Abstract
We report a case of the use of colchicine in a patient infected with SARS-CoV-2 virus. A 37-year-old man with COVID-19 presented with moderate symptoms, mild pulmonary impairment and elevated inflammatory markers, suggesting an increased risk of cytokine storm and possible worsening of clinical condition. Experimental use of colchicine resulted in an 85% decrease in C reactive protein levels 3 days after treatment initiation and a 182.6% decrease in interleukin-6 levels 8 days after treatment initiation. Due to the lack of effective therapies, it is important to search for potential compounds and compounds that focus on controlling the danger caused by systemic inflammation in COVID-19. Although further research is needed in the area of colchicine and viral infection, preliminary efficacy was observed.
Keywords: COVID-19, infectious diseases
Background
COVID-19 was first reported in Wuhan, China in December 2019.1 This disease was confirmed to be caused by the new type of enveloped RNA coronavirus B, SARS-CoV-2.2 As of April 2021, 212 countries and territories worldwide and 2 international transports have been affected by COVID-19 and there have been 164 million laboratory-confirmed cases worldwide, resulting in 3.39 million deaths. According to initial estimates, about 80% of the deaths caused by the novel coronavirus occurred in patients over 60 years of age and 75% of them had pre-existing health conditions such as cardiovascular disease and diabetes.3 In the processes of clinical diagnosis and treatment, many critically ill patients were found to have symptoms of multiple-organ dysfunction. Based on an in-depth analysis of the immune mechanism triggered by the SARS-CoV-2 virus, numerous data suggest that the exacerbation of COVID-19 is directly related to the enhanced immune system response that triggers a secondary state of severe inflammation of the organism known as cytokine storm.4 Colchicine is an alkaloid extracted from plants of the genus Colchicum. The therapeutic use of colchicine has been extensively documented in gout and Familial Mediterranean fever; it has also been used in other diseases such as Behçet’s disease, pericarditis, coronary artery disease and other inflammatory and fibrotic conditions.5 The treatment prevented the cytokine storm, as the patient had an alarming inflammatory state.
Case presentation
On 22 April 2020, a 37-year-old man working in healthcare was referred to the doctor with severe fatigue and cough for 2 days. Due to his profession, he had contact with hospitalised patients with severe COVID-19. On 24 April 2020, his RT-PCR nasopharyngeal swab tested positive. The patient’s reported symptoms were sporadic dry cough, fatigue, low-grade fever (37.5°C), loss of sense of taste and smell, muscle pain, severe eye pain and headache. Oral hydroxychloroquine was prescribed with oral azithromycin for 10 days. The complete treatment during the course of the disease and other therapeutic strategies are shown in table 1.
Table 1.
Evolutive clinical course of the disease and treatments
| Day of disease | Event | Vit C (2 g) | Zinc (66 mg) |
NAC (600 mg) |
Dipyrone (1 g) |
HCQ (400 mg) |
AZT (500 mg) |
NTZ (500 mg) |
Colchicine (0.5 mg) |
| 1 | Fever | BID | |||||||
| 2 | – | BID | |||||||
| 3 | Positive PCR | QD | QD | BID | BID | QD | BID | ||
| 4 | – | QD | QD | BID | QD | QD | BID | ||
| 5 | – | QD | QD | BID | QD | QD | BID | ||
| 6 | CI | QD | QD | BID | QD | QD | BID | ||
| 7 | CW | QD | QD | BID | QD | QD | BID | ||
| 8 | IgM + IgG - |
QD | QD | BID | QD | QD | |||
| 9 | Test results | QD | QD | QD | BID | QD | QD | ||
| 10 | – | QD | QD | QD | BID | QD | QD | ||
| 11 | – | QD | QD | QD | BID | QD | QD | ||
| 12 | – | QD | QD | QD | BID | QD | QD | ||
| 13 | Chest CT | QD | QD | QD | BID | ||||
| 14 | Test results | QD | QD | QD | BID | BID | |||
| 15 | – | QD | QD | QD | BID | QD | |||
| 16 | – | QD | QD | QD | BID | QD | |||
| 17 | Test results | QD | QD | QD | BID | QD | |||
| 18 | CI | QD | QD | QD | BID | QD | |||
| 19 | CI | QD | QD | QD | BID | QD | |||
| 20 | CI | QD | QD | QD | BID | QD | |||
| 21 | CI | QD | QD | QD | BID | QD | |||
| 22 | Test results | QD | QD | QD | BID | QD |
AZT, azithromycin; BID, twice (two times) a day; CI, clinical improvement; CW, clinical worsening; HCQ, hydroxychloroquine; NAC, N-Acetyl Cysteine; NTZ, nitazoxanide; QD, once a day.
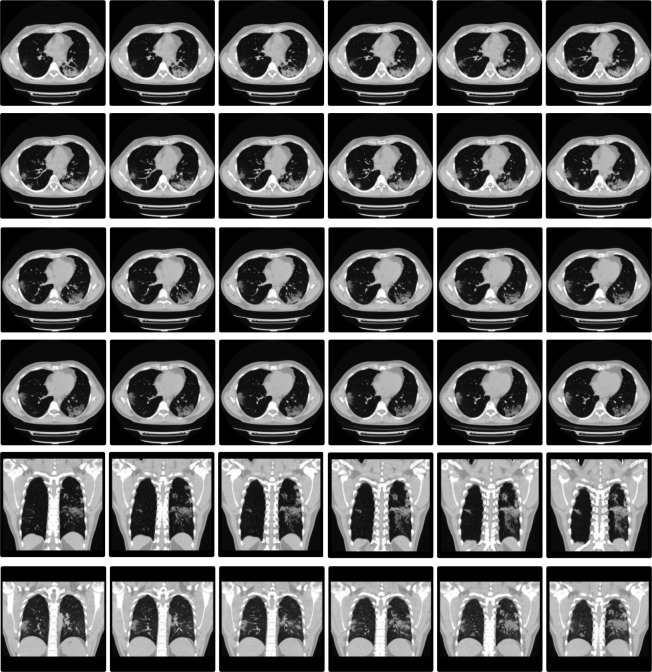
On the sixth day of the course of the disease, the patient showed great improvement of symptoms and on the seventh day the patient showed relapse of the disease with worsening of symptoms, chills and burning sensation in some parts of the body such as knees and calves. On the eighth day of illness, his anti COVID-19 IgG/IgM rapid test showed a positive result for IgM and a negative for the IgG marker. Inflammatory markers such as C reactive protein, ferritin, interleukin-6 (IL6), lactate dehydrogenase (LDH) and fibrinogen were determined during the course of the disease. Tests performed on 30 April 2020 showed elevated levels of ferritin (709 ng/dL), IL-6 (19.5 pg/mL), fibrinogen (581 mg/dL) and C reactive protein (5301 mg/dL). On 4 May, he underwent chest CT (figure 1) which showed multiple loci of diffuse ground glass opacities with predominance in the pulmonary periphery, more extensive in the posterior regions of the lower lobes exceeding 3.0 cm in length and sometimes perilobular distribution, associated with reticular opacities and septal thickening, and containing areas of denser consolidations. Extension of pulmonary involvement close to 25%. On 5 May 2020, day 14 in the disease course, the patient did the tests for the second time and the results showed a further increase in inflammatory markers: ferritin level to 920 ng/dL (29.7% increase from the previous test) and C reactive protein to 7982 mg/dL (50.5% increase from the previous test). Colchicine treatment was started after the exacerbation of symptoms and prescribed for 20 days. On the first day of treatment, the patient took 1 mg of colchicine, followed by 0.5 mg after 1 hour of the first dose. The following dosage was established in 0.5 mg two times a day for the next 19 days. On 8 May, day 17 of illness, 3 days after the first dose of colchicine, tests were performed again and showed a large decrease in ferritin levels (reduced by 25.9%–681% ng/dL), LDH (reduced by 6.7%–347 U/L) and C reactive protein (reduced by 85%–1221% mg/dL). On day 22 of illness, testing showed a further decrease in C reactive protein (0.507 mg/dL) and a significant decrease in IL-6 levels (6.9 pg/mL). All patient-reported symptoms disappeared after 4 days of colchicine use, and the patient had no side effects during treatment. In June 2020, a chest CT was performed again, which showed a positive development of lung recovery (figure 2).
Figure 1.
Chest CT performed before colchicine treatment.
Figure 2.
Chest CT performed after COVID-19.
Outcome and follow-up
The reduction in the levels of inflammatory markers was evident after a short period of treatment, and the most important marker analysed, C reactive protein, reduced by about 85% compared with the initial examination, with a progressive improvement in subsequent analyses. There was also a significant difference in symptoms, which improved the course of the disease without requiring hospitalisation.
Discussion
SARS-CoV-2 infection causes destruction of lung cells to elicit a local immune response that recruits macrophages and monocytes to respond to the infection.6 When the host is faced with a stressful condition, many defence systems are activated and several mediators modulate the production of cytokines. The complement system and the coagulation cascade can also cause the release of proinflammatory cytokines by activated macrophages, which are probably one of the main sources of cytokine production.7 In addition, cytokines produced by T lymphocytes and Th1 cells, such as interferon-y, may also promote increased release of proinflammatory cytokines. When this immune response worsens, it can lead to a condition known as cytokine storm, which causes acute lung injury and can lead to multiorgan damage.6
Since the SARS pandemic in 2002, it has been observed that severely ill patients who required intensive care in hospitals usually had higher blood plasma levels of proinflammatory cytokines and chemokines such as IL-1β, IL-2, IL-6, IL-7, IL-8, IP-10, MCP1, macrophage inflammatory protein 1α, and tumour necrosis factor (TNF).8 In addition, it is common for IL-6 levels to gradually increase over time in these patients, and these levels are relatively higher in non-survivors than in survivors.
Colchicine is an ancient drug derived from the autumn crocus, Colchicum autumnale, a poisonous flowering plant. It has been used primarily in the treatment of patients with acute gout and other inflammatory conditions such as familial Mediterranean fever.9 It is an inexpensive and potent anti-inflammatory drug that has a low rate of side effects at low doses. The mechanism of colchicine in inflammation is not fully understood, but it is thought that the drug blocks the NLRP3 inflammasome by disrupting microtubule formation and interfering with the production of IL-1β and IL-18.10 11
With the increasing number of COVID-19 in the world, many countries do not have enough hospital bed capacity to meet the high demand. The solution to alleviate this problem is to find therapeutic options that can improve the course of the disease and prevent hospitalisation in high-risk patients. Another important issue is to facilitate the monitoring of inflammatory markers in patients with COVID-19. Due to the limitations of health services in some countries for the analysis and monitoring of cytokine levels, the levels of C reactive protein (whose production is stimulated by IL-6) can be used as an indicator of IL-6 levels. IL-6 levels in peripheral blood have been used to assess the intensity of systemic cytokine responses in patients with sepsis, as IL-6 production is stimulated by TNF and IL-1β, providing an integrated signal from these two early responding cytokines.12 Acute suppression of these proinflammatory cytokines in COVID-19 patients may have important therapeutic implications.
To test the hypothesis that colchicine might attenuate the effects of the storm of inflammatory cytokines that usually occurs in the most severe cases of COVID-19, researchers at the University of São Paulo conducted a controlled, randomised, double-blind clinical trial. The clinical trial included 75 participants who were randomly divided into two groups, both of which were treated with the standard hospital therapy protocol for COVID-19. One of the groups received colchicine in addition to the standard protocol, while the other received placebo. When comparing the results of the groups, the researchers concluded that colchicine provided benefits in three parameters: it reduced the time of supplemental oxygen use, it reduced the overall length of hospital stay, and it lowered blood C reactive protein levels.13
COLCORONA, a randomised, double-blind, adaptive, placebo-controlled, multicentre study led by Montreal Heart Institute, included 4488 patients and was conducted in Brazil, Canada, Greece, South Africa, Spain and the USA. Although the study showed no significant clinical outcomes in individuals without a positive COVID-19 test, colchicine resulted in a lower rate of composite death or hospitalisation than placebo in patients with PCR-confirmed COVID-19.14
The results of these studies are promising, as therapies to reduce hospitalisations and deaths from COVID-19 have been constantly sought since the beginning of the pandemic. Several drugs are being tested for these purposes, but a large proportion of these tend not to be widely used because they are costly drugs, unlike colchicine, an effective, safe drug that can be easily adopted as a treatment protocol for COVID-19 around the world.
Learning points.
The production of proinflammatory cytokines is a fundamental step in initiating the anti-infectious process, but excessive production of cytokines during severe inflammation can drive the organism to harmful consequences.
Cytokine storm is a serious complication in critically ill patients with COVID-19. It is associated with hyperactivation of the immune system and correlates with the increase of plasma ferritin and interleukin-6.
Acute inhibition of a number of key effectors in this inflammatory cascade with colchicine, an oral and inexpensive drug, can reduce hospitalisations and prevent a cytokine storm in COVID-19.
Footnotes
Contributors: RSK elaborated the project; LCL elaborated the project; RSK wrote the manuscript; TH revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.BBC . Coronavirus: window of opportunity to act, world health organisation says, 2020. [Google Scholar]
- 4.Nile SH, Nile A, Qiu J, et al. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev 2020;53:S1359-6101(20)30070-8:66–70. 10.1016/j.cytogfr.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slobodnick A, Shah B, Krasnokutsky S, et al. Update on colchicine, 2017. Rheumatology 2018;57:i4–11. 10.1093/rheumatology/kex453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19. J Infect 2020;80:607–13. 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 2014;5:491. 10.3389/fimmu.2014.00491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien J-Y, Hsueh P-R, Cheng W-C, et al. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology 2006;11:715–22. 10.1111/j.1440-1843.2006.00942.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Chetrit E, Bergmann S, Sood R. Mechanism of the anti-inflammatory effect of colchicine in rheumatic diseases: a possible new outlook through microarray analysis. Rheumatology 2006;45:274–82. 10.1093/rheumatology/kei140 [DOI] [PubMed] [Google Scholar]
- 10.Angelidis C, Kotsialou Z, Kossyvakis C, et al. Colchicine pharmacokinetics and mechanism of action. Curr Pharm Des 2018;24:659–63. 10.2174/1381612824666180123110042 [DOI] [PubMed] [Google Scholar]
- 11.Leung YY, Yao Hui LL, Kraus VB. Colchicine--Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015;45:341–50. 10.1016/j.semarthrit.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pradhan AD, Manson JE, Rifai N, et al. C-Reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–34. 10.1001/jama.286.3.327 [DOI] [PubMed] [Google Scholar]
- 13.Lopes MI, Bonjorno LP, Giannini MC, et al. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open 2021;7:e001455. 10.1136/rmdopen-2020-001455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tardif J-C, Bouabdallaoui N, L'Allier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med 2021. 10.1016/S2213-2600(21)00222-8. [Epub ahead of print: 27 May 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]