one efficacy variable at baseline and at one follow-up visit (full analysis set [FAS]). All information on eligibility, FAS, and subgroups with HLA DR3-DQ2 is shown in Fig. 1.
Figure 1.
Trial profile. Between December 2017 and April 2019, a total of 140 patients were screened for a fasting C-peptide ≥0.12 nmol/L and positivity for GAD65 antibodies (<50,000 IU/mL), and 109 underwent randomization. Of these study participants, 108 were included in the FAS, whose data were used in the analysis of clinical efficacy at 15 months. A total of 48 patients in the FAS were found to carry the HLA DR3-DQ2 haplotype. 1Information on HLA genotype was missing for one patient in the placebo group.
The baseline characteristics of the patients are shown in Table 1. Two patients had vitamin D >100 nmol/L (40 ng/mL) at screening and therefore received no vitamin D/placebo supplementation. Significant differences were observed between active and placebo treatment groups in the prespecified subgroup defined by HLA DR3-DQ2 for HbA1c (P = 0.0100) and IDAA1c (P = 0.0101); a near-significant difference was seen for C-peptide AUC (P = 0.0523).
Table 1.
Baseline characteristics of study patients, according to treatment group
| Safety set | HLA DR3-DQ2 subgroup | |||
|---|---|---|---|---|
| Characteristic | GAD-alum (n = 57) | Placebo (n = 52) | GAD-alum (n = 29) | Placebo (n = 19) |
| Age, years | 16.2 (3.8) | 16.6 (4.3) | 16.5 (4.0) | 16.3 (3.9) |
| Time from diagnosis to first treatment, days | 147.6 (54.7) | 134.9 (48.1) | 149.8 (52.0) | 131.8 (58.0) |
| Sex, n (%) | ||||
| Female | 21 (36.8) | 26 (50.0) | 10 (33.3) | 7 (36.8) |
| Male | 36 (63.2) | 26 (50.0) | 20 (66.7) | 12 (63.2) |
| BMI category (children aged ≤18 years), n (%)* | ||||
| <25th percentile | 6 (10.5) | 3 (5.8) | 3 (10.0) | 3 (15.8) |
| 25–75th percentile | 23 (40.4) | 25 (48.1) | 10 (33.3) | 9 (47.4) |
| >75th percentile | 14 (24.6) | 9 (17.3) | 8 (26.7) | 3 (15.8) |
| BMI category (adolescents and young adults aged >18 years), kg/m2, n (%)* | ||||
| ≥18.5 to <25.0 | 8 (14.0) | 8 (15.4) | 3 (10.0) | 3 (15.8) |
| ≥25.0 to <30.0 | 5 (8.8) | 5 (9.6) | 5 (16.7) | 1 (5.3) |
| >30.0 | 1 (1.8) | 2 (3.8) | 1 (3.3) | |
| Tanner puberty stage, n (%) | ||||
| 1–3 | 13 (23.2) | 7 (13.5) | 6 (20.0) | 2 (10.5) |
| 4–5 | 28 (50.0) | 32 (61.5) | 14 (46.7) | 14 (73.7) |
| Not applicable† | 15 (26.8) | 13 (25.0) | 10 (33.3) | 3 (15.8) |
| Median GADA, units/mL | 76.2 | 77.9 | 57.9 | 80.3 |
| HLA DR3-DQ2 haplotype, n (%) | ||||
| No | 27 (48.2) | 32 (61.5) | ||
| Yes | 29 (51.8) | 19 (36.5) | ||
| Fasting C-peptide, nmol/L | 0.329 (0.165) | 0.323 (0.168) | 0.320 (0.132) | 0.307 (0.130) |
| Stimulated C-peptide AUC | 0.793 (0.381) | 0.702 (0.314) | 0.819 (0.341) | 0.633 (0.263) |
| HbA1c, % | 6.39 (0.96) | 6.51 (1.13) | 6.17 (0.71) | 7.02 (1.23) |
| IDAA1c | 7.96 (1.37) | 8.16 (1.83) | 7.46 (1.09) | 8.68 (1.76) |
| Insulin dose, IU/kg body weight per day | 0.393 (0.228) | 0.411 (0.300) | 0.324 (0.191) | 0.415 (0.276) |
| Fasting plasma glucose, mmol/L | 6.34 (1.70) | 6.58 (2.07) | 5.90 (1.14) | 7.03 (2.45) |
Data are presented as mean (SD) unless otherwise indicated. HLA DR3-DQ2 haplotype, fasting C-peptide, stimulated C-peptide AUC, HbA1c, IDAA1c, insulin dose, and fasting plasma glucose are based on FAS; others are based on safety set. Safety set includes 109 patients who received at least one injection (no exclusions were made). FAS includes 108 patients from the safety set. One patient was excluded for discontinuing study 1 month after first injection and therefore did not have enough efficacy data. One patient did not have HLA DR3-DQ2 haplotype information. There were no missing data on other variables. In FAS, none of the differences between treatment groups were statistically significant. In HLA DR3-DQ2 haplotype subgroup, there were statistically significant differences between Diamyd and placebo groups in HbA1c (P = 0.0100) and IDAA1c (P = 0.0101) and near-significant difference in C-peptide AUC (P = 0.0523). Statistical testing of baseline differences between treatment groups was performed using Fisher exact test for categorical variables with two categories, and Cochran-Mantel-Haenszel test (general association, nonstratified) was used for categorical variables with more than two categories. Wilcoxon test was used for the C-peptide AUC because of skewed distribution as well as for all tests in HLA DR3-DQ2 haplotype subgroup because of small numbers. Student t test was used in all other instances.
BMI categories according to percentiles from CDC growth charts for children aged ≤18 years and according to WHO obesity categories for adolescents and young adults aged >18 years.
Young adults aged >18 years.
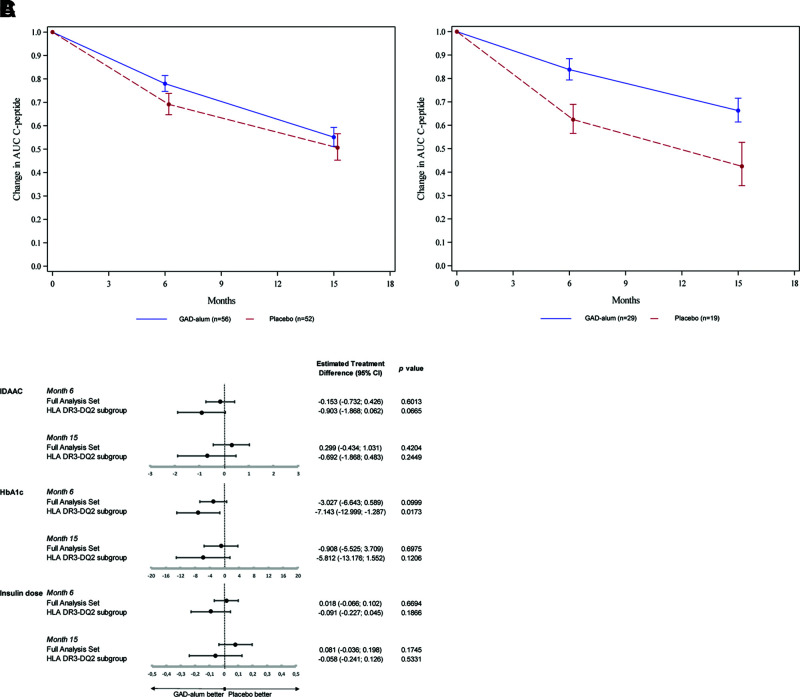
Primary end point was not reached (treatment effect ratio 1.091 [CI 0.845–1.408]; P = 0.5009). Therefore, there was no difference between the actively treated group and the placebo group in change of stimulated serum C-peptide (mean AUC over the 2-h period after an MMTT) between the baseline visit and the 15-month visit (Fig. 2A) in the FAS. There was no significant difference between the actively treated group and the placebo group in mean daily insulin dose, HbA1c, or IDAA1c.
Figure 2.
Primary and key secondary study outcomes. Relative change from baseline (back transformed from log scale model) in C-peptide AUCmean 0–120 min during an MMTT for the two treatment groups (GAD-alum and placebo) in the FAS (A) and the prespecified subgroup HLA DR3-DQ2 (B). Error bars indicate SD. C: Forest plot depicting estimated treatment difference between GAD-alum and placebo groups for key secondary end points (IDAA1c and HbA1c in nmol/mol and daily exogenous insulin dose in units/kg/24 h) observed in the FAS and in the the prespecified subgroup HLA DR3-DQ2. Error bars indicate 95% CI. P values are indicated. Primary and key secondary efficacy end point variables were analyzed using a restricted maximum likelihood–based repeated measures approach (mixed-model repeated measures), as described in Research Design and Methods.
In the prespecified subgroup of patients carrying the HLA DR3-DQ2 haplotype, there was a significantly better preservation of C-peptide AUC0–120 min during an MMTT in the actively treated group compared with the placebo group (treatment effect ratio 1.557 [CI 1.126–2.153]; P = 0.0078) (Fig. 2B). This is also illustrated in Supplementary Fig. 1, where absolute and change from baseline show that the C-peptide decline continued more rapidly up to 15 months in the placebo group than in the actively treated group. A higher proportion of actively treated patients with HLA DR3-DQ2 remained in partial remission (IDAA1c ≤9; 78.6% in active [CI 59.0–91.7] and 40.0% in placebo [CI 16.3–67.7]; P = 0.0310) and maintained a stimulated maximum C-peptide >0.2 nmol/L (96.6% in active [CI 82.2–99.9] and 70.6% in placebo [CI 44.0–89.7]; P = 0.0284) as well as stimulated 90-min C-peptide >0.2 nmol/L (96.6% in active [CI 82.2–99.9] and 64.7% in placebo [CI 38.3–85.8]; P = 0.0086) at 15 months (Fig. 2C; Supplementary Table 1). There was a trend at month 15 where actively treated patients with HLA DR3-DQ2 were within target range (70–180 mg/dL) for a median of 18.6 h per day compared with 14.8 h per day in the placebo group (P = 0.1488) Severe hypoglycemic events were only reported by one patient; therefore, analysis of this end point could not be performed.
Vitamin D levels increased for patients receiving active vitamin D throughout the supplementation period (12.4 ng/mL mean increase at 3 months) and decreased to starting levels by month 15, while these levels in placebo-treated patients changed only slightly (4.2 ng/mL maximum increase over trial period). There was no significant impact of vitamin D level (average absolute levels at month 1, 2, and 3) on primary outcome across all patients (P = 0.1186), and Pearson correlation coefficient for patients with HLA DR3-DQ2 was low (−0.0117). When patients were categorized according to low, medium, and high levels, groups became small, and influence of vitamin D on efficacy was difficult to interpret.
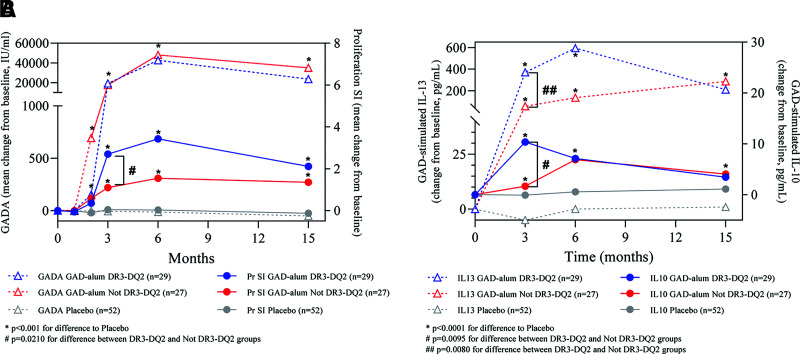
A significant increase in GADA levels was observed in individuals receiving GAD-alum treatment, both in individuals positive and in those negative for HLA DR3-DQ2 haplotype, when compared with placebo (Fig. 3A). There was no significant difference in GADA levels between actively treated patients positive and negative for the DR3-DQ2 haplotype, but patients with HLA DR3-DQ2 had both significantly increased proliferation index (Fig. 3A) and significantly increased GAD-stimulated IL-10 and IL-13 compared with those negative for HLA DR3-DQ2 or patients in the placebo group (Fig. 3B).
Figure 3.
Pharmacological outcomes. Median changes from baseline of GADA and proliferation (Pr) of PMBC (stimulation index [SI]) (A) and GAD-stimulated secretion by PBMC of IL-10 and IL-13 levels (B) for GAD-alum–treated patients with and without the DR3-DQ2 haplotype as well as placebo-treated patients. P values by Wilcoxon test are indicated.
The proportion of patients with any adverse event was 49% in the actively treated group and 58% in the placebo group (Supplementary Table 2). There were three serious adverse events, all in individuals treated with placebo. No patient discontinued the treatment regimen as a result of an adverse event. There were no physiological or neurological concerns reported. Injection site reactions were mild and similar in number in the two groups, as were results of safety laboratory analyses.
Conclusions
GAD-alum administered in the current dosing regimen did not change the disease progression of T1D across the entire trial population, but it seemed to be beneficial in a genetically selected group of patients.
Immunotherapeutic interventions in T1D patients should combine efficacy with a low-risk profile and few adverse effects to be widely accepted in clinical practice. In contrast to more burdensome interventions that suppress the immune system (5–10), GAD-alum treatment has been shown to be easy for patients and the health care system, and well-tolerated GAD-alum therapy has to date shown strong safety and patient convenience in trials involving >1,000 individuals, including children, adolescents, and adults.
Previous trials with GAD-alum revealed positive although inconsistent results, warranting an alternative approach to improve treatment efficacy (12–14,24). Animal studies have shown that intralymphatic injections induce a strong and relevant T-cell response (25,26), and clinical studies in the allergy field have shown that presentation of the antigen/allergen directly into the lymph nodes seems to be more effective than traditional administration (27). To test this hypothesis, a first-in-human pilot trial (DIAGNODE-1) with GAD-alum administered intralymphatically commenced in a small group of adults. After showing promising results (28,29) and few adverse events, authorities allowed an expansion of the trial and the inclusion of children. The full pilot trial showed encouraging results (19). To confirm these results, a larger randomized, double-blind, placebo-controlled phase II trial (DIAGNODE-2) was consequently initiated.
While DIAGNODE-2 was ongoing, a large meta-analysis showed clear positive efficacy of GAD-alum in individuals presenting with the HLA DR3-DQ2 haplotype (17), whereupon this patient subgroup was prespecified in an amendment to the study protocol. Confirming the earlier findings described in the meta-analysis, a significant preservation of β-cell function was found in this prespecified subgroup. DIAGNODE-2 was not powered to show significance for the prespecified subgroup, which constituted half of the full patient population. Even so, clear trends of improvement in several clinical parameters could be observed in individuals positive for HLA DR3-DQ2. In the prespecified patient group, there were differences in baseline HbA1c, IDAA1c, and C-peptide, but the statistical analysis adjusted for baseline differences.
Vitamin D was supplemented for 120 days in those patients with vitamin D levels <100 nmol/L, because adequate vitamin D concentrations may be important to achieve an appropriate effect on the immune system (30). Vitamin D may have beneficial effects on the preservation of β-cell function, but several studies have contradicted this (31). We were not able to see any significant effect on C-peptide decline directly related to vitamin D, and because no positive effect was seen in the actively treated HLA DR3-DQ2–negative patients, who also received supplementation with vitamin, it is unlikely that the observed efficacy was conferred by vitamin D.
Autoantigen treatment has been tried in many autoimmune diseases, with poor results. There are several problems to be solved, such as selection of autoantigen, dose, timing, and route of administration (32). This study confirmed the positive effects of intralymphatic autoantigen treatment, suggested in our previous first-in-human pilot trial (19), and this route of administration may therefore be of interest for trials in other autoimmune diseases. Compared with subcutaneous administration, intralymphatic administration allows the use of a smaller dose, reduces exposure to adjuvant, and potentially reduces immunological interference by other vaccines using traditional administration routes. It seems to be safe and easy for patients, and the required ultrasound competence needed for the injections can be found within the health care system.
There is an emerging consensus in the field that T1D is a heterogeneous disease (16). The importance of selecting the right treatment for a certain endotype of disease has already been shown in studies on the effect of immunotherapy with proinsulin peptides (33) and with methyldopa (34). Individuals carrying the HLA DR3-DQ2 haplotype are more prone to develop GADA (15). Our results show that patients with this genotype benefit from antigen immunotherapy with GAD-alum. Notably, our trial points to the crucial importance of choosing the right therapeutic antigen to treat specific endotypes of a disease (22). We observed significant differences in immune response in patients positive for HLA DR3-DQ2 compared with those negative for HLA DR3-DQ2, and further careful and extensive analysis of the immune responses in this trial will be performed to gain a deeper understanding of the mechanism of the treatment.
The value of residual insulin secretion for prevention of complications is well known, although it is generally difficult to show clinical efficacy in variables such as insulin dose or HbA1c in studies with a follow-up of <24 months in patients receiving modern intensive insulin treatment, because the study design aims for best possible metabolic control in all patients, including those who receive placebo treatment.
This study was not designed or powered to specifically evaluate individuals carrying HLA DR3-DQ2, nor was it stratified for this variable. Therefore, the number of patients in the specified subgroup was smaller, and there were some differences in the baseline characteristics of the active and placebo groups. Also, outside of the primary and key secondary end points evaluated for the total patient population, there was no adjustment for multiple end points performed in the statistical analysis of efficacy. However, the statistical model accounts for baseline values, and the subgroup in focus was prespecified based on a comprehensive meta-analysis (17) of data from previous studies and was based on a scientific rationale associated with the genetic predisposition to autoimmunity against GAD65. Therefore, the positive result in the primary end point in this subgroup supports that GAD-alum treatment is efficacious. This needs to be confirmed in future placebo-controlled studies of GAD-alum treatment in this subgroup. Thereafter, it will be natural to study whether booster doses of GAD-alum will further prolong the preservation of β-cell function.
In summary, intralymphatic administration of GAD-alum in individuals with recent-onset T1D is a relatively easy and tolerable treatment. The study did not meet its primary end point in the full patient cohort. However, in the prespecified subgroup of patients with HLA haplotype DR3-DQ2, who may constitute up to half of all individuals with GADA-positive T1D, those who received active GAD-alum treatment had preservation of β-cell function significantly better than patients who received placebo, and the clinical course of the disease tended to be improved. Because modern medicine is about matching the appropriate drugs or treatments to the patient populations most likely to benefit from them (35), this finding is promising, and if confirmed in a larger clinical trial, it could pave the way for such a precision medicine approach in T1D.
Article Information
Acknowledgments. The authors thank all the participants who agreed to participate in the study. The authors are grateful to Dr. Joachim Davidsson (Department of Radiology, Linköping University Hospital, Linköping, Sweden) for skillful performance of the needle-guided lymph node injections and instructions to other centers. The authors also thank Isabel Leiva (Malaga, Spain) and all research nurses and physicians at the sites, as well as Ingela Johansson and Gosia Smolinska (Division of Pediatrics, Department of Biomedical and Clinical Sciences, Faculty of Medicine and Health Sciences, Linköping University, Linköping, Sweden) for skillful laboratory work.
Funding. This study was supported by Barndiabetesfonden (Swedish Child Diabetes Foundation) and Diabetesfonden. Diamyd Medical provided GAD-alum, vitamin D, and matching placebos.
Neither Barndiabetesfonden nor Diabetesfonden was involved in the management of the trial, data collection, or analysis or in the decision to submit the manuscript for publication.
Duality of Interest. This study was funded by an unrestricted grant from Diamyd Medical. U.H. is employed by Diamyd Medical. No other potential conflicts of interest relevant to this article were reported.
Diamyd Medical was involved in the management of the trial, data collection, and analysis, both directly and through contract research organizations.
Author Contributions. J.L. was responsible for conceptualization, data curation, analyses, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, and visualization; wrote the original draft; and has verified the underlying data. A.N. was responsible for statistical analyses and validation. U.H. contributed to conceptualization and was responsible for data curation, analyses, funding acquisition, project administration, resources, supervision, and validation. R.C. was responsible for data curation, analyses, methodology, project administration, and validation and has verified the underlying data. All other authors contributed to investigation and acquisition of patients and data. All authors reviewed and edited the manuscript and approved the final version and submission. J.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. nos. NCT03345004, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.14427956.
References
- 1. Lind M, Pivodic A, Svensson AM, Ólafsdóttir AF, Wedel H, Ludvigsson J. HbA1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ 2019;366:l4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lind M, Svensson AM, Rosengren A. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2015;372:880–881 [DOI] [PubMed] [Google Scholar]
- 3. Rawshani A, Sattar N, Franzén S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 2018;392:477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ludvigsson J. The clinical potential of low-level C-peptide secretion. Expert Rev Mol Diagn 2016;16:933–940 [DOI] [PubMed] [Google Scholar]
- 5. Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 6. Sherry N, Hagopian W, Ludvigsson J, et al.; Protégé Trial Investigators . Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 2011;378:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quattrin T, Haller MJ, Steck AK, et al.; T1GER Study Investigators . Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N Engl J Med 2020;383:2007–2017 [DOI] [PubMed] [Google Scholar]
- 8. Haller MJ, Schatz DA, Skyler JS, et al.; Type 1 Diabetes TrialNet ATG-GCSF Study Group . Low-dose anti-thymocyte globulin (ATG) preserves β-cell function and improves HbA1c in new-onset type 1 diabetes. Diabetes Care 2018;41:1917–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rigby MR, Harris KM, Pinckney A, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest 2015;125:3285–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al.; Type 1 Diabetes TrialNet Anti-CD20 Study Group . Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Näntö-Salonen K, Kupila A, Simell S, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 2008;372:1746–1755 [DOI] [PubMed] [Google Scholar]
- 12. Ludvigsson J, Faresjö M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–1920 [DOI] [PubMed] [Google Scholar]
- 13. Wherrett DK, Bundy B, Becker DJ, et al.; Type 1 Diabetes TrialNet GAD Study Group . Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 2011;378:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ludvigsson J, Krisky D, Casas R, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med 2012;366:433–442 [DOI] [PubMed] [Google Scholar]
- 15. Krischer JP, Lynch KF, Lernmark Å, et al.; TEDDY Study Group . Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: the TEDDY study. Diabetes Care 2017;40:1194–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Battaglia M, Ahmed S, Anderson MS, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 2020;43:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hannelius U, Beam CA, Ludvigsson J. Efficacy of GAD-alum immunotherapy associated with HLA-DR3-DQ2 in recently diagnosed type 1 diabetes. Diabetologia 2020;63:2177–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Senti G, Freiburghaus AU, Larenas-Linnemann D, et al. Intralymphatic immunotherapy: update and unmet needs. Int Arch Allergy Immunol 2019;178:141–149 [DOI] [PubMed] [Google Scholar]
- 19. Casas R, Dietrich F, Barcenilla H, et al. Glutamic acid decarboxylase injection into lymph-nodes: beta cell-function and immune responses in recent onset type 1 diabetes patients. Front Immunol 2020;11:564921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al.; Type 1 Diabetes Trial Net Research Group; European C-Peptide Trial Study Group . Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;31:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Axelsson S, Chéramy M, Åkerman L, Pihl M, Ludvigsson J, Casas R. Cellular and humoral immune responses in type 1 diabetic patients participating in a phase III GAD-alum intervention trial. Diabetes Care 2013;36:3418–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mortensen HB, Hougaard P, Swift P, et al.; Hvidoere Study Group on Childhood Diabetes . New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care 2009;32:1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lachin JM, McGee PL, Greenbaum CJ, et al.; Type 1 Diabetes Trial Network . Sample size requirements for studies of treatment effects on beta-cell function in newly diagnosed type 1 diabetes. PLoS One 2011;6:e26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beam CA, MacCallum C, Herold KC, Wherrett DK, Palmer J, Ludvigsson J; Type 1 Diabetes TrialNet Study Group . GAD vaccine reduces insulin loss in recently diagnosed type 1 diabetes: findings from a Bayesian meta-analysis. Diabetologia 2017;60:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maloy KJ, Erdmann I, Basch V, et al. Intralymphatic immunization enhances DNA vaccination. Proc Natl Acad Sci USA 2001;98:3299–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johansen P, Häffner AC, Koch F, et al. Direct intralymphatic injection of peptide vaccines enhances immunogenicity. Eur J Immunol 2005;35:568–574 [DOI] [PubMed] [Google Scholar]
- 27. Senti G, Prinz Vavricka BM, Erdmann I, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci USA 2008;105:17908–17912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ludvigsson J, Wahlberg J, Casas R. Intralymphatic injection of autoantigen in type 1 diabetes. N Engl J Med 2017;376:697–699 [DOI] [PubMed] [Google Scholar]
- 29. Ludvigsson J, Tavira B, Casas R. More on intralymphatic injection of autoantigen in type 1 diabetes. N Engl J Med 2017;377:403–405 [DOI] [PubMed] [Google Scholar]
- 30. Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol 2000;164:4443–4451 [DOI] [PubMed] [Google Scholar]
- 31. Bizzarri C, Pitocco D, Napoli N, et al.; IMDIAB Group . No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care 2010;33:1962–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ludvigsson J. Autoantigen treatment in type 1 diabetes: unsolved questions on how to select autoantigen and administration route. Int J Mol Sci 2020;21:1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alhadj Ali M, Liu Y-F, Arif S, et al. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci Transl Med 2017;9:eaaf7779. [DOI] [PubMed] [Google Scholar]
- 34. Ostrov DA, Alkanani A, McDaniel KA, et al. Methyldopa blocks MHC class II binding to disease-specific antigens in autoimmune diabetes. J Clin Invest 2018;128:1888–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chung WK, Erion K, Florez JC, et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:1617–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]