Abstract
Metal–organic frameworks (MOFs) with high surface area, tunable porosity, and diverse structures are promising platforms for chemiresistors; however, they often exhibit low sensitivity, poor selectivity, and irreversibility in gas sensing, hindering their practical applications. Herein, we report that hybrids of Cu3(HHTP)2 (HHTP = 2,3,6,7,10,11-hexahydroxytriphenylene) nanoflakes and Fe2O3 nanoparticles exhibit highly sensitive, selective, and reversible detection of NO2 at 20 °C. The key parameters to determine their response, selectivity, and recovery are discussed in terms of the size of the Cu3(HHTP)2 nanoflakes, the interaction between the MOFs and NO2, and an increase in the concentration and lifetime of holes facilitated by visible-light photoactivation and charge-separating energy band alignment of the hybrids. These photoactivated MOF–oxide hybrids suggest a new strategy for designing high-performance MOF-based gas sensors.
Short abstract
Hybrid between Cu3(HHTP)2 and Fe2O3 with the type (II) heterojunction promotes photoactivated charge carrier separation and provides completely reversible chemiresistive detection of NO2.
1. Introduction
Chemiresistors, including metal oxides, transition metal dichalcogenides, and carbon-based materials, provide a simple and cost-effective method for hazardous gas detection, environmental monitoring, and exhaled breath analysis.1−4 Metal–organic frameworks (MOFs) with ultrahigh porosity, large surface area, and facile chemical tunability5−8 have been considered as viable alternatives for the design of high-performance chemiresistors owing to the recent development of electrically conductive MOFs.9 Furthermore, not only the metal ions/clusters but also the organic linkers of MOFs can interact with analyte gases, and controllable pore sizes can be used to tune the transport/sieving of gas molecules, enabling the tailored control of gas-sensing characteristics.10−12
In MOFs composed of two-dimensional (2D) ligands, delocalized charges can be generated from extended π–d conjugation between the metal node and ligand, which improve conductivity.9,13−16 Furthermore, the interaction between analyte gases and metal nodes or organic ligands can induce chemiresistive variation. Campbell et al. first reported the chemiresistive sensing of ammonia using Cu3(HITP)2 (HITP = 2,3,6,7,10,11-hexaiminotriphenylene),17 and several studies have been conducted on the design of volatile organic compound sensors by replacing metal nodes (Cu) with Ni and Co or replacing triphenylene-based ligands with phthalocyanine-based ones.18−24 Although the possibility of modulating the gas selectivity by compositional variation of MOFs has been explored, there remain many challenges such as the requirement of high sensitivity and reversibility for the implementation of MOF-based gas sensors in practical applications. In particular, most MOFs exhibit dosimetric NO2 sensing behavior;22,25 thus, the reversible detection of NO2 using chemiresistive MOFs has never been reported. Furthermore, the sensitive and selective detection of NO2 using semiconducting MOFs has rarely been reported.
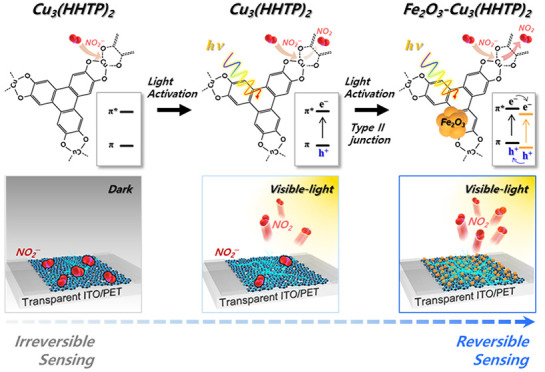
Herein, we report highly sensitive, selective, and reversible NO2 sensors using Cu3(HHTP)2 (HHTP = 2,3,6,7,10,11-hexahydroxytriphenylene) conductive MOFs composed of copper metal ions and HHTP ligands in 2D hexagonal structures (Figure 1a). To enhance the gas accessibility, fine and well-dispersed Cu3(HHTP)2-nanoflakes (NFs) were separated from coarse and agglomerated Cu3(HHTP)2-bulk (B) flakes by centrifugation. At room temperature, Cu3(HHTP)2-NFs exhibited a significantly higher response to NO2 than Cu3(HHTP)2-B flakes (Figure 1b). The visible-light photoactivation of MOFs substantially improved the recovery after NO2 sensing by promoting gas desorption at room temperature (Figure 1c). Furthermore, hybrids between Cu3(HHTP)2-NFs and Fe2O3 nanoparticles (NPs) with a charge-separating type (II) energy band alignment have been suggested to achieve complete and rapid recovery assisted by effective charge separation (Figure 1d). To the best of our knowledge, this is the first report on the design of a highly sensitive, selective, and reversible MOF-based NO2 sensor through photoactivation of the sensing/recovery reaction and the establishment of energy band alignments to prolong the lifetime of charge carriers.
Figure 1.
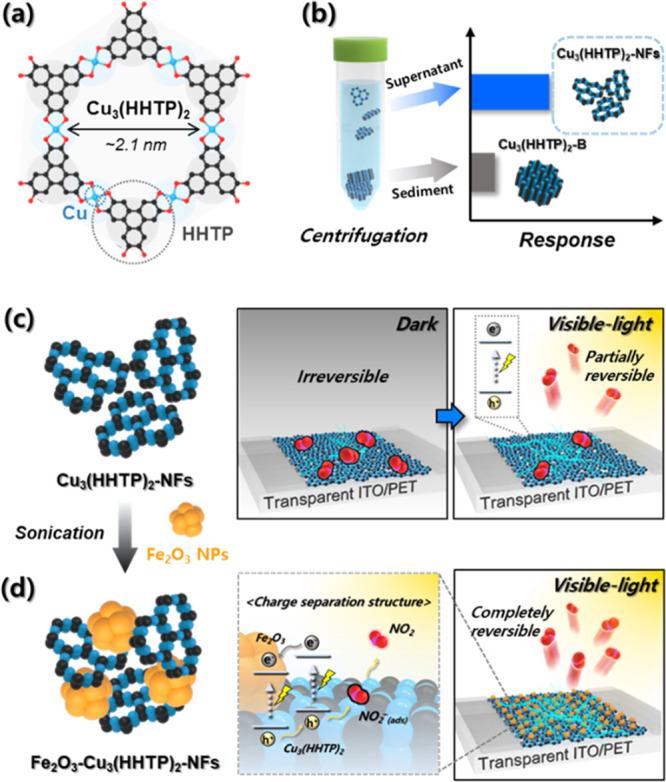
(a) Crystal structure of Cu3(HHTP)2. (b) Separation of highly sensitive Cu3(HHTP)2-NFs sensors by centrifugation. (c) Photoactivation of the recovery reaction after NO2 sensing. (d) Reversible and selective NO2 sensors using Fe2O3–Cu3(HHTP)2-NFs hybrids with charge-separating energy band alignment.
2. Results and Discussion
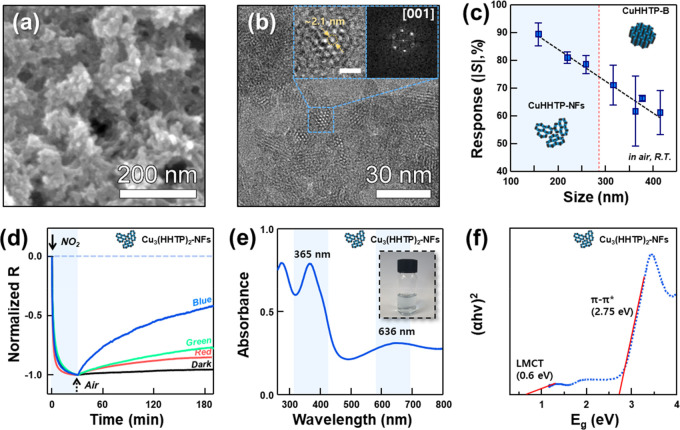
Polycrystalline Cu3(HHTP)2 flakes (referred to as Cu3(HHTP)2-Fs) were prepared by a solvothermal reaction (Figure 2a, Figure S1 in the Supporting Information). High-resolution transmission electron microscopy (TEM) image revealed a hexagonal microporous structure of Cu3(HHTP)2 (pore size: ∼2.1 nm) (Figure 2b). To systematically investigate the particle size dependence of the gas-sensing characteristics, Cu3(HHTP)2-Fs were sorted using differential velocity centrifugation (Figure S2). Centrifugation was performed at a speed of 10 000 rpm to obtain supernatants composed of small flakes (referred to as Cu3(HHTP)2-10000), and the sediments were redispersed for subsequent separation. In the same way, flakes were separated at 5000, 3000, 1000, 500, 300, 100, and 50 rpm to collect supernatants (referred to as Cu3(HHTP)2-ω, ω = 5000–50) and the remaining sediment composed of coarse flakes (referred to as Cu3(HHTP)2-0). The UV/vis spectra of the Cu3(HHTP)2-ω specimens (Figure S3) reveal a distribution of yields (Figure S4a). However, no peak shift was found in any of the specimens (Figure S3), confirming that each specimen comprised the same material at different sizes. The amount of Cu3(HHTP)2-10000 was too small to conduct a size analysis and fabricate a gas sensor. Thus, the sizes and gas-sensing characteristics of Cu3(HHTP)2-ω (ω = 5000–0) were measured and compared. The average size of Cu3(HHTP)2-5000 determined by dynamic light scattering was 158.7 ± 2.4 nm, and the particle size increased to 415.3 ± 16.9 nm as the separation speed decreased (Figure S4b). The responses [S = ΔR/Ra × 100% = (Rg – Ra)/Ra × 100%; Rg, resistance in gas; Ra, resistance in air] of Cu3(HHTP)2-ω sensors to 5 ppm of NO2 were measured at room temperature under dark conditions (Figure 2c and Figure S5). The Cu3(HHTP)2-5000 sensor demonstrated the highest response of 89.4% among the investigated sensors, and the response gradually decreased to 61.1% as the particle size increased. This is consistent with a previous report stating that the ammonia response of thin 2D triphenylene-based MOFs (∼10 layers) is superior to that of MOFs with a thicker and agglomerated configuration.26 In conductive MOFs, analyte gases are known to interact with the metal node or functional groups of ligands and change the resistance by the exchange of charges.18−21 From this perspective, the higher response of the smaller Cu3(HHTP)2 can be attributed to more interaction between the gas and the reaction sites of MOFs with high surface areas.
Figure 2.
(a) SEM images and (b) TEM images of Cu3(HHTP)2-Fs. (c) Size-dependent NO2 sensing characteristics of the Cu3(HHTP)2-ω (ω = 10000–0) sensor. The error bars were calculated from the responses of 3 different sensors. (d) Normalized sensing transients of Cu3(HHTP)2-NFs sensor to NO2 under different illumination conditions (dark, red, green, and blue). (e) UV/vis spectra and (f) Tauc plot of Cu3(HHTP)2-NFs.
To simplify the classification procedure, Cu3(HHTP)2-Fs were divided into two parts based on a fixed centrifugation speed of 1000 rpm. The Cu3(HHTP)2-NFs are the supernatants, and Cu3(HHTP)2-B are the sediments (Figure S6). The surface area of Cu3(HHTP)2-NFs (48.5 m2 g–1) was 2.4 times higher than that of Cu3(HHTP)2-B (20.3 m2 g–1) (Figure S7). The gas responses of the Cu3(HHTP)2-NFs sensor to 5 ppm of analyte gases, such as nitrogen dioxide (NO2), formaldehyde (HCHO), benzene (C6H6), carbon monoxide (CO), acetone (C3H6O), hydrogen (H2), ethanol (C2H5OH), p-xylene (C8H10), toluene (C7H8), and ammonia (NH3), were measured at room temperature under dark conditions. The sensor exhibited chemiresistive variation of p-type semiconductors: a decrease and increase in resistance upon exposure to oxidizing (NO2) and reducing gases (other gases), respectively (Figure S8b). The response to NO2 (S = −79.8%) was not only opposite to but also significantly higher than those of the other nine gases (Figure S8a), demonstrating selective NO2 detection. The high selectivity can be attributed to Lewis acid–base reactions between the metal node and the NO2. In general, the transition metal CuII accepts electrons from neutral or basic gases to fill the unoccupied d orbital.27,28 In contrast, highly acidic NO2 extracts electrons from CuI by forming a coordination complex with CuI [(1) N-nitro, (2) O-nitrito, or (3) O,O′ bidentate] if CuI is present.29,30 Thus, the NO2 adsorption reaction increases the hole concentration.
| 1 |
It is worth noting that the sensing transient of NO2 shows irreversible behavior, whereas those of other reducing gases, except NH3, return to the baseline (Figure S8b). Irreversible NO2 sensing behavior is commonly observed in all of the sensors in Figure S5, regardless of particle size, suggesting that it is an inherent sensing characteristic. This is consistent with the NO2 sensing results of most MOF-based chemiresistors in the literature.22,25 This irreversibility after NO2 sensing can be explained by the formation of a stable coordination complex between Cu and NO2,29,30 which impedes the use of Cu3(HHTP)2 as a NO2 sensor. Because NO2 sensing involves the formation of NO2(ads)–, more holes need to be provided to promote recovery. Considering the semiconducting nature and energy band gap of Cu3(HHTP)2, the photogeneration of charge carriers can be adopted to facilitate recovery. For this, NO2-sensing transients of Cu3(HHTP)2-NFs were measured under illumination by LED lamps of different colors: red (Ered = 1.97 eV; irradiance, 0.25 W m–2), green (Egreen = 2.34 eV; irradiance, 0.15 W m–2), and blue (Eblue = 2.76 eV; irradiance, 0.15 W m–2). The recovery rate of the Cu3(HHTP)2-NFs sensor in normalized sensing transients (Figure 2d) substantially increased in the order dark condition < red < green < blue light, in proportion to the photon energy, confirming the validity of photoactivation. This is supported by the fact that the sensor resistance tends to decrease with an increase in the photon energy of the light (Figure S9).
UV/vis spectra of the Cu3(HHTP)2-NFs exhibit distinct absorption peaks at 365 and 636 nm (Figure 2e). The two energy states of Cu3(HHTP)2 were determined by fitting the Tauc plot (Figure 2f). First, the state at near-infrared energy (0.60 eV) is associated with the ligand-to-metal charge transfer (LMCT) transition, which enables conduction in Cu3(HHTP)2 at room temperature. The second energy state in the visible region (2.75 eV) is related to the π–π* transition of the HHTP link,31,32 explaining the promotion of the recovery reaction under blue light illumination (Figure 2f).
For quantitative analysis of reaction kinetics, the reaction constants for the adsorption and desorption of NO2(ads)– (kads and kdes) was calculated by exponential fitting of dynamic sensing transients (S(t)) using the following eqs 2 and 3, where Smax is the maximum response, and CNO2 is the concentration of NO2. This calculation is based on the continuum site balance equations in that the response (S) is proportional to the NO2 and is explored using the mass action law of chemisorbed NO2 on Cu3(HHTP)2.33
| 2 |
| 3 |
The kdes value of the Cu3(HHTP)2-NFs sensor under blue light (1.41 × 10–4 s–1) was approximately 2.5 times higher than that under dark conditions (5.74 × 10–5 s–1) (Figure S10), further indicating that the recovery is significantly promoted by illumination with blue light. Although the NO2 response under blue light illumination is slightly lower than that under dark conditions (S = −68.9%), it is still significantly higher than those of the other nine gases (Figure S11). It should be pointed out that the Cu3(HHTP)2-NFs sensor did not exhibit complete recovery after NO2 sensing even under blue light with the highest energy. This suggests that the recombination of photogenerated electron–hole pairs occurs before promoting the desorption of NO2(ads)–, possibly due to the relatively low mobility of electrons and holes and the presence of defects in Cu3(HHTP)2.
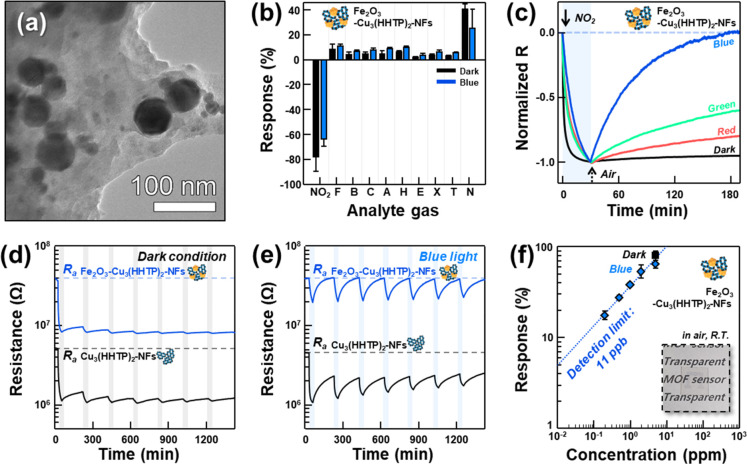
The establishment of heterostructures with charge-separating energy band structures, which are frequently employed in the design of photocatalysts, can be used to prolong the lifetimes of electrons and holes by charge separation.34 To examine this effect, a sensor using Fe2O3–Cu3(HHTP)2 heterostructures was fabricated (Figure 3a, Figure S12). Fe2O3 was chosen because it exhibits an appropriate band structure to facilitate the charge separation at the heterointerfaces. The Cu3(HHTP)2-NFs were dispersed in deionized water, which was uniformly mixed with Fe2O3 NPs (<50 nm) (Cu3(HHTP)2-NFs:Fe2O3 NPs = 2:1 by weight) by sonication (referred to as Fe2O3–Cu3(HHTP)2-NFs). The response of the Fe2O3–Cu3(HHTP)2-NFs sensor to NO2, as well as to the nine other gases, was similar to that of the Cu3(HHTP)2-NFs sensor (Figure 3b, Figure S13). The NO2 selectivity under red and green lights remained similar (Figures S14 and S15). This indicates that the chemiresistive variation of the Fe2O3–Cu3(HHTP)2-NFs sensor is primarily due to the continuous structure of Cu3(HHTP)2-NFs, while the discretely decorated Fe2O3 NPs on the Cu3(HHTP)2-NFs do not play a role in establishing an additional conduction path or contribute to the chemiresistive variation or enhancing the catalytic promotion of the sensing reaction. Interestingly, decoration by Fe2O3 NPs significantly improved the recovery of the Cu3(HHTP)2-NFs sensor under the illumination of red, green, and blue LED light (Figure 3c). In particular, the Fe2O3–Cu3(HHTP)2-NFs sensor showed completely reversible NO2 sensing characteristics (Figure 3c) and a significantly high kdes value under blue light (Figure S16). The optimized NO2 recovery was obtained when the weight ratio of Cu3(HHTP)2-NFs and Fe2O3 was 2:1 (Figure S17). The promotion of the recovery reaction by blue light is also supported by the observation that the lower light intensity led to the more sluggish recovery (Figure S18). To confirm the effect of photoactivation on the reversible sensing behaviors, the seven repetitive NO2 sensing transients of Cu3(HHTP)2-NFs and Fe2O3–Cu3(HHTP)2-NFs sensors were measured under dark conditions and blue light illumination (Figure 3d,e). Under dark conditions, the Cu3(HHTP)2-NFs and Fe2O3–Cu3(HHTP)2-NFs sensors barely recovered after the first NO2 sensing. In contrast, under blue light illumination, the Cu3(HHTP)2-NFs sensor showed improved recovery, and the Fe2O3–Cu3(HHTP)2-NFs sensor exhibited a completely reversible behavior. This clearly verifies that the heterojunction between Fe2O3 NPs and Cu3(HHTP)2-NFs promotes the desorption of NO2(ads)– by increasing the number of photoinduced holes and their lifetime. The response of the Fe2O3–Cu3(HHTP)2-NFs sensor to 0.2–5 ppm of NO2 was measured at room temperature under blue light (Figure 3f and Figure S19). The low detection limit of Fe2O3–Cu3(HHTP)2-NFs sensors to NO2 was calculated to be 11 ppb when a signal-to-noise ratio >10 was used as the criterion for gas sensing. The NO2 response in the present study was superior to those of most other sensors using mesoporous oxides, carbon-based materials, and MOFs in the literature (Table S1). It is worth noting that the particle size distribution of Cu3(HHTP)2-NFs is relatively wide, which might be a reason for the fluctuation of the gas response. In this perspective, the accuracy of the gas response can be enhanced by using Cu3(HHTP)2 with a monodisperse flake size. In addition, the sensor exhibited the mild variation of the NO2 response with changing the humidity from dry to relative humidity 50% atmosphere or varying the sensor temperature from 14 to 34 °C, demonstrating the potential of sensor operation under different ambient conditions (Figure S20).
Figure 3.
(a) TEM images of Fe2O3–Cu3(HHTP)2-NFs. (b) Gas responses of the Fe2O3–Cu3(HHTP)2-NFs sensor to 5 ppm of NO2, HCHO (F), benzene (B), CO (C), acetone (A), H2 (H), ethanol (E), p-xylene (X), toluene (T), and ammonia (N) under dark conditions and blue light illumination. (c) Normalized sensing transients of the Fe2O3–Cu3(HHTP)2-NF sensor to 5 ppm of NO2 under different illumination conditions (dark, red, green, and blue). (d, e) 7 repetitive sensing transients of the Cu3(HHTP)2-NFs and Fe2O3–Cu3(HHTP)2-NFs sensors to 5 ppm of NO2 under dark conditions and blue light illumination. (f) Response of Fe2O3–Cu3(HHTP)2-NFs sensors to 0.2–5 ppm of NO2 under blue light illumination (blue diamond) and to 5 ppm of NO2 under dark conditions. The error bars in parts b and f were calculated from the gas responses of three different sensors.
Furthermore, the static NO2 gas-sensing characteristics under natural sunlight were investigated using an acrylic chamber at 50% RH and 3 °C (Figure S21 and Video S1). Completely reversible sensing with a high response value of 30.4% upon exposure to 40 ppb of NO2 was achieved. NO2 is a representative air pollutant generated from combustion reactions and diesel engines which can cause respiratory diseases in humans and induce photochemical smog, such as ozone.35,36 The simple structure of the present sensor can be used for environmental monitoring at room temperature. Finally, the transparent (inset Figure 3f) and flexible design increases the prospects of achieving wearable gas sensors. It is worth noting that the transparency and flexibility of sensors can be enhanced further by the epitaxial growth of ultrathin 2D MOF films.24
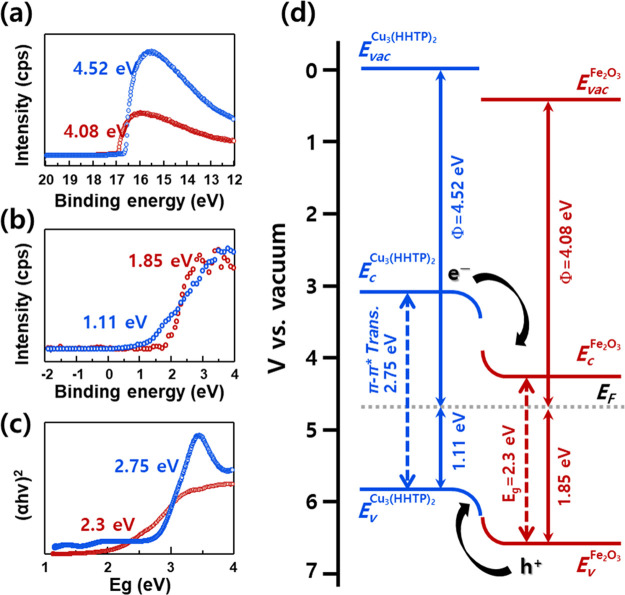
To understand the origin of the charge-separating electronic structures, the Fe2O3 NPs and Cu3(HHTP)2-NFs were analyzed by ultraviolet photoelectron spectroscopy (UPS), X-ray photoelectron spectroscopy (XPS), and UV/vis spectroscopy. The work functions of Cu3(HHTP)2-NFs and Fe2O3 NPs were calculated to be 4.50 and 4.08 eV, respectively, from the secondary electron cutoff in the UPS spectra (Figure 4a). From the XPS spectra of Cu3(HHTP)2-NFs and Fe2O3 NPs, the energy differences between the valence-band maximum and Fermi energy were determined to be 1.11 and 1.85 eV, respectively (Figure 4b). Considering the two energy states (0.60 and 2.75 eV) associated with the LMCT and π–π* transition, both type I and type II band alignments can be established across the Cu3(HHTP)2 and Fe2O3 heterojunction (Figure 4d and Figure S22). Under blue light illumination, both band alignments are advantageous for photoinduced holes to transfer from the Ev of Fe2O3 NPs to the Ev of Cu3(HHTP)2-NFs because the valence band of Cu3(HHTP)2-NFs (5.63 eV vs vacuum) is more positive than that of Fe2O3 NPs (6.37 eV vs vacuum) (Figure 4d and Figure S22). This favors the desorption of NO2(ads)–. In particular, in type II band alignments, the recombination of photogenerated charges can be suppressed by transferring the electrons from the Ec of Cu3(HHTP)2-NFs to the Ec of Fe2O3, which prolongs the lifetime of the holes in the Cu3(HHTP)2-NFs and further facilitates the desorption of NO2(ads) (Figure 4d).
Figure 4.
(a) UPS spectra, (b) XPS spectra, and (c) UV–vis spectra of Cu3(HHTP)2 and Fe2O3. (d) Schematic energy band diagram of the Cu3(HHTP)2/Fe2O3 heterojunction.
3. Conclusions
In summary, the size sorting of Cu3(HHTP)2 flakes by differential velocity centrifugation confirmed that fine nanoflakes with abundant reaction sites on the surface exhibited a higher NO2 response (89.4%) than coarse flakes (61.1%). Nanoflakes of Cu3(HHTP)2 barely reacted with nine other interference gases but exhibited irreversible NO2 sensing due to the formation of a stable coordination complex. Visible-light photoactivation has been suggested as an effective way to improve recovery. The NO2 recovery rate increased in proportion to the photon energy of the LED lamp color (red, 1.97 eV; green, 2.34; and blue, 2.76 eV). Furthermore, hybrids between Cu3(HHTP)2 and Fe2O3 with charge-separating electronic structures significantly increased the concentration and lifetime of holes in Cu3(HHTP)2, which enabled highly sensitive, selective, and reversible detection of NO2. The strategy based on the photoactivation of MOF–oxide hybrids paves the way for the design of high-performance MOF-based gas sensors with new functionalities.
4. Experimental Methods
4.1. Materials
2,3,6,7,10,11-Hexahydroxytriphenylene (H6HHTP, C18H12O6·xH2O, 95.0%) was purchased from Tokyo Chemical Industry. Copper(II) nitrate hydrate ((Cu(NO3)2·xH2O, 99.999%) and iron(III) oxide nanopowders (Fe2O3, <50 nm) were purchased from Sigma-Aldrich. All reagents were used without further purification.
4.2. Preparation of Cu3(HHTP)2-Fs
A methanol solution (3 mL) containing 25 mg of H6HHTP was mixed with 57 mL of an aqueous solution containing 60 mg of copper(II) nitrate hydrate by vigorous stirring for 5 min. This solution was transferred to a 100 mL Teflon-lined stainless-steel autoclave, and a solvothermal reaction was allowed to proceed at 70 °C for 12 h. The produced dark blue precipitate was washed twice with ethanol and three times with deionized (DI) water via centrifugation at 15 000 rpm and subsequently redispersed in DI water (3 mg mL–1).
4.3. Preparation of Cu3(HHTP)2-ω
The Cu3(HHTP)2-Fs were sorted by size using a differential velocity centrifugation method. First, 5 mL of slurry containing Cu3(HHTP)2 flakes (3 mg mL–1) was diluted with 20 mL of DI water and centrifuged at 10 000 rpm for 1 h. The supernatants were carefully collected, and the sediments were redispersed in 25 mL of DI water for subsequent centrifugation. Similarly, different sizes of Cu3(HHTP)2 particles were collected at centrifugation speeds of 5000, 3000, 1000, 500, 300, 100, and 50 rpm by collecting supernatants, and the final sediments were redispersed in 25 mL of DI water.
4.4. Preparation of Cu3(HHTP)2-NFs
The Cu3(HHTP)2-Fs were separated into two groups: Cu3(HHTP)2 nanoflakes and Cu3(HHTP)2-bulk flakes. For this, 5 mL of slurry containing Cu3(HHTP)2 flakes (3 mg mL–1) was diluted with 20 mL of DI water and centrifuged at 1000 rpm for 3 h. The supernatants (Cu3(HHTP)2-NFs) were carefully collected, and the sediments (Cu3(HHTP)2-B) were redispersed in 25 mL of DI water.
4.5. Preparation of Fe2O3–Cu3(HHTP)2-NFs
Fe2O3 nanoparticles (NPs) (1 mg) were dispersed in 10 mL of DI water by sonication for 30 min. The slurry of well-dispersed Fe2O3 NPs (1 mL, 0.1 mg mL–1) was mixed with 1 mL of Cu3(HHTP)2-NFs slurry (0.2 mg mL–1) to obtain a 2:1 weight ratio of Cu3(HHTP)2-NFs to Fe2O3 NP hybrid structures by sonication for 30 min.
4.6. Characterization Methods
The structure and morphology of the materials were investigated using field-emission scanning electron microscopy (FE-SEM, Su-70, Hitachi Co. Ltd.) and high-resolution transmission electron microscopy (HR-TEM, Titan, FEI Co. Ltd.). The phase and crystallinity of the materials were analyzed using X-ray diffraction (XRD, D/Max-2500 V/Pc, Rigaku). Absorbance spectra of the materials were measured using a UV–vis spectrophotometer (Cary 50, Agilent Technologies Inc.). The sizes of the materials were investigated by dynamic light scattering using a ζ potential and particle size analyzer (ELSZ-2000ZS, Otsuka Electronics Co. Ltd.). The specific surface areas were measured by Brunauer–Emmett–Teller analysis of N2 adsorption isotherms (BET, Tristar 3000, Micromeritics, Co. Ltd.). The electronic band structures were characterized by ultraviolet photoemission spectroscopy (UPS, AXIS-Nova, Kratos Analytical Ltd.; monochromatic He I = 21.2 eV, Ag 3d5/2 < 100 meV) and X-ray photoelectron spectroscopy (XPS, AXIS-Nova, Kratos Analytical Ltd.; monochromatic Al Kα = 1486.6 eV, Ag 3d5/2 < 0.48 eV), and a Tauc plot was established from the UV–vis spectra.
4.7. Gas-Sensing Characteristics
The slurry containing Cu3(HHTP)2-NFs (0.1 mg mL–1) was deposited onto transparent PET substrates (size: 8 mm × 8 mm) with a patterned indium tin oxide interdigitated electrode (gap: 5 μm) by drop casting. The sensors were heat-treated at 60 °C for 0.5 h to remove the solvent. The gas-sensing characteristics were measured at room temperature, and the sensors were placed in a square quartz tube (size: 3 × 3 × 3 cm3). The atmosphere was controlled using a four-way valve to ensure a constant flow rate (200 cm3 min–1) of synthetic air and analyte gases [5 ppm of nitrogen dioxide (NO2), formaldehyde (HCHO), benzene (C6H6), carbon monoxide (CO), acetone (C3H6O), hydrogen (H2), ethanol (C2H5OH), p-xylene (C8H10), toluene (C7H8), and ammonia (NH3)]. MR16 commercial LED lamps (red, green, and blue) were installed at a distance of 2 cm from the sensors, and the sensor was stabilized in air for 6 h. The two-probe direct-current resistance of the sensor was obtained using an electrometer.
Acknowledgments
This work was supported by the National Research Foundation of Korea grants funded by the Korea government (2020R1A2C3008933) and a grant from the Korea Environmental Industry & Technology Institute (2020002700011).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.1c00289.
XRD analysis, schematic experimental procedures for preparing sensing materials, UV/vis analysis, yields and size distribution of sensing materials, gas-sensing characteristics, pore size distribution, specific surface area, sensor resistances under different colors of LED lights, calculation of adsorption and desorption rate constants, SEM images, schematic images of the outdoor chamber system, and an energy band diagram (PDF)
Video S1: Static NO2 gas-sensing characteristics under natural sunlight investigated using an acrylic chamber at 50% RH and 3 °C (MP4)
The authors declare no competing financial interest.
Supplementary Material
References
- Jeong S.-Y.; Kim J.-S.; Lee J.-H. Rational Design of Semiconductor-Based Chemiresistors and their Libraries for Next-Generation Artificial Olfaction. Adv. Mater. 2020, 32, 2002075. 10.1002/adma.202002075. [DOI] [PubMed] [Google Scholar]
- Moon Y. K.; Jeong S.-Y.; Jo Y.-M.; Kang Y. C.; Lee J.-H. Highly Selective Detection of Benzene and Discrimination of Volatile Aromatic Compounds Using Oxide Chemiresistors with Tunable Rh-TiO2 Catalytic Overlayers. Adv. Sci. 2021, 8, 2004078. 10.1002/advs.202004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.; Kim T.; Lee J.; Choi Y. S.; Moon J.; Park S. Y.; Lee T. H.; Park H. K.; Lee S. A.; Kwon M. S.; Byun H.-G.; Lee J.-H.; Lee M.-G.; Hong B. H.; Jang H. W. Tailored Graphene Micropatterns by Wafer-Scale Direct Transfer for Flexible Chemical Sensor Platform. Adv. Mater. 2021, 33, 2004827. 10.1002/adma.202004827. [DOI] [PubMed] [Google Scholar]
- Wu J.; Tao K.; Guo Y.; Li Z.; Wang X.; Luo Z.; Feng S.; Du C.; Chen D.; Miao J.; Norford L. K. A 3D Chemically Modified Graphene Hydrogel for Fast, Highly Sensitive, and Selective Gas Sensor. Adv. Sci. 2017, 4, 1600319. 10.1002/advs.201600319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi N. L.; Eckert J.; Eddaoudi M.; Vodak D. T.; Kim J.; O’Keeffe M.; Yaghi O. M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129. 10.1126/science.1083440. [DOI] [PubMed] [Google Scholar]
- Furukawa H.; Ko N.; Go Y. B.; Aratani N.; Choi S. B.; Choi E.; Yazaydin A. Ö.; Snurr R. Q.; O’Keeffe M.; Kim J.; Yaghi O. M. Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329, 424–428. 10.1126/science.1192160. [DOI] [PubMed] [Google Scholar]
- Furukawa H.; Cordova K. E.; O’Keeffe M.; Yaghi O. M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- Zhou H.-C.; Long J. R.; Yaghi O. M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112, 673–674. 10.1021/cr300014x. [DOI] [PubMed] [Google Scholar]
- Xie L. S.; Skorupskii G.; Dincă M. Electrically Conductive Metal-Organic Frameworks. Chem. Rev. 2020, 120, 8536–8580. 10.1021/acs.chemrev.9b00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen I.; Burtch N.; Talin A.; Falcaro P.; Allendorf M.; Ameloot R. An Updated Roadmap for The Integration of Metal-Organic Frameworks with Electronic Devices and Chemical Sensors. Chem. Soc. Rev. 2017, 46, 3185. 10.1039/C7CS00122C. [DOI] [PubMed] [Google Scholar]
- Hu Z.; Deibert B. J.; Li J. Luminescent Metal-Organic Frameworks for Chemical Sensing and Explosive Detection. Chem. Soc. Rev. 2014, 43, 5815–5840. 10.1039/C4CS00010B. [DOI] [PubMed] [Google Scholar]
- Koo W.-T.; Jang J.-S.; Kim I.-D. Metal-Organic Frameworks for Chemiresistive Sensors. Chem. 2019, 5, 1938–1963. 10.1016/j.chempr.2019.04.013. [DOI] [Google Scholar]
- Sheberla D.; Sun L.; Blood-Forsythe M. A.; Er S.; Wade C. R.; Brozek C. K.; Aspuru-Guzik A.; Dincă M. High Electrical Conductivity in Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2, a Semiconducting Metal-Organic Graphene Analogue. J. Am. Chem. Soc. 2014, 136, 8859–8862. 10.1021/ja502765n. [DOI] [PubMed] [Google Scholar]
- Hmadeh M.; Lu Z.; Liu Z.; Gándara F.; Furukawa H.; Wan S.; Augustyn V.; Chang R.; Liao L.; Zhou F.; Perre E.; Ozolins V.; Suenaga K.; Duan X.; Dunn B.; Yamamto Y.; Terasaki O.; Yaghi O. M. New Porous Crystals of Extended Metal-Catecholates. Chem. Mater. 2012, 24, 3511–3513. 10.1021/cm301194a. [DOI] [Google Scholar]
- Dong R.; Han P.; Arora H.; Ballabio M.; Karakus M.; Zhang Z.; Shekhar C.; Adler P.; Petkov P. S.; Erbe A.; Mannsfeld S. C. B.; Felser C.; Heine T.; Bonn M.; Feng X.; Cánovas E. High-Mobility Band-Like Charge Transport in a Semiconducting Two-Dimensional Metal-Organic Framework. Nat. Mater. 2018, 17, 1027–1032. 10.1038/s41563-018-0189-z. [DOI] [PubMed] [Google Scholar]
- Huang X.; Sheng P.; Tu Z.; Zhang F.; Wang J.; Geng H.; Zou Y.; Di C.-A.; Yi Y.; Sun Y.; Xu W.; Zhu D. A Two-Dimensional π-d Conjugated Coordination Polymer with Extremely High Electrical Conductivity and Ambipolar Transport Behaviour. Nat. Commun. 2015, 6, 7408. 10.1038/ncomms8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. G.; Sheberla D.; Liu S. F.; Swager T. M.; Dincă M. Cu3(hexaiminotriphenylene)2: An Electrically Conductive 2D Metal-Organic Framework for Chemiresistive Sensing. Angew. Chem., Int. Ed. 2015, 54, 4349–4352. 10.1002/anie.201411854. [DOI] [PubMed] [Google Scholar]
- Campbell M. G.; Liu S. F.; Swager T. M.; Dincă M. Chemiresistive Sensor Arrays from Conductive 2D Metal-Organic Frameworks. J. Am. Chem. Soc. 2015, 137, 13780–13783. 10.1021/jacs.5b09600. [DOI] [PubMed] [Google Scholar]
- Smith M. K.; Mirica K. A. Self-Organized Frameworks on Textiles (SOFT): Conductive Fabrics for Simultaneous Sensing, Capture, and Filtration of Gases. J. Am. Chem. Soc. 2017, 139, 16759–16767. 10.1021/jacs.7b08840. [DOI] [PubMed] [Google Scholar]
- Meng Z.; Aykanat A.; Mirica K. A. Welding Metallophthalocyanines into Bimetallic Molecular Meshes for Ultrasensitive, Low-Power Chemiresistrive Detection of Gases. J. Am. Chem. Soc. 2019, 141, 2046–2053. 10.1021/jacs.8b11257. [DOI] [PubMed] [Google Scholar]
- Wu A.-Q.; Wang W.-Q.; Zhan H.-B.; Cao L.-A.; Ye X.-L.; Zheng J.-J.; Kumar P. N.; Chiranjeevulu K.; Deng W.-H.; Wang G.-E.; Yao M.-S.; Xu G. Layer-by-Layer Assembled Dual-Ligand Conductive MOF Nano-Films with Modulated Chemiresistive Sensitivity and Selectivity. Nano Res. 2021, 14, 438–443. 10.1007/s12274-020-2823-8. [DOI] [Google Scholar]
- Meng Z.; Stolz R. M.; Mirica K. A. Two-Dimensional Chemiresistive Covalent Organic Framework with High Intrinsic Conductivity. J. Am. Chem. Soc. 2019, 141, 11929–11937. 10.1021/jacs.9b03441. [DOI] [PubMed] [Google Scholar]
- Stassen I.; Dou J.-H.; Hendon C.; Dincă M. Chemiresistive Sensing of Ambient CO2 by an Autogenously Hydrated Cu3(hexaiminobenzene)2 Framework. ACS Cent. Sci. 2019, 5, 1425–1431. 10.1021/acscentsci.9b00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Chen J.; Wang C.; Zhou Y.; Ba K.; Xu H.; Bao W.; Xu X.; Carlsson A.; Lazar S.; Meingast A.; Sun Z.; Deng H. Metal-Organic Framework for Transparent Electronics. Adv. Sci. 2020, 7, 1903003. 10.1002/advs.201903003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo W.-T.; Kim S.-J.; Jang J.-S.; Kim D.-H.; Kim I.-D. Catalytic Metal Nanoparticles Embedded in Conductive Metal-Organic Frameworks for Chemiresistors: Highly Active and Conductive Porous Materials. Adv. Sci. 2019, 6, 1900250. 10.1002/advs.201900250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M.-S.; Lv X.-J.; Fu Z.-H.; Li W.-H.; Deng W.-H.; Wu G.-D.; Xu G. Layer-by-Layer Assembled Conductive Metal-Organic Framework Nanofilms for Room-Temperature Chemiresistive Sensing. Angew. Chem., Int. Ed. 2017, 56, 16510–16514. 10.1002/anie.201709558. [DOI] [PubMed] [Google Scholar]
- Flynn S. R.; Wass D. F. Transition metal frustrated Lewis pairs. ACS Catal. 2013, 3, 2574–2581. 10.1021/cs400754w. [DOI] [Google Scholar]
- Sibi M. P.; Cook G. R.. Copper Lewis Acids in Organic Synthesis. In Lewis Acids in Organic Synthesis; Wiley-VCH, 2000; pp 543–574. [Google Scholar]
- Timmons A. J.; Symes M. D. Converting Between the Oxides of Nitrogen using Metal-Ligand Coordination Complexes. Chem. Soc. Rev. 2015, 44, 6708. 10.1039/C5CS00269A. [DOI] [PubMed] [Google Scholar]
- Wright A. M.; Sun C.; Dincă M. Thermal Cycling of a MOF-Based NO Disproportionation Catalyst. J. Am. Chem. Soc. 2021, 143, 681–686. 10.1021/jacs.0c12134. [DOI] [PubMed] [Google Scholar]
- Sun L.; Campbell M. G.; Dincă M. Electrically Conductive Porous Metal-Organic Frameworks. Angew. Chem., Int. Ed. 2016, 55, 3566–3579. 10.1002/anie.201506219. [DOI] [PubMed] [Google Scholar]
- Rubio-Giménez V.; Galbiati M.; Castells-Gil J.; Almora-Barrios N.; Navarro-Sánchez J.; Escorcia-Ariza G.; Mattera M.; Arnold T.; Rawle J.; Tatay S.; Coronado E.; Martí-Gastaldo C. Bottom-Up Fabrication of Semiconductive Metal-Organic Framework Ultrathin Films. Adv. Mater. 2018, 30, 1704291. 10.1002/adma.201704291. [DOI] [PubMed] [Google Scholar]
- Lee C. Y.; Strano M. S. Understanding the Dynamics of Signal Transduction for Adsorption of Gases and Vapors on Carbon Nanotube Sensors. Langmuir 2005, 21, 5192–5196. 10.1021/la046867i. [DOI] [PubMed] [Google Scholar]
- Yoon J.-W.; Kim J.-H.; Kim C.; Jang H. W.; Lee J.-H. MOF-Based Hybrids for Solar Fuel Production. Adv. Energy Mater. 2021, 2003052. 10.1002/aenm.202003052. [DOI] [Google Scholar]
- Koenig J. Q.; Covert D. S.; Marshall S. G.; Belle G. V.; Pierson W. E. The Effect of Ozone and Nitrogen Dioxide on Pulmonary Function in Healthy and in Asthmatic Adolescents. Am. Rev. Respir. Dis. 1987, 136, 1152–1157. 10.1164/ajrccm/136.5.1152. [DOI] [PubMed] [Google Scholar]
- Sadanaga Y.; Yoshino A.; Kato S.; Kajii Y. Measurements of OH Reactivity and Photochemical Ozone Production in the Urban Atmosphere. Environ. Sci. Technol. 2005, 39, 8847–8852. 10.1021/es049457p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.