Abstract
OBJECTIVE
We previously reported a high (˜30%) but stable prevalence of diabetic ketoacidosis (DKA) at youth-onset diagnosis of type 1 diabetes (2002 and 2010). Given the changing demographics of youth-onset type 1 diabetes, we sought to evaluate temporal trends in the prevalence of DKA at diagnosis of type 1 diabetes from 2010 to 2016 among youth <20 years of age and evaluate whether any change observed was associated with changes in sociodemographic distribution of those recently diagnosed.
RESEARCH DESIGN AND METHODS
We calculated prevalence of DKA within 1 month of type 1 diabetes diagnosis by year and evaluated trends over time (2010–2016) (n = 7,612 incident diabetes cases; mean [SD] age 10.1 [4.5] at diagnosis). To assess whether trends observed were attributable to the changing distribution of sociodemographic factors among youth with incident type 1 diabetes, we estimated an adjusted relative risk (RR) of DKA in relation to calendar year, adjusting for age, sex, race/ethnicity, income, education, health insurance status, language, season of diagnosis, and SEARCH for Diabetes in Youth Study site.
RESULTS
DKA prevalence increased from 35.3% (95% CI 32.2, 38.4) in 2010 to 40.6% (95% CI 37.8, 43.4) in 2016 (Ptrend = 0.01). Adjustment for sociodemographic factors did not substantively change the observed trends. We observed a 2% annual increase in prevalence of DKA at or near diagnosis of type 1 diabetes (crude RR 1.02 [95% CI 1.01, 1.04] and adjusted RR 1.02 [95% CI 1.01, 1.04]; P = 0.01 for both).
CONCLUSIONS
Prevalence of DKA at or near type 1 diabetes diagnosis has increased from 2010 to 2016, following the high but stable prevalence observed from 2002 to 2010. This increase does not seem to be attributable to the changes in distribution of sociodemographic factors over time.
Introduction
Diabetic ketoacidosis (DKA) is a serious, potentially life-threatening complication of diabetes (1–3). At diabetes onset, DKA is characterized by hyperglycemia, ketosis, and acidosis, resulting from insulin deficiency and increased levels of counterregulatory hormones (glucagon, catecholamine, cortisol, and growth hormone) (4). Although DKA can be treated, serious acute morbidity (e.g., cerebral edema) and death can occur.
DKA occurs commonly at the time of diabetes diagnosis, with younger children being at greatest risk, possibly because diabetes symptoms are more likely to go unrecognized in younger children (4–8). Uninsured or underinsured children are also at increased risk, as are children from minority racial and ethnic groups (5,9,10). While children whose parents have diabetes, and who develop diabetes themselves, are less likely to be in DKA at the time of diabetes diagnosis, the overall proportion is still worrisomely high (24% vs. 41% in the Type 1 Diabetes Exchange Registry) (11).
The multisite registry of youth onset (<20 years) diabetes, the SEARCH for Diabetes in Youth Study (SEARCH), previously reported a high but stable prevalence of DKA at diagnosis of type 1 diabetes across the period 2002 through 2010 (˜30%) (12). However, in a recent study at a referral-based, single diabetes center (Barbara Davis Center for Diabetes in Colorado) (n = 2,429), DKA at diagnosis of type 1 diabetes was observed to increase from 41% in 2010 to 58% in 2017 (13). The SEARCH study has documented that the distribution of demographic characteristics of youth with newly diagnosed type 1 diabetes has changed over time (14). An increasing proportion of youth with incident type 1 diabetes in the U.S. are Hispanic and younger at diagnosis (5–9 years) than in earlier years (14). Given differences in DKA frequency at type 1 diabetes onset between demographic groups, we hypothesized that an increase in DKA at diagnosis could be explained, at least in part, by the shift in demographic characteristics of youth diagnosed with diabetes.
Given the changing demographics of youth-onset type 1 diabetes, we sought to evaluate temporal trends in the prevalence of DKA at diagnosis of type 1 diabetes from 2010 to 2016 among youth <20 years of age and evaluate whether any change observed was associated with changes in sociodemographic distribution of those recently diagnosed.
Research Design and Methods
The SEARCH registry study is an ongoing population-based surveillance effort and study of incident and prevalent youth-onset (<20 years) diabetes (15). The process of case ascertainment has been previously described (14). Briefly, cases of type 1 diabetes are identified based on clinical assessment of a physician as documented in the medical record, with systematic ascertainment across four geographic regions (Washington, South Carolina, Ohio, and Colorado) and among enrollees in a managed health care plan in California. Capture-recapture assessments have indicated that 99% of type 1 diabetes cases are consistently ascertained within the catchment areas of the SEARCH sites (14). At the time of ascertainment of incident diabetes cases, demographic characteristics and key elements related to diagnosis, including date (month and year) of diabetes diagnosis, diabetes type, and presence of DKA at diagnosis, were abstracted from clinical notes in the medical record. Dates of diabetes diagnosis are limited to month and year only; thus, diagnosis of DKA is defined based on the documentation of one or more of the following in the medical record within ˜1 month of the diabetes diagnostic month: serum bicarbonate <15 mmol/L, or pH <7.25 (venous) or <7.3 (arterial or capillary), or provider documentation of diagnosis of DKA (5). Race and ethnicity, sex, parent/guardian education, insurance status, household income, and primary language spoken in the household are obtained from a survey completed by individuals aged ≤18 years with diabetes or parents/caregivers of youth <18 years of age. If race and ethnicity, or sex, were not reported on the survey, information from the medical record was used. Local Institutional Review Boards at each of the five SEARCH sites reviewed and approved the study protocol.
Using date of diagnosis and diabetes type, we calculated the prevalence of DKA within 1 month of diagnosis of type 1 diabetes for each year from 2010 through 2016 and examined trends in prevalence of DKA across the 7-year period. Prevalence was calculated as the proportion of incident cases presenting in DKA at or near the time of type 1 diabetes diagnosis for each time interval examined (i.e., in DKA at or near incident diabetes diagnosis/number of cases with incident diabetes diagnosis). Descriptive characteristics were summarized using counts (percentages), and differences in distribution of sociodemographic factors across years were tested using χ2 tests. Trends in DKA prevalence were assessed overall and by age, sex, race/ethnicity, insurance status, season of diagnosis, and study site. To evaluate trends over time, the total follow-up period of 7 years was divided into four equal, consecutive periods (21 months), and Ptrend was calculated from generalized linear models (log link and Poisson distribution) regressing time period, across the four periods, on DKA overall and within age, sex, race/ethnicity, insurance status, season, and SEARCH site strata.
Next, we evaluated whether observed trends in DKA prevalence were attributable to shifts in the distribution of sociodemographic characteristics of registered cases. Using generalized linear models (log link and Poisson distribution) we estimated the crude and adjusted relative risk (RR) of DKA according to calendar year (2010–2016), adjusting for the covariates of interest: age group (0–4 years, 5–9 years, and 10–14 years vs. 15–19 years), sex (female vs. male), race/ethnicity (other vs. non-Hispanic white), income (<$25,000, $25,000–49,000, and $50,000–74,000 vs. ≥$75,000/year), highest parental/caregiver education (less than high school diploma vs. high school diploma or higher), insurance status (public/other/none vs. private), and primary language (non-English vs. English). Additionally, we adjusted for SEARCH site (Washington, California, Ohio, and Colorado vs. South Carolina) and season of diabetes diagnosis (spring [months March through May] vs. other seasons). Categorization of covariates was based on categories established in previously reported estimates for DKA prevalence in the SEARCH study and by combining categories was based on qualitative examination of the distribution of the data to ensure adequate sample counts within categories. Crude and adjusted estimates were obtained through complete case analysis, with the crude estimates obtained from those cases with complete data on covariates included in the multivariable model. Robust SEs were used for calculation of estimate 95% CIs. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
A total of 7,743 youth with type 1 diabetes were registered from 2010 through 2016, of which 7,612 (98.3%) had nonmissing information to determine DKA status at or near diabetes diagnosis. Youth with type 1 diabetes were predominantly non-Hispanic White (n = 4,961; 65.3%) but were socioeconomically diverse, with 32.9% of families reporting incomes ≤$49,000/year and 32.5% reporting public insurance coverage (Medicaid or Medicare) (Table 1). Overall, of the 7,612 cases eligible for this analysis, 2,929 had DKA at or within 1 month of diabetes diagnosis (38.5 out of 100 [95% CI 37.4, 39.6]). The highest prevalence of DKA at or near diabetes diagnosis was among youth aged 0–4 at diagnosis (46.7% [95% CI 43.8, 49.5]) as compared with 37.7% (95% CI 35.7, 39.6) for 5–9 years of age, 39.5% (95% CI 37.6, 41.3) for 10–14 years of age, and 29.7% (95% CI 27.1, 32.3) for ages ≥15 years. Those with nonprivate insurance coverage also had a higher prevalence of DKA at or near diabetes diagnosis (44.2% [95% CI 42.2, 46.2] as compared with 34.8% [95% CI 33.3, 36.2] for private insurance). Across racial and ethnicity groups, those of Hispanic ethnicity had the highest prevalence of DKA at or near diabetes diagnosis (43.6 out of 100 cases [95% CI 40.8, 46.3]), but this was only statistically significantly higher than non-Hispanic Whites (36.9% [95% CI 35.5, 38.2]). The lowest prevalence of DKA was observed among youth registered in South Carolina (31.5 out of 100 cases [95% CI 29.2, 33.8]), although this was only significantly different when compared against Colorado, California, and Washington. No significant differences were observed when comparing males and females or season of type 1 diabetes diagnosis (Supplementary Tables 1–6).
Table 1.
Demographic characteristics of incident type 1 diabetes cases in the SEARCH for Diabetes in Youth study (2010–2016) (n = 7,612)*
| Characteristic | N | Count (%) |
|---|---|---|
| Age at diagnosis (years) | 7,611** | |
| 0–4 | 1,189 (15.6) | |
| 5–9 | 2,400 (31.5) | |
| 10–14 | 2,824 (37.1) | |
| ≥15 | 1,198 (15.7) | |
| Sex | 7,612 | |
| Female | 3,493 (45.9) | |
| Male | 4,119 (54.1) | |
| Race/ethnicity | 7,600 | |
| Non-Hispanic White | 4,961 (65.3) | |
| Hispanic | 1,251 (16.5) | |
| African American | 1,033 (13.6) | |
| Asian Pacific Islander | 254 (3.3) | |
| American Indian | 29 (0.4) | |
| Other | 72 (0.9) | |
| Household income+ | 6,511 | |
| <$25,000 | 974 (15.0) | |
| $25–49,000 | 1,168 (17.9) | |
| $50–74,000 | 929 (14.3) | |
| ≥$75,000 | 2,464 (37.8) | |
| Do not know/refused | 976 (15.0) | |
| Highest parental education+ | 6,570 | |
| Less than high school | 257 (3.9) | |
| High school or higher | 6,313 (96.1) | |
| Insurance type+ | 6,606 | |
| Private | 4,206 (63.7) | |
| Medicaid/Medicare | 2,145 (32.5) | |
| Other/none | 255 (3.9) | |
| Primary language at home+ | 6,444 | |
| English | 6,332 (98.3) | |
| Non-English | 112 (1.7) | |
| Season of diagnosis | 7,612 | |
| Summer (June–August) | 1,749 (23.0) | |
| Fall (September–November) | 1,780 (23.4) | |
| Winter (December–February) | 2,112 (27.7) | |
| Spring (March–May) | 1,971 (25.9) |
Excludes n = 131 with missing documentation of DKA status.
n = 1 participant with missing age at diagnosis. +Data available only for participants who completed the survey of registered cases.
Trends in DKA Prevalence at Diagnosis
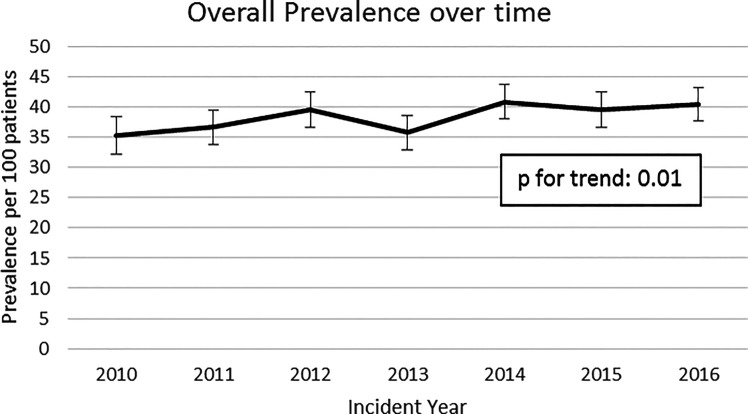
We observed an increase in the prevalence of DKA at or near diabetes diagnosis, from 35.3 out of 100 (95% CI 32.2, 38.4) in 2010 to 40.6 out of 100 (95% CI 37.8, 43.4) in 2016 (Fig. 1) (Ptrend = 0.01). Within subgroups, youth ages 10–14 years at type 1 diabetes diagnosis (Ptrend = 0.05), males (Ptrend = 0.05), those diagnosed in the spring season (Ptrend = 0.01), and those registered in South Carolina (Ptrend = 0.05) demonstrated a significant increase in prevalence of DKA across the study period (Supplementary Tables 1–6).
Figure 1.
Trends in prevalence of DKA at type 1 diabetes diagnosis in the SEARCH for Diabetes in Youth study (2010–2016). *P value for trend (0.0110) generated by creating four equal time periods.
Analysis Accounting for Shifting Sociodemographic Factors Over Time
Examination of missing data indicated that 19.6% of registered cases were missing data on one or more key covariates that could explain any change in trends in DKA at or near to diabetes onset. Over the study period, we observed an increase in the proportion of registered cases with higher household income and with Medicaid or Medicare insurance (P < 0.05) (Supplementary Fig. 1).
In our complete case analysis, evaluating trends over time while accounting for changes in sociodemographic factors, season of diagnosis, and study site, we observed a 2% annual increase (adjusted RR 1.02 [95% CI 1.01, 1.04]; P = 0.01) in the prevalence of DKA at or near to the time of diabetes diagnosis across the study period. Crude (RR 1.02 [95% CI 1.01, 1.04]; P = 0.01) and adjusted estimates were very similar, suggesting that shifting sociodemographic factors had little influence on the trends observed. Crude estimates obtained from the complete case analysis sample were identical to the crude estimates obtained from the full sample (RR 1.02 [95% CI 1.01, 1.04]) (Table 2).
Table 2.
Relative annual increase in prevalence of DKA at type 1 diabetes diagnosis in the SEARCH for Diabetes in Youth study (2010–2016)
| N | DKA at diagnosis (n) | RR (95% CI) 1-year increase | P | |
|---|---|---|---|---|
| Model 1: complete case, unadjusted | 6,121 | 2,326 | 1.02 (1.01, 1.04) | 0.0064 |
| Model 2: complete case, adjusted* | 6,121 | 2,326 | 1.02 (1.01, 1.04) | 0.0052 |
| Model 3: complete sample, unadjusted | 7,612 | 2,929 | 1.02 (1.01, 1.04) | 0.0045 |
Adjusted for age group, sex, race/ethnicity, income, parent/caregiver education, insurance status, primary language, SEARCH site, and season.
Conclusions
While the distribution of demographic factors for incident type 1 diabetes is changing (14), we report that the proportion of children with incident type 1 diabetes presenting with DKA at or near to the time of diabetes diagnosis is also increasing and that changes in incident type 1 diabetes demographics do not explain this increase. Across the period 2010–2016, we observed that DKA at or near diabetes diagnosis of youth-onset type 1 diabetes, increased at a rate of 2% per year. Overall, 38.5% of case subjects with type 1 diabetes presented with DKA at or near to the time of diagnosis, an increase from our previously estimated prevalence of ˜30% from 2002 to 2010. Consistent with earlier reports from SEARCH, we observed the highest proportion of DKA at or near type 1 diabetes diagnoses among younger children (5). Still, it has been noted that adolescents (10–14 years of age) also may be at higher risk of DKA at diagnosis (16). In the current study, youth aged 10–14 had a higher prevalence of DKA at type 1 diabetes diagnosis as compared with older adolescents ≥15 years (Supplementary Table 1). Youth with Hispanic ethnicity identity and from families without private insurance also had higher prevalence of DKA as compared with their non-Hispanic White and privately insured peers. Within subgroups, we noted increasing trends in prevalence of DKA at or near type 1 diabetes diagnosis for youth aged 10–14 years, those diagnosed in the spring season, and those registered in South Carolina.
Studies from other developed countries have reported a broad range of prevalence estimates for DKA at diagnosis of type 1 diabetes (17), specifically countries with higher health care provision experiencing the lowest prevalence of DKA (17,18). Recent reports indicate prevalence may be increasing (19–21). However, in the SEARCH study from 2002 to 2010, we found no evidence of a change in DKA prevalence at or near youth-onset type 1 diabetes diagnosis (30.2% in 2002–2003, 29.1% in 2004–2005, and 31.1% in 2008–2010; Ptrend = 0.42) (12). In the U.S. specifically, reports from a referral-based, single center suggest increases (13,22). The current study confirms these observations, in a more diverse, population-based registry of youth-onset type 1 diabetes.
While we observed a changing distribution in sociodemographic factors among cases over time (Supplementary Fig. 1), this did not explain the increase in DKA prevalence across the study period. Although income was not adjusted for inflation, and income appeared to increase over the period of observation, the overall proportion of those on Medicaid also increased across this same time period. Of note, our findings are also consistent with a recent population-based study among youth (≤17 years) in Quebec, where DKA at diagnosis has increased from 22% in 2001% to 30% in 2014, with a relative increase of 2% each year (RR 1.02 [95% CI 1.01–1.03]) (23). Canada has universal health care; however, it has been noted that access to primary care has decreased over time and that access to primary care is associated with decreased risk of DKA at diagnosis (24).
While beyond the scope of the current study, future evaluations may want to consider the potential influence of the changing health insurance coverage landscape in the U.S. In the U.S., health insurance coverage varies, with some individuals bearing high out-of-pocket and co-pay costs, even with insurance coverage. While the proportion of adults reporting insurance coverage, having usual health care providers, and using health care and screening for diabetes has increased (27), there has also been an increase in high-deductible, employer-sponsored health plans, in which out-of-pocket deductible costs for employees have more than doubled (28). However, how these changes have translated to health care utilization among children and adolescents and any potential impact on delayed diagnosis of diabetes are unknown (27,28).
Our report is the largest, contemporary population-based study of type 1 youth-onset diabetes in the U.S.; however, our sample size is likely still too small for assessing differences in trends across sociodemographic subgroups. Furthermore, while we had a high proportion of cases with complete data, with just 19.5% of registered cases missing one or more covariates, our multivariable analysis accounting for sociodemographic factors in the association between calendar time and DKA could reflect selection bias. However, reassuringly, the crude estimates obtained from the complete sample and the complete case analysis sample were nearly identical; thus, informative loss of data, in a manner that could introduce selection bias, is unlikely (Supplementary Table 7). Still, it remains possible that missing data could explain the changing prevalence in DKA at or near type 1 diagnosis if the data missing were indeed informative.
Additionally, our assumption is that an absence of information on DKA documented in the medical record is consistent with not having DKA at diagnosis. The period from 2010 to 2016 was a period in which many clinical sites were undergoing changes in documentation of medical records, either transitioning to electronic systems or to new platforms for electronic medical record management. Our review of how DKA criteria were met across the observation period found an increasing proportion of DKA cases with documentation of laboratory values consistent with DKA as opposed to documentation of DKA in clinical notes only. We also observed an increase in the proportion of DKA diagnosis based on two or more of the diagnostic criteria being met (from 55% in 2010 to 79% in 2016). However, the proportion with provider documentation of DKA remained consistent across time, suggesting that while there may have been improvements in documentation of laboratory values, documentation of provider assessment was consistent throughout his time period, with 99–100% of those determined to have DKA having evidence of a provider note indicating presence of DKA across the entire period under study. Further, because date of diabetes diagnosis is documented by month and year, we cannot rule out the possibility that a portion of the DKA cases occurred after diabetes diagnosis (but within 1 month of diagnosis). Of note, we applied the same approach to characterizing DKA as has been previously applied in SEARCH, thus allowing for comparison across time in burden. Because data on DKA status were abstracted retrospectively from patient medical records, with variation in the documentation of laboratory values, details on DKA severity were not available.
In conclusion, in a multisite, population-based U.S. study of youth onset diabetes, we observed a significant increase in the prevalence of DKA at or near diagnosis of type 1 diabetes over a 7-year time period (2010–2016). This increase is in contrast to our previous findings indicating a high but static prevalence of DKA across the 9-year period from 2002 to 2010 (12). The increase observed was not explained by changes in the distribution of sociodemographic factors among individuals newly diagnosed with type 1 diabetes over time, but the increase in DKA was found to be significant in subgroups such as 10–14-year-old children and males, who may represent risk groups for development of DKA at diagnosis. The increase in DKA at or near type 1 diabetes diagnosis is of considerable concern, as DKA is not only associated with acute complications and mortality (1,2), but also with risk of long-term poorly managed diabetes, diabetes-associated complications and comorbidities, and significant health care costs (25,26,29–32). Additional study is needed to understand these trends. Examination of the contribution of the changing health insurance landscape in the U.S. is one potential area of investigation, but other factors may also contribute. Understanding the role of these factors is critical to mitigating risk of DKA and could inform other risk mitigation strategies, such as education and screening of children at increased risk (9,33–36).
The increase in prevalence of DKA at or near diabetes diagnosis reported in this study, as well as in other recent reports described above, is likely not explained by changes in population distribution of genetic susceptibility factors. Identifying children at risk, increasing surveillance of these children, and ensuring barriers to care are minimized, particularly for populations at greatest risk of marginalization, may be critical for reducing DKA at diagnosis.
Article Information
Acknowledgments. The SEARCH for Diabetes in Youth Study is indebted to the many youth, their families, and health care providers, whose participation made this study possible. The authors thank Kaiser Permanente Southern California’s Clinical Research Center (funded by Kaiser Foundation Health Plan and supported in part by the Southern California Permanente Medical Group), the South Carolina Clinical & Translational Research Institute at the Medical University of South Carolina, National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) grants UL1 TR000062 and UL1 TR001450; Seattle Children’s Hospital and the University of Washington, NIH/NCATS grant number UL1 TR00423; University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS grant UL1 TR000154; the Barbara Davis Center for Diabetes at the University of Colorado at Denver (Diabetes Endocrinology Research Center NIH grant P30 DK57516); the University of Cincinnati, NIH/NCATS grants UL1 TR000077 and UL1 TR001425; and the Children with Medical Handicaps program managed by the Ohio Department of Health for involvement in this study. This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of this study or its conclusions.
Funding. The SEARCH for Diabetes in Youth Cohort Study is funded by the NIH, National Institute of Diabetes and Digestive and Kidney Diseases (1UC4DK108173-01) and supported by the Centers for Disease Control and Prevention (CDC). The Population Based Registry of Diabetes in Youth Study is funded by the CDC (RFP DP15-002) and supported by the NIH, National Institute of Diabetes and Digestive and Kidney Diseases. Funding for the study sites is as follows: Kaiser Permanente Southern California (U18DP006133, U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U18DP006139, U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Cincinnati’s Children’s Hospital Medical Center (U18DP006134, U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U18DP006138, U48/CCU419249, U01 DP000254, and U18DP002708), Seattle Children’s Hospital (U18DP006136, U58/CCU019235-4, U01 DP000244, and U18DP002710-01), and Wake Forest University School of Medicine (U18DP006131, U48/CCU919219, U01 DP000250, and 200-2010-35171).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC and the National Institute of Diabetes and Digestive and Kidney Diseases.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.T.J. and D.D. led the study design. E.T.J. and R.B.D’A. provided oversight to the study analyses. J.M.S. conducted study analyses. E.T.J. prepared the manuscript. L.M.D., J.M.L., E.J.M.-D., C.P., and D.D. oversaw data collection. All other authors provided critical input into study design and review of the manuscript. E.T.J. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented at the 55th Annual Meeting of the European Association for the Study of Diabetes, Barcelona, Spain, 16–20 September 2019.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14489001.
References
- 1. Desai D, Mehta D, Mathias P, Menon G, Schubart UK. Health care utilization and burden of diabetic ketoacidosis in the U.S. over the past decade: a nationwide analysis. Diabetes Care 2018;41:1631–1638 [DOI] [PubMed] [Google Scholar]
- 2. Gibb FW, Teoh WL, Graham J, Lockman KA. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia 2016;59:2082–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mays JA, Jackson KL, Derby TA, et al. An evaluation of recurrent diabetic ketoacidosis, fragmentation of care, and mortality across Chicago, Illinois. Diabetes Care 2016;39:1671–1676 [DOI] [PubMed] [Google Scholar]
- 4. Wolfsdorf JI, Allgrove J, Craig ME, et al.; International Society for Pediatric and Adolescent Diabetes . ISPAD Clinical Practice Consensus Guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes 2014;15(Suppl. 20):154–179 [DOI] [PubMed] [Google Scholar]
- 5. Rewers A, Klingensmith G, Davis C, et al. Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the Search for Diabetes in Youth Study. Pediatrics 2008;121:e1258–e1266 [DOI] [PubMed] [Google Scholar]
- 6. Bui H, To T, Stein R, Fung K, Daneman D. Is diabetic ketoacidosis at disease onset a result of missed diagnosis? J Pediatr 2010;156:472–477 [DOI] [PubMed] [Google Scholar]
- 7. de Vries L, Oren L, Lazar L, Lebenthal Y, Shalitin S, Phillip M. Factors associated with diabetic ketoacidosis at onset of type 1 diabetes in children and adolescents. Diabet Med 2013;30:1360–1366 [DOI] [PubMed] [Google Scholar]
- 8. Maahs DM, Hermann JM, Holman N, et al.; National Paediatric Diabetes Audit and the Royal College of Paediatrics and Child Health, the DPV Initiative, and the T1D Exchange Clinic Network . Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care 2015;38:1876–1882 [DOI] [PubMed] [Google Scholar]
- 9. Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers 2020;6:40. [DOI] [PubMed] [Google Scholar]
- 10. Rewers A, Chase HP, Mackenzie T, et al. Predictors of acute complications in children with type 1 diabetes. JAMA 2002;287:2511–2518 [DOI] [PubMed] [Google Scholar]
- 11. Fox LA, Mubasher M, Wolfsdorf JI, et al.; T1D Exchange Clinic Network . Characteristics of youth with type 1 diabetes (T1D) with and without a parent with T1D in the T1D exchange clinic registry. J Diabetes 2016;8:834–838 [DOI] [PubMed] [Google Scholar]
- 12. Dabelea D, Rewers A, Stafford JM, et al.; SEARCH for Diabetes in Youth Study Group . Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics 2014;133:e938–e945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alonso GT, Coakley A, Pyle L, Manseau K, Thomas S, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado children, 2010-2017. Diabetes Care 2020;43:117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamman RF, Bell RA, Dabelea D, et al.; SEARCH for Diabetes in Youth Study Group . The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care 2014;37:3336–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hekkala A, Reunanen A, Koski M, Knip M; Finnish Pediatric Diabetes Register . Age-related differences in the frequency of ketoacidosis at diagnosis of type 1 diabetes in children and adolescents. Diabetes Care 2010;33:1500–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia 2012;55:2878–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grosse J, Hornstein H, Manuwald U, Kugler J, Glauche I, Rothe U. Incidence of diabetic ketoacidosis of new-onset type 1 diabetes in children and adolescents in different countries correlates with Human Development Index (HDI): an updated systematic review, meta-analysis, and meta-regression. Horm Metab Res 2018;50:209–222 [DOI] [PubMed] [Google Scholar]
- 19. Manuwald U, Schoffer O, Hegewald J, et al. Ketoacidosis at onset of type 1 diabetes in children up to 14 years of age and the changes over a period of 18 years in Saxony, Eastern-Germany: a population based register study. PLoS One 2019;14:e0218807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality - United States, 2000-2014. MMWR Morb Mortal Wkly Rep 2018;67:362–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cherubini V, Grimsmann JM, Åkesson K, et al. Temporal trends in diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes between 2006 and 2016: results from 13 countries in three continents. Diabetologia 2020;63:1530–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rewers A, Dong F, Slover RH, Klingensmith GJ, Rewers M. Incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado youth, 1998-2012. JAMA 2015;313:1570–1572 [DOI] [PubMed] [Google Scholar]
- 23. Robinson ME, Li P, Rahme E, Simard M, Larocque I, Nakhla MM. Increasing prevalence of diabetic ketoacidosis at diabetes diagnosis among children in Quebec: a population-based retrospective cohort study. CMAJ Open 2019;7:E300–E305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakhla M, Rahme E, Simard M, Larocque I, Legault L, Li P. Risk of ketoacidosis in children at the time of diabetes mellitus diagnosis by primary caregiver status: a population-based retrospective cohort study. CMAJ 2018;190:E416–E421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Okoro CA, Zhao G, Fox JB, Eke PI, Greenlund KJ, Town M. Surveillance for health care access and health services use, adults aged 18-64 years - behavioral risk factor surveillance system, United States, 2014. MMWR Surveill Summ 2017;66:1–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sommers BD, Blendon RJ, Orav EJ, Epstein AM. Changes in utilization and health among low-income adults after Medicaid expansion or expanded private insurance. JAMA Intern Med 2016;176:1501–1509 [DOI] [PubMed] [Google Scholar]
- 27. Duca LM, Reboussin BA, Pihoker C, et al. Diabetic ketoacidosis at diagnosis of type 1 diabetes and glycemic control over time: The SEARCH for diabetes in youth study. Pediatr Diabetes 2019;20:172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fredheim S, Johannesen J, Johansen A, et al.; Danish Society for Diabetes in Childhood and Adolescence . Diabetic ketoacidosis at the onset of type 1 diabetes is associated with future HbA1c levels. Diabetologia 2013;56:995–1003 [DOI] [PubMed] [Google Scholar]
- 29. Aye T, Mazaika PK, Mauras N, et al.; Diabetes Research in Children Network (DirecNet) Study Group . Impact of early diabetic ketoacidosis on the developing brain. Diabetes Care 2019;42:443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cameron FJ, Scratch SE, Nadebaum C, et al.; DKA Brain Injury Study Group . Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care 2014;37:1554–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gregg EW, Hora I, Benoit SR. Resurgence in diabetes-related complications. JAMA 2019;321:1867–1868 [DOI] [PubMed] [Google Scholar]
- 32. Saydah SH, Shrestha SS, Zhang P, Zhou X, Imperatore G. Medical costs among youth younger than 20 years of age with and without diabetic ketoacidosis at the time of diabetes diagnosis. Diabetes Care 2019;42:2256–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hekkala AM, Ilonen J, Toppari J, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes: Effect of prospective studies with newborn genetic screening and follow up of risk children. Pediatr Diabetes 2018;19:314–319 [DOI] [PubMed] [Google Scholar]
- 34. Marigliano M, Morandi A, Maschio M, et al. Diabetic ketoacidosis at diagnosis: role of family history and class II HLA genotypes. Eur J Endocrinol 2012;168:107–111 [DOI] [PubMed] [Google Scholar]
- 35. Ziegler AG, Kick K, Bonifacio E, et al.; Fr1da Study Group . Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA 2020;323:339–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Elding Larsson H, Vehik K, Bell R, et al.; TEDDY Study Group; SEARCH Study Group; Swediabkids Study Group; DPV Study Group; Finnish Diabetes Registry Study Group . Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care 2011;34:2347–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]