Abstract
SARS-CoV-2 vaccine roll-out has been successful in the UK and other parts of the world; however, there are increasing concerns about adverse events. A 44-year-old woman presented to a UK hospital with left upper arm pain at the vaccine site a couple of days after receiving the Pfizer-BioNTech mRNA vaccine, which progressed to fever, diarrhoea and abdominal pain over the next few days. She had an erythematous rash on the chest with subcutaneous oedema. Her C reactive protein was 539 mg/L, white cell count of 17×109/L (1.8–7.5), troponin-T of 1013 ng/L and creatine kinase of 572 u/L. She developed an unprovoked pulmonary embolism with acute kidney injury. After administration of intravenous methylprednisolone, the muscle oedema, skin rashes and acute kidney injury resolved. Although multisystem inflammatory syndrome (MIS) is described in children (MIS-C) and adults (MIS-A) following SARS-CoV-2 infection, we highlight the first reported MIS-V case after the SARS-CoV-2 vaccine.
Keywords: COVID-19, immunological products and vaccines, vaccination/immunisation, intensive care
Background
Available literature data suggest that SARS-CoV-2 vaccines are generally safe and well tolerated.1–3 However, there is an increasing focus on adverse events following immunisation (AEFI) after the SARS-CoV-2 vaccine recently. In the UK, over 43 million of the adult population have been vaccinated so far in the fight to end the pandemic.4 Reported data indicate that 1 in 15 patients may develop mild to moderate self-limiting side effects for few days, mimicking influenza or low-grade COVID-19 infection following SARS-CoV-2 vaccination. The most common side effects are sore arm, localised swelling, headache, fever, swollen glands, muscle pains and fatigue.5 Reassuringly, severe AEFI is rare with COVID-19 vaccines, but recent reports suggest that a small number of individuals have sustained severe adverse events.
Pfizer-BioNTech m-RNA SARS-CoV-2 vaccination is known to cause rare allergic reactions, ranging from hives, tongue swelling and life-threatening anaphylaxis (1 in 100 000), particularly in individuals with known allergies.3 6 Recently, new concerns have been raised about thromboembolism (TE) risk following the AstraZeneca-Oxford SARS-CoV-2 vaccine.7–9 The Medicines and Healthcare products Regulatory Agency revealed in April 2021 that 7 out of 18 million adults in the UK have died from TE after receiving the AstraZeneca SARS-CoV-2 vaccine. Further reports in the global media highlighting cerebrovenous sinus thromboses (CVST) and potential causal relationship with the AstraZeneca-Oxford SARS-CoV-2 vaccine have caused worldwide concern. Current evidence suggests that the risk of CVST is overall low, with an estimated risk of 13.2 cases per million per year.10
Multisystem inflammatory syndrome (MIS) associated with COVID-19 infection also generated much interest, mainly in children (MIS-C) and, more recently, adults (MIS-A).11–15
We highlight a first reported case of an MIS following the Pfizer-BioNTech m-RNA SARS-Cov-2 vaccine, successfully treated with intravenous corticosteroid therapy. There is currently limited information about the prevalence of MIS-A. Therefore, we feel it is essential to report an MIS case due to the SARS-CoV-2 vaccine. We will explain the disease’s course, discuss the differences compared with MIS-A and MIS-C, and its management in our patient. The available literature is reviewed.
Case presentation
A 44-year-old woman with a history of mild asthma was admitted to our local hospital with left upper arm/chest pain in January 2021, 2 days after receiving the Pfizer-BioNTech m-RNA SARS-CoV-2 vaccine. She reported constant left arm pain, which was worse on limb movement. Symptoms progressed to involve the left axilla and abdominal wall over the next few days. She reported that she was unable to lift her left arm due to pain. She also had bilious vomiting, loose stools and chest tightness. During the hospital admission, the patient developed an erythematous skin rash on the left chest wall (figure 1). The initial suspicion was that this could be a herpes zoster rash, but it was felt unlikely following a dermatology review.
Figure 1.
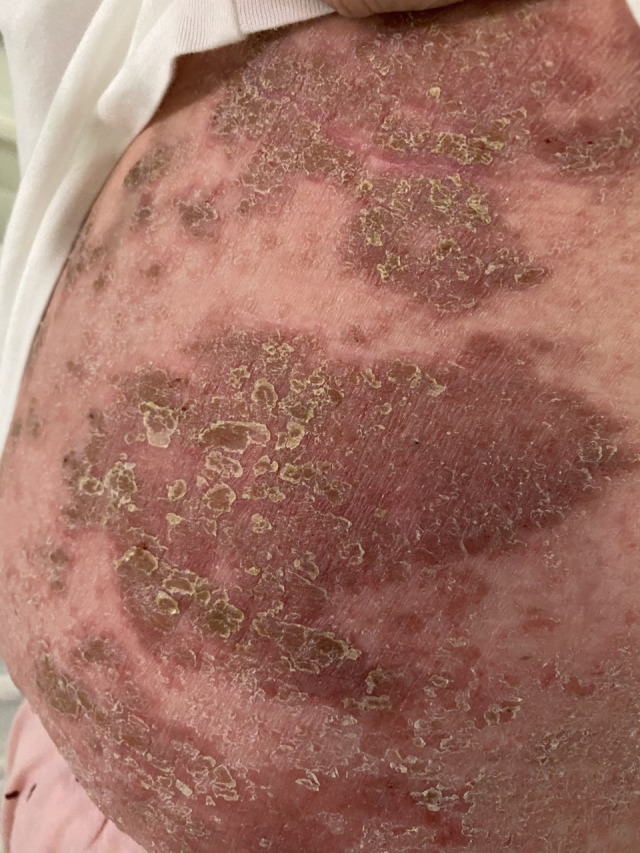
Erythematous skin rash on the abdomen and left lower chest
The patient denied any history of skin rashes, Raynaud’s, photosensitivity or mouth ulcers before hospital admission. She reported being well before receiving the SARS-CoV-2 vaccine. She denied any nose bleeds, ear discharge or hearing loss. She previously had influenza and yellow fever vaccines without any reaction. The patient did not have any allergies. She had three first trimester miscarriages and one ectopic pregnancy before she delivered her healthy full-term first child. The patient denied having a history of TE.
She was admitted to the intensive therapy unit (ITU) with a temperature of 38°C. She was hypotensive with a blood pressure of 81/38 mm Hg and a pulse rate of 100 beats/min. The patient had tenderness in the left axilla, chest wall and loin. Left-arm abduction was limited due to pain, with weakness of 4/5 on the Medical Research Council scale. She had normal heart sounds and her chest was clear to auscultation. The patient continued to have temperature spikes >38°C.
Investigations
Her admission electrocardiography (ECG) showed no evidence of ischaemia. Serum creatinine was 193 μmol/L (54–79) with an estimated glomerular filtration rate of 27 mL/min/1.73 m (normal >60). The white cell count was 17.1×109/L (4.1–11.0) with a neutrophil count of 8×109/L (1.8–7.5). Lymphocyte and eosinophil counts were normal. The C reactive protein (CRP) was elevated at 539 mg/L (0–5), and troponin-T was 1013 ng/L (0–15). D-dimer was raised at 2564 units (n<540) (table 1). The patient had a negative SARS CoV-2 real-time reverse transcriptase-PCR test following a nasopharyngeal swab, including tests for influenza and respiratory syncytial virus. The creatinine kinase (CK) was moderately elevated at 572 u/L (0–200), which peaked at 865 u/L. The patient also had a positive varicella immunoglobulin G (IgG) test. CT of the chest revealed left chest wall muscle oedema (figure 2). Her antinuclear antibodies (ANAs) were negative with double-stranded DNA, and complements and anti-neutrophil cytoplasmic antibody (ANCA) were in normal range. The patient had a normal anti-cardiolipin IgG <1 u/mL (negative <10), IgM antibodies were at 1.1 u/mL (negative <10 units) and her beta-2 glycoprotein-1 antibodies were 1.7 u/mL (negative <7 units). The laboratory did not process the patient’s lupus anticoagulant as the patient was on anticoagulation.
Table 1.
Blood results on admission, during the hospital stay and at discharge
| Investigation | Before hospital admission | Day 1 | Day 4 | Day 8 | Day 12 | At discharge | 2 weeks after discharge |
| White cell count (×109/L) | 9.7 | 17.1 | 27 | 14.7 | 14.7 | 12.8 | 11.3 |
| CRP (mg/L) | <4 | 539 | 132 | 88 | 53 | 19 | 11 |
| Creatinine (μmol/L) | 60 | 193 | 179 | 156 | 107 | 76 | 73 |
| e-GFR (mL/min) | >90 | 27 | 29 | 35 | 55 | 82 | 87 |
| Albumin (g/L) | 42 | 33 | 28 | 24 | 31 | 42 | 43 |
| D-dimer (ng/mL) | – | 2564 | |||||
| Troponin (ng/L) | – | 1013 | 1347 | ||||
| CK (u/L) | – | 572 | 865 | 123 |
CK, creatine kinase; CRP, C reactive protein; e-GFR, estimated glomerular filtration rate.
Figure 2.
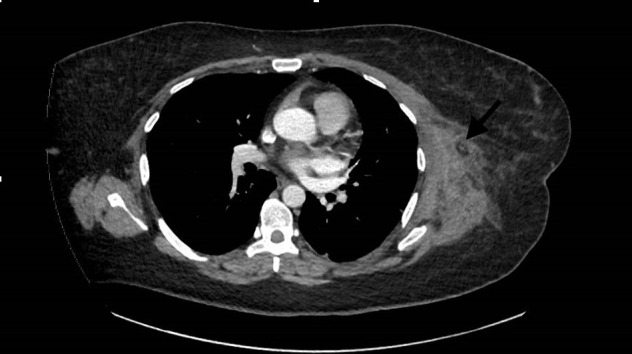
CT of the chest illustrates left chest wall muscle oedema with subcutaneous fat stranding.
Urine analysis showed 1+ protein and 3+ blood without myoglobinuria. Bedside transthoracic echocardiogram showed no evidence of vegetations but demonstrated a 5 mm pericardial effusion. A plain radiograph of the chest showed no consolidation.
Differential diagnosis
The first impression was that of septic shock in view of hypotension and raised CRP. Elevated CK and acute kidney injury raised the possibility of rhabdomyolysis. However, urine was visibly clear and repeat CK was not significantly elevated. Due to the presence of fever, COVID-19 infection was sought. A CT evidence of subsegmental pulmonary embolism and elevated troponin also strengthened this suspicion. However, the patient did not have any typical respiratory symptoms, such as cough and breathlessness, had multiple negative SARS-CoV-2 nasopharyngeal swabs and antibody tests, and the CT of the chest showed no evidence of COVID-19 pneumonitis. Finally, with the elevated CK and the CT scan evidence of muscle oedema, we explored the possibility of inflammatory myositis. Due to the lack of symmetrical upper and lower limb proximal muscle involvement and normal connective tissue disease screen, including ANA and extractable-nuclear antigens, we ruled this out. Due to the patient’s normal ferritin, macrophage activation syndrome was also ruled out.
Overall, the patient’s clinical features and biochemical markers were consistent with the Centers for Disease Control and Prevention (CDC) definition of MIS.16 Although to date, MIS has been described in children and adults in the context of infection with COVID-19, it is a syndrome that can have various presentations. Our patient had a fever, elevated inflammatory markers and illness requiring in-hospital admission with multisystem organ involvement (cardiac, renal, gastrointestinal, dermatological and vascular). Although the CDC criteria for MIS require the presence of current or recent infection with SARS-CoV-2 infection or exposure, our patient had received a Pfizer-BioNTech SARS-CoV-2 mRNA vaccine just 2 days before this illness. Hence, this patient meets the criteria drawn recently by the Brighton Collaboration Network definition of vaccine-induced MIS.17 There was no alternative diagnosis to explain our patient’s symptoms.
Treatment
The patient was treated with intravenous antibiotics (piperacillin/tazobactam 4.5 g three times a day and teicoplanin 12 mg/kg once daily for a week) and intravenous fluids during the admission. Despite broad-spectrum antibiotics, the patient had no improvement in her clinical condition. Multiple blood cultures grew no organism. With suspicion of MIS, the patient was treated with intravenous methylprednisolone 1 g/day for 3 days, leading to rapid improvement of her clinical condition and inflammation markers. The patient received therapeutic enoxaparin during her hospital stay. Subsequently, she was commenced on apixaban 5 mg two times per day for 6 months. However, the patient needed a prolonged in-hospital stay for her rehabilitation.
Outcome and follow-up
The patient made a gradual recovery after discharge with significant improvement in her inflammatory markers and oedema. However, she has not been able to return to work yet.
Discussion
MIS, a post-inflammatory syndrome that affects multiple organs, typically develops 2–6 weeks after recovery from COVID-19 infection in children. First cases of MIS-C were reported in Europe and subsequently in the USA between March and July 2020.18 19 MIS-C can present with many symptoms, including abdominal pain, vomiting, diarrhoea, skin rashes, hypotension and mucocutaneous involvement. Most children with MIS-C require in-hospital admission, with many needing care in ITU for multiorgan support.20–22
More recently, MIS has been reported in adults following COVID-19 infection.23 Published case series and reports highlighted that, like MIS-C, MIS-A also has variable presentation and can affect multiple organs. MIS-A commonly presents with circulatory shock, cardiac dysfunction, skin rashes and often high inflammatory markers. In contrast with MIS-C, lack of severe respiratory involvement and a positive test result for current or previous SARS-CoV-2 infection (nucleic acid, antigen or antibody) in the last 12 weeks are mandatory to diagnose MIS-A.23 The mainstay of treatment for MIS-C and MIS-A is a combination of supportive measures, immunomodulatory drugs and intravenous immunoglobulins.21–23
Although both MIS-C and MIS-A have now been clearly described as complications after COVID-19 infection, their pathophysiology and mechanism of injury are unclear. So far, there are no reports of either MIS-C or MIS-A following vaccination with any of the vaccines developed against COVID-19. There are now several vaccines against COVID-19 that are being used in vaccination programmes worldwide, and more are in the process of development. Therefore, it is essential to identify and report such patients with MIS-C or MIS-A that arise due to vaccination against COVID-19. Both MIS-C and MIS-A share common clinical features with Kawasaki disease. A systematic review by the Brighton Collaboration and several case studies have reported instances of Kawasaki disease associated with vaccines such as diphtheria-tetanus-pertussis, influenza and hepatitis vaccines.24 The Brighton Collaboration network recently recommended criteria to identify MIS cases in adults and children associated with SARS-CoV-2 vaccines. As per these criteria, to diagnose SARS-CoV-2 vaccination-induced MIS, patients should have onset of MIS symptoms within 4–6 weeks of SARS-CoV-2 vaccination for MIS-C and up to 12 weeks for MIS-A.17
A study from NEJM reports that 12 individuals developed delayed hypersensitivity reactions, called the ‘COVID-19 arm’, after receiving Moderna m-RNA SARS-CoV-2 vaccine.25 As demonstrated in the present case, the patient’s symptoms started as a ‘COVID-19 arm’ which evolved as MIS. This emphasises that MIS can manifest following SARS-CoV-2 vaccination. Unlike the longer duration between acute COVID-19 and MIS-C and MIS-A in reported cases so far, our patient’s symptoms suggest MIS-V (MIS due to vaccination) developing within a week after an m-RNA, SARS-CoV-2 vaccine. However, we suspect similar pathophysiology seen in MIS-C and MIS-A may play a key role in patients with MIS-V, causing immune system dysregulation and cytokine storm, leading to multiorgan dysfunction.26 As demonstrated in managing this patient, a diagnosis of MIS-V is a decision of exclusion that requires a high index of clinical suspicion. Hyperinflammatory syndromes such as acute COVID-19, macrophage activation syndrome and sepsis must be ruled out before considering MIS-V in patients who receive SARS-CoV-2 vaccinations. Being aware of the higher prevalence of MIS in children than adults, it remains to be seen whether SARS-CoV-2 vaccination in children would pose a greater risk of MIS-V.
The ideal management of MIS-V will become clearer once more cases are detected and reported. The fact that our patient responded exceptionally well to high-dose intravenous steroids raises the possibility that the mechanism of MIS-V is probably similar to MIS-C and MIS-A. Since steroids, intravenous immunoglobulins and other immunomodulatory medications have all been successfully used to treat cases of MIS-C and MIS-A, we anticipate further studies into their use for treating future cases of MIS-V.
We hope that our case sets a precedent for clinicians globally to identify and report cases of MIS associated with immunisation against SARS-CoV-2 infection. Recently published guidelines to define MIS-V should help raise awareness among clinicians to recognise MIS-V early during the patients’ illness and distinguish this new syndrome from similar conditions. We envisage that the CDC will play a key role in collating the suspected cases of multisystem hyperinflammatory syndromes with circulatory shock to identify MIS-V cases, helping understand the pathophysiology and natural course of illness. This will help to find suitable treatments and better clinical outcomes in patients with MIS-V.
Learning points.
Multisystem inflammatory syndrome (MIS) is widely reported in children but less common in adults. However, SARS-CoV-2 vaccination-induced MIS is not well known. We report the first known case of MIS-V due to a SARS-CoV-2 mRNA vaccine.
We emphasise this case to raise awareness that a SARS-CoV-2 vaccine can cause MIS, highlighting its clinical course.
A high dose of intravenous corticosteroids helped the rapid recovery of our patient. However, further research is required to understand the pathogenesis and treatment options for MIS caused by the SARS-CoV-2 vaccination.
Footnotes
Contributors: AN has conceptualised and designed the work. AN, KPI, CG and AEA have contributed individually and as a group to writing this case report regarding the planning, conduct, reporting and acquisition of data or analysis and interpretation of data.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020;383:2439–50. 10.1056/NEJMoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med 2021;384:643–9. 10.1056/NEJMra2035343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Who coronavirus disease (COVID-19) Dashboard, 2020. Available: https://covid19.who.int/ [Accessed 10 Apr 2021].
- 5.Remmel A. COVID vaccines and safety: what the research says. Nature 2021;590:538–40. 10.1038/d41586-021-00290-x [DOI] [PubMed] [Google Scholar]
- 6.Frank A, Radparvar S, Manasia A, et al. Prolonged anaphylaxis to pfizer coronavirus disease 2019 vaccine: a case report and mechanism of action. Crit Care Explor 2021;3:e0397. 10.1097/CCE.0000000000000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124–30. 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldenburg J, Klamroth R, Langer F. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie 2021;41. [DOI] [PubMed] [Google Scholar]
- 9.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–101. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the heart failure association of the European Society of cardiology. Eur J Heart Fail 2019;21:1169–86. 10.1002/ejhf.1531 [DOI] [PubMed] [Google Scholar]
- 11.Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 2020;183:e7:968–81. 10.1016/j.cell.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020;369:m2094.:m2094. 10.1136/bmj.m2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabeerdoss J, Pilania RK, Karkhele R, et al. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int 2021;41:19–32. 10.1007/s00296-020-04749-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deza Leon MP, Redzepi A, McGrath E, et al. COVID-19-Associated pediatric multisystem inflammatory syndrome. J Pediatric Infect Dis Soc 2020;9:407–8. 10.1093/jpids/piaa061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 2020;20:e276–88. 10.1016/S1473-3099(20)30651-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention . Emergency Preparedness and Response: Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Health Alert Network, 2020. Available: https://emergency.cdc.gov/han/2020/han00432.asp [Accessed 10 Apr 2021].
- 17.Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021;39:3037–49. 10.1016/j.vaccine.2021.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-associated multisystem inflammatory syndrome in children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep 2020;69:1074–80. 10.15585/mmwr.mm6932e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020;324:259–69. 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020;142:429–36. 10.1161/CIRCULATIONAHA.120.048360 [DOI] [PubMed] [Google Scholar]
- 21.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020;383:334–46. 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med 2020;383:347–58. 10.1056/NEJMoa2021756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris SB, Schwartz NG, Patel P, et al. Case Series of Multisystem Inflammatory Syndrome in Adults Associated with SARS-CoV-2 Infection - United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep 2020;69:1450–6. 10.15585/mmwr.mm6940e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phuong LK, Bonetto C, Buttery J, et al. Kawasaki disease and immunisation: a systematic review. Vaccine 2017;35:1770–9. 10.1016/j.vaccine.2016.09.033 [DOI] [PubMed] [Google Scholar]
- 25.Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med 2021;384:1273–7. 10.1056/NEJMc2102131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta A, Gill A, Sharma M, et al. Multi-System inflammatory syndrome in a child mimicking Kawasaki disease. J Trop Pediatr;395. 10.1093/tropej/fmaa060 [DOI] [PMC free article] [PubMed] [Google Scholar]


