Abstract
OBJECTIVE
Environmental microbial exposures have been implicated to protect against immune-mediated diseases such as type 1 diabetes. Our objective was to study the association of land cover around the early-life dwelling with the development of islet autoimmunity and type 1 diabetes to evaluate the role of environmental microbial biodiversity in the pathogenesis.
RESEARCH DESIGN AND METHODS
Association between land cover types and the future risk of type 1 diabetes was studied by analyzing land cover types classified according to Coordination of Information on the Environment (CORINE) 2012 and 2000 data around the dwelling during the first year of life for 10,681 children genotyped for disease-associated HLA-DQ alleles and monitored from birth in the Type 1 Diabetes Prediction and Prevention (DIPP) study. Land cover was compared between children who developed type 1 diabetes (n = 271) or multiple diabetes-associated islet autoantibodies (n = 384) and children without diabetes who are negative for diabetes autoantibodies.
RESULTS
Agricultural land cover around the home was inversely associated with diabetes risk (odds ratio 0.37, 95% CI 0.16–0.87, P = 0.02 within a distance of 1,500 m). The association was observed among children with the high-risk HLA genotype and among those living in the southernmost study region. Snow cover on the ground seemed to block the transfer of the microbial community indoors, leading to reduced bacterial richness and diversity indoors, which might explain the regional difference in the association. In survival models, an agricultural environment was associated with a decreased risk of multiple islet autoantibodies (hazard ratio [HR] 1.60, P = 0.008) and a decreased risk of progression from single to multiple autoantibody positivity (HR 2.07, P = 0.001) compared with an urban environment known to have lower environmental microbial diversity.
CONCLUSIONS
The study suggests that exposure to an agricultural environment (comprising nonirrigated arable land, fruit trees and berry plantations, pastures, natural pastures, land principally occupied by agriculture with significant areas of natural vegetation, and agroforestry areas) early in life is inversely associated with the risk of type 1 diabetes. This association may be mediated by early exposure to environmental microbial diversity.
Introduction
Type 1 diabetes is considered a chronic autoimmune disease caused by the destruction of the insulin-producing β-cells in the pancreatic islets leading to a life-long need for insulin replacement therapy. Autoantibodies against β-cell proteins are found in the peripheral circulation months to years before the symptomatic disease appears, serving as markers of the ongoing autoimmune process and predicting the onset of the disease. Genetic and environmental factors both contribute to the pathogenesis of type 1 diabetes (1).
The incidence of type 1 diabetes has increased during the past 70 years in the developed countries paralleling similar increase in other immune-mediated diseases such as allergies and asthma (2,3). The rapid increase, together with the conspicuous variation in incidence rates between countries, supports the role of environmental factors in the pathogenesis. Overall, the incidence rate tends to be high in countries located in the north, although exceptions to this trend exist (4).
Living in an agricultural environment and contacts with farm animals and pets at home has been associated with a higher microbial diversity indoors and a decreased risk of allergic diseases (5–8). Although the mechanisms of this phenomenon are not fully understood, several lines of evidence suggest that exposure to environmental microbial diversity and direct soil contacts may play a role (9–11). This, in turn, could lead to the activation of immunoregulatory pathways suppressing overreactive immune responses, as presented by the biodiversity hypothesis (6,9). A wide exposure of the skin and mucosal surfaces to all kinds of microbes, including bacteria, viruses, and eukaryotes, regardless of whether they are infecting or colonizing humans, could provide constant immunological stimulation to the immune system, which is needed for the development of healthy immune regulation (12).
As with allergic diseases, type 1 diabetes is also associated with failure to control hyperreactive immune responses. In type 1 diabetes, these immune responses target β-cell autoantigens instead of allergens. Analogously, it could be hypothesized that an early exposure to a rich environmental microbiome could reduce the disease risk. In support of this, many studies imply that microbial exposure might influence the pathogenesis of type 1 diabetes. Microbial exposures prevent the development of autoimmune diabetes in the NOD mouse model (13,14), and exposure to an indoor dog or pets during the first year of life is inversely associated with the development of type 1 diabetes and islet autoantibodies in children (15,16). Moreover, the rates of both type 1 diabetes and IgE-mediated sensitization are several folds higher in Finland than in the neighboring Karelian Republic of Russia, where children are exposed to microbes substantially more frequently (17,18), and alterations in the intestinal microbiome of young children who later develop type 1 diabetes have been reported (19,20).
The association of an urban and rural living environment with type 1 diabetes has previously been addressed with conflicting results (21–24). However, these studies have defined the environment mainly by indirect factors, such as population density, and the spatial resolution of the data have been relatively low (e.g., municipality-level data), thus dismissing the effect of the immediate environment surrounding the home and exposure to environmental microbial biodiversity. Moreover, most of the studies have been performed after the diagnosis of diabetes without regarding early-life exposures, which are considered important for the development of the immune system and the succession of microbiota (25,26). The β-cell–damaging process has often already started during the first 3 years of life, emphasizing the importance of early-life exposures in type 1 diabetes (27,28).
The effect of the living environment on the disease risk could be mediated by environmental microbes that are mainly limited to the microbes transferred indoors during early life. Although there are indications of seasonal variation in this transfer (7,8), the effect of snow cover on the transferred microbiota is poorly understood. Consequently, we studied whether snow cover could block the connection between the outdoor environment and indoor microbiota and thus act as a potential inducer of regional differences in the connection between the living environment and type 1 diabetes risk.
This is the first prospective study performed in a large cohort of children monitored from birth to analyze the association between early-life exposure to environmental microbial diversity and the development of childhood type 1 diabetes. The study focuses on biotypes that surround the dwelling during the first year of life and are able to influence the microbial exposure of these infants (7,8). The impact of the biotypes on the risk of aggressive β-cell autoimmunity and the progression of the β-cell–damaging process was evaluated. Meteorological data and microbial analyses of doormat samples were used to analyze the effect of snow cover on the transfer of the outdoor microbial community indoors as a possible cause of regional variation in the observed associations.
Research Design and Methods
Study Design
The study subjects were participants in the Finnish Type 1 Diabetes Prediction and Prevention (DIPP) study. Families with children born in three university hospitals (Oulu, Tampere, and Turku) and confirmed to carry increased genetic risk for type 1 diabetes based on HLA-DQ typing from cord blood were invited to participate in prospective follow-up starting from birth (ClinicalTrials.gov NCT03269084) (29). Blood samples were drawn at the ages of 3, 6, 12, 18, and 24 months and once (Oulu and Tampere) or twice (Turku) a year thereafter until the age of 15 years or the diagnosis of type 1 diabetes. Samples were screened for three disease-associated biochemical autoantibodies to insulin (IAA), GAD antibody (GADA), and the tyrosine phosphatase-related insulinoma-associated 2 molecule (IA-2A), as well as nonbiochemical islet cell antibodies (ICA), as described earlier (30). In children born before 2003, ICA was used as a primary screening marker. If the child turned ICA positive, biochemical autoantibodies (IAA, GADA, and IA-2A) were analyzed from all available samples from that child. Clinical type 1 diabetes was diagnosed using the World Health Organization criteria.
The guardians of all participating children gave written informed consent for genetic screening and the follow-up. The study adhered to the principles of the Declaration of Helsinki and was approved by the Ethical Committees of the Pirkanmaa Hospital District (Tampere), Northern Ostrobothnia Hospital District (Oulu), and the Hospital District of Southwest Finland (Turku). Data for dwelling coordinates were acquired from the Finnish Population Register Centre.
The children for the current study were selected from the entire DIPP cohort using the criteria of living at the same address (their dwelling coordinates did not change) from birth until the age of 12 months and being born during the years 1994 to 2013. The average follow-up time was 6.6 years (median 5.9, range 0.1–18.7 years). The details of the DIPP cohort follow-up and the drop-up rates have been described previously (31), showing that altogether, 56% of the children who started the follow-up completed the entire 15-year follow-up, and 3.5% progressed to type 1 diabetes at the time their follow-up ended (89% were in the follow-up at the age of 5 years and 71% at the age of 10 years).
Because the study aimed at analyzing the association of environmental microbial diversity with the first signs of aggressive islet autoimmunity that portends type 1 diabetes, the development of multiple biochemical diabetes-associated autoantibodies was used as the case child criterion (positivity for a single autoantibody is a poor predictor of diabetes) (32), and an additional end point was clinical type 1 diabetes. The current study cohort included 10,681 children monitored from birth and born in the university hospitals of Oulu (30.9%), Tampere (34.8%), and Turku (34.2%) in Finland. The cohort comprised 384 children who turned positive for multiple biochemical islet autoantibodies, 271 children who developed type 1 diabetes (of whom 253 were multiple autoantibody positive), and 10,279 autoantibody-negative control children. Among the multiple-autoantibody-positive children, 243 children turned initially positive only for a single autoantibody (131 for IAA, 96 for GADA, and 16 for IA-2A). The average age of appearance of the first autoantibody was 2.9 years (median 2.0, range 0.4–14.0 years) and that of multiple autoantibodies 3.9 years (median 2.9, range 0.26–15.0 years). There were 141 children who already had multiple autoantibody positivity in the first autoantibody-positive sample. The time of the first detection of autoantibodies (single or multiple) among these multiple-autoantibody-positive children was used as the time of auto-antibody seroconversion in the analyses. The average age of developing type 1 diabetes was 6.8 years (median 6.0, range 0.9–18.7 years).
Land Cover Data
The proportion of agricultural areas, built environment, forests and seminatural areas, wetlands, and water bodies surrounding the dwellings were analyzed within 50 m, 100 m, 250 m, 500 m, and 1,500 m of the dwelling coordinates using the preclassified Coordination of Information on the Environment (CORINE) Land Cover 2012 20-m raster data. The analysis was repeated using CORINE Land Cover 2000 data from the year 2000 to confirm the results and to eliminate the possible effect of changing land cover patterns over the years 1994–2014 (33). CORINE land cover data are produced by national institutes using the criteria determined by the European Environment Agency. The data are produced by automated visual interpretation of high-resolution satellite imagery complemented with national in situ data, satellite image processing, and geographic information system integration and generalization, together with European Union-wide standardized land cover classifications (33,34). The CORINE 2012 land cover classification is presented in Supplementary Table 1.
Meteorological Data
The meteorological data were acquired from the Finnish Meteorological Institute (35). The annual number of days with snow cover was calculated using daily snow depth data collected from the airport of each study site for regional comparisons. For doormat sample comparisons of the daily snow depth, data were obtained from the meteorological station closest to the dwelling (maximum distance was 33 km) to confirm that the ground was covered by snow in the area during the February doormat sample collection.
Microbial Analyses
The effect of snow cover on the ground on the environmental microbial exposure transferred indoors was analyzed using doormat samples. Environmental microbial communities transferred indoors were characterized by analyzing samples collected from standardized polythene doormats kept inside and adjacent to the main door for 2 weeks in August 2015 and again in February 2016. The guardians of 22 study participants collected the debris deposited on the doormats, as previously described (7). The February samples were collected after a minimum of 1 month of continuous snow cover on the ground confirmed by using local meteorological data. DNA was extracted and sequenced using the Illumina 16S sequencing platform, and sequences were processed and analyzed as previously described (7) using mothur (version 1.39.5) (36,37). The samples were rarified to 6,359 sequences for comparison of abundance, species richness (number of species), and bacterial diversity (Shannon diversity index considering both the number of species and the inequality between species abundances) from an even sampling depth. Raw sequence reads are available in the Sequence Read Archive at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/sra) under accession numbers SAMN13087634–SAMN13087655.
Statistical Analyses
The association of type 1 diabetes with the proportion of land cover types was determined using generalized linear models with binomially distributed errors (logistic regression) in R software (38). The Kruskal-Wallis test was used to analyze differences in the annual duration of snow cover between the study regions. Doormat diversity measures were calculated using package vegan and compared between the seasons using the paired Student t test in R. These P values were corrected using the Bonferroni correction method. The differences in the bacterial abundances between February and August doormat samples were determined using the Kruskal-Wallis test, and the P values were corrected for multiple comparisons using the Benjamini-Hochberg method in R. A Cox proportional hazards regression model was computed using package survival in R.
Results
Agricultural Land Cover Is Inversely Associated With the Risk of Type 1 Diabetes, and Built Environment Is Associated With the Risk of Islet Autoimmunity
Living in an agricultural environment during the first year of life was inversely associated with the risk of type 1 diabetes. The inverse association was seen with the 1,500-m radius around the dwelling using the year 2012 land cover data set (odds ratio [OR] 0.37, 95% CI 0.16–0.87, P = 0.02) and a similar but nonsignificant trend was seen when the analysis was repeated with smaller radii (Table 1). Similar results were obtained when the analysis was performed with an earlier version of the land cover data produced in 2000 showing significant associations also when radii shorter than 1,500 m were used (Table 1). On the other hand, a higher coverage of built environment within a 1,500-m radius around the home was associated with an increased risk of islet autoimmunity (OR 1.69, 95% CI 1.03–2.79, P = 0.04), with a similar trend when smaller radii were analyzed (Table 1). Again, this association was significant also with shorter radii when CORINE 2000 data were used in the analyses (Table 1). There were no such associations with the other land cover classes.
Table 1.
The association of type 1 diabetes and islet autoantibody positivity with agricultural and built environment land cover around the home during the first year of life
| Land cover class | Area radius* | Multiple autoantibody positivity | Type 1 diabetes | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| CORINE 2012 data | ||||||
| Agriculture | ||||||
| 50 m | 0.17 (0.03–1.13) | 0.07 | 0.16 (0.02–1.52) | 0.11 | ||
| 100 m | 0.64 (0.28–1.44) | 0.28 | 0.49 (0.18–1.37) | 0.18 | ||
| 250 m | 0.64 (0.34–1.23) | 0.18 | 0.46 (0.20–1.04) | 0.06 | ||
| 500 m | 0.68 (0.36–1.27) | 0.23 | 0.51 (0.24–1.12) | 0.09 | ||
| 1,500 m | 0.54 (0.27–1.06) | 0.08 | 0.37 (0.16–0.87) | 0.02 | ||
| Built environment | ||||||
| 50 m | 1.21 (0.63–2.30) | 0.57 | 1.04 (0.49–2.22) | 0.91 | ||
| 100 m | 1.15 (0.75–1.77) | 0.53 | 1.06 (0.64–1.76) | 0.83 | ||
| 250 m | 1.30 (0.85–1.98) | 0.22 | 1.18 (0.72–1.94) | 0.52 | ||
| 500 m | 1.32 (0.85–2.04) | 0.22 | 1.30 (0.77–2.18) | 0.33 | ||
| 1,500 m | 1.69 (1.03–2.79) | 0.04 | 1.66 (0.92–3.02) | 0.09 | ||
| CORINE 2000 data | ||||||
| Agriculture | ||||||
| 50 m | 0.37 (0.12–1.10) | 0.07 | 0.39 (0.11–1.39) | 0.15 | ||
| 100 m | 0.44 (0.21–0.94) | 0.03 | 0.39 (0.16–0.98) | 0.045 | ||
| 250 m | 0.51 (0.28–0.95) | 0.03 | 0.43 (0.20–0.91) | 0.03 | ||
| 500 m | 0.56 (0.31–1.02) | 0.06 | 0.50 (0.24–1.04) | 0.06 | ||
| 1,500 m | 0.56 (0.29–1.08) | 0.08 | 0.44 (0.20–0.99) | 0.046 | ||
| Built environment | ||||||
| 50 m | 2.00 (1.17–3.41) | 0.01 | 1.52 (0.85–2.72) | 0.16 | ||
| 100 m | 1.55 (1.01–2.37) | 0.045 | 1.43 (0.87–2.36) | 0.16 | ||
| 250 m | 1.29 (0.88–1.90) | 0.19 | 1.20 (0.77–1.89) | 0.42 | ||
| 500 m | 1.37 (0.92–2.03) | 0.12 | 1.33 (0.83–2.12) | 0.24 | ||
| 1,500 m | 1.67 (1.07–2.62) | 0.02 | 1.61 (0.95–2.74) | 0.08 | ||
The bold data are statistically significant (P < 0.05). The proportion of agricultural area and built area around the dwelling was analyzed as a predictor of autoantibody positivity and type 1 diabetes using land cover data from the year 2012 and 2000 (CORINE 2012 and CORINE 2000 data, respectively). Children who developed multiple biochemical islet autoantibodies (n = 384) and type 1 diabetes (n = 271) were compared with autoantibody-negative children using logistic regression (n = 10,279).
Radius of the analyzed area around the home.
The 2000 and 2012 land cover data sets were both compatible with the range of birth years of these children, but the resolution of the data produced in 2012 was higher. Therefore, further analyses were performed using the 2012 data set. These analyses showed that the trend was also seen in both sexes and in the two endotypes of type 1 diabetes characterized by the appearance of IAA or GADA as the first-appearing single autoantibody (27,28) (Supplementary Tables 2 and 3). The statistical model was also adjusted for the duration of exclusive and total breastfeeding (data were available for 5,743 and 7,635 children, respectively), but neither of these influenced the original associations.
The Association Between the Living Environment and Type 1 Diabetes Is Modified by HLA-Dependent Genetic Risk of Type 1 Diabetes
To evaluate whether the association of living environment with type 1 diabetes is modulated by disease-associated HLA-DQ genes, we categorized children into two groups carrying the high-risk genotype (HLA-DQB1*02/*03:02) or the moderate-risk genotypes (HLA-DQB1 03:02/x; x ≠ DQB1*02, *03:01, *0602). A small proportion of the children carried low-risk (HLA-DQB1 *02/x DQA1*05; x ≠ DQB1*0301, *0602, *06:03), neutral, or protective genotypes, and they were not included in this analysis (Fig. 1). Agricultural land cover within a distance of 1,500 m around the home was inversely associated with clinical disease and islet autoantibodies in children with the high-risk genotype, whereas no significant association was seen in those carrying moderate-risk genotypes. A significant risk association was also seen between built environment and clinical disease as well as islet autoantibodies among children carrying the high-risk HLA genotype (Table 2).
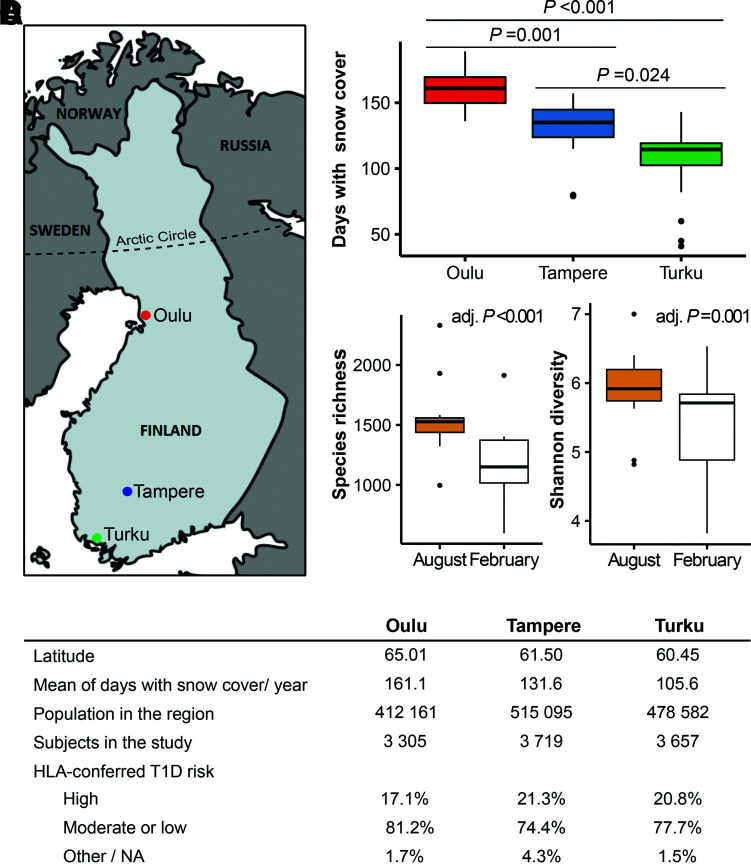
Figure 1.
The study regions differ in the annual number of days with snow cover on the ground, and the snow cover reduces the microbial diversity transferred indoors. A: Subjects included in the study were born in university hospitals in three cities in Finland (Oulu, Tampere, and Turku). B: Annual number of days with snow cover on the ground in the study regions during the study period (1994–2014). Statistical differences were analyzed using the pairwise Wilcoxon rank sum test with Bonferroni correction for multiple comparisons. C: Comparison of α diversity measures between doormat samples collected after at least 1 month of snow cover on the ground (February) and a snowless period (August) from the dwelling of 22 DIPP families. The statistical difference for several diversity measures was analyzed using the paired Student t test, and the P values were adjusted with Bonferroni correction for multiple comparisons. Adjusted P values (adj. P) are indicated in the graphs. Box-and-whisker plots show a horizontal line indicating median value, a box representing the interquartile range, and whiskers showing the 95% confidence interval. D: Demographic data of the study sites. NA, not available; T1D, type 1 diabetes.
Table 2.
Associations of agricultural and built environment with the development of type 1 diabetes and islet autoantibodies in children at genetic risk for type 1 diabetes
| Land cover class | Area radius* | Multiple autoantibody positivity | Type 1 diabetes | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| High HLA-conferred risk† | ||||||
| Agriculture | ||||||
| 50 m | 0.08 (0.00–3.31) | 0.18 | 0.15 (0.00–7.04) | 0.34 | ||
| 100 m | 0.37 (0.08–1.81) | 0.22 | 0.42 (0.08–2.37) | 0.33 | ||
| 250 m | 0.36 (0.10–1.30) | 0.12 | 0.33 (0.08–1.40) | 0.13 | ||
| 500 m | 0.51 (0.16–1.67) | 0.27 | 0.44 (0.11–1.71) | 0.24 | ||
| 1,500 m | 0.21 (0.05–0.84) | 0.03 | 0.15 (0.03–0.75) | 0.02 | ||
| Built environment | ||||||
| 50 m | 1.14 (0.35–3.68) | 0.83 | 0.72 (0.20–2.52) | 0.60 | ||
| 100 m | 1.21 (0.56–2.63) | 0.63 | 0.83 (0.36–1.93) | 0.67 | ||
| 250 m | 1.55 (0.73–3.31) | 0.26 | 1.15 (0.50–2.62) | 0.74 | ||
| 500 m | 1.89 (0.85–4.18) | 0.12 | 1.74 (0.72–4.20) | 0.22 | ||
| 1,500 m | 3.02 (1.24–7.37) | 0.02 | 3.78 (1.40–10.25) | 0.009 | ||
| Moderate or slightly increased HLA-conferred risk‡ | ||||||
| Agriculture | ||||||
| 50 m | 0.15 (0.01–1.49) | 0.11 | 0.12 (0.01–2.34) | 0.16 | ||
| 100 m | 0.70 (0.26–1.85) | 0.47 | 0.53 (0.15–1.91) | 0.33 | ||
| 250 m | 0.70 (0.32–1.51) | 0.36 | 0.58 (0.21–1.57) | 0.28 | ||
| 500 m | 0.64 (0.30–1.36) | 0.24 | 0.57 (0.22–1.51) | 0.26 | ||
| 1,500 m | 0.58 (0.26–1.30) | 0.18 | 0.53 (0.19–1.49) | 0.23 | ||
| Built environment | ||||||
| 50 m | 1.43 (0.65–3.13) | 0.37 | 1.19 (0.45–3.14) | 0.72 | ||
| 100 m | 1.15 (0.69–1.94) | 0.59 | 1.18 (0.61–2.27) | 0.63 | ||
| 250 m | 1.20 (0.73–1.99) | 0.48 | 1.10 (0.58–2.06) | 0.77 | ||
| 500 m | 1.16 (0.68–1.96) | 0.59 | 1.03 (0.53–1.99) | 0.93 | ||
| 1,500 m | 1.25 (0.68–2.28) | 0.47 | 1.00 (0.47–2.13) | 1.00 | ||
The bold data indicate statistical significance (P < 0.05). Included were children with high or moderate/slightly increased genetic risk for type 1 diabetes as defined by different HLA-DQ allele combinations for type 1 diabetes risk. The proportion of agricultural and built area around the dwelling was analyzed as a predictor of autoantibody positivity and type 1 diabetes using CORINE 2012 data. Children who developed multiple biochemical islet autoantibodies and type 1 diabetes were compared with autoantibody-negative children within high risk (n = 1,991) and moderate or slightly increased risk (n = 8,024) subsets.
Radius of the analyzed area around the home.
HLA-DQB1*02/*03:02: 126 children with multiple autoantibodies, 101 children with type 1 diabetes.
HLA-DQB1 03:02/x; x ≠ DQB1*02,*03:01,*0602: 262 children with multiple autoantibodies, 166 children with type 1 diabetes.
The Association of Living Environment With Type 1 Diabetes Is Seen in the Region With the Highest Average Annual Temperatures and the Shortest Duration of Snow Cover
The inverse association of an agricultural environment with type 1 diabetes was significant in Turku, the southernmost study site (1,500-m radius: OR 0.25, 95% CI 0.06–0.96, P = 0.04), whereas no associations were detected in Oulu and Tampere, the two other study sites (Supplementary Table 4). Other land cover types were not associated with type 1 diabetes or islet autoimmunity in any of the regions, although there was a trend of higher diabetes and islet autoimmunity risk and built environment in Turku region (Supplementary Table 4).
The exposure to the effects of land cover diminishes during wintertime due to the snow cover on the ground, increased time spent indoors, and protection of body surfaces by clothing. The three hospital districts are located in distinct areas that differ in the duration of thermal winter and snow cover (Fig. 1). The annual duration of snow cover during the study period was significantly shorter in the Turku region compared with the Oulu region located close to the Arctic Circle (average 105.6 vs. 161.1 days per year, respectively, P < 0.001) (Fig. 1B). The Tampere region is located in between the two other areas (Fig. 1A).
Although a statistically significant inverse association between agricultural environment and diabetes risk was observed only in children with the high-risk HLA genotype and in children living in the southernmost study region, these factors did not explain the association between an agricultural environment and the disease risk when they were introduced into the regression model (HLA genotype and region adjusted OR 0.31, 95% CI 0.13–0.77, P = 0.01 within 1,500 m of the dwelling for the clinical disease) (Supplementary Table 5).
Snow Cover Affects the Diversity of Environmental Bacterial Communities Transferred Indoors and May Explain the Regional Differences in Land Cover Association
The contrast between the association of early-life agricultural and built living environment with type 1 diabetes and islet autoimmunity might be explained by differences in the environmental microbiota the children are exposed to in these environments. Considering these observations and that associations were only seen in the southernmost region with the shortest duration of snow cover, we hypothesized that snow cover on the ground blocks exposure to environmental microbiota reflected by the land cover types and thus might explain why the land cover association was seen in the southernmost area. To test this blocking hypothesis, we analyzed indoor bacterial diversity from doormat samples collected in the homes of study participants at the time when snow covered the ground (February) and in a snowless period during the summer (August). The richness and diversity of indoor bacterial communities were significantly lower in the doormat samples collected during February compared with August (Fig. 1C). The abundances of the orders Enterobacteriales (P = 0.02) and Gammaproteobacteria incertae sedis (P = 0.03) as well as of the families Enterobacteriaceae (P = 0.03) and Gilvimarinus (P = 0.047) were lower during continuous snow cover (February) compared with the snowless period (August) (Supplementary Table 6).
Progression Toward Type 1 Diabetes in Urban and Agricultural Areas
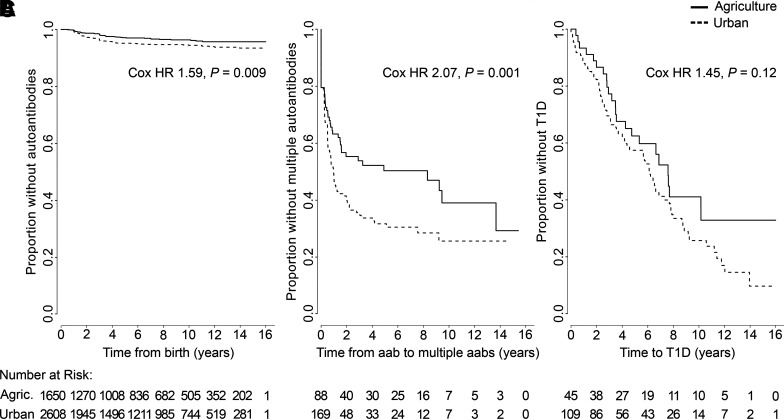
To analyze the association between early-life living environment and the disease process leading to type 1 diabetes, we formed two extreme groups based on the coverage of the associated land cover types and characterized by a “protective” living environment (the dwellings in the highest quartile of agriculture coverage [≥20%] as the agriculture subset) and a “risk” environment (the dwellings in the highest quartile of built environment coverage [≥50%] as the urban subset) using a 1,500-m radius. Five dwellings fulfilling both of these criteria were excluded from the analysis. When multiple-autoantibody-positive and control children were analyzed, the appearance of the first islet autoantibodies was significantly decreased in children living in agricultural areas compared with children in urban areas (Fig. 2A). In addition, the progression from single to multiple-autoantibody positivity reflecting the transition to a more aggressive autoimmune process occurred at a lower rate in children living in agricultural areas compared with children in urban areas (Fig. 2B). A similar but statistically nonsignificant trend was seen in the time from the first autoantibody to clinical disease (Fig. 2C).
Figure 2.
The effect of an urban and agriculture-dominated environment on the risk of developing aggressive islet autoimmunity and on the progression of the autoimmune process was compared between children living in an agricultural environment (≥20% agricultural area coverage in the analyzed area) and children living in an urban environment (≥50% built environment coverage in the analyzed area). The average follow-up time was longer in the agriculture subset (7.0 years, range 0.2–17.8 years) compared with the urban subset (6.6 years, range 0.1–18.5 years; two-sample t test, P = 0.02). A: The first detection of single or multiple autoantibodies (aabs) in children who eventually developed multiple biochemical autoantibodies and in autoantibody-negative control children (HLA genotype and region adjusted HR 1.67, P = 0.005). B: Progression time of the autoimmune process from the detection of a single autoantibody to the appearance of multiple autoantibodies (the analysis includes also the additional 103 children who remained single-autoantibody positive and did not develop multiple autoantibodies; HLA genotype and region adjusted HR 2.02, P = 0.003). C: The progression time from the appearance of the first autoantibody to type 1 diabetes (T1D) among children who developed multiple autoantibodies (HLA genotype and region adjusted HR 1.38, P = 0.19). The HRs were analyzed using Cox proportional hazards regression model and a 1,500-m area radius.
Conclusions
The current study demonstrates that living in an agricultural environment for the first 12 months of life is associated with a decreased risk of type 1 diabetes. This is the first study evaluating the association between living environment and type 1 diabetes using standardized satellite imaging and map-based land cover data. Our results are in agreement with a registry-based study suggesting an inverse association between childhood farm environment and the occurrence of diabetes (39) and a retrospective study revealing an inverse association between the consumption of vegetables from a farm and clinical diabetes (40). In addition, the finding is in line with the observations in other immune-mediated diseases, particularly allergy and asthma, which have been observed to be inversely associated with exposures to farm animals and agricultural environment (41,42).
This study is based on a large prospective birth cohort and longitudinal follow-up of children from birth and offers several important advantages. First, in contrast to retrospective studies, the current study is not affected by recall bias in the data collection.
Second, the end points were reliably identified by careful laboratory analyses of islet autoantibodies from all follow-up samples and by capturing all cases progressing to type 1 diabetes during the follow-up of these children.
Third, the prospective design enables the analysis of the association with different stages of the β-cell–damaging process, including the first signs of aggressive islet autoimmunity (first appearance of autoantibodies in multiple-autoantibody-positive children) and progression of the autoimmune process to clinical disease. The results suggest that land cover surrounding the early-life dwelling is associated with both of these stages—the risk of developing aggressive islet autoimmunity as well as the progression of the autoimmune process were both decreased in children living in an agricultural environment compared with urban areas.
Many studies have highlighted the importance of early-life environmental exposures in the development of immune-mediated diseases. The mutual/commensal microbiome and the immunogenic tolerance against harmless substances are established early in life (25,26). Furthermore, the autoimmune process leading to type 1 diabetes also often starts at an early age: in the current study, the median age of seroconversion to autoantibody positivity was 2.9 years. Thus, our findings are in line with the concept of the importance of early-life exposures. Similar results have been obtained in allergy studies, showing that land cover around the home at birth predicts atopy later in childhood whereas the residence at the time of the manifestation of atopy does not (43).
On the basis of existing knowledge, the protective association of agricultural areas with type 1 diabetes can be linked to the early exposure to a diverse microbial environment that stimulates the immunoregulatory elements of the developing immune system. Our previous studies showed that even a short-term skin exposure to environmental microbiota was able to increase the diversity of skin and gut microbiota and potentially elicit immunological effects as well (10,11). The infants not yet having direct contact with soil outdoors might still be exposed to the outdoor biodiversity because the diversity of environmental microbiota is transferred indoors (7,8). In fact, our current and previous (8) results suggest that snow cover reduces the diversity of the microbial community transferred indoors. Variation in the annual period of soil contacts could explain why the associations with agricultural and urban environments were seen, in particular, in the southernmost study region where the ground is covered by snow for a shorter time than in the other study regions. Comparing the microbiota of the children during a snowless and a snow cover period and including the land cover data into the analysis could provide more detailed information on this effect. All in all, further studies are needed to explore factors inducing the detected regional differences.
Disease-associated HLA-DQ genotypes seem to modify the association of agricultural and urban environments with type 1 diabetes because a statistically significant association was only seen among children with the high-risk HLA genotype. Interestingly, a recent study showed that an immunomodulatory anti-CD3 treatment delayed the progression of islet autoimmunity to clinical disease, particularly among children carrying the high-risk genotype (44). In addition, early-life probiotic exposure has been associated with protection against islet autoimmunity, and the association was especially strong among children with the high-risk HLA genotype (45). Thus, it is possible that the beneficial effects of immune modulations are mediated by mechanisms that are linked to the function of HLA molecules and that the children carrying the high-risk genotype could be more susceptible to the effects of such exposures.
This study has certain limitations that need to be considered when interpreting the results. Firstly, the study population is carrying HLA-DQ allele combinations that predispose to type 1 diabetes and thus does not represent the general population. However, 60–70% of Finnish children with type 1 diabetes carry one of the HLA genotypes conferring eligibility for participation in the DIPP study.
Secondly, although the study clearly implies an inverse association between an agricultural environment and diabetes risk, it cannot prove causality. Consequently, further studies are needed to clarify the mechanisms associated with these observations and to study possible effects of additional environmental factors such as pesticide use.
Thirdly, although Finland is a particularly interesting country due to its exceptionally high incidence of childhood type 1 diabetes and varying snow cover duration in different regions, the study represents a single population.
Finally, even though the study subjects represent a quite homogenous childhood population and certain potential confounding factors (breastfeeding, HLA type, and the area of residence) were included in the statistical models, it is still difficult to exclude possible effects of yet unknown confounding factors. Therefore, it is important to confirm these findings in other populations and climate zones.
In conclusion, the current study suggests that the living environment during the first year of life influences the risk of type 1 diabetes. An agricultural environment seems to contain protective factors compared with an urban environment, and exposure to environmental microbiota may be one of these factors.
Article Information
Acknowledgments. The authors thank the DIPP study participants, their families, and the DIPP study staff. The authors thank Mira Grönroos and Riikka Puhakka (University of Helsinki) for the technical guidance on microbial data processing and assistance in the microbial sample collection and EU funded HEDIMED consortium (grant agreement 864764) for providing valuable and constructive comments for the manuscript. The authors also acknowledge CSC–IT Center for Science, Finland, for computational resources.
Funding. This work is supported by the Finnish Funding Agency for Innovation (Tekes) under the Large Strategic Opening project Autoimmune Defense and Living Environment (ADELE) (grant number 40333/14) and Helsinki Institute of Life Science (HiLIFE) Proof of Concept grant. This work and the DIPP study are additionally supported by JDRF (grants 1-SRA-2016-342-M-R and 1-SRA-2019-732-M-B), Novo Nordisk Foundation, Academy of Finland (Decision No. 292538 and Centre of Excellence in Molecular Systems Immunology and Physiology Research 2012-2017 Decision No. 250114), Special Research Funds for University Hospitals in Finland, Sigrid Juselius Foundation, and the Diabetes Research Foundation (Diabetestutkimussäätiö) in Finland.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. N.N. contributed to the acquisition, analysis, and interpretation of data and drafted the manuscript. D.C. performed land cover data analyses. J.L. performed statistical analyses and contributed to the interpretation of data. A.P. and M.R. performed microbial sequencing analyses. M.L., J.I., J.T., R.V., and M.K. contributed to the study design and to the acquisition and interpretation of data. J.R., O.H.L, A.S., and H.H. contributed to the study concept and design and to the acquisition and interpretation of data. H.H. contributed to the drafting of the manuscript. All authors revised the manuscript critically for important intellectual content and gave final approval of the version to be published. H.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14356991.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Onkamo P, Väänänen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes--the analysis of the data on published incidence trends. Diabetologia 1999;42:1395–1403 [DOI] [PubMed] [Google Scholar]
- 3. Platts-Mills TAE. The allergy epidemics: 1870-2010. J Allergy Clin Immunol 2015;136:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patterson C, Guariguata L, Dahlquist G, Soltész G, Ogle G, Silink M. Diabetes in the young – a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract 2014;103:161–175 [DOI] [PubMed] [Google Scholar]
- 5. Kim H, Sitarik AR, Woodcroft K, Johnson CC, Zoratti E. Birth mode, breastfeeding, pet exposure, and antibiotic use: associations with the gut microbiome and sensitization in children. Curr Allergy Asthma Rep 2019;19:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deckers J, Lambrecht BN, Hammad H. How a farming environment protects from atopy. Curr Opin Immunol 2019;60:163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parajuli A, Grönroos M, Siter N, et al. Urbanization reduces transfer of diverse environmental microbiota indoors. Front Microbiol 2018;9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hui N, Parajuli A, Puhakka R, et al. Temporal variation in indoor transfer of dirt-associated environmental bacteria in agricultural and urban areas. Environ Int 2019;132:105069. [DOI] [PubMed] [Google Scholar]
- 9. Hanski I, von Hertzen L, Fyhrquist N, et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci U S A 2012;109:8334–8339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nurminen N, Lin J, Grönroos M, et al. Nature-derived microbiota exposure as a novel immunomodulatory approach. Future Microbiol 2018;13:737–744 [DOI] [PubMed] [Google Scholar]
- 11. Grönroos M, Parajuli A, Laitinen OH, et al. Short-term direct contact with soil and plant materials leads to an immediate increase in diversity of skin microbiota. MicrobiologyOpen 2019;8:e00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haahtela T, Holgate S, Pawankar R, et al.; WAO Special Committee on Climate Change and Biodiversity . The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ J 2013;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008;455:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. King C, Sarvetnick N. The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. PLoS One 2011;6:e17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marshall AL, Chetwynd A, Morris JA, et al. Type 1 diabetes mellitus in childhood: a matched case control study in Lancashire and Cumbria, UK. Diabet Med 2004;21:1035–1040 [DOI] [PubMed] [Google Scholar]
- 16. Virtanen SM, Takkinen HM, Nwaru BI, et al. Microbial exposure in infancy and subsequent appearance of type 1 diabetes mellitus-associated autoantibodies: a cohort study. JAMA Pediatr 2014;168:755–763 [DOI] [PubMed] [Google Scholar]
- 17. Kondrashova A, Reunanen A, Romanov A, et al. A six-fold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Ann Med 2005;37:67–72 [DOI] [PubMed] [Google Scholar]
- 18. Seiskari T, Kondrashova A, Viskari H, et al.; EPIVIR study group . Allergic sensitization and microbial load--a comparison between Finland and Russian Karelia. Clin Exp Immunol 2007;148:47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis-Richardson AG, Ardissone AN, Dias R, et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front Microbiol 2014;5:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vatanen T, Franzosa EA, Schwager R, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 2018;562:589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rytkönen M, Moltchanova E, Ranta J, Taskinen O, Tuomilehto J; SPAT Study Group; Finnish Childhood Diabetes Registry Group . The incidence of type 1 diabetes among children in Finland--rural-urban difference. Health Place 2003;9:315–325 [DOI] [PubMed] [Google Scholar]
- 22. Miller LJ, Willis JA, Pearce J, Barnett R, Darlow BA, Scott RS. Urban-rural variation in childhood type 1 diabetes incidence in Canterbury, New Zealand, 1980-2004. Health Place 2011;17:248–256 [DOI] [PubMed] [Google Scholar]
- 23. Liese AD, Puett RC, Lamichhane AP, et al. Neighborhood level risk factors for type 1 diabetes in youth: the SEARCH case-control study. Int J Health Geogr 2012;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samuelsson U, Löfman O. Geographical mapping of type 1 diabetes in children and adolescents in south east Sweden. J Epidemiol Community Health 2004;58:388–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science 2016;352:539–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhuang L, Chen H, Zhang S, Zhuang J, Li Q, Feng Z. Intestinal microbiota in early life and its implications on childhood health. Genomics Proteomics Bioinformatics 2019;17:13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ilonen J, Hammais A, Laine AP, et al. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes 2013;62:3636–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krischer JP, Lynch KF, Lernmark Å, et al.; TEDDY Study Group . Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: the TEDDY study. Diabetes Care 2017;40:1194–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Näntö-Salonen K, Kupila A, Simell S, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 2008;372:1746–1755 [DOI] [PubMed] [Google Scholar]
- 30. Knip M, Virtanen SM, Seppä K, et al.; Finnish TRIGR Study Group . Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med 2010;363:1900–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pöllänen PM, Ryhänen SJ, Toppari J, et al. Dynamics of islet autoantibodies during prospective follow-up from birth to age 15 years. J Clin Endocrinol Metab 2020;105:e4638–e4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Finnish Environment Institute . Spatial datasets. CORINE Land Cover 2012 20 m, 2014. Accessed 25 September 2017. Available from https://www.syke.fi/en-US/Open_information/Spatial_datasets/Downloadable_spatial_dataset
- 34. Copernicus Land Monitoring Service . CORINE Land Cover, 2000. Accessed 22 September 2020. Available from https://land.copernicus.eu/pan-european/corine-land-cover
- 35. Finnish Meteorological Institute . Weather in Finland. Accessed 9 July, 2019. Available from https://en.ilmatieteenlaitos.fi/
- 36. Schloss PD, Gevers D, Westcott SL. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS One 2011;6:e27310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 2013;79:5112–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: 2018. [Google Scholar]
- 39. Heikkinen SM, Pitkäniemi JM, Kilpeläinen ML, Koskenvuo MJ. Does farm environment protect against type 1 diabetes mellitus? Diab Vasc Dis Res 2013;10:375–377 [DOI] [PubMed] [Google Scholar]
- 40. Balazard F, Le Fur S, Valtat S, et al. Isis-Diab collaborative group . Association of environmental markers with childhood type 1 diabetes mellitus revealed by a long questionnaire on early life exposures and lifestyle in a case-control study. BMC Public Health 2016;16:1021–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Von Ehrenstein OS, Von Mutius E, Illi S, Baumann L, Böhm O, von Kries R. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy 2000;30:187–193 [DOI] [PubMed] [Google Scholar]
- 42. Riedler J, Braun-Fahrländer C, Eder W, et al. ALEX Study Team . Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 2001;358:1129–1133 [DOI] [PubMed] [Google Scholar]
- 43. Ruokolainen L, von Hertzen L, Fyhrquist N, et al. Green areas around homes reduce atopic sensitization in children. Allergy 2015;70:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Herold KC, Bundy BN, Long SA, et al.; Type 1 Diabetes TrialNet Study Group . An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uusitalo U, Liu X, Yang J, et al.; TEDDY Study Group . Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr 2016;170:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]