Abstract
Objective
Non-pharmacological interventions support patients with connective tissue diseases to better cope with and self-manage their diseases. This study aimed to map existing evidence on non-pharmacological interventions in patients with systemic lupus erythematosus (SLE), systemic sclerosis (SSc) and mixed connective tissue diseases regarding content, feasibility and potential suitability in an e-health setting.
Methods
A literature search was performed in eight different databases in July 2020. The intervention’s content was extracted using the ‘Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide’. A Sankey diagram and descriptive statistics were used to analyse the data and illustrate the relationships between the interventions.
Results
Of 8198 identified records, 119 papers were eligible. One hundred and four of them (87.4%) were conducted between 2000 and 2020, mainly in the USA (SLE n=24 (21.2%), SSc n=16 (14.2%)), Brazil (SLE n=8 (7.1%), SSc n=5 (4.4%)) and Italy (SLE n=0 (0%), SSc n=12 (10.6%)). Fifty-two studies (SLE n=24 (21.2%), SSc n=28 (24.8%)) used multicomponent interventions. The single interventions were physical exercises (SLE n=16 (14.2%), SSc n=17 (15.0%)), coaching/counselling (SLE n=11 (18.0%), SSc n=0 (0%)) and education (SLE n=2 (1.8%), SSc n=3 (2.7%)). Primary outcomes focused on physical function (SLE n=1 (0.9%), SSc n=15 (13.3%)), mouth opening in SSc (n=4 (5.9%)) and physical capacity (SLE n=2 (1.8%), SSc n=1 (0.9%)). No interventions for mixed connective tissue disease were found.
Conclusion
There was a great variety in the intervention’s content due to differences in body structure, activity limitations and participation restrictions in SLE and SSc. These results highlight the need for personalised, multicomponent, non-pharmacological interventions, which could be delivered as e-health interventions.
Keywords: physical therapy modalities, occupational therapy, nursing, lupus erythematosus, systemic, scleroderma, systemic
Key messages.
What is already known about this subject?
Persons suffering from autoimmune connective tissue diseases (CTD), such as systemic sclerosis (SSc), systemic lupus erythematosus (SLE) and mixed connective tissue diseases (MCTD), experience limitations in physical and mental function, activities of daily life and participation, also leading to a reduced quality of life.
What does this study add?
Consistent with the people’s limitations in their daily routines, various non-pharmacological interventions/programmes exist for SSc and SLE. We did not find non-pharmacological interventions for MCTD.
The most common interventions included patient education, self-management, physical activity/exercise and advice regarding a healthy lifestyle.
How might this impact on clinical practice or further developments?
Multicomponent, non-pharmacological interventions in people with CTDs should be personalised to increase effectiveness.
Multicomponent e-health interventions target the different needs of patients with CTDs.
E-health settings allow patients easier access to specialised health professionals.
Introduction
Connective tissue diseases (CTDs), such as systemic sclerosis (SSc),1 systemic lupus erythematosus (SLE)2 and mixed connective tissue diseases (MCTD),3 are rare chronic autoimmune diseases of unknown aetiology which affect several organ systems, such as the skin, heart, lungs, kidneys, joints, muscles and blood vessels.4 5 Consequently, people with CTDs experience fatigue, pain, reduced mobility/range of motion, shortness of breath and decreased physical function. Thus, CTDs often lead to activity limitations, participation restrictions, psychosocial and economic consequences and reduced health-related quality of life.6–10
Non-pharmacological interventions support patients with CTDs, to better cope with and self-manage their diseases, thereby increasing their quality of life. Non-pharmacological interventions are most commonly delivered by health professionals in rheumatology,11 and several studies have been conducted to establish their effectiveness. For instance, recent reviews demonstrated that being physically active and performing exercises positively impacts fatigue in people with SSc and SLE.12–15 However, these reviews focused only on a specific type of intervention (eg, exercises12–14) or restricted the study designs (eg, included only randomised controlled trials (RCTs)15). Research of interventions using other study designs, such as case studies or qualitative studies, is under-represented in these reviews. To date, a comprehensive overview of different non-pharmacological interventions is lacking. Furthermore, given the small number of patients with SLE and SSc (1–5 individuals per 10.000)16–18 and their particular needs, health professionals who are experts in CTDs are often not easily accessible for these patients especially in rural areas.19 20 Consequently, telehealth interventions or remote consultations with experts have been previously suggested.21 22 Besides, the current COVID-19 pandemic has even more increased the need for remote healthcare. However, telehealth or other remote interventions in CTDs have not yet been explicitly covered in any of the current reviews.
To define the term e-health, we used the definition of the WHO and the description of the term ‘telemedicine’ as defined in the Medical Subject Headings terms by PubMed. The WHO defines e-health as the ‘use of information and communication technologies (ICT) for health’.23 Telemedicine is defined as the ‘Delivery of health services via remote telecommunications’.24 Both definitions include interactive consultative and diagnostic services and comprise terms such as ‘mobile health’, ‘m-health’, ‘telehealth’ and ‘e-health’.
The objective of this study was to map the existing evidence of non-pharmacological interventions in patients with SLE, SSc and MCTD regarding content, feasibility and potential suitability in an e-health setting.
Materials and methods
We conducted a scoping review25 using the guidelines of the Joanna Briggs Institute’s (JBI) approach to evidence-based healthcare methodology to map and describe the content of existing non-pharmacological interventions applicable for patients with SSc, SLE and MCTD. In the second step, we conducted an analysis to provide some insights of effectiveness. Our findings are reported according to the ‘PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation’.26 The protocol of this scoping review was published on researchgate.net (DOI: 10.13140/RG.2.2.22193.43363).
Search strategy
An initial search, limited to PubMed and CINAHL, was performed (by VR, RF, EJFS) to identify the first articles on this topic to develop a search strategy. In the second step, this search strategy was used to conduct an adapted and more extensive query in eight different databases: MEDLINE (PubMed), EMBASE (OVID), CINAHL (EBSCO), PsycINFO (ProQUEST), the Cochrane Database of Systematic Reviews, OTSeeker, PEDro and SciELO (online supplemental file 1). Further, members of the research group were consulted by email to identify additional grey literature or research that had not been found through the database query. Reference lists of identified papers were searched for any additional relevant articles subjected to the same screening and selection process. There were no exclusions of papers based on publication date.
rmdopen-2021-001710supp001.pdf (71.6KB, pdf)
Study selection and inclusion criteria
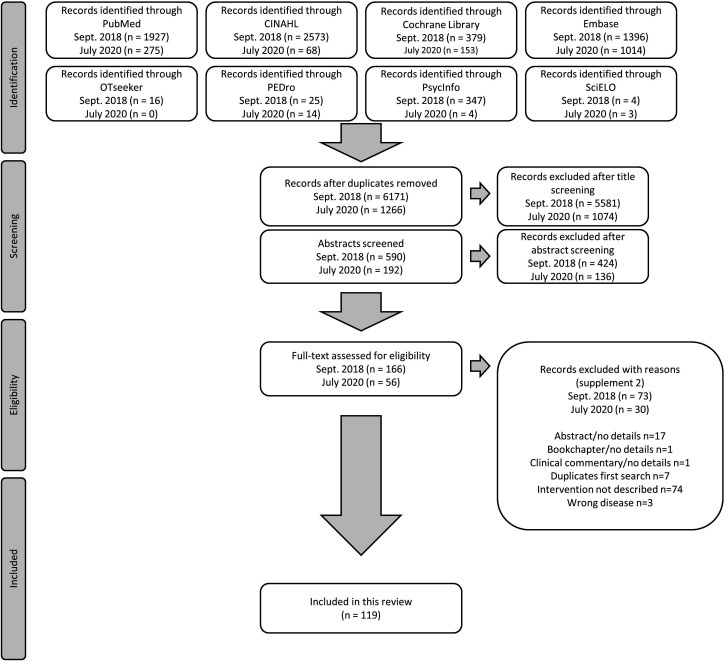
All identified studies/articles/reports in the queries were uploaded into EndNote and duplicates removed. The selection process had two phases. First, two researchers (VR, RF) independently screened the titles whether they met inclusion/exclusion criteria or not, followed by screening the abstracts. A third researcher (TAS) assessed possible disparities in selecting these titles and/or abstracts. Second, the full texts for all selected publications were retrieved and read (VR, EJ). When in doubt whether a paper met the inclusion/exclusion criteria, a third researcher (TAS) was asked to assess the paper and the decision was made by consensus. According to the PRISMA-ScR,26 reasons for the exclusion of full-text studies are provided (online supplemental file 2). The results of the selection are presented in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (figure 1).
Figure 1.
Flow chart of the search and selection process performed in September 2018 and updated in July 2020.
rmdopen-2021-001710supp002.pdf (149.7KB, pdf)
The following inclusion criteria guided the selection of publications. Following the JBI,25 the PCC (participants, concept and context) mnemonic and study design were used to screen the studies.
Participants
Studies were included in this scoping review if participants in the study had a diagnosis of SSc, SLE or MCTD.
Concept
Studies were included if any non-pharmacological, non-surgical intervention (such as exercises, education, psychosocial intervention, etc) was investigated (either the development and/or the evaluation of a non-pharmacological intervention). The intervention had to be described in sufficient detail (at least content and setting) to be included.
Context
This scoping review considered all contexts (home care, community services, primary healthcare, hospital settings, etc).
Types of studies
Any quantitative (experimental study/observational) research designs and any qualitative study/design assessing participants’ perspectives on interventions were included. Theoretical studies were excluded.
Data extraction
Study characteristics were extracted using a data extraction form as indicated by the methodology for scoping reviews developed by the JBI.25 The described interventions were further extracted using the ‘Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide’.27 Two reviewers (VR, EJ) extracted the data independently. Any disagreements that arose between these reviewers were resolved through discussion until consensus was reached.
Assessment of risk of bias and descriptive analysis
The quality of retrieved RCTs was assessed using the ‘RoB 2: a revised tool for assessing the risk of bias in randomised trials’28 (online supplemental file 3). Other study designs were used to extract information on the intervention’s content but not analysed further regarding risk of bias.
rmdopen-2021-001710supp003.pdf (168.6KB, pdf)
Due to the heterogeneity of the studies included in this review, we descriptively summarised them.25 We calculated the weighted arithmetic mean of participant age to consider the number of participants in each study in relative weighting. In addition, we used a Sankey diagram to illustrate the relationships between different studies and study characteristics graphically. In a world map, we showed the frequencies of the countries in which the studies were conducted. To summarise and display descriptively the effects of the interventions on outcomes, we created a table in which we listed the interventions in rows and the outcomes in columns. We assigned the studies to the respective combinations of interventions and outcomes (numbers shown indicate the respective study, the colour represents whether the primary outcome was significant between groups (green), significant within groups (yellow) and not significant (red)).
Results
In total, 8198 records on non-pharmacological interventions were identified from the databases. After exclusion of duplicates and title/abstract screening, 226 studies were analysed in full text. One hundred and nineteen papers comprising 113 studies (six studies were published in two articles each29–40) were used for this scoping review. The PRISMA flow chart of the study selection is depicted in figure 1. Following the RoB 2,28 4 out of 42 retrieved RCTs (published in 44 papers) had a low risk of bias, 16 moderate and 22 high (online supplemental file 3). To provide a comprehensive overview of the various forms of intervention, we prepared a detailed summary of the individual study characteristics and interventions (online supplemental files 4 and 5).
rmdopen-2021-001710supp004.pdf (472.6KB, pdf)
rmdopen-2021-001710supp005.pdf (483.3KB, pdf)
Study characteristics
Participants
In 58 of the 113 studies (51.3%), the participants were diagnosed with SLE and in 55 (48.7%) with SSc. We did not find any paper dealing specifically with MCTD and non-pharmacological interventions. In total, 5140 people were included (n=4687 (91.2%) female, n=332 (6.5%) male, n=121 (2.3%) gender not reported41–46). However, the authors of one study43 did not report the number of the patients included. Of the participants, 3484 were diagnosed with SLE (intervention groups (IG) n=2664 (77.6%), control groups (CG) n=820 (22.4%)), 1632 with SSc (IG n=1055 (66.7%), CG n=577 (33.3%)) and 58 persons were healthy controls. The allocation of 24 patients diagnosed with SSc in the experimental group and CG of Freedman et al 47 was not reported. The weighted mean (SD) age of patients with SLE was 42.0 (SD±6.8) years (weighted mean age IG 42.3 (SD±6.7); CG 41.4 (SD±6.9)) and 54.8 (SD±4.9) for patients with SSc (IG 54.3 (SD±5.1); CG 55.7 (SD±4.3)). In seven studies, the authors did not report the participant age.42 44 48–52
Date and location of publications, study designs
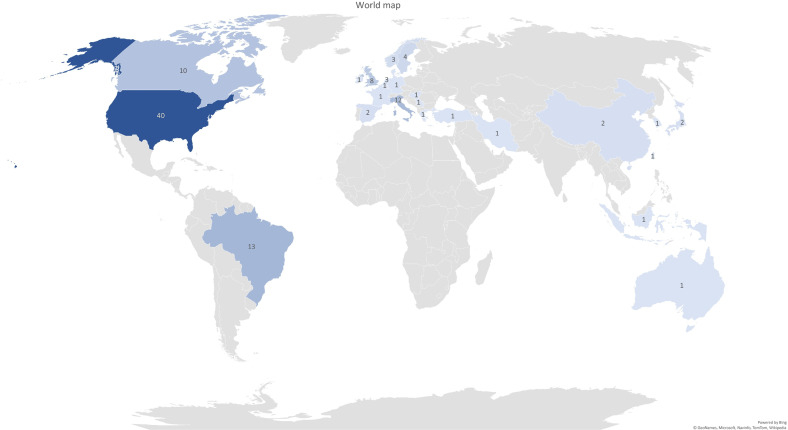
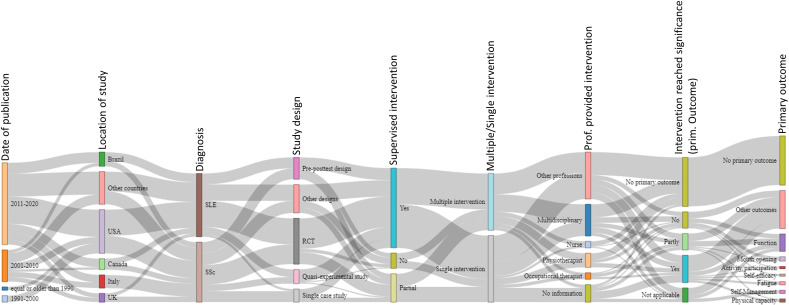
The majority of the studies were published between 2011–2020 (SLE n=36 (31.9%), SSc n=39 (34.5%)) and 2001–2010 (SLE n=16 (14.2%), SSc n=13 (11.5%)). Forty of all the studies were published in the USA (SLE n=24 (21.2%), SSc n=16 (14.2%)), 13 in Brazil (SLE n=8 (7.1%), SSc n=5 (4.2%)), 12 in Italy (SLE n=0 (0%), SSc n=12 (10.6%)), 10 in Canada (SLE n=5 (4.4%), SSc n=5 (4.4%)) and 8 in the UK (SLE n=3 (2.7%), SSc n=5 (4.4%)) (figure 2). Of the 113 studies, 42 (SLE n=24 (21.2%), SSc n=18 (15.9%)) were RCTs, 20 (SLE n=10 (8.8%), SSc n=10 (8.8%)) were one-group pre/post-test design, 13 (SLE n=7 (6.2%), SSc n=6 (5.3%)) were quasiexperimental studies and 12 (SLE n=2 (1.8%), SSc n=10 (8.8%)) were single-case studies. These study designs and other characteristics are graphically displayed in the Sankey diagram (figure 3).
Figure 2.
World map evidence representation. This graph shows the frequency of the countries in which the published studies were conducted.
Figure 3.
Sankey diagram. This diagram illustrates relationships between different studies and study characteristics graphically. The bars show the study characteristics that were compared between the studies. The grey lines between the bars are reflecting the congruencies and differences between the different studies. The wider the grey connection lines are, the more congruency exists. RCT, randomised controlled trial; SLE, systemic lupus erythematosus; SSc, systemic sclerosis.
Health professionals providing interventions
In 29 (25.7%) of the 113 articles, the interventions were performed by multidisciplinary teams. The other study interventions were performed by physiotherapists (SLE n=6 (5.3%), SSc n=7 (6.2%)), occupational therapists (SLE n=1 (0.9%), SSc n=5 (4.4%)), nurses (SLE n=4 (3.5%), SSc n=2 (1.8%)) or other health professionals (n=43 (38.1%)). In nine studies (8.0%), the interventions were performed by patients/peers (eg, peer counselling). In 16 studies (SLE n=6 (5.3%), SSc n=10 (8.8%)), the authors did not provide clear information on the profession of the health professionals delivering the intervention.7 33 46–48 53–63
Interventions, outcomes, effectiveness
In 52 of the 113 studies (SLE n=24 (21.2%), SSc n=28 (24.8%)), the researchers used multiple or multicomponent interventions to treat their patients, and in 61 studies (SLE n=34 (30.1%), SSc n=27 (23.9%)) single interventions. Of the 61 studies including a single intervention, 33 (SLE n=16 (14.2%), SSc n=17 (15.0%)) included exercises, 11 (SLE n=11 (9.7%), SSc n=0 (0.0%)) coaching/counselling (eg, goal setting, nutrition counselling, peer counselling, physical activity counselling), 5 (SLE n=2 (1.8%), SSc n=3 (2.7%)) education (eg, self-management strategies, cope with the disease) and 4 (SLE n=4 (3.5%), SSc n=0 (0%)) cognitive–behavioural interventions. In the 45 studies (39.8%; including 12 RCTs) where the authors did not clearly define a primary outcome, multiple outcomes and measurements to evaluate the effect of their studies were used (online supplemental file 4). Of the 113 articles found, in 68 (60.8%) a primary outcome was defined. The most frequent primary outcomes were physical function, such as range of motion or hand functioning (SLE n=1 (0.9%), SSc n=15 (13.3%)), mouth opening (SLE n=0 (0%), SSc n=4 (3.5%)), physical capacity (SLE n=2 (1.8%), SSc n=1 (0.9%)), fatigue (SLE n=3 (2.7%), SSc n=0 (0%)) and self-management (SLE n=1 (0.9%), SSc n=2 (1.8%)). As the primary outcome is of utmost importance to decide on the overall result of the study,64 65 we included only these in further analysis of the results. Of the 68 articles, 38 described studies with CG (RCT n=30 (44.1%), quasiexperimental designs n=8 (11.8%)). The studies including a CG and defining a primary outcome are displayed in tables 1 and 2. In these tables, the heterogeneity between the study interventions, outcomes and results becomes apparent. In SLE, 10 of the 19 RCTs (52.6%) showed a significant positive effect. In SSc, it was 7 out of 21 (33.3%). The outcomes were very different and varied greatly.
Table 1.
Interventions/outcomes in studies for people with SLE
| SLE | Outcomes | ||||||||||||||
| Stress reduction | Self-efficacy | Psychological status | Job retention | Physical capacity/activity | Quality of life | Weight management | Cytokines/lipid profile | Fatigue | Disease activity | Coping | Arterial stiffness | Hand function | |||
| Interventions | CBT | 81 | |||||||||||||
| Coaching/counselling | 82 | 83 | |||||||||||||
| Education/counselling | 43 | 84 | |||||||||||||
| Education/information | 85 | ||||||||||||||
| Exercises/coaching/diet | 86 | ||||||||||||||
| Exercise/education/coaching | 87 | ||||||||||||||
| Exercise | 41 | 7 | 56 | 55 | 88 | 30 | 73 | ||||||||
| Exercise/relaxations | 89 | ||||||||||||||
| Information/coaching | 90 | ||||||||||||||
| Psychoeducational interventions | 91 | ||||||||||||||
| Self-management | 45* | ||||||||||||||
To summarise and display descriptively the effects of the interventions on outcomes, we created a table in which we listed the interventions in rows and the outcomes in columns. Only studies which had defined a primary outcome are listed. We assigned the studies to the respective combinations of interventions and outcomes (numbers shown indicate the respective study, the colour represents whether the primary outcome was significant between groups (green), significant within groups (yellow) and not significant (red)).
*Significant after the intervention, not significant after 6 and 12 months.
CBT, cognitive–behavioural therapy; SLE, systemic lupus erythematosus.
Table 2.
Interventions/outcomes in studies for people with SSc
| SSc | Outcomes | ||||||||
| Microcirculation | Activities of daily living | Physical functioning | Mouth opening | Aerobic capacity | Self-efficacy | Gingival health | |||
| Interventions | Autogenic training/biofeedback | 47 | |||||||
| Education/counselling | 92* | 22 | |||||||
| Education | 93 | ||||||||
| Exercise/splints | 94† | ||||||||
| Exercise | 33 54 | 46 | 70 | 40 95† 44‡ | 57 | ||||
| Information | 22 | ||||||||
| Massage/mobilisation/exercise | 62 | ||||||||
| Mobilisation/exercises | 96 | ||||||||
| Oral hygiene/exercises | 40 | ||||||||
| Thermal treatment/exercises | 48 97 98 | ||||||||
| Thermal treatment/exercises/physiotherapy | 99 | ||||||||
To summarise and display descriptively the effects of the interventions on outcomes, we created a table in which we listed the interventions in rows and the outcomes in columns. Only studies which had defined a primary outcome are listed. We assigned the studies to the respective combinations of interventions and outcomes (numbers shown indicate the respective study, the colour represents whether the primary outcome was significant between groups (green), significant within groups (yellow) and not significant (red)).
*Intention-to-treat analysis not significant, per-protocol analysis significant.
†Only short-term effects.
‡Pilot randomised controlled trial (RCT), authors stated that it was underpowered.
SSc, systemic sclerosis.
Setting and tailoring the intervention to the patient’s needs
In the 113 articles, 98 (86.7%) of the interventions were either partly (SLE n=5 (4.4%), SSc n=21 (18.6%)) or completely supervised (SLE n=46 (40.7%), SSc n=26 (23.0%)). Supervised in this context refers to face-to-face communication or contact. Partly supervised interventions had an initial face-to-face component, or direct support was provided at the beginning of the treatment. In the course of treatment, this support was constantly reduced. Only 15 of the study interventions (SLE n=7 (6.2%), SSc n=8 (7.1%)) were designed and used as full-distance intervention programmes.38 42 52 66–76
In total, 52 (SLE n=32 (28.3%), SSc n=20 (17.7%)) of the interventions were tailored to the patient’s needs and 29 (SLE n=16 (14.2%), SSc n=13 (11.5%)) to the patient’s physical condition/fitness. ‘Tailored to the needs of the patient’ in this context means that either individual coaching/counselling as part of the intervention or that the measures taken were adapted to the patient’s life situation. ‘Tailored to the patient’s physical condition/fitness’ were interventions, mainly exercises, that were adapted to the patient’s personal ability/capacity, but not to the patient’s life circumstances or preferences. The interventions in 21 studies (SLE n=3 (2.7%), SSc n=18 (15.9%)) were not tailored. In nine (SLE n=5 (4.4%), SSc n=4 (3.5%)) studies, the authors did not provide information on tailoring or individualisation of the interventions. Of the 15 studies where distance intervention programmes were used, only 4 (26.7%) were tailored to the patient’s needs, 2 (13.3%) to the patient’s physical condition/fitness and 8 (53.3%) of the interventions were not tailored.
Discussion
This scoping review with a descriptive analysis provides a comprehensive overview of non-pharmacological interventions for people diagnosed with SLE and SSc regarding content, feasibility and potential suitability in an e-health setting. CTDs impact people’s lives in multifaceted, complex ways. Thus, there is no single non-pharmacological intervention that can simultaneously help these patients reduce pain and fatigue, increase physical function (eg, range of motion, grip strength) and enhance health-related quality of life and cope with the disease. Consequently, the use of multicomponent interventions, regardless of whether in an e-health setting or not, seems essential and clinically relevant. Education, counselling and/or exercises were part of most of the interventions described. In systematic reviews of exercise/physical activity, it has been shown that being more physically active reduces, for example, fatigue, pain and depressive symptoms in people with SLE.77
On purpose, we did not exclude papers based on countries (where the studies were conducted) or publication date to avoid limiting ourselves to intervention types that are culturally specific or that might have changed over time. Our results show that the majority of articles (87.4%) were published between 2000 and 2020. Potential reasons might be the generally increased number of studies on non-pharmacological interventions after 2000 and the growing focus on facilitating more active involvement of patients in managing their healthcare. Thus, interventions requiring active participation, such as patient education, instructions for self-management, physical activity/exercise and advice regarding a healthy lifestyle, have been created and evaluated for feasibility and/or effectiveness.
Most of the interventions described in the studies were either partly or entirely supervised by healthcare providers and/or patients/peers (n=98 (87%)). Thus, these interventions’ feasibility and suitability in an e-health setting are questionable and should be evaluated in further studies. Non-pharmacological interventions are associated with low adherence rates because they often involve lifestyle modifications and require changes in behaviour and daily routine habits, which are challenging to achieve.78 Therefore, interventions that have been developed in a supervised setting cannot be transferred to an unsupervised setting without further validation.78 79 It might be possible that the supervision per se is an important trigger for the people with CTDs to adhere to the treatment and, hence, reach a good/better clinical outcome.
The studies in our review which included tele/e-health interventions focused mainly on patient education and information and were not individually tailored to patients’ needs. In 2021, we would potentially consider a larger variety of interventions suitable for e-health, including supervised non-pharmacological interventions, such as physical exercises, functional training, activity pacing advice, etc. We assume that this is partly due to the fact that the COVID-19 pandemic has substantially advanced the tools, but also our skills in digital healthcare delivery. However, we need to consider equity and access to technologies to not exclude certain groups of people systematically.
People with CTD experience specific clinical manifestations of their disease, and therefore certain limitations in everyday life. To account for this individuality, the interventions should be tailored to the patient’s needs.80 In our results, 81 (72%) of the described face-to-face interventions showed such a tailored approach. However, only 7 out of 15 (47%) e-health programmes were tailored, which indicates a need for development of innovative tools and strategies to facilitate personalisation of these interventions. Artificial intelligence applications might facilitate that e-health interventions become smart. For instance, based on some patient’s characteristics or responses, specific contents might be available or not.
The scoping review methodology allowed us to focus on the content, feasibility and potential suitability in an e-health setting. Compared with a classical analysis of RCTs, the strength of a scoping review is the possibility to include a heterogeneous body of literature, conveying a large number and variety of patients with such particular clinical conditions that would otherwise not be available. By descriptively mapping the content of the studies, we were able to identify research gaps and bring together literature from different disciplines, including intervention programmes with emerging evidence.25
The Sankey diagram analysis shows that in most studies the interventions were partially or fully supervised. Furthermore, it highlights that studies on SSc and SLE are balanced in numbers, but studies on non-pharmacological interventions in people with MCTD are entirely lacking. However, Sankey diagrams do not present meaningful differentiations or comparisons as the widths of the connection lines are similar. Consequently, it was difficult to depict the associations between specific interventions and their outcomes in the diagram. For this purpose, we used the representation in table form. In the tables, we directly contrasted outcomes and types of interventions which is another way to represent knowledge gaps or research needs visually. For example, tables 1 and 2 show that exercise as a single intervention was evaluated in several studies focusing on different outcomes. Other non-pharmacological programmes and interventions were assessed in each case with different outcomes. Because of this heterogeneity, it is difficult to compare the studies and effects with each other. For instance, a self-management programme was assessed only in relation to quality of life but not in relation to other outcomes.45 Further research is needed in this area to make more reliable statements about effectiveness and efficacy.
However, we acknowledge that our review has certain limitations. We focused only on SLE, SSc and MCTD. Furthermore, conclusions on efficacy cannot be made with a scoping review design. While we used a comprehensive search strategy, we excluded studies that did not describe or evaluate a specific intervention. The qualitative studies that we found did not evaluate or describe interventions. They were almost all preliminary studies focused on developing an intervention. Thus, they were excluded because they did not meet the inclusion criteria.
Conclusion
Our results underscore the complexity of treating people with non-pharmacological interventions in CTDs such as SLE and SSc. An interdisciplinary approach tailored to the patient’s needs is essential to support people with SLE and SSc holistically and comprehensively. Education, counselling/coaching and (promoting) exercises/physical activity are important parts of non-pharmacological interventions in people with SLE and SSc and are also suitable for e-health interventions.
Footnotes
Twitter: @FerreiraRJO, @EduardoJFSantos
Contributors: VR, TAS, CB and CvdE wrote the manuscript. VR, RJOF, EJ and EJFS performed the search strategies and selected the studies. VR and EJ assessed the risk of bias of RCTs and extracted the data of all studies, and synthesised the results. TAS, CB and CvdE reviewed the processes and excluded articles and tailored the synthesis reports. All other authors suggested and agreed upon the research questions, read the report prior to the manuscript, discussed the results and made contributions to the text. All authors approved the final version of the manuscript.
Funding: This project was funded by EULAR (project number HPR038).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Smith V, Scirè CA, Talarico R, et al. Systemic sclerosis: state of the art on clinical practice guidelines. RMD Open 2019;4:e000782. 10.1136/rmdopen-2018-000782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2016;2:16039. 10.1038/nrdp.2016.39 [DOI] [PubMed] [Google Scholar]
- 3. Chaigne B, Scirè CA, Talarico R, et al. Mixed connective tissue disease: state of the art on clinical practice guidelines. RMD Open 2018;4:e000783. 10.1136/rmdopen-2018-000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Puto G, Repka I, Zurzycka P, et al. Socio-demographic determinants of the acceptance of systemic connective tissue diseases. Reumatologia 2018;56:31–6. 10.5114/reum.2018.74746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avouac J, Denton CP, Matucci-Cerinic M. Systemic sclerosis. In: Bijlma J, Hachulla E, eds. EULAR textbook on rheumatic diseases. 2nd edn. London: BMJ Publishing Group Ltd, 2015: 606–35. [Google Scholar]
- 6. Jacques T, Sudoł-Szopińska I, Larkman N, et al. Musculoskeletal manifestations of Non-RA connective tissue diseases: scleroderma, systemic lupus erythematosus, still's disease, dermatomyositis/polymyositis, Sjögren's syndrome, and mixed connective tissue disease. Semin Musculoskelet Radiol 2018;22:166–79. 10.1055/s-0038-1639473 [DOI] [PubMed] [Google Scholar]
- 7. Bogdanovic G, Stojanovich L, Djokovic A, et al. Physical activity program is helpful for improving quality of life in patients with systemic lupus erythematosus. Tohoku J Exp Med 2015;237:193–9. 10.1620/tjem.237.193 [DOI] [PubMed] [Google Scholar]
- 8. Fischer A, Zimovetz E, Ling C, et al. Humanistic and cost burden of systemic sclerosis: a review of the literature. Autoimmun Rev 2017;16:1147–54. 10.1016/j.autrev.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 9. Mouthon L, Alami S, Boisard A-S, et al. Patients' views and needs about systemic sclerosis and its management: a qualitative interview study. BMC Musculoskelet Disord 2017;18:230. 10.1186/s12891-017-1603-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stamm TA, Mattsson M, Mihai C, et al. Concepts of functioning and health important to people with systemic sclerosis: a qualitative study in four European countries. Ann Rheum Dis 2011;70:1074–9. 10.1136/ard.2010.148767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. European League Against Rheumatism . EULAR HPR: standing Committee of health professionals in rheumatology, 2019. [Google Scholar]
- 12. O'Dwyer T, Durcan L, Wilson F. Exercise and physical activity in systemic lupus erythematosus: a systematic review with meta-analyses. Semin Arthritis Rheum 2017;47:204–15. 10.1016/j.semarthrit.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 13. Liem SIE, Vliet Vlieland TPM, Schoones JW, et al. The effect and safety of exercise therapy in patients with systemic sclerosis: a systematic review. Rheumatol Adv Pract 2019;3:rkz044. 10.1093/rap/rkz044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu M-L, Yu K-H, Tsai J-C. The effectiveness of exercise in adults with systemic lupus erythematosus: a systematic review and meta-analysis to guide evidence-based practice. Worldviews Evid Based Nurs 2017;14:306–15. 10.1111/wvn.12221 [DOI] [PubMed] [Google Scholar]
- 15. Fangtham M, Kasturi S, Bannuru RR, et al. Non-pharmacologic therapies for systemic lupus erythematosus. Lupus 2019;28:703–12. 10.1177/0961203319841435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pellar RE, Pope JE. Evidence-based management of systemic sclerosis: navigating recommendations and guidelines. Semin Arthritis Rheum 2017;46:767–74. 10.1016/j.semarthrit.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 17. Denton CP, Khanna D. Systemic sclerosis. Lancet 2017;390:1685–99. 10.1016/S0140-6736(17)30933-9 [DOI] [PubMed] [Google Scholar]
- 18. Dörner T, Furie R. Novel paradigms in systemic lupus erythematosus. The Lancet 2019;393:2344–58. 10.1016/S0140-6736(19)30546-X [DOI] [PubMed] [Google Scholar]
- 19. Joachim G, Acorn S. Life with a rare chronic disease: the scleroderma experience. J Adv Nurs 2003;42:598–606. 10.1046/j.1365-2648.2003.02663.x [DOI] [PubMed] [Google Scholar]
- 20. Ackerman IN, Buchbinder R, Osborne RH. Factors limiting participation in arthritis self-management programmes: an exploration of barriers and patient preferences within a randomized controlled trial. Rheumatology 2013;52:472–9. 10.1093/rheumatology/kes295 [DOI] [PubMed] [Google Scholar]
- 21. Poole JL, Khanna D, Skipper B. Taking charge of systemic sclerosis: a pilot study of an Internet self-management program. Arthritis Rheum 2012;64:S1111–2. [DOI] [PubMed] [Google Scholar]
- 22. Khanna D, Serrano J, Berrocal VJ. A randomized controlled trial to evaluate an Internet-based self-management program in systemic sclerosis. Arthritis Care Res 2018;71:435–47. 10.1002/acr.23595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organisation . eHealth at WHO: World health organisation; 2021.
- 24. PubMed . Telemedicine, 1993. Available: https://www.ncbi.nlm.nih.gov/mesh/?term=telemedicine
- 25. Peters MDJ, Godfrey CM, Khalil H, et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015;13:141–6. 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 26. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 27. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 28. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;2:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 29. Carrera BG, Vargas-Hitos JA, Morillas-De-Laguno P. FRI0236 might a 12-week aerobic exercise intervention improve patient-reported outcomes in women with systemic lupus erythematosus? Ann Rheumatic Dis 2019;78:798. [Google Scholar]
- 30. Soriano-Maldonado A, Morillas-de-Laguno P, Sabio JM, et al. Effects of 12-week aerobic exercise on arterial stiffness, inflammation, and cardiorespiratory fitness in women with systemic lupus erythematosus: non-randomized controlled trial. J Clin Med 2018;7:477. 10.3390/jcm7120477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cunningham NR, Fussner LM, Moorman E, et al. Development and pilot testing of the treatment and education approach for childhood-onset lupus (TEACH): a cognitive behavioral treatment. Pediatr Rheumatol 2019;17. 10.1186/s12969-019-0307-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cunningham NR, Fussner LM, Moorman E, et al. Development and pilot testing of the treatment and education approach for childhood-onset lupus (teach): a cognitive behavioral treatment. Pediatr Rheumatol Online J 2019;17:9. 10.1186/s12969-019-0307-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitropoulos A, Gumber A, Akil M, et al. Exploring the microcirculatory effects of an exercise programme including aerobic and resistance training in people with limited cutaneous systemic sclerosis. Microvasc Res 2019;125:103887. 10.1016/j.mvr.2019.103887 [DOI] [PubMed] [Google Scholar]
- 34. Mitropoulos A, Gumber A, Crank H, et al. Exploring the feasibility of an exercise programme including aerobic and resistance training in people with limited cutaneous systemic sclerosis. Clin Rheumatol 2020;39:1889–98. 10.1007/s10067-019-04921-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Twumasi AA, Shao A, Dunlop-Thomas C, et al. Exploring the perceived impact of the chronic disease self-management program on self-management behaviors among African American women with lupus: a qualitative study. ACR Open Rheumatol 2020;2:147–57. 10.1002/acr2.11117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Twumasi AA, Shao A, Dunlop-Thomas C, et al. Health service utilization among African American women living with systemic lupus erythematosus: perceived impacts of a self-management intervention. Arthritis Res Ther 2019;21:155. 10.1186/s13075-019-1942-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams EM, Dismuke CL, Faith TD, et al. Cost-effectiveness of a peer mentoring intervention to improve disease self-management practices and self-efficacy among African American women with systemic lupus erythematosus: analysis of the peer approaches to lupus self-management (PalS) pilot study. Lupus 2019;28:937–44. 10.1177/0961203319851559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams EM, Egede L, Oates JC, et al. Peer approaches to self-management (PALS): comparing a peer mentoring approach for disease self-management in African American women with lupus with a social support control: study protocol for a randomized controlled trial. Trials 2019;20:529. 10.1186/s13063-019-3580-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yuen HK, Marlow NM, Reed SG, et al. Effect of orofacial exercises on oral aperture in adults with systemic sclerosis. Disabil Rehabil 2012;34:84–9. 10.3109/09638288.2011.587589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yuen HK, Weng Y, Bandyopadhyay D, et al. Effect of a multi-faceted intervention on gingival health among adults with systemic sclerosis. Clin Exp Rheumatol 2011;29:S26–32. [PMC free article] [PubMed] [Google Scholar]
- 41. Prado DML, Benatti FB, de Sá-Pinto AL, et al. Exercise training in childhood-onset systemic lupus erythematosus: a controlled randomized trial. Arthritis Res Ther 2013;15:R46. 10.1186/ar4205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Daltroy LH, Robb-Nicholson C, Iversen MD, et al. Effectiveness of minimally supervised home aerobic training in patients with systemic rheumatic disease. Br J Rheumatol 1995;34:1064–9. 10.1093/rheumatology/34.11.1064 [DOI] [PubMed] [Google Scholar]
- 43. Allaire SH, Jingbo Niu, LaValley MP. Employment and satisfaction outcomes from a job retention intervention delivered to persons with chronic diseases. Rehabil Couns Bull 2005;48:100–9. 10.1177/00343552050480020401 [DOI] [Google Scholar]
- 44. Naylor WP, Douglass CW, Mix E. The nonsurgical treatment of microstomia in scleroderma: a pilot study. Oral Surg Oral Med Oral Pathol 1984;57:508–11. 10.1016/0030-4220(84)90309-8 [DOI] [PubMed] [Google Scholar]
- 45. Berdal G, Bø I, Dager TN, et al. Structured goal planning and supportive telephone follow-up in rheumatology care: results from a pragmatic, Stepped-Wedge, cluster-randomized trial. Arthritis Care Res 2018;70:1576–86. 10.1002/acr.23520 [DOI] [PubMed] [Google Scholar]
- 46. Filippetti M, Cazzoletti L, Zamboni F, et al. Effect of a tailored home-based exercise program in patients with systemic sclerosis: a randomized controlled trial. Scand J Med Sci Sports 2020;30:1675–84. 10.1111/sms.13702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Freedman RR, Ianni P, Wenig P. Behavioral treatment of Raynaud's phenomenon in scleroderma. J Behav Med 1984;7:343–53. 10.1007/BF00845268 [DOI] [PubMed] [Google Scholar]
- 48. Sandqvist G, Åkesson A, Eklund M. Evaluation of paraffin bath treatment in patients with systemic sclerosis. Disabil Rehabil 2004;26:981–7. 10.1080/09638280410001702405 [DOI] [PubMed] [Google Scholar]
- 49. Kusnanto K, Sari NPWP, Harmayetty H, et al. Self-care model application to improve self-care agency, self-care activities, and quality of life in patients with systemic lupus erythematosus. J Taibah Univ Med Sci 2018;13:472–8. 10.1016/j.jtumed.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martin MM. Effects of the myofascial release in diffuse systemic sclerosis. J Bodyw Mov Ther 2009;13:320–7. 10.1016/j.jbmt.2008.04.042 [DOI] [PubMed] [Google Scholar]
- 51. Horton R, Peterson MGE, Powell S, et al. Users evaluate lupusline a telephone peer counseling service. Arthritis Care Res 1997;10:257–63. 10.1002/art.1790100407 [DOI] [PubMed] [Google Scholar]
- 52. Young SP, Henderson E, Cheseldine DL, et al. Development and assessment of a world wide web site for systemic lupus erythematosus patient information. Lupus 2002;11:478–84. 10.1191/0961203302lu225oa [DOI] [PubMed] [Google Scholar]
- 53. Clarke-Jenssen A-C, Fredriksen PM, Lilleby V, et al. Effects of supervised aerobic exercise in patients with systemic lupus erythematosus: a pilot study. Arthritis & Rheumatism 2005;53:308–12. 10.1002/art.21082 [DOI] [PubMed] [Google Scholar]
- 54. Mitropoulos A, Gumber A, Crank H, et al. The effects of upper and lower limb exercise on the microvascular reactivity in limited cutaneous systemic sclerosis patients. Arthritis Res Ther 2018;20:112. 10.1186/s13075-018-1605-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Benatti FB, Miossi R, Passareli M, et al. The effects of exercise on lipid profile in systemic lupus erythematosus and healthy individuals: a randomized trial. Rheumatol Int 2015;35:61–9. 10.1007/s00296-014-3081-4 [DOI] [PubMed] [Google Scholar]
- 56. Perandini LA, Sales-de-Oliveira D, Mello SBV, et al. Exercise training can attenuate the inflammatory milieu in women with systemic lupus erythematosus. J Appl Physiol 2014;117:639–47. 10.1152/japplphysiol.00486.2014 [DOI] [PubMed] [Google Scholar]
- 57. Oliveira NC, dos Santos Sabbag LM, de Sá Pinto AL, et al. Aerobic exercise is safe and effective in systemic sclerosis. Int J Sports Med 2009;30:728–32. 10.1055/s-0029-1224180 [DOI] [PubMed] [Google Scholar]
- 58. Reis LK, Trevisani VF. Aerobic Exercise on Improving Sleep in Systemic Lupus Erythematosus. (EFEXO) [ClinicalTrials.gov identifier: NCT02037971]: ClinicalTrials.gov 2014.
- 59. Greco CM. Reducing Depressive Symptoms in Systemic Lupus Erythematosus [ClinicalTrials.gov identifier: NCT01120652]: ClinicalTrials.gov 2020.
- 60. Sheffield Hallam University . The Effects of Exercise in Patients With Systemic Sclerosis [ClinicalTrials.gov identifier: NCT03058887]: ClinicalTrials.gov 2020.
- 61. Thombs BD, Dyas L, Pépin M, et al. Scleroderma patient-centered intervention Network-Scleroderma support group leader education (SPIN-SSLED) program: non-randomised feasibility trial. BMJ Open 2019;9:e029935. 10.1136/bmjopen-2019-029935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bongi SM, Del Rosso A, Galluccio F, et al. Efficacy of connective tissue massage and Mc Mennell joint manipulation in the rehabilitative treatment of the hands in systemic sclerosis. Clin Rheumatol 2009;28:1167–73. 10.1007/s10067-009-1216-x [DOI] [PubMed] [Google Scholar]
- 63. McNearney TA, Sallam HS, Hunnicutt SE, et al. Prolonged treatment with transcutaneous electrical nerve stimulation (TENS) modulates neuro-gastric motility and plasma levels of vasoactive intestinal peptide (VIP), motilin and interleukin-6 (IL-6) in systemic sclerosis. Clin Exp Rheumatol 2013;31:140–50. [PubMed] [Google Scholar]
- 64. Choudhary D, Garg PK. Primary outcome in a randomized controlled trial: a critical issue. Saudi J Gastroenterol 2011;17:369. 10.4103/1319-3767.84504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stanley K. Design of randomized controlled trials. Circulation 2007;115:1164–9. 10.1161/CIRCULATIONAHA.105.594945 [DOI] [PubMed] [Google Scholar]
- 66. Neville C, Da Costa D, Rochon M, et al. Development of the lupus interactive navigator as an empowering web-based eHealth tool to facilitate lupus management: users perspectives on usability and acceptability. JMIR Res Protoc 2016;5:e44. 10.2196/resprot.4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Herschman J, Kasenberg T, Levy D, et al. Development of a smartphone app for adolescents with lupus: a collaborative meeting-based methodology inclusive of a wide range of stakeholders. Rev Panam Salud Publica 2014;35:471–6. [PubMed] [Google Scholar]
- 68. Poole JL, Skipper B, Mendelson C. Evaluation of a mail-delivered, print-format, self-management program for persons with systemic sclerosis. Clin Rheumatol 2013;32:1393–8. 10.1007/s10067-013-2282-7 [DOI] [PubMed] [Google Scholar]
- 69. Poole JL, Mendelson C, Skipper B, et al. Taking charge of systemic sclerosis: a pilot study to assess the effectiveness of an Internet self-management program. Arthritis Care Res 2014;66:778–82. 10.1002/acr.22192 [DOI] [PubMed] [Google Scholar]
- 70. Piga M, Tradori I, Pani D, et al. Telemedicine applied to kinesiotherapy for hand dysfunction in patients with systemic sclerosis and rheumatoid arthritis: recovery of movement and telemonitoring technology. J Rheumatol 2014;41:1324–33. 10.3899/jrheum.130912 [DOI] [PubMed] [Google Scholar]
- 71. Mouthon L, Thombs BD. Scleroderma Patient-centered Intervention Network (SPIN) Hand Program (SPIN-HAND) [ClinicalTrials.gov identifier: NCT03419208]: ClinicalTrials.gov 2020.
- 72. Carrier M-E, Kwakkenbos L, Nielson WR, et al. The scleroderma patient-centered intervention network self-management program: protocol for a randomized feasibility trial. JMIR Res Protoc 2020;9:e16799. 10.2196/16799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Keramiotou K, Anagnostou C, Kataxaki E, et al. The impact of upper limb exercise on function, daily activities and quality of life in systemic lupus erythematosus: a pilot randomised controlled trial. RMD Open 2020;6:e001141. 10.1136/rmdopen-2019-001141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Landim SF, Bertolo MB, Marcatto de Abreu MF, et al. The evaluation of a home-based program for hands in patients with systemic sclerosis. J Hand Ther 2019;32:313–21. 10.1016/j.jht.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 75. F Landim S, B Bertolo M, Del Rio AP, et al. Sustained efficacy of a Concise self-management programme for hands in systemic sclerosis: a longitudinal case-control observational study. Rheumatology 2020;59:3330–9. 10.1093/rheumatology/keaa140 [DOI] [PubMed] [Google Scholar]
- 76. Sheikh SZ, Kaufman K, Gordon B-B, et al. Evaluation of the self-directed format of walk with ease in patients with systemic lupus erythematosus: the Walk-SLE pilot study. Lupus 2019;28:764–70. 10.1177/0961203319846387 [DOI] [PubMed] [Google Scholar]
- 77. Alexanderson H, Boström C. Exercise therapy in patients with idiopathic inflammatory myopathies and systemic lupus erythematosus - A systematic literature review. Best Pract Res Clin Rheumatol 2020;34:101547. 10.1016/j.berh.2020.101547 [DOI] [PubMed] [Google Scholar]
- 78. Ritschl V, Stamm TA, Aletaha D, et al. Prevention, screening, assessing and managing of non-adherent behaviour in people with rheumatic and musculoskeletal diseases: systematic reviews Informing the 2020 EULAR points to consider. RMD Open 2020;6:e001432. 10.1136/rmdopen-2020-001432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ritschl V, Lackner A, Boström C, et al. I do not want to suppress the natural process of inflammation: new insights on factors associated with non-adherence in rheumatoid arthritis. Arthritis Res Ther 2018;20:234. 10.1186/s13075-018-1732-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ritschl V, Stamm TA, Aletaha D, et al. 2020 EULAR points to consider for the prevention, screening, assessment and management of non-adherence to treatment in people with rheumatic and musculoskeletal diseases for use in clinical practice. Ann Rheum Dis 2020:annrheumdis-2020-218986. 10.1136/annrheumdis-2020-218986 [DOI] [PubMed] [Google Scholar]
- 81. Navarrete-Navarrete N, Peralta-Ramírez MI, Sabio-Sánchez JM, et al. Efficacy of cognitive behavioural therapy for the treatment of chronic stress in patients with lupus erythematosus: a randomized controlled trial. Psychother Psychosom 2010;79:107–15. 10.1159/000276370 [DOI] [PubMed] [Google Scholar]
- 82. Karlson EW, Liang MH, Eaton H, et al. A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis Rheum 2004;50:1832–41. 10.1002/art.20279 [DOI] [PubMed] [Google Scholar]
- 83. Maisiak R, Austin JS, West SG, et al. The effect of person-centered counseling on the psychological status of persons with systemic lupus erythematosus or rheumatoid arthritis: a randomized, controlled trial. Arthritis Care Res 1996;9:60–6. 10.1002/art.1790090111 [DOI] [PubMed] [Google Scholar]
- 84. Li LC, Feehan LM, Xie H, et al. Efficacy of a physical activity counseling program with use of a wearable Tracker in people with inflammatory arthritis: a randomized controlled trial. Arthritis Care Res 2020;72:1755–65. 10.1002/acr.24199 [DOI] [PubMed] [Google Scholar]
- 85. Ng P, Chan W. Group psychosocial program for enhancing psychological well-being of people with systemic lupus erythematosus. J Soc Work Disabil Rehabil 2007;6:75–87. 10.1300/J198v06n03_05 [DOI] [PubMed] [Google Scholar]
- 86. Rimmer JH, Wang E, Pellegrini CA. Telehealth weight management intervention for adults with physical disabilities: a randomized controlled trial. Am J Phys Med Rehabil 2013;92:1084–94. 10.1097/PHM.0b013e31829e780e [DOI] [PubMed] [Google Scholar]
- 87. Boström C, Elfving B, Dupré B, et al. Effects of a one-year physical activity programme for women with systemic lupus erythematosus - a randomized controlled study. Lupus 2016;25:602–16. 10.1177/0961203315622817 [DOI] [PubMed] [Google Scholar]
- 88. Avaux M, Hoellinger P, Nieuwland-Husson S, et al. Effects of two different exercise programs on chronic fatigue in lupus patients. Acta Clin Belg 2016;71:403–6. 10.1080/17843286.2016.1200824 [DOI] [PubMed] [Google Scholar]
- 89. Tench CM, McCarthy J, McCurdie I, et al. Fatigue in systemic lupus erythematosus: a randomized controlled trial of exercise. Rheumatology 2003;42:1050–4. 10.1093/rheumatology/keg289 [DOI] [PubMed] [Google Scholar]
- 90. Edworthy SM, Dobkin PL, Clarke AE, et al. Group psychotherapy reduces illness intrusiveness in systemic lupus erythematosus. J Rheumatol 2003;30:1011–6. [PubMed] [Google Scholar]
- 91. Haupt M, Millen S, Jänner M, et al. Improvement of coping abilities in patients with systemic lupus erythematosus: a prospective study. Ann Rheum Dis 2005;64:1618–23. 10.1136/ard.2004.029926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Uras C, Mastroeni S, Tabolli S, et al. A comparison between two educational methods in the rehabilitation of the microstomia in systemic sclerosis: a randomized controlled trial. Clin Rehabil 2019;33:1747–56. 10.1177/0269215519858395 [DOI] [PubMed] [Google Scholar]
- 93. Zanatta E, Rodeghiero F, Pigatto E, et al. Long-Term improvement in activities of daily living in women with systemic sclerosis attending occupational therapy. Br J Occup Ther 2017;80:417–22. 10.1177/0308022617698167 [DOI] [Google Scholar]
- 94. Rannou F, Boutron I, Mouthon L, et al. Personalized physical therapy versus usual care for patients with systemic sclerosis: a randomized controlled trial. Arthritis Care Res 2017;69:1050–9. 10.1002/acr.23098 [DOI] [PubMed] [Google Scholar]
- 95. Pizzo G, Scardina GA, Messina P. Effects of a nonsurgical exercise program on the decreased mouth opening in patients with systemic scleroderma. Clin Oral Investig 2003;7:175–8. 10.1007/s00784-003-0216-5 [DOI] [PubMed] [Google Scholar]
- 96. Maddali-Bongi S, Landi G, Galluccio F, et al. The rehabilitation of facial involvement in systemic sclerosis: efficacy of the combination of connective tissue massage, Kabat's technique and kinesitherapy: a randomized controlled trial. Rheumatol Int 2011;31:895–901. 10.1007/s00296-010-1382-9 [DOI] [PubMed] [Google Scholar]
- 97. Kristensen LQ, Oestergaard LG, Bovbjerg K, et al. Use of paraffin instead of Lukewarm water prior to hand exercises had no additional effect on hand mobility in patients with systemic sclerosis: a randomized clinical trial. Hand Therapy 2019;24:13–21. 10.1177/1758998318824346 [DOI] [Google Scholar]
- 98. Gregory WJ, Wilkinson J, Herrick AL. A randomised controlled trial of wax baths as an additive therapy to hand exercises in patients with systemic sclerosis. Physiotherapy 2019;105:370–7. 10.1016/j.physio.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 99. Horváth J, Bálint Z, Szép E, et al. Efficacy of intensive hand physical therapy in patients with systemic sclerosis. Clin Exp Rheumatol 2017;35(Suppl 106):159–66. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-001710supp001.pdf (71.6KB, pdf)
rmdopen-2021-001710supp002.pdf (149.7KB, pdf)
rmdopen-2021-001710supp003.pdf (168.6KB, pdf)
rmdopen-2021-001710supp004.pdf (472.6KB, pdf)
rmdopen-2021-001710supp005.pdf (483.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request.