Abstract
Background
Pectus excavatum (PE) and pectus carinatum (PC) have generally been considered an aesthetic issue, although there is growing evidence of associated cardiopulmonary function (CPF) impairment, especially in PE patients. The study goal was to determine any correlation between pectus malformations and cardiopulmonary symptoms and function based on systematic assessment of CPF and thoracic measurements, such as Haller Index (HI) and sternal torsion angle (STA).
Methods
Data from 76 adolescent patients with PE (n=30) or PC (n=46) were retrospectively collected referred between January 2015 and April 2018. CPF measurements and thoracic imaging were performed in all patients. HI and STA correction indexes were measured in all patients.
Findings
Medical records from 76 patients (PE n=30; PC n=46) were analysed. Patients were predominantly male (>93.3%), and aged between 13 and 14½ old. PE was associated with airway obstruction, with a forced expiratory volume in 1 s value under the lower limit of normal in 13% of cases (p<0.001). Restrictive syndrome was observed in 23% of cases (p<0.001), with a Z score for total lung capacity under the lower limit of normal. In PC, pulmonary function was not affected. All patients showed slightly decreased values of left and right ejection fraction and cardiac index at rest, although values were within normal range. There were no significant correlations between pulmonary and cardiac functions or between low CPF and thoracic measurements.
Interpretation
Our results confirm the modest impact of pectus malformations on CPF at rest, without correlation with anamnestic dyspnoea on exertion, nor with chest pain or anatomical measurements. Validation of new correction indexes could be helping characterise these malformations and choose optimal therapeutic management.
Keywords: imaging/CT MRI etc, paediatric lung disaese, rare lung diseases, thoracic surgery, lung physiology
Key messages.
What is the impact on cardiopulmonary function (CPF) of chest wall deformity, such as pectus excavatum (PE) and pectus carinatum (PC): can we assume that these two chest wall malformations have no impact on CPF, even in clinically symptomatic patients, and do we have clinical measures or correction indexes predicting the CPF results?
Independently of chest wall deformity, pulmonary and cardiac functions remained within the normal range, although several measures were clutering around the lower limit of the norm. Even though symptoms at rest were present, these functional anomalies did not correlate with clinical impairment, dyspnoea on exertion, neither chest pain nor correction indices measurements at rest.
This study is the first to concomitantly report on pulmonary and cardiac function in adolescent patients with either PE or PC. We compared the type of thorax deformity with cardiopulmonary symptoms based on the systematic assessment of CPF and thoracic imaging, in addition to the Haller Index and sternal torsion angle that were measured with a standardised method in all patients.
Introduction
Pectus excavatum (PE) and pectus carinatum (PC) are the most frequent chest wall deformities, representing 95%–97% of all thoracic morphological anomalies.1 PE is an anterior chest wall depression resulting from a dorsal deviation of the sternum and adjacent costal cartilage and/or ribs, with an estimated prevalence of 1.275%.2 PC is an anterior protrusion of the sternum and associated costal cartilage with a prevalence of 0.6%.3
Although the precise pathophysiology behind these heterogeneous malformations remains unclear, current hypotheses point toward underlying metabolic defects and premature maturation of sternocostal cartilage.1 Associated scoliosis is observed in 5%–21% of PE cases and 8%–32% of PC.4 5 A family history of chest wall deformity is present in up to 43% of PE and 25% of PC patients, pointing toward a probable genetic aetiological predisposition.1 In isolated cases, Marfan or Noonan syndromes should be ruled out.
For many years, these deformities were considered primarily an aesthetic issue with no significant functional impairment. Quality of life and self-esteem are nevertheless diminished in these patients,6 whose most frequent complaint relates to aesthetic considerations. Nonetheless, in the last decades evidence of functional involvement has been accumulating, and numerous studies have shown some degree of lower airway obstruction in children, increasing with age, as well as a variable proportion of restrictive patterns.3 7 Some studies have also suggested an impact on cardiovascular function, in PE patients, notably decreased ejection fraction and limited exercise tolerance associated with lesser maximum stroke volume on MRI evaluations due to thoracic compression.8 9 Minimal invasive surgical procedures to treat PE were introduced in 1997, and many studies have shown that levels of improvement in cardiopulmonary function (CPF) after repair of PE depend on the degree of malformation.10 11 For PC, very little data are available on clinical benefits of conservative versus surgical treatment.
Based on these considerations, the current research project aimed to systematically study CPF in a cohort of patients with PE or PC, using the widely normalised values previously described.12 13 We first analysed the correlation between pulmonary and cardiac functional parameters, and then the correlation between cardiopulmonary and patient morphological parameters, such as the Haller Index (HI) and sternal torsion angle (STA).
Methods
We retrospectively reviewed data of 108 patients with thoracic deformity referred between January 2015 and April 2018 for multidisciplinary assessment at the University Center of Pediatric Surgery of Western Switzerland. All patients were systematically evaluated based on pulmonary function tests (PFT), orthopaedic assessment and cardiac MRI. Inclusion criteria were: presence of isolated congenital PE or PC, age under 16 years and absence of previous conservative or surgical treatment. Exclusion criteria were: pulmonary or cardiological malformation or underlying pathology (based on medical history and formal spirometry to exclude asthma), syndromic PE/PC, missing data or PFT with non-reproductible or uninterpretable values. Patients were not involved in the study design and development.
Orthopedic assessment
Anthropometric measures were recorded and Beighton/Marfan scores calculated.14 15 Full spine low-dose X-ray was performed using a two-dimensional EOS system (Biospace Med, Paris, France). HI was measured on MRI as previously described.16 The described upper limit of normal (ULN) value for HI is 2.7 (range between 2.5 and 2.7).17 We also calculated HI in PC to assess the degree of sternal protrusion, as previously published.18 STA was measured to evaluate pectus severity in both PE and PC.19
Pulmonary assessment
All patients underwent PFT using Medisoft BodyBox 5500. Spirometry including forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC ratio, forced expiratory flow between 25% and 75% of FVC curve (FEF25-75), as well as lung volumes including total lung capacity (TLC) and vital capacity (VC) and lung diffusing capacity were performed. Global lung initiative (GLI) reference values were used for spirometric and lung diffusing capacity results.13 Single-breath technique for lung diffusing capacity of carbon monoxide was used. Results were expressed in percentage of expected value and in Z scores according to GLI references. For TLC, Rosenthal equations were used to obtain percentage of expected value and Z scores.20 These results were normalised using reference values recently published for mean, lower limit of normal (LLN) and ULN.21
Cardiac assessment
Functional cardiac MRI was obtained with a clinical Siemens 3T PRISMA FIT and analysed using the SyngoVia (Siemens Healthcare, Erlangen, Ge) or the CVI42 (Circle, Calgary, California, USA) software. Left and right ventricle cardiac index (LVCI and RVCI), left and right ventricle ejection fraction (LVEF and RVEF), left and right ventricle telediastolic volume (LVTDV and RVTDV) and left and right ventricle telesystolic volume (LVTSV and RVTSV) were recorded from the MRI exam. For cardiac function parameters, standard cine in left and right chambers and short axis views were acquired. Results were normalised using reference values recently published for mean, LLN and ULN.12
Statistical data analysis
Patient characteristics were described using mean (SD) or frequencies and were compared between PE and PC using the t-test or Fisher’s exact test.
Cardiac and PFT were formulated as means and SD. In PE and PC, t tests were applied to test the null hypothesis that the mean of Z scores equals zero, which is the value expected if patients’ cardiac and PFT values were similar to the average population. The mean level of Z scores was compared between PE and PC patients with t tests. Associations with sternal angle and HI were assessed with Spearman’s correlation coefficients. For each Z score, the proportion of patients with a value lower or higher than the reference values (±1.64 for pulmonary functions and ±1.96 for cardiac functions) was assessed. As usually defined in the respective dedicated literature about the PFT and cardiac functional values.in Z score, the expected proportions of observations out of the reference intervals are: 5% lower than −1.64% and 5% higher than +1.64 for PFT, 2.5% lower than −1.96 and 2.5% higher than +1.96 for cardiac functions. The null hypothesis that the probability that an observation of a patient falls lower than the lower reference value equals 0.05 (or 0.025), as expected in a normal population. Binomial tests were used. Similar analyses were conducted for the upper reference value. The type 1 error was 0.05 two sided for all statistical analyses. Analyses were conducted with the software R V.4.0.2 (Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Medical records from 108 patients (46 PE, 62 PC) were reviewed. Data from 76 subjects were analysable, 30 with PE and 46 with PC (online supplemental figure 1). The characteristics of these 76 patients are summarised in table 1. We observed male preponderance (93%) in a higher degree than previously published.22 A high proportion of patients presented chest pain, 20% of PE and 15% of PC possibly linked to unusual muscular and nerve insertion. None of the patients presented Marfan or Beighton scores high enough to suspect hyperlaxity.
Table 1.
Patient characteristics
| Population | Pectus excavatum (n=30) | Pectus carinatum (n=46) | P value |
| Male gender, n (%) | 28 (93.3) | 43 (93.5) | >0.99* |
| Age in years, mean (±SD) | 13.8 (±2.0) | 14.5 (±1.7) | 0.15# |
| Height for age in Z score, mean (±SD) | 0.93 (±1.01) | 0.84 (±0.92) | 0.69# |
| Weight for age in Z score, mean (±SD) | −0.001 (±0.76) | 0.01 (±0.86) | 0.92# |
| Span in cm, mean (±SD) | 169.8 (±17.0) | 172.7 (±11.4) | 0.42# |
| Span/height ratio, mean (±SD) | 1.01 (±0.02) | 1.01 (±0.03) | 0.40# |
| Spinal deformity, n (%) | 5 (16.6) | 14 (30.4) | |
| Scoliosis, n (%) | 3 (10.0) | 8 (17.4) | 0.51* |
| Kyphosis, n (%) | 2 (6.6) | 6 (13) | 0.47* |
| Familial history of pectus, n (%) | 13 (43) | 16 (34.8) | 0.48* |
| Pectus characteristics | |||
| Anteroposterior measure in cm, mean (±SD) | 5.7 (±1.4) | 10.1 (±1.3) | <0.001# |
| Transverse measure in cm, mean (±SD) | 24.5 (±2.5) | 24.2 (±1.7) | 0.53# |
| Haller index, mean (±SD) | 4.62 (±1.33) | 2.44 (±0.32) | <0.001# |
| Sternal angle in degrees, mean (±SD) | 12.22 (±9.77) | 8.49 (±6.38) | 0.09# |
| Thoracic symptoms | 10 (33) | 14 (30.43) | 0.80* |
| Pain at rest, n (%) | 6 (20.0) | 7 (15.2) | 0.76* |
| Exertion dyspnoea, n (%) | 4 (13.3) | 8 (17.4) | 0.75* |
Comparison between the groups was performed with the Fisher’s exact test (*) or with the t-test (#).
bmjresp-2021-001020supp001.pdf (1.1MB, pdf)
Pulmonary functions
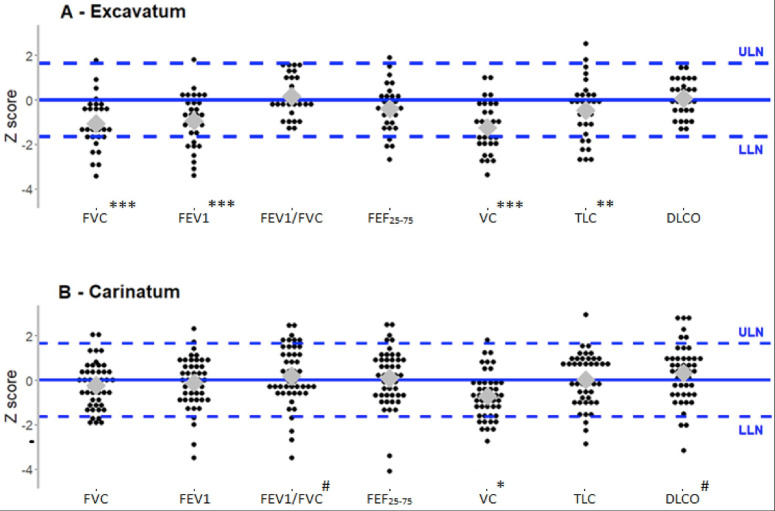
In PE patients, the Z scores for FVC, FEV1 and FEF25-75 were significantly lower than reference values. In PC patients, no significant difference was observed compared with reference values (table 2). However, the proportion of PE patients presenting FVC and FEV1 values below the LLN (respectively 30%, n=9% and 27%, n=8) was higher than the 5% expected in a normal population (respectively p<0.001 and p=0.001). Thirteen percent (n=4) of PE patients showed FEF25-75 values under the LLN, but the number of patients was not significantly different from the expected percentage of 5% (p=0.06) (figure 1A). In PC patients, no significant differences in lung flow were observed, although lower values for FVC and FEV1 were measured, with 4% of patients under the LLN (figure 1B). The FEV1/FVC ratio was similar to the expected values both for PE and PC, and no statistically significant value was under the LLN.
Table 2.
Pulmonary function
| Pectus excavatum (n=30) |
Pectus carinatum (n=46) |
|||||
| % of predicted | Z score | P value (Z score) |
% of predicted | Z score | P value (Z score) |
|
| Mechanics | ||||||
| FVC | 87.9 (±13.4) | −1.08 (±1.16) | <0.0001 | 97.7 (±11.7) | −0.23 (±1.01) | 0.12 |
| FEV1 | 87.2 (±16.7) | −0.94 (±1.19) | 0.0002 | 98.7 (±13.8) | −0.13 (±1.15) | 0.45 |
| FEV1/FVC | 100.6 (±6.3) | 0.15 (±0.92) | 0.38 | 100.5 (±9.7) | 0.19 (±1.33) | 0.35 |
| FEF25-75 | 92.09 (±22.9) | −0.40 (±1.06) | 0.05 | 103.5 (±28.3) | 0.10 (±1.32) | 0.61 |
| Volumes | ||||||
| VC | 85.5 (±13.1) | −1.24 (±1.15) | <0.0001 | 92.1 (±11.8) | −0.71 (±1.03) | <0.001 |
| TLC | 96.07 (±13.52) | −0.48 (±1.36) | 0.06 | 99.5 (±13.6) | 0.09 (±1.25) | 0.64 |
| Diffusion | ||||||
| DLCO | 100.6 (±12.1) | 0.06 (±0.79) | 0.68 | 106.0 (±18.9) | 0.31 (±1.3) | 0.11 |
Results are expressed in Z score or in % of predicted values; mean (±SD).
P values are calculated with for Z scores and express the difference between the analysed group and the reference values.
DLCO, diffusing capacity for carbon monoxide; FEF25-75, forced expiratory flow from 25% to 75% of expiration; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; TLC, total lung capacity; VC, vital capacity.
Figure 1.
Distributions of z-scores of pulmonary functions in patients with pectus excavatum (n=30) (A) and in patients with pectus carinatum (n=46) (B). Black circles represent individual observations; the grey diamonds the median values and the dashed lines the reference values (z-scores of −1·64 and +1·64 corresponding to 5% and 95% percentiles in control population). All p results are measured for medians values. *P<0.05 for under LLN values; **p<0.001 for under LLN values; ***p<0.0001 for under LLN values. #P<0.05 for above ULN value. DLCO, diffusing capacity of the lung for carbon monoxide; FEF25-75, forced expiratory flow from 25% to 75% of expiration; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LLN, lower limit of normal; TLC, total lung capacity; ULN, upper limit of normal; VC, vital capacity.
Concerning lung volumes, the mean Z score for VC was significantly lower than expected in both PE and PC groups (p<0.0001 and p<0.001, respectively) (table 2). In PE patients, Z scores below the LLN were found in 23% (n=7, p<0.001) for TLC (figure 1A). We also observed that variation in TLC was not statistically significant in this group (figure 1B).
Cardiac functions
Patients with PE or PC had Z scores lower than expected according to reference mean values for LVCI, LVEF, RVCI and RVEF (table 3).
Table 3.
Cardiac function
| Pectus excavatum (n=30) |
P value | Pectus carinatum (n=46) |
P value | |
| Left ventricle | ||||
| TDV | 0.20 (±1.24) | 0.39 | 0.24 (±1.31) | 0.21 |
| TSV | 0.90 (±1.44) | 0.002 | 0.46 (±1.33) | 0.03 |
| CI | −1.07 (±0.76) | <0.0001 | −0.82 (±1.03) | <0.0001 |
| EF | −0.88 (±0.90) | <0.0001 | −0.51 (±1.05) | 0.002 |
| TDMM | −0.13 (±0.92) | 0.44 | 0.15 (±0.95) | 0.19 |
| Right ventricle | ||||
| TDV | 0.57 (±1.65) | 0.07 | 0.33 (±1.35) | 0.10 |
| TSV | 1.49 (±1.73) | <0.0001 | 0.71 (±1.29) | 0.0005 |
| CI | −1.11 (±0.76) | <0.0001 | −0.86 (±1.07) | <0.0001 |
| EF | −1.81 (±1.10) | <0.0001 | −0.82 (±1.42) | 0.0003 |
Results are expressed in Z score; mean (±SD).
P values express the difference between the analysed group and the reference values.
CI, Cardiac Index; EF, ejection fraction; TDMM, telediastolic myocardic mass; TDV, telediastolic volume; TSV, telesystolic volume.
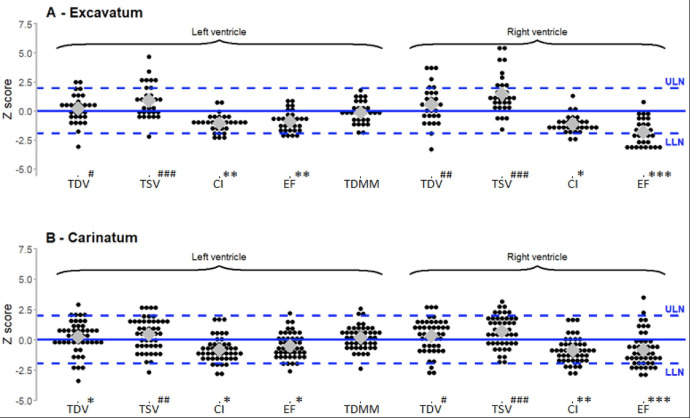
In PE patients, the proportion of Z scores under the LLN was higher than expected for LVCI (16.7% of patients, n=5, p<0.001) and LVEF (13.3% of patients, n=4, p=0.006) (figure 2A). Right heart function was also impaired and the results were below the LLN in 10% of the patients (n=3, p=0.004) for RVCI and in 53% (n=16, p<0.0001) for RVEF. No patient with PE showed LVEF or RVEF values below 50%.
Figure 2.
Distributions of z-scores of cardiac functions in patients with pectus excavatum (n=30) (A) and in patients with pectus carinatum (n=46) (B). TSV: telesystolic volume, TDMM: telediastolic myocardic mass, l, ULN: upper limit of normal. Black circles represent individual observations; the grey diamonds the median values and the blue dashed lines the reference values (z-scores of −1·96 and +1·96 corresponding to 2.5% and 97.5% percentiles in the general population. All p results are measured for medians values.*: p<0.05 for under LLN values; **: p<0.001 for under LLN values; ***: p<0.0001 for under LLN values#: p<0.05 for above ULN values; ##: p<0.001 for above ULN values; ###: p<0.0001 above ULN value. CI, cardiac index; EF, ejection fraction; LLN, lower limit of norma; TDV: telediastolic volume;
Z scores below the LLN were also found in PC patients: 10.9% (n=5, p=0.006) for LVCI, 8.7% (n=4, p=0.03) for LVEF, 13.0% (n=6, p=0.001) for RVCI and 26.1% (n=12, p<0.0001) for RVEF (figure 2B). Similarly to PE patients, no measure below 50% was noted for RVEF in PC patients.
Interestingly, in PE patients, Z scores were above the ULN in LVTDV and LVTSV patients. A similar pattern of results was found for right heart evaluation, with Z scores above ULN for RVTDV and for RVTSV.
In patients with PC, LVTST had Z scores above the ULN. For LVTDV, no significant difference was observed compared with reference values. Similarly, PC patients had Z scores above the ULN for RVTSV, while no statistically significant data were observed concerning RVTDV.
Correlation between orthopedic assessment and CPF markers
We compared the HI and the STA measurements with all CPF measurements and we did not find any correlations.
Discussion
PE and PC continue to be widely considered primarily as an aesthetic issue even though evidence of CPF impact is accumulating, also for the older population.23 Previous work mainly reported results in relatively small patient samples where cardiac and pulmonary impact had been considered independently from each other.3 8 9 To our knowledge, there is no published data coupling cardiac and pulmonary function evaluation for the same cohort of paediatric subjects presenting PE or PC. In addition, most of the studies reported small series of patients with only little detailed data on CPF.
In our study, the prevalence of a positive family history as well as spinal deformity is concordant with previous publications. Our results show a clear tendency towards lower CPF values in both PE and PC patients. For pulmonary function, PE patients were more affected than PC patients, whereas cardiac functional values were equally impacted among both groups.
Regarding pulmonary results, we observed that even if symptoms were reported at rest, they had little effect on clinical evaluation, with mild functional anomalies. Indeed, only PE patients presented decreased lung flow measured by spirometry, but both pectus subtypes had a tendency to decreased lung volumes.
Lung function in PE patients was associated with mean Z scores for FVC, FEV1 and FEF25-75 within the normal range, although scores were lower than in the general population. This confirms previous studies on adult subjects in which obstructive patterns have been observed.24 Nonetheless, the FEV1/FVC ratio in PE patients was similar to the general population. Decreased mid expiratory flows (FEF25-75) suggests obstruction on small airways and is also associated with asthma symptoms. We reported values under the LLN for FVC (30%), FEV1 (27%) and FEF 25–75 (13.3%) of PE patients corresponding to previous studies where 5.6% to 41% of obstructive syndrome was reported.3 7 Lung volumes were associated with a restrictive syndrome in 23% of PE patients, consistent with previous studies.3 7 These results can be explained by a deformed thoracic cage in PE and PC that decreases mechanical compliance and certainly impacts respiratory muscle efficiency. As abnormal development of the sternocostal cartilage is thought to be a determinant in pectus formation, increased thoracic wall stiffness is also possibly present. However, in PC patients, only VC was significantly decreased, as previously described.3 25 Considering the scarcity of lung function data in PC paediatric patients, this represents an important learning that differs clearly from PE data.
A proportion of PE patients complained of dyspnoea on exertion (13.3%) and chest pain at rest (20%), although rates were lower compared with previously published data. A large multicentric study reported a prevalence 62% for shortness of breath, and 32% for chest pain at rest.26 Casar Berazaluce et al recently published a similar prevalence of 41% of shortness of breath and 62% for chest pain at rest in 345 patients with PE.27 Even if the precise prevalence of thoracic symptoms in PC patients is not well documented, progressive symptoms of dyspnoea or reduced endurance improving after surgical repair were described in moderate to severe thoracic deformity.28 Our results confirm these tendencies, with 29% of PC patients reporting thoracic symptoms at rest or during physical activity without correlation with pectus severity. Unfortunately, we lacked a prospective quantifiable value to evaluate pain level and duration.
For both pectus types, these symptoms could have been partially associated with a relative exercise deconditioning secondary to embarrassment to undress in public.29 30 In addition, asthmatic patients were excluded, which could also have decreased the number of patients with pulmonary symptoms.
As for pulmonary function, cardiac function was also close to normal range. A set of reference values for cardiac function evaluated by MRI in children exists and has Z scores with ULN and LLN defined as mean ±2 SD.12 It is worth noting that these values are only normalised for sex and total body surface and can therefore not be used as strict normative values. To date, percentage of EF remains the main tool to evaluate cardiac function, with abnormal values below 55%. Applying these values to our PE and PC patients, around 50% and 25% had an RVEF under the LLN, respectively. Nonetheless, no RVEF value was recorded below 50% either for PE or PC, highlighting the absence of an argument for significant cardiac functional impairment at rest in these patients.
Consistent with these observations, the mean values for LVCI, LVEF and RVCI in PE and PC patients were significantly lower than expected, but within normal range. Here again, despite no clearly defined pathological patterns, observations confirmed a shift towards lower normal values for the major indicators of cardiac function at rest in pectus patients consistent with previously published data.8 31 However, no correlation was found between EF and HI severity.
Our results confirm a tendency toward lower RVEF in PE and attest the possibility of a same effect on LVEF. Our data also show elevated ventricular end-diastolic and end-systolic volumes in both types of pectus. This reinforces the probability of an anatomically driven bilateral relative impairment of myocardial contractility due to thoracic and sternal cardiac compression. Such a mechanism was previously assumed in the presence of lower RVEF and decreased RV circumferential strain magnitude in PE compared with controls.32
Both pectus groups showed pulmonary and cardiac functions within the normal range, with a tendency toward statistically significant lower values. We did not find any association between cardiac and pulmonary function. Even though symptoms were present at rest, these functional anomalies are not a proper reflection of clinical impairment, which depends, among other factors, on physiological adaptation to exercise and training. Previous studies evaluating the relation between PE and CPF during physical effort using treadmill or cycle ergometer exercise testing showed improved maximal oxygen consumption after surgical repair.9 11 CPF parameters have yet to be precisely and directly observed during exercise, in particular using functional cardiac MRI during or directly after physical effort.
Finally, previous publications report a possible association between severity of HI and reduction of pulmonary function in PE.33 In our cohort, we did not find any statistically significant association between HI and STA with pulmonary or cardiac functional parameters in PE and PC patients, reinforcing the need of an accurate cardiopulmonary evaluation of these patients.
According to previously published work, even though CPF impairment is more likely to be observed both at rest and during exercise in PE patients with increased thoracic deformity, modest pectus can also be accompanied by thoracic symptoms or functional limitations. Conversely, some patients more severely affected according to standard morphological evaluation tools, such as HI, display no major impairment of pulmonary function. It is worth noting that a significant 47% of overlapping HI values between PE patients and controls in a previous paediatric population.34
Full cardiopulmonary pathophysiology of pectus patients is not yet fully understood, and a more refined evaluation is needed to properly assess each situation, especially in the case of mixed deformities combining morphological measurements, such as HI and STA, with functional imaging and tests. The external three-dimensional scanner is a promising tool and has shown a certain degree of correlation with HI in PE.35
Although our study examined pectus malformation in a paediatric cohort in great detail and, to the best of our knowledge, is the first to precisely analyse the impact of PC on CPF, it does present several limitations. Indeed, the limited number of patients might have contributed toward the lack of correlation between severity of thorax deformity and diminished functional parameters. No control group was enrolled, but all parameters were normalised using published Z-score data. In addition, PE patients did not present severe HI, with a relatively dense distribution of mean values around 4.6. Regarding the relatively high frequency of associated symptoms in our cohort, the psychological aspect of chest deformities could play a significant role. Finally, our evaluation was performed before treatment and at rest. We, therefore, cannot exclude an impact of PE or PC during exercise in patients with values at the LLN. This could have explained an absence of correlation between HI and impaired functional values.
Conclusion
Taken together, our results clearly suggest that alterations of CPF in PE or PC patients are not correlated to dyspnoea on exertion, nor to chest pain or anatomical measurements (such as HI or STA). The same assessments performed during the exercise may help to better understand the anatomical role of pectus pathophysiology. In addition, the psychological aspect could play a key role in patients’ symptomatology and should be assessed in conjunction with other aspects of patient management. Finally, validation of new correction indexes could be of great help in characterising these malformations and choosing the best therapeutic management.
Acknowledgments
We would like to extend our gratitude to the medical team of the paediatric clinical research Platform (PGO) of the Geneva hospital for their help in patient recruitment and database management. We thank also Christophe Combescure for the statistical analyses.
Footnotes
Contributors: SR, PL, CB-A, IR-M contributed to conception, design and all the steps of the study. SR, JW, AT-F, ST, MB, J-PV, RC and IR-M contributed to acquisition and analyse of all data. SR, PL, CB-A, IR-M, MB, J-PV and ST have been involved in drafting and revising the manuscript. All authors have given final approval of the version to be published.
Funding: The Geneva’s Lungen Liga and the Fondation privée des Hôpitaux Universitaires de Genève support this work.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The institutional ethics committee approved this study and informed consent was obtained during scheduled hospital visits (CER 2017–01745).
References
- 1.Brochhausen C, Turial S, Müller FKP, et al. Pectus excavatum: history, hypotheses and treatment options. Interact Cardiovasc Thorac Surg 2012;14:801–6. 10.1093/icvts/ivs045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fokin AA, Steuerwald NM, Ahrens WA, et al. Anatomical, histologic, and genetic characteristics of congenital chest wall deformities. Semin Thorac Cardiovasc Surg 2009;21:44–57. 10.1053/j.semtcvs.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 3.Coskun ZK, Turgut HB, Demirsoy S, et al. The prevalence and effects of pectus excavatum and pectus Carinatum on the respiratory function in children between 7-14 years old. Indian J Pediatr 2010;77:1017–9. 10.1007/s12098-010-0155-5 [DOI] [PubMed] [Google Scholar]
- 4.Waters P, Welch K, Micheli LJ, et al. Scoliosis in children with pectus excavatum and pectus carinatum. J Pediatr Orthop 1989;9:551–6. 10.1097/01241398-198909010-00009 [DOI] [PubMed] [Google Scholar]
- 5.Tauchi R, Kawakami N, Tsuji T, et al. Evaluation of thoracic factors after scoliosis surgery in patients with both scoliosis and pectus excavatum. Eur Spine J 2018;27:381–7. 10.1007/s00586-016-4753-4 [DOI] [PubMed] [Google Scholar]
- 6.Lawson ML, Cash TF, Akers R, et al. A pilot study of the impact of surgical repair on disease-specific quality of life among patients with pectus excavatum. J Pediatr Surg 2003;38:916–8. 10.1016/S0022-3468(03)00123-4 [DOI] [PubMed] [Google Scholar]
- 7.Koumbourlis AC, Stolar CJ. Lung growth and function in children and adolescents with idiopathic pectus excavatum. Pediatr Pulmonol 2004;38:339–43. 10.1002/ppul.20062 [DOI] [PubMed] [Google Scholar]
- 8.Saleh RS, Finn JP, Fenchel M, et al. Cardiovascular magnetic resonance in patients with pectus excavatum compared with normal controls. J Cardiovasc Magn Reson 2010;12:73. 10.1186/1532-429X-12-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abu-Tair T, Turial S, Hess M, et al. Impact of pectus excavatum on cardiopulmonary function. Ann Thorac Surg 2018;105:455–60. 10.1016/j.athoracsur.2017.09.037 [DOI] [PubMed] [Google Scholar]
- 10.Sigalet DL, Montgomery M, Harder J, et al. Long term cardiopulmonary effects of closed repair of pectus excavatum. Pediatr Surg Int 2007;23:493–7. 10.1007/s00383-006-1861-y [DOI] [PubMed] [Google Scholar]
- 11.Das BB, Recto MR, Yeh T. Improvement of cardiopulmonary function after minimally invasive surgical repair of pectus excavatum (Nuss procedure) in children. Ann Pediatr Cardiol 2019;12:77–82. 10.4103/apc.APC_121_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 2015;17:29. 10.1186/s12968-015-0111-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324–43. 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr 2011;158:119–23. 10.1016/j.jpeds.2010.07.021 [DOI] [PubMed] [Google Scholar]
- 15.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476–85. 10.1136/jmg.2009.072785 [DOI] [PubMed] [Google Scholar]
- 16.Haller JA, Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg 1987;22:904–6. 10.1016/S0022-3468(87)80585-7 [DOI] [PubMed] [Google Scholar]
- 17.Daunt SW, Cohen JH, Miller SF. Age-Related normal ranges for the Haller index in children. Pediatr Radiol 2004;34:326–30. 10.1007/s00247-003-1116-1 [DOI] [PubMed] [Google Scholar]
- 18.Lain A, Garcia L, Gine C, et al. New methods for imaging evaluation of chest wall deformities. Front Pediatr 2017;5:257. 10.3389/fped.2017.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egan JC, DuBois JJ, Morphy M, et al. Compressive orthotics in the treatment of asymmetric pectus carinatum: a preliminary report with an objective radiographic marker. J Pediatr Surg 2000;35:1183–6. 10.1053/jpsu.2000.8724 [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal M, Cramer D, Bain SH, et al. Lung function in white children aged 4 to 19 years: II--Single breath analysis and plethysmography. Thorax 1993;48:803–8. 10.1136/thx.48.8.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culver BH, Graham BL, Coates AL, et al. Recommendations for a standardized pulmonary function report. An official American thoracic Society technical statement. Am J Respir Crit Care Med 2017;196:1463–72. 10.1164/rccm.201710-1981ST [DOI] [PubMed] [Google Scholar]
- 22.Biavati M, Kozlitina J, Alder AC, et al. Prevalence of pectus excavatum in an adult population-based cohort estimated from radiographic indices of chest wall shape. PLoS One 2020;15:e0232575. 10.1371/journal.pone.0232575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kragten HA, Siebenga J, Höppener PF, et al. Symptomatic pectus excavatum in seniors (SPES): a cardiovascular problem? Neth Heart J 2011;19:73–8. 10.1007/s12471-010-0067-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly RE, Obermeyer RJ, Nuss D. Diminished pulmonary function in pectus excavatum: from denying the problem to finding the mechanism. Ann Cardiothorac Surg 2016;5:466–75. 10.21037/acs.2016.09.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ateş O, Karakuş OZ, Hakgüder G, et al. Pectus carinatum: the effects of orthotic bracing on pulmonary function and gradual compression on patient compliance. Eur J Cardiothorac Surg 2013;44:e228–32. 10.1093/ejcts/ezt345 [DOI] [PubMed] [Google Scholar]
- 26.Kelly RE, Shamberger RC, Mellins RB, et al. Prospective multicenter study of surgical correction of pectus excavatum: design, perioperative complications, pain, and baseline pulmonary function facilitated by Internet-based data collection. J Am Coll Surg 2007;205:205–16. 10.1016/j.jamcollsurg.2007.03.027 [DOI] [PubMed] [Google Scholar]
- 27.Casar Berazaluce AM, Jenkins TM, Garrison AP, et al. The chest wall gender divide: females have better cardiopulmonary function and exercise tolerance despite worse deformity in pectus excavatum. Pediatr Surg Int 2020;36:1281–6. 10.1007/s00383-020-04738-5 [DOI] [PubMed] [Google Scholar]
- 28.Fonkalsrud EW. Surgical correction of pectus carinatum: lessons learned from 260 patients. J Pediatr Surg 2008;43:1235–43. 10.1016/j.jpedsurg.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 29.Lomholt JJ, Jacobsen EB, Thastum M, et al. A prospective study on quality of life in youths after pectus excavatum correction. Ann Cardiothorac Surg 2016;5:456–65. 10.21037/acs.2016.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Frari B, Sigl S, Schwabegger AH, et al. Impact of surgical treatment of pectus carinatum on cardiopulmonary function: a prospective study. Eur J Cardiothorac Surg 2021;59:382–8. 10.1093/ejcts/ezaa335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Töpper A, Polleichtner S, Zagrosek A, et al. Impact of surgical correction of pectus excavatum on cardiac function: insights on the right ventricle. A cardiovascular magnetic resonance study†. Interact Cardiovasc Thorac Surg 2016;22:38–46. 10.1093/icvts/ivv286 [DOI] [PubMed] [Google Scholar]
- 32.Truong VT, Li CY, Brown RL, et al. Occult RV systolic dysfunction detected by CMR derived RV circumferential strain in patients with pectus excavatum. PLoS One 2017;12:e0189128. 10.1371/journal.pone.0189128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawson ML, Mellins RB, Paulson JF, et al. Increasing severity of pectus excavatum is associated with reduced pulmonary function. J Pediatr 2011;159:256–61. 10.1016/j.jpeds.2011.01.065 [DOI] [PubMed] [Google Scholar]
- 34.St Peter SD, Juang D, Garey CL, et al. A novel measure for pectus excavatum: the correction index. J Pediatr Surg 2011;46:2270–3. 10.1016/j.jpedsurg.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 35.Glinkowski W, Sitnik R, Witkowski M, et al. Method of pectus excavatum measurement based on structured light technique. J Biomed Opt 2009;14:044041. 10.1117/1.3210782 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2021-001020supp001.pdf (1.1MB, pdf)
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.