Abstract
OBJECTIVE
Insulin icodec (icodec) is a novel once-weekly basal insulin analog. This trial investigated two approaches for switching to icodec versus once-daily insulin glargine 100 units/mL (IGlar U100) in people with type 2 diabetes receiving daily basal insulin and one or more oral glucose-lowering medications.
RESEARCH DESIGN AND METHODS
This multicenter, open-label, treat-to-target phase 2 trial randomized (1:1:1) eligible basal insulin–treated (total daily dose 10–50 units) people with type 2 diabetes (HbA1c 7.0–10.0% [53.0–85.8 mmol/mol]) to icodec with an initial 100% loading dose (in which only the first dose was doubled [icodec LD]), icodec with no loading dose (icodec NLD), or IGlar U100 for 16 weeks. Primary end point was percent time in range (TIR; 3.9–10.0 mmol/L [70–180 mg/dL]) during weeks 15 and 16, measured using continuous glucose monitoring. Key secondary end points included HbA1c, adverse events (AEs), and hypoglycemia.
RESULTS
Estimated mean TIR during weeks 15 and 16 was 72.9% (icodec LD; n = 54), 66.0% (icodec NLD; n = 50), and 65.0% (IGlar U100; n = 50), with a statistically significant difference favoring icodec LD versus IGlar U100 (7.9%-points [95% CI 1.8–13.9]). Mean HbA1c reduced from 7.9% (62.8 mmol/mol) at baseline to 7.1% (54.4 mmol/mol icodec LD) and 7.4% (57.6 mmol/mol icodec NLD and IGlar U100); incidences and rates of AEs and hypoglycemic episodes were comparable.
CONCLUSIONS
Switching from daily basal insulin to once-weekly icodec was well tolerated and provided effective glycemic control. Loading dose use when switching to once-weekly icodec significantly increased percent TIR during weeks 15 and 16 versus once-daily IGlar U100, without increasing hypoglycemia risk.
Introduction
Although many individuals with type 2 diabetes require insulin therapy at some point (1,2), glycemic control remains inadequate in many patients (3). This could be attributed to the need for frequent injections resulting in poor adherence and concerns about complex dosing, the adverse effects of hypoglycemia and weight gain, and treatment costs (2,4–8). Once-daily basal insulin analogs have addressed some of these concerns (9,10), but research suggests that people with type 2 diabetes would value a further reduction in dosing frequency of injectable therapies to once weekly, which may improve convenience, adherence, and quality of life. In turn, these improvements may lead to better glycemic control (11).
Insulin icodec (icodec) is a novel basal insulin analog for subcutaneous administration for the treatment of diabetes. It has stable pharmacokinetic and pharmacodynamic profiles, with a half-life of ∼1 week, supporting once-weekly administration (12). The long half-life of icodec can be attributed to its strong, reversible albumin binding, reduced enzymatic degradation, and slow receptor-mediated clearance (13). After subcutaneous injection and absorption into the circulation, icodec monomers bind to albumin to form an essentially inactive depot, from which icodec molecules slowly reach insulin receptors at target tissues to stimulate glucose lowering. With each weekly injection, the pool of albumin-bound icodec gradually increases, until steady state is reached after 3–4 weeks when the full glucose-lowering effect is achieved and insulin clearance matches administered insulin dose. At steady state, a slow, continuous release of icodec from the inactive albumin-bound depot provides effective glucose lowering throughout the week (13), which was shown to be near evenly distributed across a 1-week dosing interval (12). In a phase 2 trial in insulin-naive individuals with type 2 diabetes, once-weekly icodec resulted in a similar glucose-lowering effect and safety profile compared with once-daily insulin glargine 100 units/mL (IGlar U100) (14).
Theoretically, individuals switching from a daily basal insulin regimen may require additional insulin to maintain glycemic control during the initial weeks after switch, until steady state is reached. Pharmacokinetic and pharmacodynamic modeling data, based on clinical pharmacology studies of icodec, suggest that when switching from daily to once-weekly basal insulin, adding a supplemental dose of icodec (a “loading dose”) to the first dose may prevent any transient deterioration in fasting glucose levels before steady state is achieved without jeopardizing safety, as this additional dose would add to the inactive albumin-bound reservoir of icodec.
The aim of the current trial was to evaluate the effect on glycemic control and safety of two different approaches of switching to once-weekly icodec, with and without a loading dose, versus once-daily IGlar U100 in people with type 2 diabetes inadequately controlled on basal insulin plus at least one oral glucose-lowering medication.
Research Design and Methods
Research Design
This exploratory, multicenter, open-label, randomized, active-controlled, parallel-group, treat-to-target phase 2 trial was conducted in 34 centers located in Canada, Czech Republic, Germany, Italy, and the U.S. The trial consisted of a 2-week screening period, 16 weeks of randomized treatment, and 5 weeks of follow-up (Supplementary Fig. 1).
The trial was conducted in accordance with the guidelines for Good Clinical Practice of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and the Declaration of Helsinki. The protocol, consent form, and other relevant documents were approved by the appropriate independent review boards or independent ethics committees.
Patients
The trial population comprised basal insulin–treated individuals with type 2 diabetes recruited between 9 May 2019 and 15 August 2019 with the last follow-up occurring on 27 January 2020. Eligible participants, aged 18–75 years with a BMI of ≤40 kg/m2 and a centrally assessed HbA1c of 7.0–10.0% (53.0–85.8 mmol/mol), were treated for at least 90 days before screening with stable doses of metformin, with or without concomitant use of dipeptidyl peptidase 4 inhibitors and/or sodium–glucose cotransporter 2 inhibitors (SGLT2is), along with basal insulin (once or twice daily) with a total daily dose of 10–50 units. Full details of inclusion and exclusion criteria are provided in Supplementary Table 1. All participants provided written informed consent to participate in the trial.
Randomization
After screening, participants were randomized (1:1:1) to once-weekly icodec with an initial 100% loading dose (icodec LD), once-weekly insulin icodec with no loading dose (icodec NLD), or once-daily IGlar U100. Randomization was performed centrally using an interactive web response system. Participants were assigned to the next available treatment according to the randomization schedule and stratified based on SGLT2i use (yes or no) and type of pretrial insulin therapy (i.e., receiving once-daily basal insulin, or recieving twice-daily basal insulin or once-daily IGlar 300 units/mL [U300]).
Procedures
The initial dose of the trial products was based on the pretrial insulin daily doses. Participants who had been receiving once-daily basal insulin (except for those receiving IGlar U300) at baseline underwent a “unit to unit” switch to icodec (700 units/mL; prefilled pen injector) (Novo Nordisk, Bagsvaerd, Denmark) or IGlar U100 (100 units/mL) (SoloSTAR prefilled pen injector; Sanofi, Paris, France) based on their daily dose; to derive the equivalent once-weekly dose for the icodec LD and NLD groups, the daily dose was multiplied by 7. For those who had been receiving twice-daily basal insulin or once-daily IGlar U300 at baseline, starting doses of IGlar U100 were decreased by 20% to minimize the postswitch hypoglycemia risk; for the icodec LD and NLD groups, the dose was similarly decreased by 20% and then multiplied by 7 to derive the once-weekly dose. Participants in the icodec LD group received an initial 100% loading dose with the first dose of icodec (i.e., the first weekly dose was doubled), and then it was reverted to the calculated weekly dose at week 2 (Supplementary Fig. 1B). Icodec had to be taken subcutaneously once weekly on the same day each week, while IGlar U100 had to be taken subcutaneously once daily at any time of the day but at the same time every day.
A treat-to-target approach was used to ensure optimal titration of insulin; doses of icodec and IGlar U100 were titrated weekly to achieve a prebreakfast self-measured blood glucose (SMBG) target of 4.4–7.2 mmol/L (80–130 mg/dL). Participants measured SMBG levels (FreeStyle Precision/Optium Neo Meter; Abbott GmbH & Co. KG, Abbott Diabetes Care) each day before breakfast. Doses were uptitrated weekly by 4 units/day (IGlar U100) or 28 units/week (icodec LD and icodec NLD) if the mean of the three most recent prebreakfast SMBG values in a week was >7.2 mmol/L (>130 mg/dL). However, if any one of the three prebreakfast SMBG values was <4.4 mmol/L (<80 mg/dL), the insulin doses were downtitrated 4 units/day (IGlar U100) or 28 units/week (icodec LD and icodec NLD).
Background oral glucose-lowering drugs were continued unchanged throughout the trial unless there were safety concerns in the opinion of the investigator.
Outcomes
Participants wore a continuous glucose monitoring (CGM) system (Dexcom G6; Dexcom, Inc., San Diego, CA) throughout the screening and treatment periods; sensors were changed weekly. CGM data were blinded for both participants and investigators and were not used for insulin dose titration or reporting of hypoglycemic episodes. Participants were asked to measure SMBG if they experienced symptoms suggestive of hypoglycemia at any time of the day.
The primary end point was percent time in range (TIR; 3.9–10.0 mmol/L [70–180 mg/dL]), measured by CGM, during weeks 15 and 16 of treatment. Supportive secondary efficacy end points were changes in HbA1c, fasting plasma glucose (FPG), and body weight from baseline to week 16 and weekly insulin doses during weeks 15 and 16 of treatment. Safety end points were number of on-treatment adverse events (AEs) from baseline to week 21 and number of self-reported hypoglycemic events documented by SMBG or by requirement for external assistance for recovery. Hypoglycemic events were classified as: level 1 (blood glucose <3.9 mmol/L and ≥3.0 mmol/L [<70 mg/dL and ≥54 mg/dL]); a combination of level 2 (blood glucose <3.0 mmol/L [<54 mg/dL]) and level 3 (hypoglycemia with severe cognitive impairment requiring external assistance for recovery); or level 3 alone (15). AEs of special interest were major cardiovascular AEs, hypersensitivity and injection-site reactions, and death; these were reviewed by a blinded independent adjudication committee.
Statistical Analysis
The sample size was chosen as the number of patients required for the 95% CI for TIR 3.9–10.0 mmol/L (70–80 mg/dL) of any pairwise comparison having a width of 2.5 h/24 h (corresponding to a difference of 10%-points), with a probability of 80%, based on an assumed SD of 3.0 h/24 h (corresponding to 12%); based on this, 50 participants per treatment arm were required. This trial was exploratory in nature and intended to inform the design of the phase 3 program; therefore, the power was not calculated.
TIR during weeks 15 and 16 for each individual was calculated as the number of recorded measurements in the range 3.9–10.0 mmol/L (70–180 mg/dL) divided by the total number of recorded measurements over 14 days, multiplied by 100.
The primary estimand (trial product estimand) was defined as the mean difference in the primary end point between each icodec group and IGlar U100 for all randomized participants, if they had adhered to the randomized insulin treatment without initiation of rescue medication (initiation of insulin treatment other than the randomized treatment) and had recorded ≥70% of the planned CGM measurements during weeks 15 and 16. A more detailed explanation is provided in the Supplementary Material. For TIR, the response throughout the last 2 weeks of treatment (weeks 15 and 16) was analyzed using an ANCOVA model with randomized treatment, pretrial insulin treatment, and SGLT2i use as fixed factors and baseline TIR as covariate. Missing end point values were imputed by multiple imputation using data from participants in the same randomized group based on an ANCOVA model with baseline TIR as a covariate. Each imputed data set was analyzed separately, and estimates were then combined using Rubin’s rule (16). Supportive secondary efficacy end points were analyzed in the same way as the primary end point, except for the mean weekly insulin dose during the last 2 weeks of treatment, which was log-transformed and analyzed using an ANOVA model. Missing data for secondary end points were imputed based on data from participants in the same randomized group using a sequential conditional regression approach including all postbaseline values; Markov chain Monte-Carlo methods were used for imputing intermittent missing values. Because this is a phase 2 trial and it is exploratory in nature, no adjustments were made for multiplicity. The number of hypoglycemic events was analyzed using a negative binomial regression model with log link. The model included treatment, pretrial insulin treatment, and SGLT2i use as fixed factors and the logarithm of the time period for which the hypoglycemic episodes were considered as an offset.
The on-treatment period was defined as the period from the date of first trial drug dose until the last follow-up visit or the last dosing day of randomized treatment plus 5 weeks (for IGlar U100) or 6 weeks (for icodec), whichever came first, and represents the time period during which participants are considered to be exposed to the trial product.
The full analysis set consisted of all randomized participants. The safety analysis set consisted of all participants exposed to at least one dose of trial product. Data were analyzed using SAS software 9.4. No data monitoring committee oversaw the trial. This trial was registered on the ClinicalTrials.gov website (NCT03922750).
Data and Resource Availability
The data sets generated during and/or analyzed during the current trial are available from the corresponding author on reasonable request.
Results
Of 222 people screened, 154 individuals were randomized (icodec LD, n = 54; icodec NLD, n = 50; and IGlar U100, n = 50), and 152 completed the 16-week treatment period (Supplementary Fig. 2). All 154 trial participants were included in the efficacy and safety analyses. Eight participants had missing or <70% CGM measurements during the last 2 weeks of treatment (weeks 15 and 16) and required imputation of data for the primary end point: one for icodec LD, five for icodec NLD, and two for IGlar U100. Three participants started insulin aspart as a rescue medication during the trial; two participants (one each for icodec NLD and IGlar U100) started insulin aspart on the same day or after the last dose of trial drug, and one participant (icodec NLD) started insulin aspart on day 30. Separately, one participant (IGlar U100) discontinued sitagliptin/metformin treatment during the trial.
Baseline demographics and clinical characteristics were generally similar among groups (Table 1 and Supplementary Table 2): most participants were male (72.1%); the mean age was 61.7 years; the mean duration of type 2 diabetes was 15.1 years; the mean ± SD HbA1c was 7.9% ± 0.7 (62.8 ± 7.7 mmol/mol); the mean ± SD FPG was 8.0 ± 2.1 mmol/L (144 ± 37 mg/dL); and the majority of participants (57.8%) were taking more than one oral glucose-lowering drug. IGlar U100 was the most used basal insulin at screening (59.7%).
Table 1.
Baseline demographics and clinical characteristics
| Icodec LD (n = 54) | Icodec NLD (n = 50) | IGlar U100 (n = 50) | Total (N = 154) | |
|---|---|---|---|---|
| Age, years | 62.4 ± 7.2 | 62.1 ± 8.2 | 60.5 ± 7.9 | 61.7 ± 7.8 |
| Male, n (%) | 39 (72.2) | 39 (78.0) | 33 (66.0) | 111 (72.1) |
| Duration of type 2 diabetes, years | 13.8 ± 7.7 | 16.8 ± 8.2 | 14.8 ± 8.1 | 15.1 ± 8.1 |
| Ethnicity, n (%) | ||||
| White | 46 (85.2) | 39 (78.0) | 44 (88.0) | 129 (83.8) |
| Asian | 4 (7.4) | 9 (18.0) | 3 (6.0) | 16 (10.4) |
| Black or African American | 3 (5.6) | 2 (4.0) | 3 (6.0) | 8 (5.2) |
| Native Hawaiian or other Pacific Islander | 1 (1.9) | 0 | 0 | 1 (0.6) |
| BMI, kg/m2 | 30.2 ± 4.3 | 29.0 ± 4.1 | 30.3 ± 5.0 | 29.8 ± 4.5 |
| FPG, mmol/L | 7.9 (1.9)* | 8.0 (2.3) | 8.2 (2.0)† | 8.0 (2.1) |
| FPG, mg/dL | 142 ± 34* | 144 ± 41 | 148 ± 36† | 144 ± 37 |
| HbA1c, % | 7.8 (0.7) | 7.9 (0.7) | 7.9 (0.7) | 7.9 (0.7) |
| HbA1c, mmol/mol | 62.0 (7.4) | 63.3 (8.0) | 63.2 (7.8) | 62.8 (7.7) |
| TIR at baseline, % | 58.9 (23.2) | 54.5 (20.2) | 58.7 (21.5) | 57.4 (21.7) |
| Total insulin dose‡ (CV%), units | 22.5 (61.0) | 24.5 (47.7) | 24.0 (49.2) | 23.6 (52.8) |
| Basal insulin at screening, n (%) | ||||
| Insulin degludec | 4 (7.4) | 15 (30.0) | 7 (14.0) | 26 (16.9) |
| Insulin detemir | 9 (16.7) | 3 (6.0) | 1 (2.0) | 13 (8.4) |
| Insulin glargine U100 | 32 (59.3) | 23 (46.0) | 37 (74.0) | 92 (59.7) |
| Insulin glargine U300 | 9 (16.7) | 8 (16.0) | 5 (10.0) | 22 (14.3) |
| NPH (isophane) insulin | 0 | 1 (2.0) | 0 | 1 (0.6) |
Data are mean (SD) unless otherwise indicated. All percentages are subject to rounding. CV%, coefficient of variation.
n = 52.
n = 49.
Geometric mean.
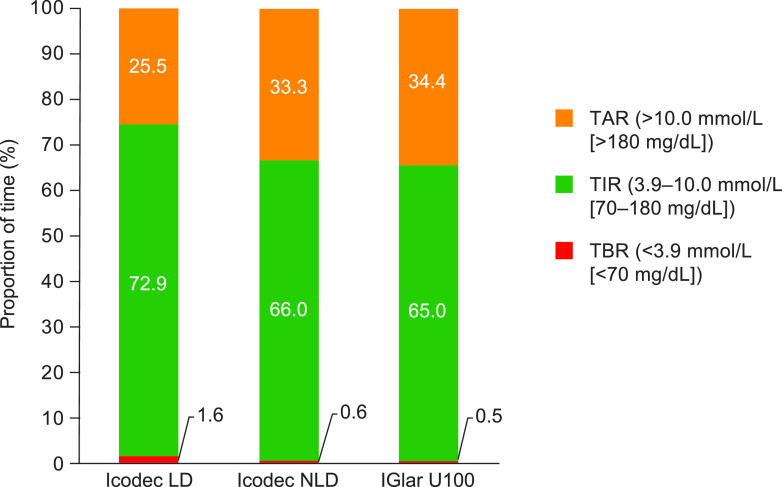
The estimated mean TIR during weeks 15 and 16 was 72.9% for icodec LD, 66.0% for icodec NLD, and 65.0% for IGlar U100, representing estimated changes from baseline in TIR of 15.4%-points, 8.6%-points, and 7.6%-points for icodec LD, icodec NLD, and IGlar U100, respectively. These correspond to estimated changes from baseline in TIR of ∼3 h 42 min/day, 2 h 4 min/day, and 1 h 49 min/day, respectively. The primary end point of TIR was statistically significantly greater for the icodec LD group compared with the IGlar U100 group (estimated treatment difference [ETD], 7.88%-points [95% CI 1.83–13.93]; P = 0.01) and similar between the icodec NLD group and the IGlar U100 group (ETD, 1.01%-points [95% CI –5.33 to 7.5]; P = nonsignificant [NS]) (Fig. 1).
Figure 1.
TIR during the last 2 weeks of the treatment period (full analysis set). TIR was the primary end point. TAR, time above range; TBR, time below range.
HbA1c levels reduced in all treatment groups during the trial (Fig. 2A). At week 16, estimated mean HbA1c levels were 7.1% (54.4 mmol/mol), 7.4% (57.6 mmol/mol), and 7.4% (56.9 mmol/mol) in the icodec LD, icodec NLD, and IGlar U100 groups, respectively. The estimated mean change from baseline at week 16 was –0.8%-points (–8.4 mmol/mol) in the icodec LD group, –0.5%-points (–5.2 mmol/mol) in the icodec NLD group, and –0.5%-points (–5.9 mmol/mol) in the IGlar U100 group, with no statistically significant differences between the icodec groups and IGlar U100 group (ETD, icodec LD vs. IGlar U100, –0.23%-points [95% CI –0.49 to 0.02] [–2.53 mmol/mol (95% CI –5.33 to 0.27)], P = NS; ETD, icodec NLD vs. IGlar U100, 0.07%-points [95% CI –0.19 to 0.33] [0.73 mmol/mol (95% CI –2.11 to 3.57)], P = NS). After 16 weeks of treatment, the proportions of participants achieving a target HbA1c of <7.0% (53 mmol/mol) were 44.2%, 25.0%, and 30.6% for the icodec LD, icodec NLD, and IGlar U100 groups, respectively (no statistical analyses were performed).
Figure 2.
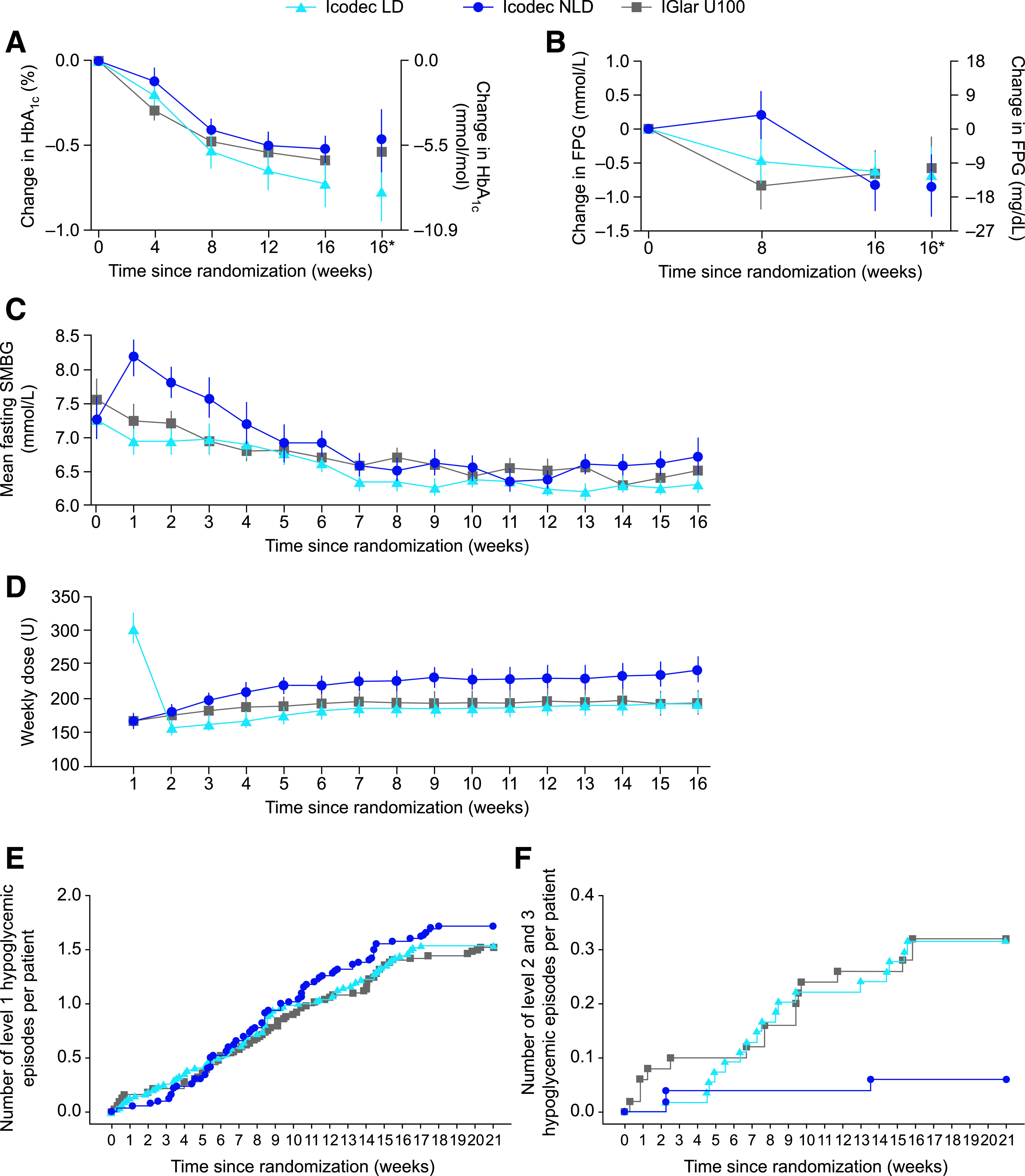
Key parameters during the trial. A: Mean change in HbA1c from baseline to week 16 (FAS). B: Mean change in FPG from baseline to week 16 (FAS). C: Mean prebreakfast SMBG levels over time (FAS). D: Mean weekly insulin dose over time (FAS). E: Cumulative number of level 1 (“alert” value) hypoglycemic episodes per patient (SAS). F: Cumulative number of level 2 (clinically significant) or level 3 (severe) hypoglycemic episodes per patient (SAS). Observed data. A–C: Mean ± SEM. D: Geometric mean ± SEM on log-scale back transformed. For C, SMBG was assessed with a blood glucose meter as plasma equivalent of capillary whole blood glucose. For D, the dose for a given visit represents the total dose during the preceding week, and weekly IGlar U100 doses were derived as seven times the average daily dose during the preceding week. *Estimated mean values and the corresponding CI at week 16 derived based on multiple imputation. FAS, full analysis set; SAS, safety analysis set; U, unit.
Compared with baseline, FPG was lower at week 16 in all treatment groups; estimated mean values at week 16 were 7.3 mmol/L (132 mg/dL), 7.2 mmol/L (129 mg/dL), and 7.4 mmol/L (134 mg/dL) for icodec LD, icodec NLD, and IGlar U100, respectively (Fig. 2B). The estimated mean change from baseline was similar between the icodec LD and IGlar U100 groups (–0.7 mmol/L vs. –0.6 mmol/L: ETD, –0.12 mmol/L [95% CI –0.74 to 0.50], P = NS [–12 mg/dL vs. –10 mg/dL: ETD, –2.20 mg/dL (95% CI –13.33 to 8.94), P = NS]) and also between the icodec NLD and IGlar U100 groups (–0.8 mmol/L vs. –0.6 mmol/L: ETD, –0.26 mmol/L [95% CI –0.90 to 0.38], P = NS [–15 mg/dL vs. –10 mg/dL: ETD, –4.74 mg/dL (95% CI –16.28 to 6.80), P = NS]). At week 8, mean observed FPG values were similar between the icodec LD and IGlar U100 groups (7.4 mmol/L vs. 7.3 mmol/L [133 mg/dL vs. 132 mg/dL]) but slightly higher for the icodec NLD group (8.2 mmol/L [144 mg/dL]). No statistical analyses at week 8 were performed.
For comparison, the weekly changes in fasting (prebreakfast) SMBG levels (measured as part of the titration process and not as an end point) are shown in Fig. 2C. SMBG levels decreased in all treatment groups over the course of the trial; however, in the icodec NLD group, mean SMBG levels were seen to increase slightly versus baseline during the first 3 weeks before reverting to levels similar to those for the icodec LD and IGlar U100 groups at about week 5.
Insulin dose gradually increased during the trial in all groups (Fig. 2D). During the last 2 weeks of treatment (weeks 15 and 16), the estimated mean weekly doses were 191 units (2.18 units/kg), 242 units (2.81 units/kg) and 196 units (2.27 units/kg) for icodec LD, icodec NLD, and IGlar U100, respectively (estimated treatment ratio, icodec LD vs. IGlar U100, 0.98 units [95% CI 0.78–1.23], P = NS [0.96 units/kg (95% CI 0.78–1.19), P = NS]; estimated treatment ratio, icodec NLD vs. IGlar U100, 1.24 units [95% CI 0.98–1.56], P = NS [1.24 units/kg (95% CI 1.00–1.54), P = 0.047]).
The change in body weight during the trial is shown in Supplementary Fig. 3. Estimated mean changes from baseline were 0.6 kg, 1.3 kg, and 0.1 kg in the icodec LD, icodec NLD, and IGlar U100 groups, respectively. Although the mean change in body weight from baseline was not statistically significantly different between the icodec LD and IGlar U100 groups (ETD, 0.51 kg [95% CI –0.44 to 1.47]; P = NS), a statistically significantly greater increase in body weight was seen with icodec NLD compared with IGlar U100 (ETD, 1.22 kg [95% CI 0.24–2.2]; P = 0.01).
Hypoglycemic events over 21 weeks (on-treatment period) are summarized in Table 2. The percentage of participants with one or more level 2 or level 3 hypoglycemic events during this period was 7.4%, 4.0%, and 12.0% in the icodec LD, icodec NLD, and IGlar U100 groups, respectively, with rates per patient-year of exposure of 0.78, 0.15, and 0.79, respectively. No level 3 (severe) hypoglycemic events were reported in the trial. Similar results were observed for the period from baseline to week 16 (data not shown). The rate and pattern of level 1 hypoglycemia (Fig. 2E) over the on-treatment period were similar across all groups. The rate and pattern of combined level 2 and level 3 hypoglycemic episodes (Fig. 2F) were similar between the icodec LD and IGlar U100 groups and appeared to be lower for the icodec NLD group than for the IGlar U100 group. No initial increase in level 1 or combined level 2 and level 3 hypoglycemia was observed in the icodec LD group.
Table 2.
On-treatment hypoglycemic episodes and AEs, including AEs of special interest (SAS; N = 154)
| Icodec LD (n = 54, 21.8 PYE) | Icodec NLD (n = 50, 20.3 PYE) | IGlar U100 (n = 50, 20.2 PYE) | RR (95% CI)* icodec LD/IGlar U100 | RR (95% CI)* icodec NLD/IGlar U100 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Events | Rate* | n (%) | Events | Rate | n (%) | Events | Rate | |||
| Hypoglycemic episodes | |||||||||||
| Hypoglycemia “alert” value (level 1)† | 19 (35.2) | 83 | 3.809 | 26 (52.0) | 87 | 4.29 | 22 (44.0) | 76 | 3.77 | 0.94 (0.44–1.98) | 1.10 (0.51–2.35) |
| Clinically significant (level 2)‡ or severe (level 3) hypoglycemia | 4 (7.4) | 17 | 0.78 | 2 (4.0) | 3 | 0.15 | 6 (12.0) | 16 | 0.79 | 0.87 (0.18–4.24) | 0.39 (0.05–2.80) |
| Severe hypoglycemia (level 3)§ | 0 | 0 | 0 | ||||||||
| AEs | |||||||||||
| All AEs | 28 (51.9) | 85 | 3.90 | 30 (60.0) | 77 | 3.80 | 23 (46.0) | 76 | 3.77 | ||
| Serious AEs | 2 (3.7) | 7‖ | 32.1 | 0 | 1 (2.0) | 1¶ | 0.05 | ||||
| Fatal AEs | 0 | 0 | 0 | ||||||||
| Severe AEs | 1 (1.9) | 1 | 4.6 | 1 (2.0) | 1 | 0.05 | 0 | ||||
| AEs leading to withdrawal | 1 (1.9) | 1 | 4.6 | 0 | 0 | ||||||
| AEs probably related to basal insulin | 1 (1.9) | 2 | 9.2 | 2 (4.0) | 5 | 0.247 | 3 (6.0) | 3 | 0.15 | ||
| AEs possibly related to basal insulin | 0 | 1 (2.0) | 1 | 0.05 | 2 (4.0) | 2 | 0.10 | ||||
| AEs of special interest | |||||||||||
| Injection-site reaction# | 1 (1.9) | 2 | 9.2 | 1 (2.0) | 4 | 0.20 | 2 (4.0) | 3 | 0.15 | ||
| Hypersensitivity event# | 2 (4.0) | 2 | 9.9 | ||||||||
| MACE# | 1 (1.9) | 1‖** | 4.6 | 0 | 1 (2.0) | 1¶** | 0.05 | ||||
The on-treatment period represents the time period during which participants are considered to be exposed to the trial product. MACE, major adverse cardiovascular event; PYE, patient-years of exposure; RR, rate ratio; SAS, safety analysis set.
The number of hypoglycemic events was analyzed post hoc using a negative binomial regression model with log link. The model included treatment, pretrial insulin treatment, and SGLT2i use as fixed factors and the logarithm of the time period for which the hypoglycemic episodes were considered as an offset.
Rate: number of events per patient-year of exposure.
Hypoglycemia “alert” value (level 1): blood glucose value <3.9 mmol/L (<70 mg/dL) and ≥3.0 mmol/L (≥54 mg/dL) confirmed by blood glucose meter.
Clinically significant hypoglycemia (level 2): blood glucose value <3.0 mmol/L (<54 mg/dL).
Severe hypoglycemia (level 3): hypoglycemia with severe cognitive impairment requiring external assistance for recovery. ‖Acute myocardial infarction (confirmed by adjudication), fall, joint injury, and upper limb and two facial bone fractures in one patient; muscle abscess in one patient.
Myocardial infarction (confirmed by adjudication).
Independently adjudicated and confirmed.
Also included as serious AEs.
As summarized in Table 2, the incidence and rates of AEs were similar across treatment groups. Most AEs were mild in severity, and only a small number of AEs in each group were possibly or probably related to treatment, none of which were serious. The most frequent AEs are provided in Supplementary Table 3.
Two participants reported seven serious AEs in the icodec LD group (including one event of acute myocardial infarction), and one participant reported one serious AE (acute myocardial infarction) in the IGlar U100 group (Table 2). Both acute myocardial infarction events were confirmed by adjudication and assessed as unlikely to be related to the trial products. The participant in the icodec LD group who experienced acute myocardial infarction was withdrawn from the trial; no other discontinuations due to AEs occurred. None of the AEs led to a reduction in the dose of trial medication.
No deaths were reported during the trial. Two participants (4.0%) in the icodec NLD group had hypersensitivity reactions confirmed by adjudication, which were reported as not being related to the trial treatment (one participant had a skin rash with irritation attributed to an adhesive bandage and another had a skin rash at the CGM insertion site). All injection-site reactions reported in the trial were mild (Table 2).
Conclusions
The results of the current trial suggest that, in people with type 2 diabetes receiving daily basal insulin therapy, switching to once-weekly icodec is effective and well tolerated. Compared with switching to once-daily basal insulin IGlar U100, switching to once-weekly icodec displayed similar or greater efficacy regarding the primary end point of TIR during weeks 15 and 16. Switching to icodec using an initial 100% LD (doubling of the first dose) resulted in statistically significantly longer TIR than switching to IGlar U100. Furthermore, doubling the initial dose did not increase the risk of hypoglycemia at any point during the trial period and prevented the mild, transient prebreakfast SMBG elevation observed in those randomized to icodec without a loading dose during the immediate postswitch period; that could lead clinicians or patients to inadvertently perceive that the once-weekly insulin may not be as effective as the previous basal insulin.
Notably, a greater change from baseline in TIR (15.4%-points, corresponding to ∼3 h 42 min/day) was observed during weeks 15 and 16 in the icodec LD group (vs. 8.6%-points [2 h 4 min/day] in the icodec NLD group and 7.6%-points [1 h 49 min/day] in the IGlar U100 group); this is an important observation given that a 5% increase in TIR is considered a clinically significant improvement in glycemic control (17) and that TIR improvement has been associated with clinically significant benefits for people with type 2 diabetes (17–22). Moreover, it is important to note that a mean TIR of >70%, a target recommended by the International Consensus on Time in Range (17), was achieved in the icodec LD group during the last 2 weeks of treatment. Notably, despite the short trial duration limiting the time patients had to reach target, the HbA1c reductions and proportion of participants who achieved HbA1c <7.0% (<53.0 mmol/mol) with icodec are consistent with the observed increase in percent TIR. There was a statistically significant but small increase in body weight at the end of the trial in the icodec NLD group compared with the IGlar U100 group; this may reflect the numerically higher insulin dose requirement in the former.
In this trial, administration of a loading dose of icodec when switching from daily basal insulin seemed to have prevented the mild and transient elevation of prebreakfast SMBG levels seen in the icodec NLD group. This may be explained by the molecular characteristics and pharmacokinetic and pharmacodynamic properties of icodec. While the long half-life of icodec makes it suitable for once-weekly dosing, it also extends the time to steady state (by ∼3–4 weeks). This could consequently produce an initial mild deterioration in fasting glucose levels in those switching from a prior basal insulin therapy who will have an initial treatment deficit. Pharmacokinetic and pharmacodynamic modeling indicated that using a loading dose of icodec could offset this by ensuring the availability of an adequate albumin-bound inactive reservoir; slow release of icodec molecules would provide effective glucose lowering without an increased risk of hypoglycemia versus once-daily basal insulin. This hypothesis is supported by the results of the current trial, because the rate and pattern of hypoglycemic episodes, irrespective of level, were similar between the icodec LD and IGlar U100 groups both immediately postswitch and throughout the treatment period.
The results from this trial suggest that switching from an existing basal insulin to icodec, with or without a loading dose, provides effective glycemic control with comparable risk of hypoglycemia. It was reassuring that not a single episode of severe hypoglycemia (level 3) was reported for any treatment group throughout the trial duration and that the time spent below range (<3.9 mmol/L [<54 mg/dL]) during weeks 15 and 16 was well below the 4% target recommended by the International Consensus on Time in Range across all treatment groups.
Results for the other safety parameters measured also show that icodec LD was well tolerated. Most AEs across treatment groups were mild in severity, and most (including the small number of serious AEs) were assessed to be unrelated to treatment. None of the AEs required a reduction in the dose of trial medication, and there were few AEs of special interest.
The strengths of this trial include its randomized, multicenter design and the low treatment discontinuation rate. Additionally, this trial used CGM and measured TIR as a primary end point in a patient population with type 2 diabetes, which is quite unique for comparative insulin studies. TIR is a clinically recognized parameter, and internationally accepted TIR targets now exist to guide diabetes management (15,17,19). Limitations of the trial include its relatively short duration and the modest sample size. In addition, the trial used an open-label design; however, the CGM recordings used to analyze the primary end point of TIR were blinded to both the investigator and trial participant. The observed differences in TIR at weeks 15 and 16 could be due to minor differences in patient characteristics between treatment groups and the low patient numbers concerned. Although individuals taking metformin and/or dipeptidyl peptidase 4 inhibitors and/or SGLT2is were eligible to participate, those who were taking other glucose-lowering agents and patients with certain comorbidities were precluded from participation, limiting the generalizability of the results.
The potential to administer basal insulin once weekly, thereby reducing the number of injections, has important implications for diabetes management. Research shows that people with diabetes would welcome such a reduction to improve convenience and quality of life; consequently, it may help to improve adherence and overall glycemic control further (11). In a study by Peyrot et al. (6), 27.6% of people with diabetes (n = 1,530) described “taking insulin at prescribed time or with meals every day” as difficult, and 92.5% of them expressed a wish for good control with “insulin [that did] not [need to be] injected every day.” Data from studies evaluating glucagon-like peptide 1 receptor agonists demonstrate that once-weekly administration of these agents is associated with improved treatment satisfaction and medication-taking behavior (23–25). In type 2 diabetes, medication adherence and timely insulin initiation are critical for maintaining glycemic control and reducing diabetes-related complications and associated health care resource utilization (26–30).
Overall, the safety and glycemic efficacy of icodec seen in this first phase 2 trial with icodec in basal insulin–treated participants reflect the findings of two completed treat-to-target trials with icodec in insulin-naive people with type 2 diabetes (ClinicalTrials.gov identifiers NCT03751657 and NCT03951805) (14). Taken together, these phase 2 trial results suggest effective glycemic control and comparable safety of icodec versus IGlar U100 in a broad patient population with type 2 diabetes, with the major clinical value of reducing the number of weekly basal insulin injections from seven to only one.
In conclusion, in a patient population with type 2 diabetes receiving daily basal insulin therapy, the use of a loading dose when switching to once-weekly icodec resulted in effective glycemic control without a transient elevation of fasting glucose levels during the switch and without increasing the risk of clinically relevant hypoglycemia compared with IGlar U100. These results will be considered when switching to once-weekly insulin icodec in the phase 3 clinical development program.
Article Information
Acknowledgements. The authors thank Dr. Gemma Rogers of Oxford PharmaGenesis (Oxford, U.K.) (supported by Novo Nordisk) for providing writing assistance.
Duality of Interest. H.S.B. has received speaking fees from AstraZeneca, Eli Lilly and Company, Janssen Pharmaceuticals, Merck, and Novo Nordisk and research funding paid to LMC Healthcare for serving as principal investigator on clinical trials from Amgen, AstraZeneca, Boehringer Ingelheim, Ceapro Inc., Eli Lilly and Company, Gilead Sciences, Inc., Janssen Pharmaceuticals, Kowa Pharmaceuticals Co. Ltd., Madrigal Pharmaceuticals, Merck, Novo Nordisk, Sanofi, and Tricida, Inc. R.M.B. has received research support from, consulted for, or has been on a scientific advisory board for Abbott Diabetes Care, Ascensia, Dexcom, Inc., Eli Lilly and Company, Hygieia Biological Laboratories, Johnson & Johnson, Medtronic, Novo Nordisk, Onduo, Roche, Sanofi, and UnitedHealthcare (R.M.B.’s employer, nonprofit HealthPartners Institute, contracts for his services, and no personal income goes to him). M.J.D. has acted as a consultant, advisory board member, and speaker for AstraZeneca, Boehringer Ingelheim, Janssen Pharmaceuticals, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; served as an advisory board member for Gilead Sciences, Inc. and Servier; served as a speaker for Mitsubishi Tanabe Pharma Corporation, Napp Pharmaceuticals, and Takeda Pharmaceuticals International Inc.; and has received grants in support of investigator and investigator-initiated trials from AstraZeneca, Boehringer Ingelheim, Janssen Pharmaceuticals, Eli Lilly and Company, Novo Nordisk, and Sanofi. I.L. received research funding, advisory/consulting fees, and/or other support from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GI Dynamics, Intarcia Therapeutics, Intercept Pharmaceuticals, Janssen Pharmaceuticals, MannKind Corporation, Merck, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi, Target PharmaSolutions, Inc., Valeritas, Inc., and Zealand Pharma. P.A.S. received research support to his institution from Allergan, Novo Nordisk, and ViaCyte; as chair of Diabetes Canada Clinical Practice Guidelines, he does not provide consulting or speaking services to, and receives no personal fees from, industry partners. R.J.S. serves on speakers bureaus for AstraZeneca and Novo Nordisk and serves on advisory panels for Novo Nordisk. R.T. received lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Novo Nordisk, and Sanofi and has participated in advisory panels for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Novo Nordisk, and Sanofi. J.R. has participated in advisory panels for Applied Therapeutics, Boehringer Ingelheim, Eli Lilly and Company, Intarcia Therapeutics, Janssen Pharmaceuticals, Novartis, Novo Nordisk, Oramed Pharmaceuticals, and Sanofi and has received research support from Eli Lilly and Company, Genentech, GlaxoSmithKline, Intarcia Therapeutics, Janssen Pharmaceuticals, Merck & Co., Novo Nordisk, Oramed Pharmaceuticals, Pfizer, and Sanofi. A.C., A.G., and J.I. are employees of Novo Nordisk. A.C. and A.G. hold share options and/or shares of Novo Nordisk. Novo Nordisk funded the trial and was responsible for trial design and data analysis. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors had full access to all data, were responsible for data interpretation and manuscript preparation, and had final responsibility for the decision to submit for publication. H.S.B. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in e-poster form at the 56th Annual Meeting of the European Association for the Study of Diabetes, 21–25 September 2020.
Footnotes
Clinical trial reg. no. NCT03922750, clinicaltrials.gov
See accompanying articles, pp. 1459 and 1595.
This article contains supplementary material online at https://doi.org/10.2337/figshare.14043746.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2020 [published correction appears in Diabetes Care 2020;43:1979]. Diabetes Care 2020;43(Suppl. 1):S98–S110 [DOI] [PubMed] [Google Scholar]
- 2. Nyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism 2011;60:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin 2011;27(Suppl. 3):13–20 [DOI] [PubMed] [Google Scholar]
- 4. Escalada J, Orozco-Beltran D, Morillas C, et al. Attitudes towards insulin initiation in type 2 diabetes patients among healthcare providers: a survey research. Diabetes Res Clin Pract 2016;122:46–53 [DOI] [PubMed] [Google Scholar]
- 5. Karter AJ, Subramanian U, Saha C, et al. Barriers to insulin initiation: the translating research into action for diabetes insulin starts project. Diabetes Care 2010;33:733–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med 2012;29:682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okemah J, Peng J, Quiñones M. Addressing clinical inertia in type 2 diabetes mellitus: a review. Adv Ther 2018;35:1735–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donnelly LA, Morris AD; DARTS/MEMO collaboration . Adherence to insulin and its association with glycaemic control in patients with type 2 diabetes. QJM 2007;100:345–350 [DOI] [PubMed] [Google Scholar]
- 9. Cheng AYY, Patel DK, Reid TS, Wyne K. Differentiating basal insulin preparations: understanding how they work explains why they are different. Adv Ther 2019;36:1018–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hernando VU, Pablo FJ. Efficacy and safety of the second generation basal insulin analogs in type 2 diabetes mellitus: a critical appraisal. Diabetes Metab Syndr 2019;13:2126–2141 [DOI] [PubMed] [Google Scholar]
- 11. Polonsky WH, Fisher L, Hessler D, Bruhn D, Best JH. Patient perspectives on once-weekly medications for diabetes. Diabetes Obes Metab 2011;13:144–149 [DOI] [PubMed] [Google Scholar]
- 12. Hövelmann U, Brønsted L, Kristensen NR, et al. Insulin icodec, an insulin analog suited for once-weekly dosing in type 2 diabetes (Abstract). Diabetes 2020;69(Suppl. 1):237-OR [Google Scholar]
- 13. Nishimura E, Kjeldsen T, Hubalek F, et al. Molecular and biological properties of insulin icodec, a new insulin analog designed to give a long half-life suitable for once-weekly dosing (Abstract). Diabetes 2020;69(Suppl. 1):236-OR [Google Scholar]
- 14. Rosenstock J, Bajaj HS, Janež A, et al.; NN1436-4383 Investigators . Once-weekly insulin for type 2 diabetes without previous insulin treatment. N Engl J Med 2020;383:2107–2116 [DOI] [PubMed] [Google Scholar]
- 15. American Diabetes Association . 6. Glycemic targets: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S61–S70 [DOI] [PubMed] [Google Scholar]
- 16. Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York, John Wiley & Sons, 1987 [Google Scholar]
- 17. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol 2019;13:614–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther 2020;22:72–78 [DOI] [PubMed] [Google Scholar]
- 21. Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018;41:2370–2376 [DOI] [PubMed] [Google Scholar]
- 22. Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21:81–85 [DOI] [PubMed] [Google Scholar]
- 23. Drucker DJ, Buse JB, Taylor K, et al.; DURATION-1 Study Group . Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 [DOI] [PubMed] [Google Scholar]
- 24. Nguyen H, Dufour R, Caldwell-Tarr A. Glucagon-like peptide-1 receptor agonist (GLP-1RA) therapy adherence for patients with type 2 diabetes in a medicare population. Adv Ther 2017;34:658–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takase T, Nakamura A, Yamamoto C, et al. Improvement in treatment satisfaction after switching from liraglutide to dulaglutide in patients with type 2 diabetes: a randomized controlled trial. J Diabetes Investig 2019;10:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Banerji MA, Dunn JD. Impact of glycemic control on healthcare resource utilization and costs of type 2 diabetes: current and future pharmacologic approaches to improving outcomes. Am Health Drug Benefits 2013;6:382–392 [PMC free article] [PubMed] [Google Scholar]
- 27. Boye KS, Curtis SE, Lage MJ, Garcia-Perez LE. Associations between adherence and outcomes among older, type 2 diabetes patients: evidence from a Medicare Supplemental database. Patient Prefer Adherence 2016;10:1573–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther 2013;4:175–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes 2017;11:3–12 [DOI] [PubMed] [Google Scholar]
- 30. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]